Abstract
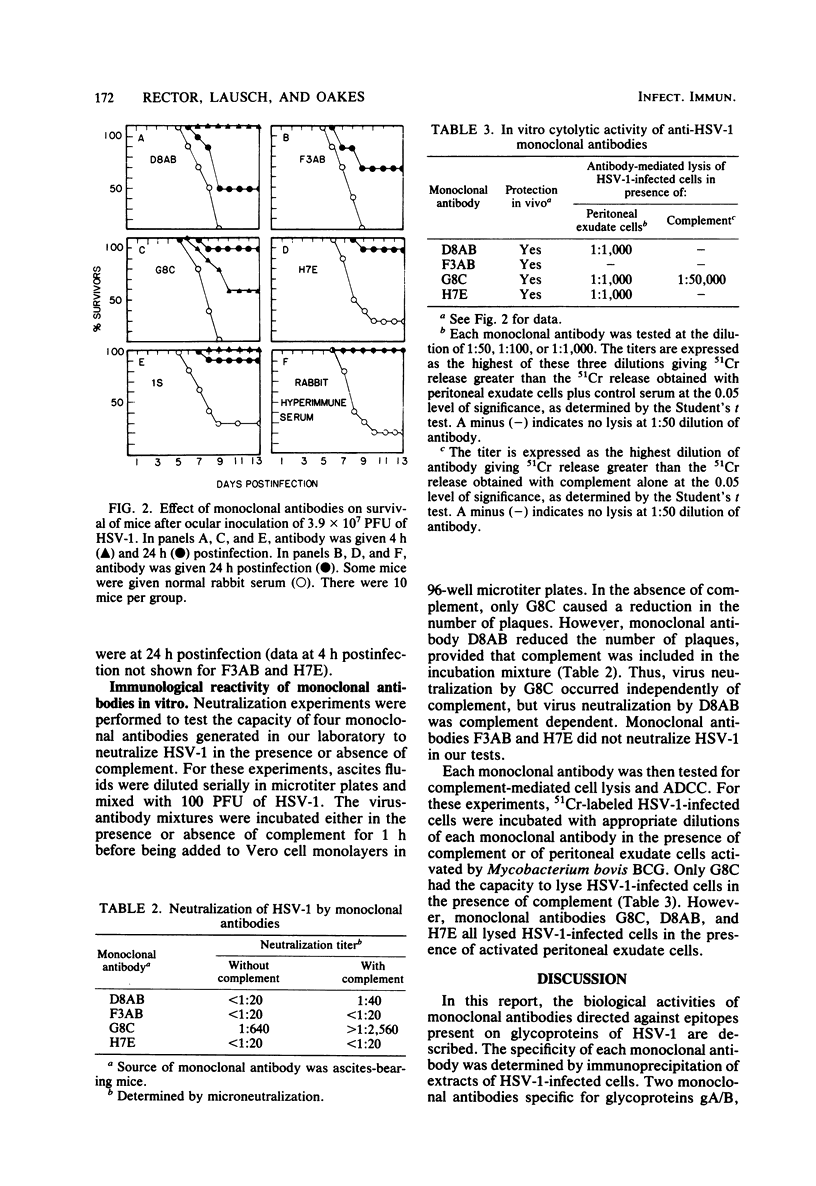
Monoclonal antibodies specific for the five major glycoproteins of herpes simplex virus type 1 (HSV-1) were tested for their capacity to mediate immunity to ocular HSV-1 infection. The specificity of the immunoglobulin made by each monoclone was determined by immunoprecipitation of [14C]glucosamine-labeled polypeptides from detergent-solubilized HSV-1-infected cells. Of the five monoclonal antibodies studied, two immunoprecipitated glycoproteins gA/B, one immunoprecipitated glycoprotein gC, one immunoprecipitated glycoprotein gD, and one immunoprecipitated glycoprotein gE. All five were effective in passively transferring immunity to mice when they were given 4 to 24 h after HSV-1 infection on an abraded cornea. Four of the monoclonal antibodies were also evaluated for their capacity to neutralize HSV-1 and to promote complement-mediated cell lysis and antibody-dependent cellular cytotoxicity. It was found that none of these in vitro assays correlated with the protective activity of the antibodies in vivo. In fact, one of the monoclonal antibodies was unreactive in all three immunological reactions, even though it was highly effective in promoting recovery from HSV-1 induced ocular disease in vivo. The results suggest that antibodies can interact in vivo with virus-specific glycoproteins gA/B, gC, gD, and gE to initiate recovery from HSV-1-induced ocular disease, and that the therapeutic effectiveness of a specific monoclonal antibody does not correlate with its immunological reactivity in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baucke R. B., Spear P. G. Membrane proteins specified by herpes simplex viruses. V. Identification of an Fc-binding glycoprotein. J Virol. 1979 Dec;32(3):779–789. doi: 10.1128/jvi.32.3.779-789.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brier A. M., Wohlenberg C., Rosenthal J., Mage M., Notkins A. L. Inhibition or enhancement of immunological injury of virus-infected cells. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3073–3077. doi: 10.1073/pnas.68.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Katze M., Hydrean-Stern C., Eisenberg R. J. Type-common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular-weight envelope glycoprotein. J Virol. 1978 Jul;27(1):172–181. doi: 10.1128/jvi.27.1.172-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis W. B., Taylor J. A., Oakes J. E. Ocular infection with herpes simplex virus type 1: prevention of acute herpetic encephalitis by systemic administration of virus-specific antibody. J Infect Dis. 1979 Oct;140(4):534–540. doi: 10.1093/infdis/140.4.534. [DOI] [PubMed] [Google Scholar]
- Dix R. D., Pereira L., Baringer J. R. Use of monoclonal antibody directed against herpes simplex virus glycoproteins to protect mice against acute virus-induced neurological disease. Infect Immun. 1981 Oct;34(1):192–199. doi: 10.1128/iai.34.1.192-199.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. Preparation and characterization of specific antisera to individual glycoprotein antigens comprising the major glycoprotein region of herpes simplex virus type 1. J Virol. 1980 Sep;35(3):902–917. doi: 10.1128/jvi.35.3.902-917.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle R., Courtney R. J. gA and gB glycoproteins of herpes simplex virus type 1: two forms of a single polypeptide. J Virol. 1980 Dec;36(3):665–675. doi: 10.1128/jvi.36.3.665-675.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Ponce de Leon M., Cohen G. H. Comparative structural analysis of glycoprotein gD of herpes simplex virus types 1 and 2. J Virol. 1980 Aug;35(2):428–435. doi: 10.1128/jvi.35.2.428-435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Pathogenesis of herpetic encephalitis in mice after ophthalmic inoculation. J Infect Dis. 1974 Jul;130(1):16–27. doi: 10.1093/infdis/130.1.16. [DOI] [PubMed] [Google Scholar]
- Kohl S., Cahall D. L., Walters D. L., Schaffner V. E. Murine antibody-dependent cellular cytotoxicity to herpes simplex virus-infected target cells. J Immunol. 1979 Jul;123(1):25–30. [PubMed] [Google Scholar]
- Kohl S., Loo L. S., Pickering L. K. Protection of neonatal mice against herpes simplex viral infection by human antibody and leukocytes from adult, but not neonatal humans. J Immunol. 1981 Oct;127(4):1273–1275. [PubMed] [Google Scholar]
- Liddell F. D. Evaluation of survival in challenge experiments. Microbiol Rev. 1978 Mar;42(1):237–249. doi: 10.1128/mr.42.1.237-249.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machtiger N. A., Pancake B. A., Eberle R., Courtney R. J., Tevethia S. S., Schaffer P. A. Herpes simplex virus glycoproteins: isolation of mutants resistant to immune cytolysis. J Virol. 1980 May;34(2):336–346. doi: 10.1128/jvi.34.2.336-346.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Davis W. B., Taylor J. A., Weppner W. A. Lymphocyte reactivity contributes to protection conferred by specific antibody passively transferred to herpes simplex virus-infected mice. Infect Immun. 1980 Aug;29(2):642–649. doi: 10.1128/iai.29.2.642-649.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes J. E., Lausch R. N. Role of Fc fragments in antibody-mediated recovery from ocular and subcutaneous herpes simplex virus infections. Infect Immun. 1981 Jul;33(1):109–114. doi: 10.1128/iai.33.1.109-114.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Baucke R. B., Spear P. G. Glycoprotein gE of herpes simplex virus type 1: effects of anti-gE on virion infectivity and on virus-induced fc-binding receptors. J Virol. 1982 Jan;41(1):129–136. doi: 10.1128/jvi.41.1.129-136.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Para M. F., Goldstein L., Spear P. G. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J Virol. 1982 Jan;41(1):137–144. doi: 10.1128/jvi.41.1.137-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Dondero D., Norrild B., Roizman B. Differential immunologic reactivity and processing of glycoproteins gA and gB of herpes simplex virus types 1 and 2 made in Vero and HEp-2 cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5202–5206. doi: 10.1073/pnas.78.8.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather S. O., Geller R. W., Lausch R. N. Inability of antiserum active in antibody-dependent cellular cytotoxicity and arming tests to protect against simian virus 40 tumor cell challenge. J Natl Cancer Inst. 1979 May;62(5):1273–1277. [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. S., Hayden M., Langley S., Kaliss N., Smith M. R. Antibody-mediated suppression of grafted lymphoma. III. Evaluation of the role of thymic function, non-thymus-derived lymphocytes, macrophages, platelets, and polymorphonuclear leukocytes in syngeneic and allogeneic hosts. J Immunol. 1975 Apr;114(4):1255–1263. [PubMed] [Google Scholar]
- Shore S. L., Black C. M., Melewicz F. M., Wood P. A., Nahmias A. J. Antibody-dependent cell-mediated cytotoxicity to target cells infected with type 1 and type 2 herpes simplex virus. J Immunol. 1976 Jan;116(1):194–201. [PubMed] [Google Scholar]
- Shore S. L., Nahmias A. J., Starr S. E., Wood P. A., McFarlin D. E. Detection of cell-dependent cytotoxic antibody to cells infected with herpes simplex virus. Nature. 1974 Sep 27;251(5473):350–352. doi: 10.1038/251350a0. [DOI] [PubMed] [Google Scholar]
- Showalter S. D., Zweig M., Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981 Dec;34(3):684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]