Abstract
Given an ever-increasing risk of nuclear and radiological emergencies, there is a critical need for development of medical radiation countermeasures (MRCs) that are safe, easily administered, and effective in preventing and/or mitigating the potentially lethal tissue damage caused by acute high-dose radiation exposure. Because the efficacy of MRCs for this indication cannot be ethically tested in humans, development of such drugs is guided by the Food and Drug Administration's Animal Efficacy Rule. According to this rule, human efficacious doses can be projected from experimentally established animal efficacious doses based on the equivalence of the drug's effects on efficacy biomarkers in the respective species. Therefore, identification of efficacy biomarkers is critically important for drug development under the Animal Efficacy Rule. CBLB502 is a truncated derivative of the Salmonella flagellin protein that acts by triggering Toll-like receptor 5 (TLR5) signaling and is currently under development as a MRC. Here, we report identification of two cytokines, granulocyte colony-stimulating factor (G-CSF) and interleukin-6 (IL-6), as candidate biomarkers of CBLB502's radioprotective/mitigative efficacy. Induction of both G-CSF and IL-6 by CBLB502 1) is strictly TLR5-dependent, 2) occurs in a CBLB502 dose-dependent manner within its efficacious dose range in both nonirradiated and irradiated mammals, including nonhuman primates, and 3) is critically important for the ability of CBLB502 to rescue irradiated animals from death. After evaluation of CBLB502 effects on G-CSF and IL-6 levels in humans, these biomarkers will be useful for accurate prediction of human efficacious CBLB502 doses, a key step in the development of this prospective radiation countermeasure.
Introduction
Because of nuclear proliferation and terrorist activity, there is an ever-increasing risk of military, civilian, and emergency responder exposure to lethal doses of ionizing radiation. The lethality of high-dose total body irradiation (TBI) is caused by acute radiation syndrome (ARS), which is caused by massive apoptotic death of radiosensitive cells predominantly of the hematopoietic (HP) (starting at lower TBI doses) and gastrointestinal (joining at higher TBI doses) systems (Waselenko et al., 2004).
A number of compounds with diverse chemical structures and mechanisms of action have been explored as MRCs, including thiols (Grdina et al., 2002), cytokines (Waddick et al., 1991; Singh and Yadav, 2005), steroids (Whitnall et al., 2001), antioxidants (Weiss and Landauer, 2000), and nutraceuticals (Srinivasan and Weiss, 1992; Weiss and Landauer, 2000); however, to date none have been approved by the FDA as an MRC against ARS (Mettler and Voelz, 2002). To identify novel countermeasures against radiation and other tissue-damaging stresses, we looked toward the modulation of nuclear factor κ-B (NF-κB), a transcriptional activator of multiple genes encoding: 1) antiapoptotic proteins (for review, see Karin and Lin, 2002), 2) cytokines and growth factors that induce proliferation and survival of HP and other stem cells (Hayden et al., 2006), and 3) reactive oxygen species-scavenging antioxidants (Xu et al., 1999). To obtain NF-κB activators, we took advantage of natural factors produced by microbes of the human microflora that activate NF-κB via the stimulation of specific Toll-like receptors (TLRs). The TLR5 agonist flagellin was of particular interest because: 1) TLR5 activation is well tolerated in mammals (Takeda et al., 2003; Rakoff-Nahoum et al., 2004); 2) TLR5 is expressed in the HP and gastrointestinal tissues that are primary targets of radiation damage (Gewirtz et al., 2001; Maaser et al., 2004; Caron et al., 2005; Honko and Mizel, 2005; Tsujimoto et al., 2005; Uematsu et al., 2006; He et al., 2009; van den Berk et al., 2009; Dearman et al., 2009); and 3) NF-κB activation through flagellin/TLR5 (as opposed to some other activators) does not elicit potentially dangerous inflammatory responses but rather protects against them (Vijay-Kumar et al., 2008; Carvalho et al., 2011).
After demonstration of flagellin-mediated radioprotection, these considerations led us to generate CBLB502, a proprietary pharmacologically optimized derivative of Salmonella FliC flagellin that fully retains its TLR5-dependent NF-κB-inducing activity and stability, but is substantially less immunogenic (Burdelya et al., 2008). CBLB502 was found to be safe and highly effective in reducing the risk of death after TBI in animal models (Burdelya et al., 2008) and is currently under development as an MRC under the FDA's Animal Efficacy Rule (http://www.gpo.gov/fdsys/pkg/CFR-2011-title21-vol5/xml/CFR-2011-title21-vol5-sec314-610.xml). This rule guides the development of drugs for which efficacy testing in humans would be unethical. Without human efficacy data, definition of a (projected) human efficacious dose requires determination of 1) the efficacious dose range in animal models of the disease (i.e., ARS), and 2) pharmacokinetic (PK) and/or pharmacodynamic (PD) effects of the drug in healthy and diseased (i.e., irradiated) animals and healthy humans. The identified PK/PD parameters are then suggested to be used for selection of a human dose bioequivalent to animal efficacious doses.
Although PK parameters might be acceptable for demonstrating bioequivalence between small molecules, for biologics such as CBLB502, PD parameters are more relevant. This is because CBLB502 PK assessments do not account for: 1) the activity of CBLB502-mobilized endogenous mediators, 2) the effects of drug distribution after intramuscular injection via the lymphatic system in addition to the bloodstream (Crommelin et al., 2003) where drug concentration is usually measured (although some TLR5-expressing CBLB502-responsive cells, e.g., dendritic cells, are likely to be activated by the drug in this way), 3) possible species-specific differences in ligand-receptor affinity, presence of accessory proteins, receptor density, number and distribution of receptor-expressing cells, postreceptor signal amplification, etc., and 4) NF-κB- and TLR5-regulated negative feedback loops (Fukao and Koyasu, 2003; Shih et al., 2009), which could make cells temporarily unresponsive to CBLB502 and alter the effects of the drug despite its presence in the blood.
Because CBLB502 PD effects are likely to be more accurate than PK parameters for the establishment of interspecies effective dose equivalence, we set out to identify CBLB502 PD biomarkers by fitting the following criteria that we considered important for complying with Animal Efficacy Rule requirements:
Biomarker induction should depend on the drug's mechanism of action (i.e., CBLB502-TLR5 interaction).
Biomarkers should be induced by the drug under both irradiated and nonirradiated conditions.
Biomarker expression should be induced by a range of CBLB502 doses that shows efficacy in reducing the risk of death after TBI, and the biomarker dose response should be congruent with the survival dose response.
The biological nature of the biomarkers should be relevant to the effect of CBLB502 on reducing the risk of death after TBI.
Biomarkers should be CBLB502-responsive across multiple species (animals and humans).
Biomarkers should be quantifiable by using readily available assays, in samples obtainable by simple and relatively noninvasive procedures.
Our previous animal studies demonstrated CBLB502-mediated induction of multiple cytokines (Burdelya et al., 2008). Because they are easily measurable and have relevant functions (Dainiak, 2010), cytokines are attractive candidates for CBLB502 efficacy biomarkers. Here, we demonstrate that two CBLB502-induced cytokines, G-CSF and IL-6, comply with all of the criteria described above and, hence, after confirmation in humans, can potentially serve as CBLB502's efficacy biomarkers in its development under the Animal Efficacy Rule.
Materials and Methods
CBLB502 Drug Substance and Vehicle
CBLB502 was expressed in Escherichia coli and purified to >98% purity (at SynCo BioPartners, LLC, Amsterdam, The Netherlands) by using a validated cGMP process involving two-step (ion-exchange and hydrophobic interaction) chromatographic purification followed by a dedicated ion exchange endotoxin removal column. Release testing ensures <100 EU/mg endotoxin, <5 ng/mg residual DNA, and <100 ng/mg host cell protein content in the CBLB502 drug product. The lack of additional contaminating TLR ligands was confirmed by using specific TLR-expressing cell lines. The vehicle for CBLB502 was Dulbecco's phosphate-buffered saline (Cleveland Clinic Media Core, Cleveland, OH) in initial studies or phosphate-buffered saline-0.1% Tween 80 (O'Brien Pharmacy, Mission, KS) in later studies.
Neutralizing Antibodies
Monoclonal rat anti-mouse G-CSF antibody, monoclonal rat anti-mouse IL-6 antibody, and rat isotype control IgG1 antibody were obtained from R&D Systems (Minneapolis, MN).
Animals
Mice.
Female ICR (Harlan, Indianapolis, IN) and C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME), 12 to 15 weeks old, were used in all experiments. Before experiment initiation, mice were quarantined for at least 3 days. All mouse experiments were conducted at Roswell Park Cancer Institute.
Nonhuman Primates.
For studies conducted at Armed Forces Radiobiology Research Institute (AFRRI) (one study in nonirradiated NHPs and one study in NHPs irradiated with 5 Gy of TBI and injected with CBLB502 at 45 min before TBI), healthy naive rhesus macaques were obtained from Primate Products, Inc. (Miami, FL) and quarantined for 6 weeks before the start of the experiment. Clinically healthy male and female rhesus macaques, Macaca mulatta, 2 to 5 years of age, weighing 3.8 to 7.0 kg, were housed in individual stainless-steel cages in environmentally controlled rooms set to be maintained at 18 to 29°C with a relative humidity of 30 to 70%. All NHPs were kept in rooms with a 12-h light/dark cycle. Animals were fed one or two times daily with primate diet (Harlan Teklad, Madison, WI) and fresh fruits and vegetables, and they obtained drinking water ad libitum. All animals were seronegative for herpes B, simian T-cell leukemia viruses, simian immunodeficiency virus, simian type D retrovirus, and intestinal parasites and were also negative in purified protein derivative, tuberculin tests. Animals were stratified by gender and body weight increases during the quarantine period and then assigned to different treatment groups.
For studies conducted at Frontier Biosciences (Chengdu, Sichuan Province, China), a contract research laboratory (one study in nonirradiated NHPs and two studies in NHPs irradiated with 5.2–5.75 Gy of TBI and injected with different CBLB502 doses 1 or 25 h later), healthy rhesus macaques were obtained from Ping'an Animal Breeding and Research Base (Chengdu, Sichuan Province, China). Clinically healthy male and female rhesus macaques, weighing 3 to 7 kg (2–5 years of age), were certified to be free of specific pathogenic microorganisms [such as Salmonella sp., Shigella sp., Mycobacterium tuberculosis, cercopithecine herpesvirus type I (B virus), and Toxoplasma gondii)] and received helminthicide treatment at the breeding facility. Animals were quarantined and tested for at least 3 weeks before studies. All animals were housed in individual stainless-steel cages in environment-controlled rooms, with room temperature of 21 ± 5°C, relative humidity of 55 ± 15%, and a 12-h light/dark cycle. Animals were provided with primate chow (prepared in-house) and fresh fruits and vegetables daily. Fresh drinking water was provided ad libitum. Upon completion of the prestudy period, animals meeting predefined health criteria were accepted into the study and randomly assigned to study groups based on their most recent body weights (even distribution per gender per weight).
Dogs.
Dog studies were conducted at Pharmaron Inc. (Beijing, China), a contract research organization. Healthy naive male and female beagle dogs (approximately 8–10 months of age and 5.5–10.5 kg weight) were obtained from Beijing Marshall Biotechnology, Co. LTD (Beijing, China). All animals received vaccinations against canine distemper, hepatitis, leptospirosis, parvovirus, parainfluenza, and rabies at the supplier. Animals were quarantined and tested for at least 14 days before study initiation. All animals were housed in single stainless-steel cages in environment-controlled rooms, with room temperature of 18 to 29°C, relative humidity of 50 ± 20%, and a 12-h light-dark alternation daily. Animals were provided once daily with ∼300 g of canine diet (Science Australia United Efforts Inc., Beijing, China). Fresh drinking water was provided ad libitum. Upon completion of the prestudy period, animals meeting predefined health criteria were accepted into the study and randomly assigned to study groups based on their most recent body weights (even distribution per gender per weight).
All animal studies described above (at Roswell Park Cancer Institute, AFRRI, Frontier Biosciences, and Pharmaron Inc.) were performed in Association for Assessment and Accreditation of Laboratory Animal Care International-certified facilities, based on protocols approved by the respective Institutional Animal Care and Use Committees and in compliance with the requirements of the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Injections
Injections in mice were performed either intramuscularly in the thigh muscle in a 0.05 ml volume or subcutaneously in a 0.2-ml volume in the scruff of the neck. Injections in NHPs and dogs were given in the quadriceps muscle by using a dose volume of 0.2 ml/kg.
Irradiation
Mice.
Total body gamma irradiation of mice was accomplished by using 137Cs Mark I-30 or Mark I-68 irradiators (J.L. Shepherd and Associates, San Fernando, CA) with dose rates of ∼2.2 or ∼1.4 Gy/min. Mice were irradiated in a nonshielding plastic container on a rotating platform to ensure even dose delivery to all tissues.
Nonhuman Primates.
In irradiation experiments conducted at AFRRI, ketamine-anesthetized animals (10 mg/kg i.m.; Fort Dodge Laboratories; Fort Dodge, IA) were placed in a Plexiglas restraint box (GE Plastics, Pittsfield, MA) to which they had been previously habituated and allowed to regain consciousness before irradiation. Animals were irradiated bilaterally at a dose rate of approximately 0.6 Gy/min to a total midplane dose of 5.0 Gy, by using the 60Co γ-ray source located at AFRRI. To ensure the uniformity of radiation dose among groups, abdominal circumference measurements were used to calculate the thickness of the animal to the midplane to determine exposure time to the radiation source. Dosimetry was performed by using an alanine/electron paramagnetic resonance system, with calibration factors traceable to the National Institute of Standards and Technology (Gaithersburg, MD) and confirmed by an additional check against the national standard 60Co source of the UK National Physics Laboratory (Middlesex, UK).
In irradiation experiments conducted at Frontier Biosciences, animals received 25 mg/kg pentobarbital sodium in the animal room to achieve mild anesthesia before transportation and an additional 8 to 10 mg/kg ketamine injection in transit to the irradiation facility. Sedated animals were restrained in plastic irradiation chairs. Animals were irradiated bilaterally by using the 60Co γ-ray source located at the Sichuan Atomic Energy Institute (Chengdu, Sichuan Province, China). In the first study, the dose rate was ∼0.8 Gy/min, and animals received a total surface dose of 5.2 Gy (approximately equivalent to a midplane dose of 5.0 Gy for ∼3- to 4-kg animals). In a second study after source upgrade, the dose rate was ∼1.0 Gy/min, and animals received a total surface dose of 5.75 Gy (approximately equivalent to a midplane dose of 5.3 Gy for ∼3- to 4-kg animals). Individual animal dosimetry was performed by using thermoluminescent dosimeter sets provided and evaluated by Global Dosimetry Solutions, Inc. (Irvine, CA). Calibration of the radiation field of the source was performed periodically based on the Chinese National Standard [standard method for using the ferrous sulfate (Fricke) dosimeter to measure absorbed dose in water].
Blood Collection for Cytokine Level Measurements
Mice.
Blood was collected via cardiac puncture from three to six mice per time point (freshly euthanized by CO2 inhalation or deeply anesthetized by using ketamine/xylazine).
Nonhuman Primates.
Peripheral blood from NHPs was drawn from ketamine-anesthetized (AFRRI) or nonanesthetized, briefly restrained (Frontier Biosciences) animals, via a saphenous vein.
Dogs.
Peripheral blood was collected via cephalic vein from nonanesthetized, briefly held animals.
For all three animal species, blood was collected with K2EDTA as an anticoagulant and processed to prepare plasma. Blood plasma samples were aliquoted and stored at −80°C until analysis.
Analysis of Plasma Cytokine Levels
Mice.
Cytokine levels in plasma samples from earlier ICR and all C57BL/6J experiments were measured at AFRRI by Luminex multiplex assays, using a Luminex-100 Multiplex Bio-Assay Analyzer or Luminex-200 dual-laser flow analyzer (Luminex Corporation, Austin, TX) according to the manufacturer's instructions. Calculations were performed by using Bio-Plex Manager software version 5.0 (Bio-Rad Laboratories, Hercules, CA). In earlier ICR mouse studies, multiplex assays from Bio-Rad Laboratories (Bio-Plex Pro Mouse Cytokine group 8-plex Assay) were used for analysis of IL-1β, IL-6, IL-10, IL-12p70, G-CSF, granulocyte-macrophage colony-stimulating factor (GM-CSF), keratinocyte chemoattractant (KC), and tumor necrosis factor α (TNF-α), and reagents from Invitrogen (Carlsbad, CA) were used for the measurement of interferon γ-induced protein 10kDa (IP-10). Stem cell factor (SCF) and thrombopoietin (TPO) levels were measured by using mouse enzyme-linked immunosorbent assay quantikine kits for SCF and TPO (R&D Systems) based on the multiple-antibody sandwich principle. For studies in C57BL/6J mice, levels of IL-6, IL-10, IL-12p40, G-CSF, KC, monocyte chemotactic protein-1 (MCP-1), TNF-α, monokine induced by interferon γ (MIG), and macrophage inflammatory protein-2 (MIP-2) were measured by using cytokine analysis kits custom-ordered from Bio-Rad Laboratories. Samples from later ICR mouse studies were evaluated at the Oklahoma Medical Research Foundation Proteomics Core (Oklahoma City, OK) by using a Luminex-100 Multiplex Bio-Assay Analyzer and Luminex assays for mouse G-CSF, IL-6, KC, IL-1α, IL-10, eotaxin, and TNF-α from Millipore Corporation (Billerica, MA). All assays were performed as recommended by the manufacturers.
Nonhuman Primates.
Plasma levels of cytokines in nonirradiated NHPs and NHPs treated with CBLB502 at 45 min before 5 Gy of irradiation were measured at AFRRI. Luminex multiplex assays were performed by using a Luminex-100 Multiplex Bio-Assay Analyzer. Human-targeted assays from Millipore Corporation were used to analyze IL-8, IL-12p70, and IP-10, whereas assays from R&D Systems were used to measure G-CSF, GM-CSF, IL-1β, IL-6, and IL-10 according to the manufacturers' instructions. The monkey C-reactive protein enzyme-linked immunosorbent assay kit (Alpha Diagnostics International Inc., San Antonio, TX) was used for C-reactive protein analysis according to the manufacturer's instructions.
Plasma cytokine levels in NHPs treated with CBLB502 at 1 h after 5.2 Gy of irradiation were measured at the Baylor Institute for Immunology Research (Dallas, TX), and in NHPs treated with CBLB502 at 25 h after 5.75 Gy of irradiation they were measured at the Millipore Corporation facility in St. Charles, MO. In both cases, Luminex Multiplex immunological assays were used (according to the manufacturer's directions). The MILLIPLEX MAP Nonhuman Primate Cytokine Panel (Millipore Corporation) was used for all cytokines with the exception of IL-10, which was analyzed by using an assay from R&D Systems. Luminex-100 Multiplex Bio-Assay Analyzer instruments were used for the analysis.
Dogs.
Levels of IL-6, IL-8, IL-10, and TNFα in beagle dog plasma were measured at the University of Illinois at Chicago Toxicology Research Laboratory (Chicago, IL) by using electrochemiluminescence technology from Meso Scale Discovery (Gaithersburg, MD) and a custom MULTI-SPOT 96 4-Spot Prototype assay kit that contained antibodies and standards also present in the Canine IL-10 Ultra-Sensitive Kit and the Canine ProInflammatory Panel 3 Ultra-Sensitive Kit (IL-6, IL-8, and TNFα) spotted on MULTI-SPOT 96-well 4 Spot Cytokine Panel 16 US Plates (all from Meso Scale Discovery, Gaithersburg, MD).
Data Analysis
Area under the Curve.
Area under the curve (AUC) values were calculated by using the trapezoid rule over the first 24 h after treatment (AUC(0–24)). To exclude the influence of basal cytokine levels, AUC(0–24) values were background-adjusted by subtracting the minimum observed factor concentration [for the same individual animal (large animals) or per sampling set within the same dose group (mice)] multiplied by 24 h.
Statistical Analysis.
p values <0.05 were considered to be statistically significant. Error bars in graphs represent S.D. Dashed lines in figures represent linear regression trend lines (in log-log coordinates).
Kaplan-Meier Curves.
Kaplan-Meier curves are shown to illustrate the kinetics of survival after irradiation. Numbers of survivors (at 30 days after irradiation) were compared pairwise by using Fisher's exact test (two-tailed). Kinetics of mortality (for decedents only) was compared among groups by using the log rank test (two-tailed). For analysis of the effect of CBLB502 treatment on survival, the natural logarithm of odds ratio of survival (odds of survival in the treatment group divided by that in control group) was chosen as the metric. The odds associated with a probability p were defined as p/(1 − p). For a group of size n with 100% survival, odds were defined as (n − 0.5)/n; for groups with 0% survival they were 0.5/n.
Sigmoid Curve Fitting and ED50 Calculation.
Dependence of CBLB502-induced increase in log odds of survival on drug dose was analyzed by using BioDataFit 1.02 (http://www.changbioscience.com/stat/ec50.html), using the four-parameter sigmoid regression model. The 50% efficacious dose (ED50) was calculated on the obtained four-parameter sigmoid model.
Results
Establishment of the Efficacious Dose Range of CBLB502 for Protection from and Mitigation of Acute Radiation Syndrome in Mice.
Our previous experiments demonstrated potent radioprotective effects of CBLB502 in both mice and NHPs (Burdelya et al., 2008). However, these experiments were conducted with a single drug dose that was most likely on its efficacy plateau. Therefore, to identify potential biomarkers of CBLB502 anti-ARS efficacy, it was first necessary to fully define its efficacious dose range. To simplify the logistics involved, such experiments were initially conducted in mice.
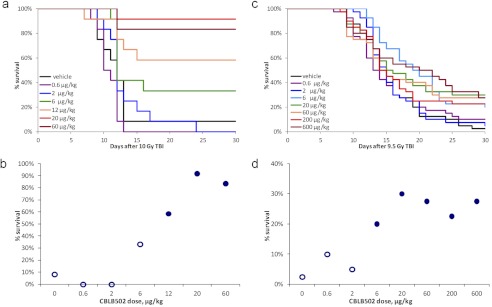
To establish the radioprotective dose range of CBLB502, groups of 10 to 20 female ICR mice were injected intramuscularly with a CBLB502 dose within a wide range (spanning more than two logs; 0.6–200 μg/kg), and, 30 min later, they were irradiated with a lethal (∼LD90–100/30) 10-Gy dose of TBI. Control mice were similarly irradiated but received a preirradiation injection of vehicle instead of CBLB502. Mouse survival was followed for 30 days. The experiment was repeated five times to explore various dose subranges and dose steps, with one representative study shown in Fig. 1, a and b. In the experiment shown in Fig. 1, a and b, only 8% of vehicle-injected control mice (1 of 12) survived to day 30 after 10 Gy of TBI, with the majority of deaths occurring between 10 and 14 days after irradiation. The minimal tested dose of CBLB502 that provided a sizeable survival advantage of +25% over control was 6 μg/kg, whereas injection of 20 and 60 μg/kg doses led to maximal survival of ∼83 to 92% (+75–83% survival increase over the vehicle-injected control group). The observed survival advantages were statistically significant at doses >6 μg/kg (p ≤ 0.03 by two-tailed Fisher's exact test for 30-day survival versus the vehicle-treated control group). As a result of this series of experiments, the dose at the beginning of the CBLB502 radioprotective efficacy plateau was defined as ∼20 μg/kg, and the ED50 was estimated as ∼7 μg/kg (Fig. 1, a and b).
Fig. 1.
Dose-dependent effect of CBLB502 on 30-day survival of lethally irradiated mice. a, Kaplan-Meier curves illustrating mouse survival for 30 days after TBI. Female ICR mice (12/group) were injected intramuscularly with vehicle or one of the indicated doses of CBLB502 and irradiated with 10 Gy of TBI 30 min later. b, CBLB502 dose response of 30-day survival in the same study as shown in a, with filled markers indicating statistically significant difference versus vehicle control group (p < 0.05 by two-tailed Fisher's exact test). c, Kaplan-Meier curves illustrating mouse survival for 30 days after TBI. Forty female C57BL/6J mice per group were irradiated with 9.5 Gy of TBI and injected subcutaneously with either vehicle or the indicated doses of CBLB502 24 h later. d, CBLB502 dose response of 30-day survival in the same study shown in c, with filled markers indicating statistically significant difference versus vehicle control group (p < 0.05 by two-tailed Fisher's exact test).
To establish the radiomitigative (efficacy when the drug is administered after TBI) dose range of CBLB502 in mice, we used subcutaneous instead of intramuscular injection to make the effect more robust, because the small muscle mass in mice resulted in high variability/poor reproducibility of results in the preceding single intramuscular dose pilot radiomitigation experiments. A representative experiment (of three that were conducted) is shown in Fig. 1, c and d. In this experiment, groups of 40 mice received a single lethal dose of 9 Gy of TBI, and then, 24 h later, a single subcutaneous injection of CBLB502 at doses spanning a three-log range (0.6–600 μg/kg) or vehicle (negative control group). The radiomitigative efficacy of CBLB502 under these conditions was clearly more modest than its radioprotective efficacy when injected 30 min before TBI (see above). As shown in Fig. 1, c and d, the maximal level of 30-day survival after 9.5 Gy of TBI was 30% (12 of 40, achieved with 20 μg/kg CBLB502 treatment) compared with 2.5% (1 of 40) in the vehicle-treated control group. Nevertheless, the observed CBLB502-induced survival increases were statistically significant for all CBLB502 doses ≥6 μg/kg (p ≤ 0.03 by Fisher's exact test for all of these groups versus the vehicle control group). Statistically significant improvement in mouse survival was observed with injection of CBLB502 24 h after 8.5- and 9.0-Gy doses of TBI as well (Supplemental Table 1). Similar to what was found for radioprotective (before TBI) administration of CBLB502, the CBLB502 dose at the beginning of its radiomitigative efficacy plateau was defined as 10 to 20 μg/kg, and the ED50 was estimated to be ∼2 to 4 μg/kg (Fig. 1, c and d). Administration of CBLB502 intravenously at 24 h after lethal TBI resulted in slightly greater increases in survival compared with subcutaneous administration at TBI doses of 8.5 to 9.5 Gy (Supplemental Table 1).
Strict TLR5 dependence of anti-ARS activity of CBLB502 and its parental protein, flagellin, was demonstrated by their inability to rescue TLR5-deficient mice from lethal TBI (Burdelya et al., 2008; Vijay-Kumar et al., 2008) (Supplemental Fig. 1). There were no major differences in the anti-ARS efficacy of CBLB502 between male and female mice of either strain.
Identification of G-CSF and IL-6 as Two Cytokines That Show Strong, Dose-Dependent Responses to CBLB502 in Nonirradiated Mice.
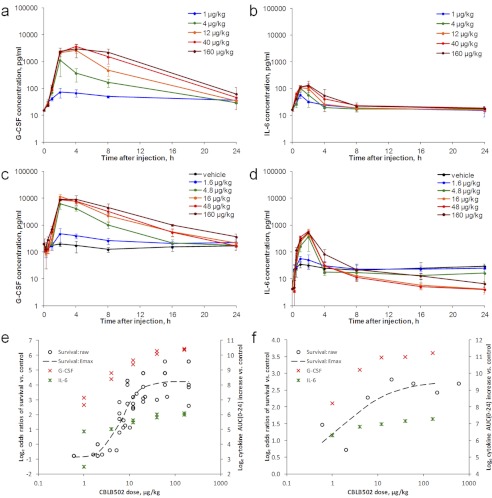
Having defined the efficacious dose range for CBLB502-mediated radioprotection and radiomitigation in mice, we next evaluated cytokine responses in nonirradiated mice treated with CBLB502 doses within this dose range. These experiments were conducted in two mouse strains that were used in previous irradiation survival experiments, ICR and C57BL/6J, and assessed plasma levels of G-CSF, IL-6, and other factors (KC) in all studies, as well as interleukin-1β (IL-1β), IL-10, IL-12p40, IL-12p70, IP-10, GM-CSF, TNFα, TPO, SCF, MCP-1, MIP-2, and MIG in some studies at 0 (noninjected animals), 0.5, 1, 2, 4, 8, and 24 h after drug or vehicle administration. There were no significant changes in plasma levels of IL-1β, IL-10, IL-12p70, GM-CSF, or TNFα between 0 and 24 h after CBLB502 injection in response to any of the tested CBLB502 doses (data not shown). KC demonstrated strong and somewhat CBLB502 dose-dependent elevation, and IL-12p40, MCP-1, MIP-2 and MIG showed modest dose-dependent increases (Supplemental Figs. 2 and 3). G-CSF and IL-6, however, stood out as the only cytokines that demonstrated both significant and CBLB502 dose-dependent elevations (Fig. 2, a-d).
Fig. 2.
G-CSF and IL-6 responses in nonirradiated mice. a and b, time-dependent changes in G-CSF (a) and IL-6 (b) plasma levels in nonirradiated female ICR mice injected intramuscularly with either vehicle or the indicated doses of CBLB502. Each data point represents the mean ± S.D. cytokine concentration measured in plasma of six mice. c and d, time-dependent changes in G-CSF (c) and IL-6 (d) plasma levels in nonirradiated female C57BL/6J mice injected subcutaneously with either vehicle or the indicated doses of CBLB502. Each data point represents the mean ± S.D. cytokine concentration measured in plasma of six mice. e and f, congruence of the CBLB502 dose response of survival and cytokine induction in ICR (e) and C57BL/6J (f) mice. For ICR mouse studies, the cumulative data from several similar experiments are presented. Cytokine data points represent natural logarithm of mean AUC(0–24) increases per dose group. Dashed line represents four-parameter sigmoid (Emax) curve fit for the survival data (represented as natural logarithm of survival odds ratio of treatment versus control group).
In ICR mice treated with CBLB502 intramuscularly, G-CSF levels peaked at 2 to 4 h after CBLB502 administration and normalized by 8 to 24 h postadministration (Fig. 2a). The duration of G-CSF elevation increased with increasing CBLB502 doses. Maximal observed plasma G-CSF levels were 3600 to 4100 pg/ml (representing ∼230- to 240-fold increases over the 16–17 pg/ml baseline level on average per dose group) at doses of 40 to 160 μg/kg CBLB502. CBLB502-induced increases in IL-6 were more modest than those observed for G-CSF, with plasma levels in treated mice reaching a maximum of 130 to 210 pg/ml (∼8- to 16-fold increases over the ∼13–17 pg/ml baseline level on average per dose group) at 40 to 160 μg/kg CBLB502 doses (Fig. 2b). Compared with G-CSF, IL-6 levels peaked earlier, at 1 to 2 h after injection, and normalized sooner, by 8 h after injection for all tested CBLB502 doses.
Similar effects were observed in C57BL/6J mice after subcutaneous injection of CBLB502 doses in the same range (Fig. 2, c and d). Plasma levels of G-CSF increased after injection, peaked at 2 to 4 h postdose, and normalized by 24 h postdose. The highest G-CSF levels observed were 8800 to 11,500 pg/ml (∼88- to 115-fold higher than the ∼100 pg/ml baseline level) after administration of 16 to 160 μg/kg doses of CBLB502. Similarly to the other tested strain (ICR) and administration route (intramuscular), IL-6 levels in subcutaneously injected C57BL/6J mice displayed a more modest elevation than G-CSF, peaked at 1 to 2 h postdose, and returned to baseline by 8 h. Maximal IL-6 levels were 490 to 570 pg/ml (120- to 140-fold higher than the ∼4–5 pg/ml baseline level) after injection of 16 to 160 μg/kg CBLB502.
None of the evaluated cytokines responded to CBLB502 administration in TLR5 knockout mice (data not shown), thus confirming previous findings with CBLB502's parent protein, flagellin (Hawn et al., 2005; Sanders et al., 2008).
To evaluate the dose dependence of G-CSF and IL-6 responses to CBLB502 quantitatively, we chose to use the CBLB502-elicited overall cytokine exposure as a single characteristic and global measure of cytokine levels and numerically expressed it, as accepted in the field, as the AUC. This approach was selected because the biological effects of cytokines depend on their systemic exposure rather than on other parameters, such as Cmax (Farese et al., 2003). As shown in Fig. 2, e and f where dose-dependent treatment effects of CBLB502 on mouse survival are plotted together with the dose-dependent effects on systemic exposure to G-CSF and IL-6, all of these CBLB502 effects are congruent and occur within the same drug dose range. The logarithm of survival odds ratio (odds of survival in a treated group divided by the odds of survival in control group) frequently used in statistical and epidemiological studies (Viera, 2008) was used as the metric of treatment effect on survival in this analysis.
G-CSF and IL-6 Show Strong, Dose-Dependent Responses to CBLB502 in Nonirradiated Rhesus Macaques and Dogs.
Having established dose dependence of CBLB502-induced G-CSF and IL-6 responses in mice, we next tested whether these responses are conserved in other mammalian animal model species, including, most importantly, species closer to humans, NHPs.
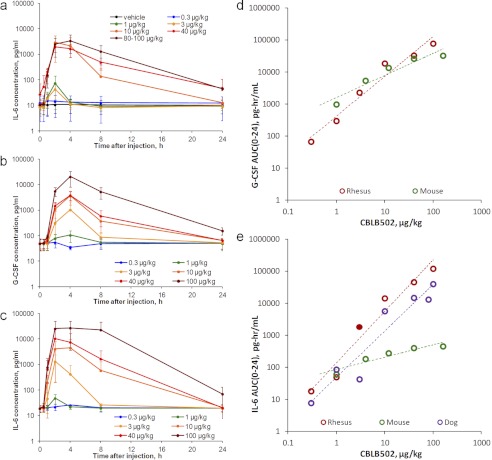
In a dog model study, groups of six beagle dogs were treated intramuscularly with either vehicle or CBLB502 over a wide dose range (0.3–100 μg/kg). Levels of cytokine biomarkers were assessed in blood plasma samples collected predose, then at 0.5, 1, 2, 4, 8, 24, 48, and 120 h postdose. IL-6 and other cytokines of biological or safety importance (IL-8, IL-10, and TNFα) were evaluated in collected samples (G-CSF was not measured because a working assay for dog G-CSF is not commercially available). IL-6 (Fig. 3a) and IL-8 (Supplemental Fig. 4) displayed strong, approximately CBLB502 dose-dependent increases, whereas IL-10 and TNFα displayed modest increases with weaker dose dependence (Supplemental Fig. 4). IL-6 levels peaked at 2 h after dosing and returned to baseline at 4 to 24 h after injection depending on the CBLB502 dose level. Maximal observed plasma IL-6 levels were ∼3000 to 4000 pg/ml on average per dose group after 10 to 100 μg/kg CBLB502 doses (∼300- to 400-fold increases over the ∼10 pg/ml baseline level).
Fig. 3.
G-CSF and IL-6 responses in rhesus macaques and dogs. a, time-dependent changes in IL-6 plasma levels in nonirradiated male and female beagle dogs (n = 6; 1:1 sex ratio) injected intramuscularly with either vehicle or the indicated doses of CBLB502. Each data point represents the mean ± S.D. b and c, time-dependent changes in G-CSF (b) and IL-6 (c) plasma levels in nonirradiated male and female rhesus macaques (n = 6; 1:1 sex ratio) injected intramuscularly with either vehicle or the indicated doses of CBLB502. Each data point represents the mean ± S.D. d and e, CBLB502 dose-dependent changes in exposures (AUC(0–24)) to G-CSF (d) and IL-6 (e) in the three treated species: mouse, dog, and rhesus macaque. Mean data per dose group are shown. Dashed lines represent linear regression trend lines (in log-log coordinates).
Two parallel independent studies were conducted to explore CBLB502 responsiveness of G-CSF, IL-6, and other cytokines in nonirradiated rhesus macaques. Groups of six (three male and three female) rhesus macaques were treated intramuscularly with a single dose of CBLB502 over a wide dose range (0.3–40 μg/kg). Levels of G-CSF, IL-6, and other factors (GM-CSF, IL-1β, IL-8, IL-10, IL-12p70, and IP-10) were assessed in blood plasma samples collected predose and at 0.5, 1, 2, 4, 8, 24, and 48 h postdose. Both G-CSF and IL-6 (Fig. 3, b and c) displayed strong CBLB502 dose-dependent increases, and a moderate increase in IL-8 was also observed (Supplemental Fig. 5). A very minor response was found for IL-10 levels (probably caused by a weakly cross-reactive human assay, as found in later studies). Levels of GM-CSF, IL-1β, IL-12(p70), and IP-10 did not change in response to CBLB502 administration (data not shown).
CBLB502-dependent induction of G-CSF and IL-6 in NHPs followed kinetics similar to that observed in mice (although somewhat delayed), with IL-6 and G-CSF levels peaking at ∼2 and ∼4 h after injection, respectively. Levels of both cytokines returned to baseline by 24 h postdose and displayed dose-dependent kinetics in their decline. Maximal induced levels were observed after injection of 40 to 100 μg/kg CBLB502 and reached for G-CSF ∼19,300 to 20,400 pg/ml (∼410- to 570-fold increase over the ∼40–50 pg/ml baseline level) and for IL-6 10,400 to 27,000 pg/ml (∼460- to 1210-fold increase over the ∼20 pg/ml baseline level) on average per dose group.
CBLB502 dose dependence of the observed increases in G-CSF and IL-6 was clearly illustrated by plotting the corresponding AUC values versus administered CBLB502 doses (Fig. 3, d and e) and was found to be better expressed in dogs and NHPs than in mice.
There were no major differences in CBLB502-elicited cytokine responses between male and female representatives of either dogs or rhesus macaques.
G-CSF and IL-6 Are Induced by CBLB502 in a Dose-Dependent Manner in Irradiated Animals.
After demonstration of CBLB502 dose dependence of G-CSF and IL-6 increases in nonirradiated animals, it was important to assess whether the same was true in irradiated animals. Such experiments were conducted in two animal species, mouse and rhesus macaque.
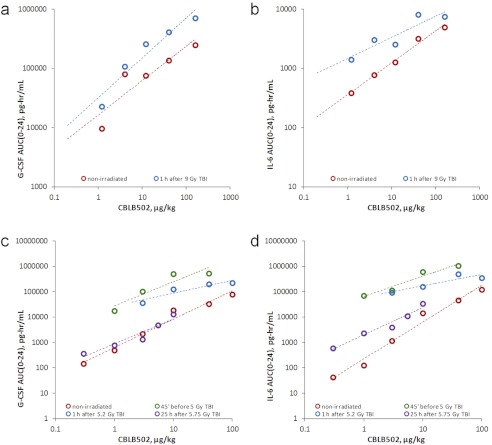
Groups of 24 female ICR mice were irradiated with 9 Gy of TBI (or left intact), and 1 h later, injected intramuscularly with vehicle or CBLB502 (single dose within a 1.2–160 μg/kg range). Cytokine levels were measured in plasma samples collected from untreated mice and at 0.25, 0.5, 1, 2, 4, 8, and 24 h after CBLB502 injection (three mice were sacrificed for this purpose per collection time point). AUC values for the resulting G-CSF and IL-6 exposures at each administered CBLB502 dose are plotted in Fig. 4, a and b. These data indicated that when CBLB502 was injected close to irradiation (1 h post-TBI) it resulted in somewhat higher cytokine exposures compared with nonirradiated mice (probably because of the combined cytokine-increasing effects of both irradiation and the drug). However, the response was dose-proportional, and slopes of CBLB502 dose-dependent increases in the two cytokines remained generally similar. Moreover, the difference in AUC values after administration of identical CBLB502 doses in irradiated and nonirradiated mice was not drastic (2- to 3-fold).
Fig. 4.
Comparison of CBLB502 dose-dependent G-CSF and IL-6 exposures in nonirradiated versus irradiated mice and rhesus macaques. a and b, mean G-CSF (a) and IL-6 (b) exposures (AUC(0–24)) at indicated CBLB502 dose levels in ICR mice. c and d, mean G-CSF (c) and IL-6 (d) exposures (AUC(0–24)) at the indicated CBLB502 dose levels in rhesus macaques. Dashed lines represent linear regression trend lines (in log-log coordinates).
The same combined effect of radiation and CBLB502 on G-CSF and IL-6 exposures was also observed in a series of NHP studies when the drug was administered in close proximity to the irradiation event, either 45 min before TBI or 1 h after TBI. In these experiments, groups of four to six animals (1:1 female/male ratio) received a predominantly nonlethal, <LD20/40 ∼5.2-Gy dose of TBI (or were left unirradiated) together with a single intramuscular injection of vehicle or CBLB502 (single dose within a range of 1–100 μg/kg) given either 45 min before TBI or 1 h after TBI. Cytokine levels (G-CSF, IL-6, and others) in plasma were measured predose and at 1, 2, 4, 8, 24, and 48 h after treatment. As shown in Fig. 4, c and d, the achieved G-CSF and IL-6 exposures resulting from combined cytokine-inducing effects of CBLB502 and TBI significantly (∼15- to 100-fold for CBLB502 dose levels eliciting substantial response, i.e., ≥3 μg/kg) exceeded those produced by the same drug doses in nonirradiated animals. However, when CBLB502 was administered at 25 h after a similar ∼LD30/40 dose of 5.75 Gy of TBI (n = 8 per dose group; CBLB502 dose range, 0.3–10 μg/kg; time points assessed, 0, 0.5, 1, 2, 4, 8, 24, and 48 h after treatment), when TBI-elicited cytokine elevation was already over, the resulting cytokine exposures (AUC) were much closer (within 3-fold for ≥3 μg/kg doses) to those seen in nonirradiated animals given the same CBLB502 doses (Fig. 4, c and d). This was particularly evident in the case of G-CSF. The residual difference in the AUCs for IL-6 seen in nonirradiated NHPs versus NHPs injected at 25 h post-TBI may be caused by interstudy/interassay variability rather than actual difference in total cytokine exposures.
CBLB502-Induced Elevation of G-CSF and IL-6 Levels Is an Important Component of the Anti-ARS Activity of CBLB502.
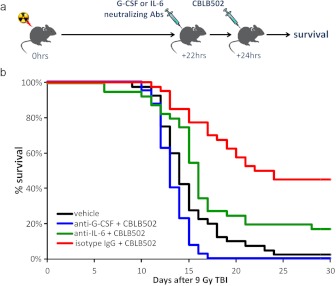
The value of a given biomarker as a predictor of a drug's efficacy greatly depends on whether the biomarker is just a witness of or a contributor to the therapeutic effect. As stated in Introduction, an ideal biomarker is an essential component of the mechanism of a drug's action or, better yet, a mediator of its effect. To determine whether G-CSF and IL-6 fit this criterion for CBLB502, we evaluated how their neutralization (using specific monoclonal antibodies) affected the ability of CBLB502 to increase the survival of irradiated mice. C57BL/6J mice (n = 40 per group) were irradiated with 9.5 Gy of TBI, and 22 h afterward, treated with either rat anti-mouse G-CSF monoclonal antibodies (IgG1), rat anti-mouse anti-IL-6 antibodies (IgG1), or a control rat anti-mouse nonspecific IgG1. All antibodies were administered intraperitoneally by using a previously established effective dose (Neta et al., 1992; Singh et al., 2010, 2011) of 500 μg/mouse. Two hours later (24 h after TBI), all mice in these groups were injected intravenously with 60 μg/kg CBLB502. An additional group of similarly irradiated mice was injected with vehicle at 24 h after TBI. Study arms with G-CSF or IL-6 alone were not included because it is known that under given conditions these cytokines do not provide survival advantage (see Neta et al., 1988; Uckun et al., 1990; and Discussion).
The results of this experiment (Fig. 5) demonstrate that neutralization of either G-CSF or IL-6 significantly reduced the radiomitigative potency of CBLB502. Only 2.5% of mice (1 of 40) survived to day 30 after TBI in the vehicle-treated negative control group, with deaths occurring between days 10 and 20. The CBLB502+IgG1-treated positive control group displayed an increase in 30-day survival of +42% over the negative control group (p < 0.0001 by Fisher's exact test; 18 of 40 versus 1 of 40 survival) as well as a significant (p = 0.015 by log rank test) increase in the mean survival time of decedents (17.2 days compared with 14.6 days in the negative control group). Neutralization of G-CSF significantly reduced both the 30-day survival rate (0% of mice survived; p < 0.0001 for comparison of 0 of 40 survived versus 18 of 40 in the positive control group) and the mean survival time of decedents (p < 0.0001 by log rank test; 13.2 days compared with 17.2 days in the positive control group). The negative consequences of IL-6 neutralization were more modest, but still statistically significant. The mean survival time of decedents was shorter in the IL-6-neutralized group (15.1 days) compared with the positive control group (17.2 days) (although not significantly; p = 0.08 by log rank test), and 30-day survival was reduced by 28% (p = 0.015 by Fisher's exact test for comparison with the positive control group). We cannot exclude, however, that the role of IL-6 in the anti-ARS activity of CBLB502 may be less prominent in mice than in primates because mice, in response to CBLB502, do not produce as levels of IL-6 as high as NHPs or dogs (Fig. 3).
Fig. 5.
Influence of G-CSF or IL-6 neutralization on the radiomitigative efficacy of CBLB502 in mice. a, experimental scheme. Groups of 40 C57BL/6J female mice were exposed to 9 Gy of TBI. At +22 h relative to irradiation, the mice were injected intraperitoneally with anti-G-CSF, anti-IL-6, or control IgG antibodies and then injected intravenously with 60 μg/kg CBLB502 2 h later. The vehicle control group received 9 Gy of TBI and intravenous injection of the vehicle at +24 h. b, Kaplan-Meier curves illustrating mouse mortality kinetics for 30 days after TBI.
As expected, induction of G-CSF and IL-6 by CBLB502 in mice depended on the presence of TLR5 (Supplemental Fig. 6).
Discussion
Development of CBLB502 under the FDA's Animal Efficacy Rule requires the identification of efficacy biomarkers that will allow the projection of a human efficacious dose based on animal efficacy data. The results described above define two cytokines induced in response to CBLB502 administration, G-CSF and IL-6 (specifically the AUC of the elicited response), as candidate biomarkers of CBLB502 efficacy that comply with the requirements of the Animal Efficacy Rule.
First, the beneficial effect of CBLB502 on survival of lethally irradiated animals is strictly TLR5-dependent and is not observed in TLR5 knockout mice. CBLB502-elicited elevation of G-CSF and IL-6 is also strictly TLR5-dependent. Therefore, the selected candidate cytokine biomarkers are not simply pharmacodynamic readouts of CBLB502 activity, but rather reflect the potency of CBLB502's interaction with its receptor, TLR5, which is the keystone of its anti-ARS activity.
Second, CBLB502 causes dose-dependent elevation of G-CSF and IL-6 in nonirradiated animals as well as irradiated animals. Although irradiation potentiates induction of G-CSF and IL-6 by CBLB502, it does not affect the dose proportionality of the responses, and its effect is short-lasting (CBLB502-induced cytokine release in NHPs at 25 h after irradiation is nearly identical to that in nonirradiated animals).
Third, both G-CSF and IL-6 elevations are induced by a range of CBLB502 doses that shows both radioprotective and radiomitigative efficacy in mice. Moreover, CBLB502-elicited changes in cytokine levels are strongly correlated with CBLB502-elicited effects on survival of lethally irradiated mice. Studies aimed at demonstrating similar relationships in additional species (NHPs) are underway and will be reported elsewhere.
Fourth, the selected biomarkers are similarly responsive across three diverse tested species (mouse, rhesus macaque, and dog). Confirmation of similar responsiveness of these biomarkers in humans is ongoing and represents a critical step for the ultimate proof of their utility for dose conversion.
Fifth, both G-CSF and IL-6 play important roles in CBLB502's mechanism of action as a radiation countermeasure. This is well illustrated by our finding that neutralization of either cytokine by specific antibodies significantly reduced the radiomitigative efficacy of CBLB502. In addition, there is a large body of literature on the biological activities of these cytokines, including anti-ARS activity, which supports the likelihood that they mediate, at least in part, the anti-ARS activity of CBLB502. The relevant literature is summarized briefly below.
G-CSF is known to increase the survival, proliferation, and differentiation of the granulocyte progenitors that produce neutrophils (Roberts, 2005). There is general agreement that G-CSF is an acceptable treatment for human subjects exposed to ≥3 Gy of TBI alone or ≥2 Gy of TBI in the presence of mechanical trauma and/or burns (combined injury). G-CSF is available as a treatment agent in current radiation countermeasure stockpiles that have been developed in the United States and by the World Health Organization (Dainiak, 2010). Recently, we found that G-CSF is a principal mediator of the radioprotective activity of tocopherol succinate (Singh et al., 2010, 2011), 5-androstenediol, and γ-tocotrienol (Singh et al., 2012; V. K. Singh and M. H. Whitnall, unpublished observations).
IL-6 accelerates multilineage HP recovery after radiation-induced HP injury (Patchen et al., 1991). It also induces proliferation of cells of the megakaryocyte lineage and platelet production and reduces thrombocytopenia (low platelet count) (Zeidler et al., 1992). It is noteworthy that thrombocytopenia was found to be a good predictor of survival in an NHP ARS model (Stickney et al., 2007). Unlike G-CSF, IL-6 cannot rescue irradiated animals on its own. However, when used in combination treatments, it was shown to greatly augment the effects of other radioprotectors, such as G-CSF and IL-1β (Neta et al., 1988; Patchen et al., 1993). The importance of IL-6 in radiation resistance of the organism is underscored by the fact that its neutralization greatly increased ARS-associated mortality in mice (Neta et al., 1992).
It is important to underscore that the anti-ARS activity of CBLB502 cannot be reduced to induction of either of these cytokines. Thus, even in its optimal treatment regimen recombinant G-CSF, which on its own was able (under certain conditions) to rescue lethally irradiated mice and NHPs (Uckun et al., 1990; Bertho et al., 2005), was less effective compared with a single injection of CBLB502. Unlike CBLB502, G-CSF was incapable of rescuing mice when administered 24 h after lethal TBI (Uckun et al., 1990), the regimen used in the present study. A single injection of CBLB502 0.5 h before supralethal (∼13 Gy) TBI was efficacious in mice with a dose modification factor of ∼1.6 (Burdelya et al., 2008), whereas G-CSF given 2 h after TBI (at time equivalent to G-CSF spike induced by pre-TBI CBLB502) failed to rescue mice from >10.5 Gy of TBI and had a dose modification factor of ≤1.2 in any regimen (Hosoi et al., 1992; Sureda et al., 1998). Another principal difference is that in NHPs CBLB502 acted primarily against thrombocytopenia (Burdelya et al., 2008), the major correlate of ARS-induced mortality in primates (Stickney et al., 2007), whereas G-CSF mitigated mostly neutropenia (MacVittie et al., 1996). It is noteworthy that previous studies indicate that cotreatment with IL-6 enhances the G-CSF-mediated acceleration of postirradiation HP recovery (Patchen et al., 1993). Therefore, G-CSF and IL-6 are likely act in concert with each other and with additional yet to be defined TLR5-induced factors.
It is also important to note that the effects of exogenously delivered cytokines on the organism's response to radiation are expected to be (and in fact are) different from those obtained with CBLB502 treatment because: 1) although G-CSF and IL-6 play crucial roles in CBLB502's mechanism of action, there are probably additional CBLB502-modulated factors involved as well; and 2) the pharmacokinetics and local tissue concentrations of exogenously delivered cytokines differ from those of endogenous cytokines produced in response to CBLB502 by specific cells in specific tissues at defined points in time. Finally, from a technical standpoint, G-CSF and IL-6 possess one more essential biomarker property: their levels can be easily and quantitatively measured by using samples obtained through a simple, relatively noninvasive procedure (peripheral blood draw) together with validated assays that are currently available.
Ongoing work is focused on validating G-CSF and IL-6 as CBLB502 efficacy biomarkers in irradiated NHPs and assessing their responses in humans. Completion of these final steps in the biomarker selection process will allow actual animal-human dose conversion, a critical step in the successful development of CBLB502 as a novel radiation countermeasure with strong potential to alter the impact of nuclear and radiological events.
Supplementary Material
Acknowledgments
We thank Grant Thomas and Stephen Wise (Armed Forces Radiobiology Research Institute) for assistance with NHP work; Margaret Kehl (Armed Forces Radiobiology Research Institute) for technical assistance in analyzing cytokines by Luminex; Patrick Gould (Armed Forces Radiobiology Research Institute) for data analysis; The Veterinary Sciences Department and Cobalt Radiation Facility of the Armed Forces Radiobiology Research Institute for support; Catherine A. Burkhart and Natalya V. Narizhneva (Cleveland BioLabs, Inc.) for assistance with mouse survival and cytokine data analyses; Patricia Baker (Cleveland BioLabs, Inc.) for editing the manuscript; and Dr. Richard M. Bittman (Bittman Biostatistics, Inc., Naples, FL) for advice on statistical analyses.
This work was supported by the U.S. Department of Defense's Defense Threat Reduction Agency [Grants H.10014_07_AR_R (to V.K.S.), HDTRA1-07-C-0021 (to E.F.), HDTRA1-11-C-0008 (to V.I.K.)]; the U.S. Department of Defense Chemical Biological Medical Systems [Grant W9113M-08-C-0151] (to Cleveland BioLabs, Inc.); the U.S. Department of Health and Human Services Biomedical Advanced Research and Development Authority [Grant HHSO100200800059C] (to A.V.G.); and the National Institutes of Health National Institute of Allergy and Infectious Diseases [Grants R01AI080446, RC2AI087616] (to A.V.G.).
V.I.K., A.N.S., F.B., Y.K., I.S., A.C., R.K.M., A.P., A.V.G., and E.F. are employees or paid consultants of Cleveland Biolabs Inc., a company that has a commercial interest in the results of this research.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- TBI
- total body irradiation
- AFRRI
- Armed Forces Radiobiology Research Institute
- ARS
- acute radiation syndrome
- AUC
- area under the curve
- FDA
- Food and Drug Administration
- G-CSF
- granulocyte colony-stimulating factor
- GM-CSF
- granulocyte-macrophage colony-stimulating factor
- HP
- hematopoietic
- IL
- interleukin
- IP-10
- interferon γ-induced protein 10kDa
- KC
- keratinocyte chemoattractant
- MCP-1
- monocyte chemotactic protein-1
- MIG
- monokine induced by interferon γ
- MIP-2
- macrophage inflammatory protein-2
- MRC
- medical radiation countermeasure
- NF-κB
- nuclear factor-κB
- NHP
- nonhuman primate
- PD
- pharmacodynamic
- PK
- pharmacokinetic
- SCF
- stem cell factor
- TLR
- Toll-like receptor
- TNFα
- tumor necrosis factor α
- TPO
- thrombopoietin.
Authorship Contributions
Participated in research design: Krivokrysenko, Shakhov, Singh, Whitnall, Gudkov, and Feinstein.
Conducted experiments: Singh, Bone, Kononov, Cheney, Krivokrysenko, Maitra, and Purmal.
Performed data analysis: Krivokrysenko, Shakhov, Singh, Shyshynova, and Feinstein.
Wrote or contributed to the writing of the manuscript: Krivokrysenko, Gudkov, and Feinstein.
References
- Bertho JM, Frick J, Prat M, Demarquay C, Dudoignon N, Trompier F, Gorin NC, Thierry D, Gourmelon P. (2005) Comparison of autologous cell therapy and granulocyte-colony stimulating factor (G-CSF) injection vs. G-CSF injection alone for the treatment of acute radiation syndrome in a non-human primate model. Int J Radiat Oncol Biol Phys 63:911–920 [DOI] [PubMed] [Google Scholar]
- Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, et al. (2008) An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science 320:226–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron G, Duluc D, Frémaux I, Jeannin P, David C, Gascan H, Delneste Y. (2005) Direct stimulation of human T cells via TLR5 and TLR7/8: flagellin and R-848 up-regulate proliferation and IFN-γ production by memory CD4+ T cells. J Immunol 175:1551–1557 [DOI] [PubMed] [Google Scholar]
- Carvalho FA, Aitken JD, Gewirtz AT, Vijay-Kumar M. (2011) TLR5 activation induces secretory interleukin-1 receptor antagonist (sIL-1Ra) and reduces inflammasome-associated tissue damage. Mucosal Immunol 4:102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crommelin DJ, Storm G, Verrijk R, de Leede L, Jiskoot W, Hennink WE. (2003) Shifting paradigms: biopharmaceuticals versus low molecular weight drugs. Int J Pharm 266:3–16 [DOI] [PubMed] [Google Scholar]
- Dainiak N. (2010) Rationale and recommendations for treatment of radiation injury with cytokines. Health Phys 98:838–842 [DOI] [PubMed] [Google Scholar]
- Dearman RJ, Cumberbatch M, Maxwell G, Basketter DA, Kimber I. (2009) Toll-like receptor ligand activation of murine bone marrow-derived dendritic cells. Immunology 126:475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese AM, Yang BB, Roskos L, Stead RB, MacVittie TJ. (2003) Pegfilgrastim, a sustained-duration form of filgrastim, significantly improves neutrophil recovery after autologous marrow transplantation in rhesus macaques. Bone Marrow Transplant 32:399–404 [DOI] [PubMed] [Google Scholar]
- Fukao T, Koyasu S. (2003) PI3K and negative regulation of TLR signaling. Trends Immunol 24:358–363 [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. (2001) Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol 167:1882–1885 [DOI] [PubMed] [Google Scholar]
- Grdina DJ, Murley JS, Kataoka Y. (2002) Radioprotectants: current status and new directions. Oncology 63 (Suppl 2):2–10 [DOI] [PubMed] [Google Scholar]
- Hawn TR, Wu H, Grossman JM, Hahn BH, Tsao BP, Aderem A. (2005) A stop codon polymorphism of Toll-like receptor 5 is associated with resistance to systemic lupus erythematosus. Proc Natl Acad Sci U S A 102:10593–10597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, West AP, Ghosh S. (2006) NF-κB and the immune response. Oncogene 25:6758–6780 [DOI] [PubMed] [Google Scholar]
- He XX, Bai H, Yang GR, Xue YJ, Su YN. (2009) [Expression of Toll-like receptors in human bone marrow mesenchemal stem cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi 17:695–699 [PubMed] [Google Scholar]
- Honko AN, Mizel SB. (2005) Effects of flagellin on innate and adaptive immunity. Immunol Res 33:83–101 [DOI] [PubMed] [Google Scholar]
- Hosoi Y, Kurishita A, Ono T, Sakamoto K. (1992) Effect of recombinant human granulocyte colony-stimulating factor on survival in lethally irradiated mice. Acta Oncol 31:59–63 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Karin M, Lin A. (2002) NF-κB at the crossroads of life and death. Nat Immunol 3:221–227 [DOI] [PubMed] [Google Scholar]
- Maaser C, Heidemann J, von Eiff C, Lugering A, Spahn TW, Binion DG, Domschke W, Lugering N, Kucharzik T. (2004) Human intestinal microvascular endothelial cells express Toll-like receptor 5: a binding partner for bacterial flagellin. J Immunol 172:5056–5062 [DOI] [PubMed] [Google Scholar]
- MacVittie TJ, Farese AM, Herodin F, Grab LB, Baum CM, McKearn JP. (1996) Combination therapy for radiation-induced bone marrow aplasia in nonhuman primates using synthokine SC-55494 and recombinant human granulocyte colony-stimulating factor. Blood 87:4129–4135 [PubMed] [Google Scholar]
- Mettler FA, Jr, Voelz GL. (2002) Major radiation exposure–what to expect and how to respond. N Engl J Med 346:1554–1561 [DOI] [PubMed] [Google Scholar]
- Neta R, Perlstein R, Vogel SN, Ledney GD, Abrams J. (1992) Role of interleukin 6 (IL-6) in protection from lethal irradiation and in endocrine responses to IL-1 and tumor necrosis factor. J Exp Med 175:689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta R, Vogel SN, Sipe JD, Wong GG, Nordan RP. (1988) Comparison of in vivo effects of human recombinant IL 1 and human recombinant IL 6 in mice. Lymphokine Res 7:403–412 [PubMed] [Google Scholar]
- Patchen ML, Fischer R, MacVittie TJ. (1993) Effects of combined administration of interleukin-6 and granulocyte colony-stimulating factor on recovery from radiation-induced hemopoietic aplasia. Exp Hematol 21:338–344 [PubMed] [Google Scholar]
- Patchen ML, MacVittie TJ, Williams JL, Schwartz GN, Souza LM. (1991) Administration of interleukin-6 stimulates multilineage hematopoiesis and accelerates recovery from radiation-induced hematopoietic depression. Blood 77:472–480 [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. (2004) Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118:229–241 [DOI] [PubMed] [Google Scholar]
- Roberts AW. (2005) G-CSF: a key regulator of neutrophil production, but that's not all! Growth Factors 23:33–41 [DOI] [PubMed] [Google Scholar]
- Sanders CJ, Moore DA, 3rd, Williams IR, Gewirtz AT. (2008) Both radioresistant and hemopoietic cells promote innate and adaptive immune responses to flagellin. J Immunol 180:7184–7192 [DOI] [PubMed] [Google Scholar]
- Shih VF, Kearns JD, Basak S, Savinova OV, Ghosh G, Hoffmann A. (2009) Kinetic control of negative feedback regulators of NF-κB/RelA determines their pathogen- and cytokine-receptor signaling specificity. Proc Natl Acad Sci U S A 106:9619–9624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Wise SY, Ducey EJ, Brown DS, Singh VK. (2011) Radioprotective efficacy of tocopherol succinate is mediated through granulocyte-colony stimulating factor. Cytokine 56:411–421 [DOI] [PubMed] [Google Scholar]
- Singh VK, Brown DS, Kao TC. (2010) α-Tocopherol succinate protects mice from gamma-radiation by induction of granulocyte-colony stimulating factor. Int J Radiat Biol 86:12–21 [DOI] [PubMed] [Google Scholar]
- Singh VK, Ducey EJ, Brown DS, Whitnall MH. (2012) A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int J Radiat Biol 88:296–310 [DOI] [PubMed] [Google Scholar]
- Singh VK, Yadav VS. (2005) Role of cytokines and growth factors in radioprotection. Exp Mol Pathol 78:156–169 [DOI] [PubMed] [Google Scholar]
- Srinivasan V, Weiss JF. (1992) Radioprotection by vitamin E: injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int J Radiat Oncol Biol Phys 23:841–845 [DOI] [PubMed] [Google Scholar]
- Stickney DR, Dowding C, Authier S, Garsd A, Onizuka-Handa N, Reading C, Frincke JM. (2007) 5-androstenediol improves survival in clinically unsupported rhesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol 7:500–505 [DOI] [PubMed] [Google Scholar]
- Sureda A, Kádár E, Valls A, García-López J. (1998) Granulocyte colony-stimulating factor administered as a single intraperitoneal injection modifies the lethal dose95/30 in irradiated B6D2F1 mice. Haematologica 83:863–864 [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. (2003) Toll-like receptors. Annu Rev Immunol 21:335–376 [DOI] [PubMed] [Google Scholar]
- Tsujimoto H, Uchida T, Efron PA, Scumpia PO, Verma A, Matsumoto T, Tschoeke SK, Ungaro RF, Ono S, Seki S, et al. (2005) Flagellin enhances NK cell proliferation and activation directly and through dendritic cell-NK cell interactions. J Leukoc Biol 78:888–897 [DOI] [PubMed] [Google Scholar]
- Uckun FM, Souza L, Waddick KG, Wick M, Song CW. (1990) In vivo radioprotective effects of recombinant human granulocyte colony-stimulating factor in lethally irradiated mice. Blood 75:638–645 [PubMed] [Google Scholar]
- Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, et al. (2006) Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol 7:868–874 [DOI] [PubMed] [Google Scholar]
- van den Berk LC, Jansen BJ, Siebers-Vermeulen KG, Netea MG, Latuhihin T, Bergevoet S, Raymakers RA, Kögler G, Figdor CC, Adema GJ, et al. (2009) Toll-like receptor triggering in cord blood mesenchymal stem cells. J Cell Mol Med 13:3415–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viera AJ. (2008) Odds ratios and risk ratios: what's the difference and why does it matter? South Med J 101:730–734 [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Sanders CJ, Frias A, Sloane VM, Xu J, Neish AS, Rojas M, Gewirtz AT. (2008) Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol 180:8280–8285 [DOI] [PubMed] [Google Scholar]
- Waddick KG, Song CW, Souza L, Uckun FM. (1991) Comparative analysis of the in vivo radioprotective effects of recombinant granulocyte colony-stimulating factor (G-CSF), recombinant granulocyte-macrophage CSF, and their combination. Blood 77:2364–2371 [PubMed] [Google Scholar]
- Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, et al. (2004) Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med 140:1037–1051 [DOI] [PubMed] [Google Scholar]
- Weiss JF, Landauer MR. (2000) Radioprotection by antioxidants. Ann NY Acad Sci 899:44–60 [PubMed] [Google Scholar]
- Whitnall MH, Elliott TB, Landauer MR, Jackson WE, 3rd, Wilhelmsen CL, McKinney L, Kumar KS, Srinivasan V, Ledney GD, Seed TM. (2001) In vivo protection against gamma-irradiation with 5-androstenediol. Exp Biol Med (Maywood) 226:625–627 [DOI] [PubMed] [Google Scholar]
- Xu Y, Kiningham KK, Devalaraja MN, Yeh CC, Majima H, Kasarskis EJ, St Clair DK. (1999) An intronic NF-κB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-α and interleukin-1β. DNA Cell Biol 18:709–722 [DOI] [PubMed] [Google Scholar]
- Zeidler C, Kanz L, Hurkuck F, Rittmann KL, Wildfang I, Kadoya T, Mikayama T, Souza L, Welte K. (1992) In vivo effects of interleukin-6 on thrombopoiesis in healthy and irradiated primates. Blood 80:2740–2745 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.