Abstract
Cannabinoid receptor agonists produce reliable antinociception in most preclinical pain assays but have inconsistent analgesic efficacy in humans. This disparity suggests that conventional preclinical assays of nociception are not sufficient for the prediction of cannabinoid effects related to clinical analgesia. To extend the range of preclinical cannabinoid assessment, this study compared the effects of the marijuana constituent and low-efficacy cannabinoid agonist Δ9-tetrahydrocannabinol (THC) and the high-efficacy synthetic cannabinoid agonist 3-(2-hydroxy-4-(1,1-dimethylheptyl)phenyl)-4-(3-hydroxypropyl)cyclohexanol (CP55940) in assays of pain-stimulated and pain-depressed behavior. Intraperitoneal injection of dilute lactic acid (1.8% in 1 ml/kg) stimulated a stretching response or depressed intracranial self-stimulation (ICSS) in separate groups of male Sprague-Dawley rats. THC (0.1–10 mg/kg) and CP55940 (0.0032–0.32 mg/kg) dose-dependently blocked acid- stimulated stretching but only exacerbated acid-induced depression of ICSS at doses that also decreased control ICSS in the absence of a noxious stimulus. Repeated THC produced tolerance to sedative rate-decreasing effects of THC on control ICSS in the absence of the noxious stimulus but failed to unmask antinociception in the presence of the noxious stimulus. THC and CP55940 also failed to block pain-related depression of feeding in rats, although THC did attenuate satiation-related depression of feeding. In contrast to the effects of the cannabinoid agonists, the clinically effective analgesic and nonsteroidal anti-inflammatory drug ketoprofen (1 mg/kg) blocked acid-stimulated stretching and acid-induced depression of both ICSS and feeding. The poor efficacy of THC and CP55940 to block acute pain-related depression of behavior in rats agrees with the poor efficacy of cannabinoids to treat acute pain in humans.
Introduction
The marijuana plant has been used for centuries in an effort to treat pain, and cannabinoid receptor agonists such as the marijuana constituent Δ9-tetrahydrocannabinol (THC) and the synthetic compound 3-(2-hydroxy-4-(1,1-dimethylheptyl)phenyl)-4-(3-hydroxypropyl)cyclohexanol (CP55940) produce antinociception in nearly all preclinical assays of acute and chronic pain-related behavior (Rice, 2006; Karst et al., 2010). However, in contrast to the reliable antinociceptive efficacy of cannabinoids in preclinical studies, controlled clinical trials in humans have found that cannabinoids are ineffective against acute pain and have weak efficacy and a narrow therapeutic window for the treatment of many types of chronic pain (Raft et al., 1977; Rice, 2006; Karst et al., 2010; Kraft, 2012). This discrepancy suggests that existing preclinical assays of antinociception in animals overestimate the analgesic potential of cannabinoids in humans and may be insufficient for preclinical behavioral assessment of candidate cannabinoid analgesics.
The goal of this study was to compare the antinociceptive effects of THC and CP55940 in rats by using assays of acute pain-stimulated and pain-depressed behavior that have been used previously to examine effects of opioid and nonopioid compounds (Pereira Do Carmo et al., 2009; Negus et al., 2010a,b, 2011). Pain-stimulated behaviors are defined as behaviors (e.g., withdrawal responses) that increase in rate or intensity after delivery of a noxious stimulus. In assays of pain-stimulated behavior, antinociception is indicated by decreases in the target behavior. However, decreases in pain-stimulated behavior can be produced either by a reduction in sensory sensitivity to the noxious stimulus (true analgesia) or nonselective behavioral depressant effects (sedation, motor impairment) that limit the subject's ability to respond. Sedative drugs such as cannabinoid agonists are especially prone to producing false-positive antinociception in assays of pain-stimulated behavior (De Vry et al., 2004; Finn et al., 2004). In contrast, pain-depressed behaviors are defined as behaviors such as feeding, locomotion, or operant behavior that decrease in rate or intensity after delivery of a noxious stimulus. Assays of pain-depressed behavior have two attributes important to the assessment of candidate analgesics. First, antinociception is indicated by increases in the target behavior, and, as a result, assays of pain-depressed behavior are not vulnerable to false-positive effects caused by nonselective behavioral depression (Negus et al., 2010a,b, 2011). Second, assays of pain-depressed behavior may model pain-related functional impairment and/or depressed mood used to assess pain in both human and veterinary medicine (Cleeland and Ryan, 1994; National Research Council, 2003; Dworkin et al., 2005) and thus may provide insight into the effects of candidate analgesics on these clinically relevant components of pain (Negus et al., 2006, 2010a). In view of these attributes, we have argued that assays of pain-depressed behavior may complement conventional assays of pain-stimulated behavior and increase the predictive validity of preclinical candidate analgesic assessment (Negus et al., 2006, 2010a). Given the poor efficacy of THC and other cannabinoid receptor agonists to treat acute pain in humans (Raft et al., 1977; Buggy et al., 2003; Naef et al., 2003; Beaulieu, 2006; Kraft et al., 2008; Klooker et al., 2011) we predicted that THC and CP55940 would not produce antinociception in assays of acute pain-depressed behavior in rats despite the apparent efficacy of these drugs in standard assays of pain-stimulated behavior.
In the present study, intraperitoneal injection of dilute acid served as an acute noxious stimulus to stimulate stretching (a pain-stimulated behavior) and depress intracranial self-stimulation (ICSS; a pain-depressed operant behavior in which subjects respond on a lever to receive pulses of electrical stimulation delivered via electrodes implanted in the brain's “reward pathway”) (Pereira Do Carmo et al., 2009; Negus et al., 2010a,b, 2011). Initial experiments indicated that the cannabinoid agonists THC and CP55940 failed to produce antinociception in the assay of acid-depressed ICSS. Two follow-up studies were conducted to further evaluate conditions under which THC and/or CP55940 might be effective. First, previous studies with another drug class (δ-opioid agonists) showed that the expression of antinociception in the assay of acid-depressed ICSS could be obscured by rate-decreasing effects, but repeated drug treatment could produce selective tolerance to rate-decreasing effects and unmask antinociception (Negus et al., 2012b). Accordingly, THC effects in assays of acid-stimulated stretching and acid-depressed ICSS were evaluated during chronic THC administration to test the hypothesis that repeated THC might produce selective tolerance to rate-decreasing effects and unmask antinociception in the assay of acid-depressed ICSS. Second, the effects of THC and CP55940 were evaluated in an assay of acid-induced depression of feeding (Stevenson et al., 2006). Feeding is reliably stimulated by THC and other cannabinoid agonists in the absence of pain (Williams et al., 1998; Miller et al., 2004; Järbe and DiPatrizio, 2005; Farrimond et al., 2011), suggesting that cannabinoids might be more effective in blocking acid-induced depression of feeding than acid-induced depression of ICSS. The nonsteroidal anti-inflammatory drug (NSAID) and clinically effective analgesic ketoprofen (Flecknell, 2009; Sarzi-Puttini et al., 2010) was tested as a positive control in assays of acid-stimulated and acid-depressed behavior.
Materials and Methods
Subjects
Seventy-nine male Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing approximately 300 to 320 g (age 10–11 weeks) at the time of surgery and/or delivery, were individually housed and maintained on a 12-h light/dark cycle with lights on from 6:00 AM to 6:00 PM. Rats had free access to food and water except during testing. Animal maintenance and research were in compliance with the National Institutes of Health's Guide for the Use and Care of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and adhered to guidelines of the Committee for Research (National Research Council, 2003) and Ethical Issues of the International Association for the Study of Pain (Zimmermann, 1983). All animal use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Assay of Lactic Acid-Stimulated Stretching
Behavioral Procedure.
Twenty-six rats that failed to meet the criteria for ICSS within 4 weeks (see below) were used for studies of lactic acid-stimulated stretching as described previously (Pereira Do Carmo et al., 2009; Negus et al., 2010b). During test sessions, rats were placed into an acrylic test chamber (31.0 × 20.1 × 20.0 cm) for a 30-min observation period that began immediately after the injection of dilute lactic acid (1.8% in a volume of 1 ml/kg). A stretch was operationally defined as a contraction of the abdomen followed by a stretching of the hind limbs, and the number of stretches during the observation period was counted.
Studies with acute THC were conducted in four phases. First, a THC dose-effect curve was determined by administering THC (0.32–10 mg/kg or vehicle) 30 min before acid. Doses were delivered in a Latin-square dose order across rats and separated by at least 1 week. Second, the time course of effects produced by 3.2 mg/kg THC was determined by varying the interval between administration of THC and acid (10, 30, 100, and 300 min and 24 h). A dose of 3.2 mg/kg THC was chosen for time-course studies because it was the lowest dose to significantly decrease acid-stimulated stretching during dose-effect testing. Each pretreatment time was tested in a different test session in randomized order, and test sessions were separated by at least 1 week. Third, to assess the role of cannabinoid 1 receptors in mediating THC effects, THC-induced antinociception was evaluated for its sensitivity to antagonism by the cannabinoid 1 receptor antagonist rimonabant. For these studies, rimonabant (0.01–1.0 mg/kg or vehicle) was administered 20 min before THC (3.2 mg/kg), and acid was administered 30 min after THC. All THC and rimonabant doses were delivered in a Latin-square dose order across rats and separated by at least 1 week.
Finally, to assess the potential for antinociceptive tolerance to repeated THC, acid-stimulated stretching was evaluated after chronic treatment with THC (3.2 mg/kg/day). Initially a vehicle test was conducted in which rats were administered THC vehicle before treatment with acid. Beginning 1 week later, THC (3.2 mg/kg) was administered once daily for 22 days. On days 1, 8, 15, and 22, acid (1.8% in 1 ml/kg) was administered 30 min after THC, and the stretching response was evaluated. The effects of 3.2 mg/kg THC on acid-stimulated stretching were redetermined one additional time 2 weeks after the termination of chronic THC.
To provide a comparison for results with THC, two additional groups of rats were used to evaluate the high-efficacy cannabinoid 1 receptor agonist CP55940 (0.0032–0.1 mg/kg or vehicle) and the NSAID ketoprofen (1 mg/kg or saline). In both cases, the test drug was administered 30 min before acid, and tests were separated by 1 week.
Data Analysis.
Drug effects on acid-stimulated stretching were evaluated by repeated-measures one-way analysis of variance (ANOVA) or t test as appropriate. A significant ANOVA was followed by Newman Keul's or Dunnett's post hoc test, and the criterion for significance was set at p < 0.05.
Assay of Intracranial Self-Stimulation
Surgery.
All rats were anesthetized with isoflurane (2.5–3% in oxygen; Webster Veterinary, Phoenix, AZ) for implantation of stainless-steel electrodes (Plastics One, Roanoke, VA). One pole (the cathode) of each bipolar electrode was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip, and the other pole (the anode) was 0.125 mm in diameter and uninsulated. The cathode was implanted in the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to bregma, 1.7 mm lateral from the midsagittal suture, and 8.8 mm below the skull). The anode was wrapped around one of the three skull screws to serve as the ground, and the skull screws and electrode assembly were secured to the skull with orthodontic resin. The animals were allowed to recover for at least 7 days before commencing ICSS training.
Apparatus.
Experiments were conducted in sound-attenuating boxes that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever (4.5 cm wide, extended 2 cm through the center of one wall, 3 cm off the floor), stimulation lights (three lights colored red, yellow, and green; positioned 7.6 cm directly above the response lever), a 2-W house light, and an ICSS stimulator (MED Associates, St. Albans, VT). Electrodes were connected to the stimulator via a swivel connector (model SL2C; Plastics One). The stimulator was controlled by computer software that also controlled programming parameters and data collection (MED Associates).
Behavioral Procedure.
After initial shaping of lever press responding, rats were trained under a continuous reinforcement schedule of brain stimulation by using procedures similar to those described previously (Carlezon and Chartoff, 2007; Do Carmo et al., 2009; Negus et al., 2010a). During sessions lasting 30 to 60 min, each lever press resulted in the delivery of a 0.5-s train of square wave cathodal pulses (0.1-ms pulse duration), and stimulation was accompanied by illumination of the stimulus lights over the lever. Responses during the 0.5-s stimulation period did not earn an additional stimulation. Initially, the frequency of stimulation was held constant at 158 Hz, and the stimulation intensity for each rat was adjusted gradually to the lowest value that would sustain a high rate of reinforcement (> 30 stimulations/min). This intensity (100–280 μA across rats) was then held constant for the remainder of the study, and frequency manipulations were introduced. Sessions involving frequency manipulations consisted of sequential 10-min components. During each component, a descending series of 10 current frequencies (158–56 Hz in 0.05-log increments) was presented, with each frequency available during sequential 1-min frequency trials. Each frequency trial began with a 10-s timeout, during which responding had no scheduled consequences. During the last 5 s of this timeout, five noncontingent “priming” stimulations were delivered at the frequency available during that trial, and the lever lights were illuminated during each stimulation. Noncontingent stimulations were separated by intervals of 0.5 s. This noncontingent stimulation was then followed by a 50-s “response” phase, during which responding produced electrical stimulation under the continuous reinforcement schedule. Training continued with presentation of three to six sequential components per day until rats reliably responded for only the first four to six frequency trials of all components for at least 3 consecutive days. In general, rats were implanted with electrodes and trained on ICSS procedures in groups of 10 to 12. The first six rats in each group to meet training criteria were then advanced to testing, and the remaining rats were assigned to assays of acid-stimulated stretching as described above.
Once training was completed, testing was initiated. The first component of each test session was considered to be an acclimation component, and data from this component were discarded. Data from the second and third “baseline” components were used to calculate baseline parameters of the frequency-rate curves for that session (see Data Analysis). Drugs were administered immediately after removing the subjects from the operant chamber after the third baseline component. Studies of THC effects on ICSS were conducted in four phases. In the first phase, the effects of acute THC on ICSS were studied in two separate groups of rats. In the first group of rats, THC effects on ICSS were studied in the absence of the noxious stimulus (control ICSS). Subjects were placed in their home cages after administration of THC (0.32–10 mg/kg or vehicle) and then transferred back to the operant chambers at designated times (30, 100, 180, 300 min) for two consecutive “test” components, totaling 20 min at each time point. In the second group of rats, THC effects on ICSS were studied in the presence of the noxious stimulus (acid-depressed ICSS). Subjects were administered THC (0.32–3.2 mg/kg or vehicle) 30 min before lactic acid (1.8% in a volume of 1 ml/kg), which was administered immediately before two consecutive test components. THC and acid doses were administered in Latin-square order and separated by at least 1 week. The second phase examined the effects of 3.2 mg/kg THC administered 180 and 300 min before acid treatment. These times were selected because initial results indicated that treatment with 3.2 mg/kg THC significantly decreased acid-stimulated stretching after 180 and 300 min but did not significantly decrease ICSS at these pretreatment times in the absence of a noxious stimulus. For these experiments, subjects were placed in their home cages after THC administration and then injected with acid and transferred back to the operant chamber at the designated time (180 or 300 min) for two consecutive test components. Each pretreatment time/dose combination was tested in a different test session in randomized order, and test sessions were separated by at least 1 week. In the third phase, the ability of rimonabant to block THC effects on ICSS was investigated. In these experiments, rimonabant (1 mg/kg or vehicle) was administered 50 min before testing and 20 min before THC (3.2 mg/kg or vehicle). THC and rimonabant doses were administered in Latin-square order and separated by at least 1 week. In phases 1 to 3, training and test sessions were conducted Monday through Friday for the duration of the experiment, with test sessions conducted on Thursdays or Fridays.
The final phase of studies with THC examined the effects of chronic THC on two separate groups of rats. In the first group, the effects of chronic THC were studied in the absence of the acid noxious stimulus (control ICSS). For these experiments, subjects initially received chronic treatment of THC vehicle (1 ml/kg/day) for 3 weeks while being tested with THC (1–10 mg/kg or vehicle) once/week in a Latin-square dose order. After this treatment regimen, subjects were treated for 11 days with 1 mg/kg/day THC, 11 days with 3.2 mg/kg/day THC, and 11 days with 10 mg/kg/day THC (33 days of total THC treatment). On the last 4 days of treatment with each dose of chronic THC, subjects were tested with THC (1–10 mg/kg or vehicle) one dose/day in a Latin-square dose order. Subjects that received test THC doses lower than the chronic THC dose for that day were administered the difference of the test and chronic doses at the end of the test session. In the second group of rats, the effects of chronic THC were studied in the presence of the noxious stimulus (acid-depressed ICSS). For these experiments, THC vehicle was initially administered before acid vehicle. One week later, THC vehicle was administered before acid. Beginning 1 week later, THC (3.2 mg/kg) was administered once daily for 22 days. On days 1, 8, 15, and 22, acid (1.8% in 1 ml/kg) was administered 30 min after THC, and ICSS was evaluated as described above. The effects of 3.2 mg/kg THC on acid-depressed ICSS were redetermined one additional time 2 weeks after the termination of chronic THC. In both groups of rats, training and test sessions were conducted 7 days/week.
In addition to these studies with THC, two groups of rats were tested with either CP55940 or ketoprofen. For these studies, one group was treated with CP55940 (0.01–0.32 mg/kg or vehicle) and the other with ketoprofen (1 mg/kg or saline) 30 min before treatment with 1.8% lactic acid or lactic acid vehicle (sterile water). Training and test sessions were conducted Monday through Friday for the duration of the experiment, with test sessions conducted on Thursdays or Fridays.
Data Analysis.
The primary dependent variable in this ICSS procedure was the reinforcement rate in stimulations per minute during each frequency trial. To normalize these data, raw reinforcement rates from each trial in each rat were converted to percentage of maximum control rate (%MCR), with the MCR defined as the mean of the maximal rates observed during the second and third baseline components for that session in that rat. Thus, %MCR values for each trial were calculated as (reinforcement rate during a frequency trial ÷ maximum control rate) × 100. For each test session, data from the second and third components were averaged to yield a baseline frequency-rate curve. Data from each test (two consecutive test components) were averaged for each test for each rat. Baseline and test curves were then averaged across rats to yield mean baseline and test curves for each manipulation. For statistical analysis, results were compared by repeated-measures two-way ANOVA, with treatment and ICSS frequency as the two factors. A significant ANOVA was followed by Holm-Sidak post hoc test, and the criterion for significance was set at p < 0.05.
To provide an additional summary of ICSS performance, the total number of stimulations per component was calculated as the sum of stimulations delivered across all 10 frequency trials of each component. Test data were then normalized to individual baseline data by using the equation percentage of baseline total stimulations per component = (mean total stimulations per test component ÷ mean total stimulations per baseline component) × 100. Data were then averaged across rats in each experimental condition and compared by repeated-measures one-way ANOVA or two-way ANOVA where appropriate. A significant one-way ANOVA was followed by Newman Keul's or Dunnett's post hoc test, a significant two-way ANOVA was followed by Holm-Sidak post hoc test, and the criterion for significance was set at p < 0.05.
Assay of Lactic Acid- and Prefeeding-Depressed Feeding
Behavioral Procedure.
Sixteen rats were used for feeding studies. During test sessions, rats were placed into an acrylic test chamber (31 × 20.1 × 20 cm) within a sound- and light-attenuating cabinet for a 30-min feeding session. Rodent 45-mg purified food pellets (Bio-Serv, Frenchtown, NJ) were delivered in preweighed glass Petri dishes (60 × 15 mm) (Corning Life Sciences, Lowell, MA) securely taped to the bottom left corner of the test chamber. Spilled pellets/dust were collected at the end of the session and added back to the Petri dish, which was then reweighed. Percentage of body weight food consumed after each session was determined with the following equation: [(presession dish weight − postsession dish weight) ÷ [daily subject's weight (g)] × 100]. Initial baseline feeding sessions were conducted for 2 weeks until stable feeding baselines were achieved. Studies were conducted in four phases. First, pain-related depression of feeding was established by administering dilute lactic acid (0.56–1.8% or vehicle in a volume of 1 ml/kg) immediately before the test session. For these studies, drug vehicle was also administered 30 min before acid. Second, THC and ketoprofen effects on pain-related depression of feeding were determined by administering THC (0.32–3.2 mg/kg or vehicle) or ketoprofen (1 mg/kg or vehicle) 30 min before the injection of lactic acid (1.8% or vehicle in a volume of 1 ml/kg), which was administered immediately before the test session. Third, satiation-related depression of feeding was established by exposing rats to a 60-min prefeeding session 30 min before the test session. Prefeeding sessions were similar to test sessions, except that they were conducted in separate chambers not housed in sound- and light-attenuated cabinets. Finally, THC and ketoprofen effects on satiation-related depression of feeding were determined by administering THC (0.1–1 mg/kg or vehicle) or ketoprofen (1 mg/kg or vehicle) immediately after a 60-min prefeeding session and 30 min before the test session. THC doses were delivered in a Latin-square dose order across rats and separated by at least 1 week. Ketoprofen was tested after THC. Rats were housed in their home cages with free access to food and water at all times except during feeding sessions.
In addition to these studies with THC and ketoprofen, a group of rats was tested with CP55940 under conditions of acid- and satiation-related depression of feeding. In this study, animals were treated with CP55940 (0.0032–0.032 mg/kg or vehicle) 30 min before the feeding session by using procedures identical to those with THC and ketoprofen. In all feeding studies, rats were housed in their home cages with free access to food and water at all times except during feeding sessions. Training and test sessions were conducted Monday through Friday, with test sessions conducted on Wednesdays or Fridays.
Data Analysis.
Drug effects on acid- and satiation-related depression of feeding were evaluated by repeated-measures one-way ANOVA. A significant ANOVA was followed by Newman Keul's or Dunnett's post hoc test, and the criterion for significance was set at p < 0.05.
Drugs
Lactic acid was purchased from Sigma (St. Louis, MO). THC, CP55940 (see Lichtman and Martin, 1991 for structure information), and rimonabant were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Ketoprofen was purchased from Spectrum Chemical Co. (New Brunswick, NJ). Lactic acid was prepared in sterile water. THC, CP55940, and rimonabant were prepared in a vehicle consisting of ethanol, Emulphor EL-620 (Rhone-Poulenc, Princeton, NJ), and sterile saline in a ratio of 1:1:18, respectively. Ketoprofen was prepared in sterile saline except in feeding tests, in which it was prepared in the same vehicle as THC. All solutions were injected intraperitoneally in a volume of 1 ml/kg.
Results
Effects of THC on Acid-Stimulated Stretching.
Figure 1 shows that THC produced dose-dependent, time-dependent, and rimonabant-reversible antinociception in the assay of acid-stimulated stretching. Intraperitoneal administration of acid (1.8% lactic acid in a volume of 1 ml/kg) stimulated approximately 30 stretches after administration of THC vehicle. Figure 1a shows that stretching was significantly lower 30 min after the administration of 3.2 and 10 mg/kg THC than after THC vehicle. Figure 1b shows that 3.2 mg/kg THC produced a significant reduction in acid-stimulated stretching from 10 to 300 min with recovery after 24 h. Figure 1c shows that rimonabant dose-dependently blocked the antinociceptive effect of 3.2 mg/kg THC, and significant antagonism was achieved at a dose of 1.0 mg/kg rimonabant.
Fig. 1.
THC produced dose-dependent, time-dependent, and rimonabant-reversible blockade of lactic acid-stimulated stretching. a, effects of THC (0.1–10 mg/kg) or its vehicle administered 30 min before acid treatment. Abscissa, dose of THC in milligrams per kilogram. Ordinates, number of stretches observed during a 30-min observation period. b, effects of THC (3.2 mg/kg) administered 10 min to 24 h before acid treatment. Effects of vehicle administered 30 min before acid treatment are included for comparison. Abscissa, time after THC or vehicle administration. c, the effects of 50-min pretreatment with rimonabant (0.01–1.0 mg/kg) or its vehicle and 30 min pretreatment with THC (3.2 mg/kg) before acid treatment. Effects of rimonabant vehicle + THC vehicle + acid are included for comparison. Abscissa, dose rimonabant in milligrams per kilogram. One-way ANOVA indicated significant main effects of THC treatment in a (F5,25 = 6.63; p < 0.001), time in b (F5,25) = 7.65; p < 0.001), and rimonabant dose in c (F4,20 = 11.21; p < 0.001). *, significantly different from vehicle + acid. $, significantly different from 30-min pretreatment with THC (3.2 mg/kg) in b and THC (3.2 mg/kg) + rimonabant vehicle in c as determined by Newman-Keuls post hoc test; p < 0.05. All bars show mean ± S.E.M. in six rats.
Effects of THC on ICSS in the Absence of a Noxious Stimulus.
Figure 2 shows that THC produced a dose-dependent, time-dependent, and rimonabant-reversible decrease in ICSS in the absence a noxious stimulus. During each test session, a baseline frequency-rate curve was determined before testing to permit determination of the MCR for that session. Over the course of the entire study for this group of rats, the average MCR was 59.7 ± 2.6 stimulations/trial, and the average baseline total stimulations was 266.6 ± 56.8. Reinforcement rates during each frequency trial of a session were then expressed as a percentage of that session's MCR. The average baseline frequency-rate curve for studies with THC is shown in Fig. 2a as a gray line. Rats generally did not respond at frequencies of 56 to 89 Hz, and reinforcement rates increased across a frequency range of 89 to 158 Hz. Maximum reinforcement rates were usually observed at the highest stimulation frequencies. When administered 30 min before an ICSS session, THC produced a dose-dependent rightward and downward shift in the ICSS frequency-rate curve (Fig. 2a). Low doses of 0.32 and 1 mg/kg THC had no effect on control ICSS in the absence of the acid noxious stimulus; however, treatment with 3.2 mg/kg THC significantly decreased reinforcement rates at a single frequency of 89 Hz, and treatment with 10 mg/kg THC significantly decreased reinforcement rates at frequencies of 89 to 158 Hz compared with treatment with THC vehicle. THC also produced a dose-dependent and time-dependent decrease in total stimulations (Fig. 2b). Low doses of 0.32 and 1 mg/kg THC had no effect on total stimulations at any time point, but 3.2 mg/kg THC significantly decreased total stimulations at a pretreatment time of 30 min, and 10 mg/kg THC significantly decreased total stimulations at pretreatment times of 30 to 180 min. THC-induced decreases in ICSS were also blocked by pretreatment with rimonabant (Fig. 2c). Rimonabant (1 mg/kg) administered 50 min before testing had no effect on ICSS alone, but significantly blocked decreases in ICSS induced by 30 min pretreatment with 3.2 mg/kg THC.
Fig. 2.
THC produced dose-dependent, time-dependent, and rimonabant-reversible depression of ICSS in the absence of a noxious stimulus. a, ICSS frequency-rate curves determined 30 min after treatment with THC (0.32–10 mg/kg) or its vehicle. Abscissa, frequency of electrical brain stimulation in hertz (log scale). Ordinate, %MCR. The average baseline ICSS frequency-rate curve for the entire study in this group of rats is shown by the gray line for comparison, but these data were not included in statistical analysis. Two-way ANOVA indicated a significant main effect of THC treatment (F4,20 = 11.78; p < 0.001), a significant main effect of frequency (F9,45 = 19.31; p < 0.001), and a significant frequency × treatment interaction (F36,180 = 4.77; p < 0.001). Filled symbols indicate frequencies at which reinforcement rates after THC treatment were significantly lower than rates after THC vehicle treatment as determined by Holm-Sidak post hoc test; p < 0.05. All data show mean ± S.E.M. in six rats. b, the total number of stimulations per component expressed as a percentage of baseline stimulations per component after treatment with THC (0.32–10.0 mg/kg) or its vehicle at various pretreatment times. Abscissa, time after THC or vehicle administration. Ordinate, percentage of baseline total number of stimulations per component. Two-way ANOVA indicated a significant main effect of THC treatment (F4,20 = 12.35; p < 0.001) and a significant treatment × time interaction (F12,60 = 5.93; p < 0.001). Filled symbols indicate significantly lower than vehicle treatment at the indicated times as determined by Holm-Sidak post hoc test; p < 0.05. All data show mean ± S.E.M. in six rats. c, the total number of stimulations per component expressed as a percentage of baseline stimulations per component after 50-min pretreatment with rimonabant (1 mg/kg) or its vehicle and 30-min pretreatment with THC (3.2 mg/kg) or its vehicle. Abscissa, dose of rimonabant in milligrams per kilogram. Ordinate, percentage of baseline total number of stimulations per component. One-way ANOVA indicated a significant main effect of treatment (F3,12 = 17.04; p < 0.001). *, significantly different from rimonabant vehicle + THC vehicle. $, significantly different from rimonabant vehicle + THC (3.2 mg/kg) as determined by Newman-Keuls post hoc test; p < 0.05. All bars show mean ± S.E.M. in five rats.
Effects of THC on Acid-Induced Depression of ICSS.
Figure 3 shows that the same noxious stimulus used in the stretching assay (intraperitoneal injection of 1.8% lactic acid in 1 ml/kg) depressed ICSS. Treatment with acid vehicle had little effect on the frequency-rate curve; however, treatment with 1.8% lactic acid depressed ICSS, producing a significant rightward shift in the frequency-rate curve and a decrease in total stimulations delivered across all frequencies. Figure 4 shows that THC failed to produce antinociception in the assay of acid-depressed ICSS. Rather, when administered 30 min before acid treatment, THC produced a further dose-dependent depression of ICSS. Low doses of 0.32 and 1 mg/kg THC significantly decreased ICSS at a single frequency of 141 Hz, and treatment with 3.2 mg/kg THC significantly decreased ICSS at frequencies of 126, 141, and 158 Hz compared with treatment with THC vehicle (Fig. 4a). In addition, treatment with THC (0.32–3.2 mg/kg) or its vehicle 30 min before acid administration significantly decreased total stimulations delivered across all frequencies, and there was a trend for treatment with THC to exacerbate acid-induced decreases in total stimulations, but this trend did not achieve statistical significance (Fig. 4b). A dose of 3.2 mg/kg THC also failed to block acid-induced depression of ICSS when it was administered 180 or 300 min before acid treatment (Fig. 4c). These were times at which this dose of THC significantly decreased acid-stimulated stretching (Fig. 1b) but did not significantly decrease ICSS in the absence of a noxious stimulus (Fig. 2b).
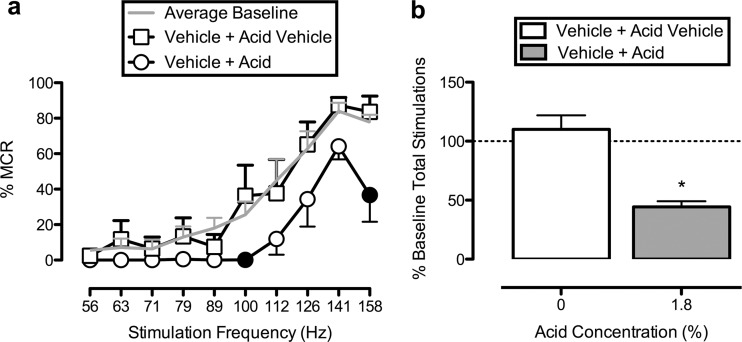
Fig. 3.
Lactic acid depresses ICSS. a, ICSS frequency-rate curves determined after treatment with THC vehicle 30 min before lactic acid vehicle or 1.8% lactic acid administration. Abscissa, frequency of electrical brain stimulation in hertz (log scale). Ordinate, %MCR. The average baseline ICSS frequency-rate curve for the entire study in this group of rats is shown by the gray line for comparison, but these data were not included in statistical analysis. Two-way ANOVA indicated a significant main effect of frequency (F9,36 = 23.92; p < 0.001) and a significant frequency × treatment interaction (F9,36 = 2.40; p = 0.030). Filled symbols indicate frequencies at which reinforcement rates after acid treatment were significantly lower than rates after acid vehicle treatment as determined by Holm-Sidak post hoc test; p < 0.05. b, the total number of stimulations per component expressed as a percentage of baseline stimulations per component determined after treatment with THC vehicle 30 min before lactic acid vehicle or 1.8% lactic acid administration. Abscissa, lactic acid concentration. Ordinate, percentage of baseline total number of stimulations per component. *, 1.8% lactic acid significantly depressed ICSS compared with 0% lactic acid (i.e., lactic acid vehicle) as determined by paired t test (t4 = 6.95; p = 0.002). All bars show mean ± S.E.M. in five rats.
Fig. 4.
THC exacerbates lactic acid-induced depression of ICSS. a, ICSS frequency-rate curves determined after treatment with THC (0.32–3.2 mg/kg) or its vehicle 30 min before acid administration. Abscissa, frequency of electrical brain stimulation in hertz (log scale). Ordinate, %MCR. The THC vehicle + acid vehicle frequency-rate curve is shown by the gray line for comparison, but these data were not included in statistical analysis. Two-way ANOVA indicated a significant main effect of THC treatment (F3,12 = 5.16; p = 0.016), a significant main effect of frequency (F9,36 = 6.68; p < 0.001), and a significant frequency × treatment interaction (F27,108 = 4.62; p < 0.001) Filled symbols indicate frequencies at which reinforcement rates after THC + acid treatment were significantly lower than after vehicle + acid treatment as determined by Holm-Sidak post hoc test; p < 0.05. All data show mean ± S.E.M. in five rats. b, the total number of stimulations per component expressed as a percentage of baseline stimulations per component after treatment with THC (0.32–3.2 mg/kg) or its vehicle 30 min before acid administration. Abscissa, dose THC in milligrams per kilogram. Ordinate, percentage of baseline total number of stimulations per component. One-way ANOVA indicated a significant main effect of treatment (F4,16 = 19.26; p < 0.001). *, treatment with THC vehicle + acid or THC + acid significantly depressed ICSS compared with treatment with THC vehicle + acid vehicle as determined by Newman Keul's post hoc test; p < 0.05. All bars show mean ± S.E.M. in five rats. c, the total number of stimulations per component expressed as a percentage of baseline stimulations per component after treatment with THC vehicle 30 min before acid vehicle or acid administration or THC (3.2 mg/kg) 30 to 300 min before acid administration. Abscissa, time after THC or vehicle administration. Ordinate, percentage of baseline total number of stimulations per component. One-way ANOVA indicated a significant main effect of treatment (F4,12 = 14.43; p < 0.001). *, treatment with THC vehicle + acid or THC (3.2 mg/kg) + acid significantly depressed ICSS compared with treatment with THC vehicle + acid vehicle as determined by Newman Keul's post hoc test; p < 0.05. All bars show mean ± S.E.M. in four rats.
Effects of CP55940 and Ketoprofen on Acid-Stimulated Stretching and ICSS in the Absence or Presence of Acid.
Figure 5, a and b shows that CP55940, like THC, produced antinociception in the assay of acid-stimulated stretching but not in the assay of acid-depressed ICSS. However, unlike THC, CP55940 was approximately 10-fold more potent to produce antinociception in the assay of acid-stimulated stretching than to decrease control ICSS in the absence of the acid stimulus. In particular, doses of 0.01 and 0.032 mg/kg CP55940 produced significant antinociception in the assay of acid-stimulated stretching (Fig. 5a), whereas 10-fold higher doses of 0.1 to 0.32 mg/kg were required to decrease control ICSS (Fig. 5b, white bars). Despite this evidence for selective antinociception, CP55940 still failed to block acid-induced depression of ICSS. Rather, CP55940 only exacerbated acid-induced depression of ICSS (Fig. 5b, black bars) at the same doses of 0.1 and 0.32 mg/kg that decreased control ICSS (Fig. 5b, white bars). Figure 5, c and d shows that, in contrast to THC and CP55940, the NSAID ketoprofen produced antinociception in assays of both acid-stimulated stretching and acid-depressed ICSS. Thus, a dose of 1.0 mg/kg ketoprofen significantly reduced acid-stimulated stretching (Fig. 5c). The same dose of 1.0 mg/kg ketoprofen had no effect on control ICSS, but significantly blocked acid-induced depression of ICSS (Fig. 5d).
Fig. 5.
Effects of CP55940 (a and b) and ketoprofen (c and d) on lactic acid-stimulated stretching and lactic acid-induced depression of ICSS. Abscissae, drug dose in milligram/kilogram. a and c ordinates, number of stretches observed during 30-min observation periods. b and d ordinates, percentage of baseline total number of stimulations per component. a, CP55940 dose-dependently blocked acid-stimulated stretching (F4,16 = 38.80; p < 0.001) as indicated by one-way ANOVA. b, two-way ANOVA on ICSS data in the presence and absence of acid treatment indicated a significant main effect of CP55940 dose (F4,16 = 34.44; p < 0.01) and a significant main effect of acid treatment (F1,4 = 15.69; p = 0.017), but no significant interaction. c, ketoprofen blocked acid-induced stimulation of stretching (t3 = 4.43; p = 0.021) as indicated by t test. d, two-way ANOVA on ICSS data in the presence and absence of acid treatment indicated a significant main effect of ketoprofen dose (F1,3 = 16.95; p = 0.026) and a significant main effect of acid treatment (F1,3 = 13.71; p = 0.034), but no significant interaction. In a and c, *, significant difference from a 0 drug dose (i.e., vehicle) + lactic acid as determined by one-way ANOVA followed by Dunnett's post hoc test (CP55940) or t test (ketoprofen); p < 0.05. In b and d, *, significant difference from a 0 drug dose (i.e., vehicle) + lactic acid vehicle. $, significant difference from a 0 drug dose + lactic acid. #, significant depression of ICSS by lactic acid as determined by two-way ANOVA followed by Holm-Sidak post hoc test; p < 0.05. All bars show mean ± S.E.M. in five rats (CP55940) or four rats (ketoprofen).
Effects of Chronic THC Treatment on Acid-Stimulated Stretching and ICSS in the Absence or Presence of Acid.
Chronic THC treatment produced a dose-dependent tolerance to THC-induced rate-decreasing effects on control ICSS. Specifically, complete tolerance was observed to the rate-decreasing effects of 3.2 mg/kg THC after chronic treatment with 3.2 and 10 mg/kg/day THC but not after 1 mg/kg/day THC (Fig. 6). In the assay of acid-stimulated stretching, chronic THC treatment (3.2 mg/kg/day) produced duration-dependent partial tolerance to THC-induced antinociceptive effects. Partial tolerance to THC-induced antinociceptive effects was observed on day 15 of chronic THC treatment (Fig. 7a). No greater tolerance was produced by an additional 7 days of treatment, and full THC antinociception recovered 2 weeks after termination of chronic THC. In contrast, chronic THC treatment (3.2 mg/kg/day) did not produce antinociception in the assay of acid-depressed ICSS (Fig. 7b) at treatment durations that produced significant antinociception in the assay of acid-stimulated stretching (Fig. 7a) and complete tolerance to THC-induced rate-decreasing effects on control ICSS (Fig. 6). Analysis of frequency-rate curves (data not shown) indicated that THC initially exacerbated acid-induced depression of ICSS on days 1 and 8 of chronic THC treatment, and tolerance to this effect developed by days 15 and 22 of chronic THC treatment. No greater tolerance was produced by an additional 7 days of treatment, and THC exacerbation of acid-induced depression of ICSS recovered 2 weeks after termination of chronic THC.
Fig. 6.
Chronic administration of THC produces tolerance to its rate-decreasing effects on ICSS in the absence of a noxious stimulus. Abscissa, THC challenge dose (milligram/kilogram). Ordinate, percentage of baseline total number of stimulations per component. Two-way ANOVA indicated a significant main effect of chronic THC dose (F3,12 = 16.27; p < 0.001), a significant main effect of THC challenge dose (F3,12 = 55.33; p < 0.001), and a significant interaction (F9,36 = 3.58; p = 0.003). Filled symbols indicate chronic THC + THC challenge dose combinations after which reinforcement rates were significantly higher than rates after the same THC challenge administered during chronic vehicle, as determined by Holm-Sidak post hoc test; p < 0.05. All bars show mean ± S.E.M. in five rats.
Fig. 7.
Chronic administration of THC produces partial tolerance to its antinociceptive effects in the assay of acid-stimulated stretching but does not unmask antinociceptive effects in the assay of acid-depressed ICSS. Abscissae, day of THC (3.2 mg/kg/day) administration. a ordinate, number of stretches observed during 30-min observation periods. b ordinate, percentage of baseline total number of stimulations per component. Effects of a 2-week washout period after chronic THC administration are also shown for comparison but were not included in the statistical analysis. a, the effects of chronic administration of THC in the assay of acid-stimulated stretching. One-way ANOVA indicated a significant main of THC treatment duration (F4,20 = 9.41; p < 0.001). *, treatment with THC produced significant antinociception compared with treatment with THC vehicle on days 1, 8, 15, and 22 of chronic THC administration. $, significant tolerance to this antinociceptive effect on day 15 compared with day 1 of chronic THC administration as determined by Newman-Keul's post hoc test; p < 0.05. b, the effects of chronic administration of THC in the assay of acid-depressed ICSS. One-way ANOVA indicated a significant main effect of treatment (F5,20 = 10.08; p < 0.001). *, treatment with acid significantly depressed ICSS compared with treatment with lactic acid vehicle as determined by Newman-Keul's post hoc test; p < 0.05. Chronic THC administration failed to alter THC effects on acid-induced depression of ICSS. All bars show mean ± S.E.M. in six rats (stretching) or five rats (ICSS).
Effects of THC, Ketoprofen, and CP55940 on Feeding Depressed by Acid or Prefeeding.
Lactic acid produced a concentration-dependent decrease in food consumption, and exposing rats to a 60-min prefeeding session before testing also significantly decreased food consumption by approximately the same extent as 1.8% lactic acid (Fig. 8a). Neither THC nor ketoprofen significantly altered food consumption in the absence of acid or prefeeding (Fig. 8b). Ketoprofen, but not THC, significantly blocked acid-induced depression of feeding (Fig. 8c), and in contrast, THC, but not ketoprofen, significantly attenuated prefeeding-induced depression of feeding (Fig. 8d). CP55940 did not produce significant effects on acid-induced (Fig. 8e) or prefeeding-induced (Fig. 8f) depression of feeding, although an intermediate dose of 0.01 mg/kg CP55940 did more than double mean food consumption after prefeeding. Higher CP55940 doses were not tested in the feeding assays because they significantly decreased both control and acid-depressed ICSS (Fig. 5b).
Fig. 8.
THC and ketoprofen effects on feeding depressed by acid or prefeeding. a, the effects of lactic acid vehicle, lactic acid (0.56–1.8%), or a 60-min prefeeding session on feeding. Abscissa, percentage of acid concentration. Ordinates, percentage of body weight food consumed in grams during a 30-min feeding session. One-way ANOVA indicated a significant main effect of treatment (F4,20 = 16.30; p < 0.001). *, lactic acid (1–1.8%) or a 60-min prefeeding session significantly decreased feeding as determined by Dunnett's post hoc test; p < 0.05. All bars show mean ± S.E.M. in six rats. b, effects of THC (0.32–3.2 mg/kg), ketoprofen (1 mg/kg), or vehicle administered 30 min before acid vehicle treatment on control feeding. Abscissa, drug dose in milligrams per kilogram. THC and ketoprofen did not significantly alter feeding in the absence of lactic acid or during a 60-min prefeeding session. All bars show mean ± S.E.M. in seven rats. c, the effects of THC (0.32–1 mg/kg), ketoprofen (1 mg/kg), or vehicle administered 30 min before 1.8% lactic acid. Abscissa, drug dose in milligrams per kilogram. One-way ANOVA indicated a significant main effect of treatment (F4,24 = 10.46; p < 0.001). *, ketoprofen significantly blocked acid-induced depression of feeding as determined by Dunnett's post hoc test; p < 0.05. All bars show mean ± S.E.M. in seven rats. d, the effects of THC (0.1–1 mg/kg), ketoprofen (1 mg/kg), or vehicle administered immediately after a 60-min prefeeding session and 30 min before the test session. Abscissa, drug dose in milligrams per kilogram. One-way ANOVA indicated a significant main effect of treatment (F4,28 = 2.88; p = 0.041). *, THC significantly blocked prefeeding-induced depression of feeding as determined by Dunnett's post hoc test; p < 0.05. All bars show mean ± S.E.M. in eight rats. e, the effects of CP55940 (0.0032–0.032 mg/kg or vehicle) administered 30 min before 1.8% lactic acid. One-way ANOVA indicated a significant main effect of acid treatment (F4,28 = 19.64; p < 0.001). *, lactic acid (1.8%) significantly decreased feeding as determined by Newman-Keuls post hoc test; p < 0.05. All means ± S.E.M. represent eight rats. f, the effects of CP55940 (0.0032–0.032 mg/kg or vehicle) administered immediately after a 60-min prefeeding session and 30 min before the test session. One-way ANOVA indicated a significant main effect of prefeeding treatment (F4,28 = 4.25; p = 0.008). *, a 60-min prefeeding session significantly decreased feeding versus CP55940 vehicle alone as determined by Newman-Keuls post hoc test; p < 0.05. CP55940 did not alter feeding under either condition tested. All means ± S.E.M. represent eight rats.
Discussion
The purpose of this study was to assess the effects of the cannabinoid receptor agonists THC and CP55940 in assays of pain-stimulated and pain-depressed behavior in rats. There were four main findings. First, in agreement with the literature on antinociceptive effects of cannabinoid agonists in assays of pain-stimulated behavior (Rice, 2006; Karst et al., 2010), THC and CP55940 dose-dependently decreased acid-stimulated stretching. Second, THC and CP55940 also decreased control ICSS in the absence of the acid noxious stimulus; however, depression of control ICSS was shorter in duration (THC) or occurred at lower doses (CP55940) than depression of acid-stimulated stretching. Furthermore, chronic administration of THC produced complete tolerance to THC-induced depression of control ICSS and only produced partial tolerance to THC-induced antinociception in the assay of acid-stimulated stretching. These findings suggest that nonselective behavioral depression may have contributed to, but could not account entirely for, cannabinoid antinociception in the assay of acid-stimulated stretching. Third, despite evidence for antinociception in the assay of acid-stimulated stretching, both acute and chronic THC and acute CP55940 failed to produce antinociception in the assay of acid-depressed ICSS. Finally, THC and CP55940 also failed to produce antinociception in the assay of acid-depressed feeding. Collectively, these findings demonstrate that although cannabinoid agonists are effective in producing antinociception in assays of pain-stimulated behavior, they are ineffective in assays of acute pain-depressed behavior. The effects of THC and CP55940 contrast with the antinociceptive efficacy of clinically effective analgesics such as ketoprofen (Negus et al., 2012a; present study) and morphine (Negus et al., 2006; Pereira Do Carmo et al., 2009) in these assays of pain-depressed behavior. Moreover, the lack of cannabinoid efficacy in these assays of acute pain-depressed behavior in rats agrees with the general lack of efficacy of cannabinoids in treating acute pain in humans (Raft et al., 1977; Rice, 2006; Karst et al., 2010; Kraft, 2012). Taken together, these results do not support the use of cannabinoid agonists to treat the behavioral depressant effects of acute pain and further suggest that preclinical assays of pain-depressed behavior may be useful during cannabinoid drug development for predicting clinical drug effects on pain in humans.
Cannabinoid Agonist Effects on Pain-Stimulated Behavior.
In assays of pain-stimulated behavior, delivery of a noxious stimulus increases the rate or intensity of the target behavior, and drug-induced antinociception is inferred from drug-induced decreases in the target behavior (Negus et al., 2010a). In the present study, acid stimulated a stretching response in rats, and THC and CP55940 produced antinociception insofar as they decreased acid-stimulated stretching. The antinociceptive effects of THC and CP55940 in the present study agree with a large literature showing that cannabinoid agonists produce antinociception in nearly all assays of pain-stimulated behavior (for recent reviews, see Rice, 2006; Karst et al., 2010). For example, previous studies in rodents have shown that cannabinoid agonists decreased stretching elicited by intraperitoneal acid administration (Sofia et al., 1975; Anikwue et al., 2002; Booker et al., 2009), first and second phases of nociceptive behavior elicited by intraplantar formalin injection (Finn et al., 2004; Khodayar et al., 2006), tail-flick/paw-withdrawal responses elicited by noxious heat (Lichtman and Martin, 1991; De Vry et al., 2004; Wiley et al., 2007), and hypersensitive withdrawal responses elicited by thermal/mechanical stimuli in inflammatory or neuropathic pain models (Cheng and Hitchcock, 2007; Elikkottil et al., 2009; Sain et al., 2009). As in the present study, cannabinoid antinociception is often shown to be dose- and/or time-dependent, and sensitivity to rimonabant antagonism or genetic knockout of cannabinoid 1 receptors has been interpreted as evidence of cannabinoid 1 receptor mediation (Monory et al., 2007; Booker et al., 2009). It is generally appreciated that nonselective behavioral depression may confound measures of cannabinoid antinociception in assays of pain-stimulated behavior (De Vry et al., 2004; Finn et al., 2004), and in the present study, THC and CP55940 produced evidence of nonselective behavioral depression insofar as they decreased ICSS in the absence of pain. However, THC-induced depression of acid-stimulated stretching was longer lasting than THC-induced depression of control ICSS. Furthermore, chronic THC administration produced complete tolerance to THC-induced depression of control ICSS, whereas only partial tolerance developed to THC-induced depression of acid-stimulated stretching. Finally, CP55940 produced antinociception in the assay of acid-stimulated stretching at doses that produced no effect on control ICSS. These findings provide evidence for a selective antinociceptive effect of cannabinoids in assays of pain-stimulated behavior. Likewise, other studies of pain-stimulated behavior on THC and CP55940 have found comparable dose selectivity for antinociception versus nonselective behavioral depression (Fox et al., 2001; Booker et al., 2009). Overall, the robust and reliable antinociceptive effects of cannabinoid agonists in preclinical assays of pain-stimulated behavior have encouraged development of cannabinoids as candidate analgesics.
Cannabinoid Agonist Effects on Pain-Depressed Behavior.
In assays of pain-depressed behavior, delivery of a noxious stimulus decreases the rate or intensity of the target behavior, and drug-induced antinociception is inferred from drug-induced increases in the target behavior (Negus et al., 2010a). In the present study, acid-induced depression of ICSS and feeding served as assays of pain-depressed behavior, and in these assays, THC and CP55940 failed to produce antinociception. Rather, THC and CP55940 only exacerbated acid-induced depression of ICSS and were ineffective in the assay of acid-depressed feeding. This lack of cannabinoid antinociception cannot be attributed to a lack of assay sensitivity. In the present study and a previous study (Negus et al., 2012a), the NSAID ketoprofen blocked acid-stimulated stretching and acid-induced depression of ICSS and feeding, and these data agree with the clinical efficacy of ketoprofen for the treatment of acute pain in animals (Flecknell, 2009) and humans (Sarzi-Puttini et al., 2010). Likewise, the μ-opioid receptor agonist and clinically effective analgesic morphine also blocked acid-stimulated stretching and acid-induced depression of ICSS in rats (Pereira Do Carmo et al., 2009; Negus et al., 2010b) and acid-induced depression of feeding in mice (Stevenson et al., 2006). Both NSAID and μ-opioid analgesics have also been shown to block other examples of pain-depressed behavior including acid-induced depression of locomotion and wheel running in mice (Stevenson et al., 2009; Miller et al., 2011), laparotomy-induced depression of locomotion and food-maintained operant responding in rats (Martin et al., 2007), and depression of locomotion and wheel running induced by bilateral inflammation of the knee joints by complete Freund's adjuvant in rats (Matson et al., 2007; Cobos et al., 2012). Taken together, these results suggest that cannabinoid agonist effects on pain-depressed behavior are opposite to those produced by clinically effective NSAID and opioid analgesics.
Determinants of the poor efficacy of cannabinoids in assays of pain-depressed behavior remain to be understood. However, three points warrant mention. First, cannabinoids can produce general behavioral depressant effects manifested in this study as decreases in control ICSS, and such general behavioral depressant effects could obscure the expression of antinociception in assays of pain-depressed behavior (Negus et al., 2010a). However, several findings argue against a major influence of this factor. For example, THC/CP55940 failed to block acid-induced depression of ICSS even at times/doses that did not decrease control ICSS but did block acid-stimulated stretching. Moreover, in contrast to previous results with a δ-opioid receptor agonist (Negus et al., 2012b), chronic administration of THC did not unmask antinociceptive effects of THC in the assay of acid-depressed ICSS, despite producing complete tolerance to THC-induced rate-decreasing effects on control ICSS and only partial tolerance to THC-induced antinociception in the assay of acid-stimulated stretching. Finally, THC failed to block pain-related depression of feeding at a dose that did attenuate satiation-related depression of feeding in this study and stimulated feeding by rats in other studies (Williams et al., 1998; Järbe and DiPatrizio, 2005). Consequently, just as nonselective behavioral depression could not account entirely for the apparent presence of cannabinoid antinociception in the assay of pain-stimulated behavior, it also cannot account entirely for the absence of cannabinoid antinociception in the assays of pain-depressed behavior.
Second, the neural circuits that mediate acid-induced stimulation of stretching and depression of ICSS are incompletely mapped but may be dissociable (Willis, 2009). For example, noxious stimuli activate both serial and parallel spinal and supraspinal pathways, and different neural circuits have been associated with different components of pain (sensory versus affective components of pain) (Price, 2002; Borsook and Becerra, 2011). Results of the present study suggest that THC and other cannabinoids may be more effective in modulating neural circuits that mediate acid-induced stimulation of stretching than those mediating acid-induced depression of ICSS.
Finally, the present study evaluated the effects of systemic cannabinoid administration on pain-related behaviors produced by an acute chemical noxious stimulus delivered to the abdominal cavity, and poor cannabinoid antinociception may be related to these or other experimental variables. Future research would be required to assess the degree to which other routes of administration (e.g., local treatment of inflamed tissue) or other cannabinoid agonists (e.g., agonists selective for cannabinoid 2 receptors) might produce antinociception in assays of other behaviors depressed by more chronic pain states, other modalities of noxious stimuli, or noxious stimulation of other parts of the body. However, the present results demonstrate the potential for diametrically opposite effects of cannabinoid receptor agonists on pain-stimulated and pain-depressed behaviors elicited by the same noxious stimulus. Moreover, these results distinguish these cannabinoids from clinically effective analgesics and suggest that cannabinoid antinociception in assays of acute pain-stimulated behavior cannot be attributed to a simple and selective blockade of sensitivity to noxious stimuli.
Abuse-Related Effects of Cannabinoids.
In control experiments for this study, cannabinoid effects on ICSS were evaluated in the absence of the acid noxious stimulus. Drug-induced facilitation of ICSS under these conditions is often interpreted as an abuse-related effect (Carlezon and Chartoff, 2007), but THC and CP55940 produced only decreases in ICSS. These results are consistent with previous studies showing only rate-decreasing effects of cannabinoids on ICSS (Vlachou et al., 2005, 2007), although other studies have found weak facilitation of ICSS by THC under certain conditions (Gardner et al., 1988; Panagis et al., 2008). Taken together, these findings suggest that THC and related cannabinoids often fail to produce abuse-related facilitation of ICSS under conditions that are sensitive to facilitation by other classes of abused drugs.
Implications for Preclinical Strategies of Drug Development.
Preclinical assays of pain and analgesia play a critical role in analgesic drug development, but there is a growing appreciation that drug effects in conventional preclinical assays of pain-stimulated behavior are often poor predictors of clinical analgesic efficacy in humans (Blackburn-Munro, 2004; Negus et al., 2006; Whiteside et al., 2008; Mogil, 2009). Results with THC and CP55940 have illustrated this discordance insofar as cannabinoid agonists produce robust and reliable antinociception in most assays of acute pain-stimulated behavior but little or no analgesia against acute pain in humans (Rice, 2006; Karst et al., 2010; Kraft, 2012). For example, oral delivery of THC or other cannabinoids lacked analgesic efficacy or exacerbated pain in most well controlled clinical studies of postoperative or acute experimental pain (Raft et al., 1977; Buggy et al., 2003; Naef et al., 2003; Beaulieu, 2006; Kraft et al., 2008; Klooker et al., 2011); for the lone exception, see Campbell et al. (2001). Likewise, smoked marijuana at doses up to those producing untoward motor/cognitive/subjective effects produced little or no change in sensitivity to acute thermal, mechanical, or chemical noxious stimuli in clinical laboratory studies, and as with oral cannabinoids, pain ratings were sometimes worsened by smoked cannabis (Greenwald and Stitzer, 2000; Wallace et al., 2007). The present study suggests that preclinical assays of pain-depressed behavior may yield results that are more predictive of clinical cannabinoid effects than conventional assays of pain-stimulated behavior. As such, this study supports the utility of assays of pain-depressed behavior in the development of cannabinoid analgesics. It will be of particular interest for future studies to assess the efficacy of cannabinoids in assays of chronic pain-depressed behavior, because nearly all clinical evidence for cannabinoid analgesia in humans has been under conditions of chronic pain (Rice, 2006; Karst et al., 2010; Kraft, 2012).
Acknowledgments
We thank Robert O'Connell for technical assistance and Drs. Aron Lichtman and Robert Vann for gifts of THC and rimonabant.
This research was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS070715] and the National Institutes of Health National Institute on Drug Abuse [Grant F31-DA032267].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- THC
- Δ9-tetrahydrocannabinol
- ICSS
- intracranial self-stimulation
- NSAID
- nonsteroidal anti-inflammatory drug
- MCR
- maximum control rate
- %MCR
- percentage of MCR
- ANOVA
- analysis of variance
- CP55940
- 3-(2-hydroxy-4-(1,1-dimethylheptyl)phenyl)-4-(3-hydroxypropyl)cyclohexanol.
Authorship Contributions
Participated in research design: Kwilasz and Negus.
Conducted experiments: Kwilasz.
Performed data analysis: Kwilasz and Negus.
Wrote or contributed to the writing of the manuscript: Kwilasz and Negus.
References
- Anikwue R, Huffman JW, Martin ZL, Welch SP. (2002) Decrease in efficacy and potency of nonsteroidal anti-inflammatory drugs by chronic Δ9-tetrahydrocannabinol administration. J Pharmacol Exp Ther 303:340–346 [DOI] [PubMed] [Google Scholar]
- Beaulieu P. (2006) Effects of nabilone, a synthetic cannabinoid, on postoperative pain. Can J Anaesth 53:769–775 [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G. (2004) Pain-like behaviours in animals–how human are they? Trends Pharmacol Sci 25:299–305 [DOI] [PubMed] [Google Scholar]
- Booker L, Naidu PS, Razdan RK, Mahadevan A, Lichtman AH. (2009) Evaluation of prevalent phytocannabinoids in the acetic acid model of visceral nociception. Drug Alcohol Depend 105:42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsook D, Becerra L. (2011) CNS animal fMRI in pain and analgesia. Neurosci Biobehav Rev 35:1125–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. (2003) Lack of analgesic efficacy of oral Δ9-tetrahydrocannabinol in postoperative pain. Pain 106:169–172 [DOI] [PubMed] [Google Scholar]
- Campbell FA, Tramèr MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. (2001) Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ 323:13–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. (2007) Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2:2987–2995 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Hitchcock SA. (2007) Targeting cannabinoid agonists for inflammatory and neuropathic pain. Expert Opin Investig Drugs 16:951–965 [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. (1994) Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 23:129–138 [PubMed] [Google Scholar]
- Cobos EJ, Ghasemlou N, Araldi D, Segal D, Duong K, Woolf CJ. (2012) Inflammation-induced decrease in voluntary wheel running in mice: a nonreflexive test for evaluating inflammatory pain and analgesia. Pain 153:876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR, Kuhl E, Eckel G. (2004) Behavioral effects of cannabinoids show differential sensitivity to cannabinoid receptor blockade and tolerance development. Behav Pharmacol 15:1–12 [DOI] [PubMed] [Google Scholar]
- Do Carmo GP, Folk JE, Rice KC, Chartoff E, Carlezon WA, Jr, Negus SS. (2009) The selective non-peptidic Δ opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol 604:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, et al. (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113:9–19 [DOI] [PubMed] [Google Scholar]
- Elikkottil J, Elikottil J, Gupta P, Gupta K. (2009) The analgesic potential of cannabinoids. J Opioid Manag 5:341–357 [PMC free article] [PubMed] [Google Scholar]
- Farrimond JA, Mercier MS, Whalley BJ, Williams CM. (2011) Cannabis sativa and the endogenous cannabinoid system: therapeutic potential for appetite regulation. Phytother Res 25:170–188 [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA, Marsden CA, Chapman V. (2004) Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci 19:678–686 [DOI] [PubMed] [Google Scholar]
- Flecknell P. (2009) Laboratory Animal Anaesthesia, Academic Press/Elsevier, London [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. (2001) The role of central and peripheral Cannabinoid1 receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain 92:91–100 [DOI] [PubMed] [Google Scholar]
- Gardner EL, Paredes W, Smith D, Donner A, Milling C, Cohen D, Morrison D. (1988) Facilitation of brain stimulation reward by Δ9-tetrahydrocannabinol. Psychopharmacology 96:142–144 [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Stitzer ML. (2000) Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug Alcohol Depend 59:261–275 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Järbe TU, DiPatrizio NV. (2005) Δ9-THC induced hyperphagia and tolerance assessment: interactions between the CB1 receptor agonist Δ9-THC and the CB1 receptor antagonist SR-141716 (rimonabant) in rats. Behav Pharmacol 16:373–380 [DOI] [PubMed] [Google Scholar]
- Karst M, Wippermann S, Ahrens J. (2010) Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs 70:2409–2438 [DOI] [PubMed] [Google Scholar]
- Khodayar MJ, Shafaghi B, Naderi N, Zarrindast MR. (2006) Antinociceptive effect of spinally administered cannabinergic and 2-adrenoceptor drugs on the formalin test in rat: possible interactions. J Psychopharmacol 20:67–74 [DOI] [PubMed] [Google Scholar]
- Klooker TK, Leliefeld KE, Van Den Wijngaard RM, Boeckxstaens GE. (2011) The cannabinoid receptor agonist Δ9-tetrahydrocannabinol does not affect visceral sensitivity to rectal distension in healthy volunteers and IBS patients. Neurogastroenterol Motil 23:30–35, e2 [DOI] [PubMed] [Google Scholar]
- Kraft B. (2012) Is there any clinically relevant cannabinoid-induced analgesia? Pharmacology 89:237–246 [DOI] [PubMed] [Google Scholar]
- Kraft B, Frickey NA, Kaufmann RM, Reif M, Frey R, Gustorff B, Kress HG. (2008) Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology 109:101–110 [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. (1991) Spinal and supraspinal components of cannabinoid-induced antinociception. J Pharmacol Exp Ther 258:517–523 [PubMed] [Google Scholar]
- Martin TJ, Kim SA, Buechler NL, Porreca F, Eisenach JC. (2007) Opioid self-administration in the nerve-injured rat: relevance of antiallodynic effects to drug consumption and effects of intrathecal analgesics. Anesthesiology 106:312–322 [DOI] [PubMed] [Google Scholar]
- Matson DJ, Broom DC, Carson SR, Baldassari J, Kehne J, Cortright DN. (2007) Inflammation-induced reduction of spontaneous activity by adjuvant: a novel model to study the effect of analgesics in rats. J Pharmacol Exp Ther 320:194–201 [DOI] [PubMed] [Google Scholar]
- Miller CC, Murray TF, Freeman KG, Edwards GL. (2004) Cannabinoid agonist, CP 55,940, facilitates intake of palatable foods when injected into the hindbrain. Physiol Behav 80:611–616 [DOI] [PubMed] [Google Scholar]
- Miller LL, Picker MJ, Schmidt KT, Dykstra LA. (2011) Effects of morphine on pain-elicited and pain-suppressed behavior in CB1 knockout and wildtype mice. Psychopharmacology 215:455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS. (2009) Animal models of pain: progress and challenges. Nat Rev Neurosci 10:283–294 [DOI] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, Schütz G, Wotjak CT, Lutz B, Marsicano G. (2007) Genetic dissection of behavioural and autonomic effects of Δ9-tetrahydrocannabinol in mice. PLoS Biol 5:e269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R. (2003) The analgesic effect of oral Δ9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain 105:79–88 [DOI] [PubMed] [Google Scholar]
- National Research Council (2003) Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research, National Academies Press, Washington, DC: [PubMed] [Google Scholar]
- Negus SS, Bilsky EJ, Do Carmo GP, Stevenson GW. (2010a) Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia. Methods Mol Biol 617:79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. (2010b) Effects of κ opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 210:149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, O'Connell R, Morrissey E, Cheng K, Rice KC. (2012a) Effects of peripherally restricted κ opioid receptor agonists on pain-related stimulation and depression of behavior in rats. J Pharmacol Exp Ther 340:501–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Rosenberg MB, Altarifi AA, O'Connell RH, Folk JE, Rice KC. (2012b) Effects of the δ opioid receptor agonist SNC80 on pain-related depression of intracranial self-stimulation (ICSS) in rats. J Pain 13:317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. (2006) Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther 319:507–514 [DOI] [PubMed] [Google Scholar]
- Panagis G, Vlachou S, Nomikos GG. (2008) Behavioral pharmacology of cannabinoids with a focus on preclinical models for studying reinforcing and dependence-producing properties. Curr Drug Abuse Rev 1:350–374 [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. (2009) Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain 144:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD. (2002) Central neural mechanisms that interrelate sensory and affective dimensions of pain. Mol Interv 2:392–403, 339 [DOI] [PubMed] [Google Scholar]
- Raft D, Gregg J, Ghia J, Harris L. (1977) Effects of intravenous tetrahydrocannabinol on experimental and surgical pain. Psychological correlates of the analgesic response. Clin Pharmacol Ther 21:26–33 [DOI] [PubMed] [Google Scholar]
- Rice A. ed (2006) Wall and Melzack's Textbook of Pain, Churchill Livingstone, Philadelphia, PA [Google Scholar]
- Sain NM, Liang A, Kane SA, Urban MO. (2009) Antinociceptive effects of the non-selective cannabinoid receptor agonist CP 55,940 are absent in CB1(−/−) and not CB2(−/−) mice in models of acute and persistent pain. Neuropharmacology 57:235–241 [DOI] [PubMed] [Google Scholar]
- Sarzi-Puttini P, Atzeni F, Lanata L, Bagnasco M, Colombo M, Fischer F, D'Imporzano M. (2010) Pain and ketoprofen: what is its role in clinical practice? Reumatismo 62:172–188 [DOI] [PubMed] [Google Scholar]
- Sofia RD, Vassar HB, Knobloch LC. (1975) Comparative analgesic activity of various naturally occurring cannabinoids in mice and rats. Psychopharmacologia 40:285–295 [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS. (2006) Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain 7:408–416 [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. (2009) Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci 85:309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Panagis G. (2005) CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology 179:498–508 [DOI] [PubMed] [Google Scholar]
- Vlachou S, Nomikos GG, Stephens DN, Panagis G. (2007) Lack of evidence for appetitive effects of Δ9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav Pharmacol 18:311–319 [DOI] [PubMed] [Google Scholar]
- Wallace M, Schulteis G, Atkinson JH, Wolfson T, Lazzaretto D, Bentley H, Gouaux B, Abramson I. (2007) Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology 107:785–796 [DOI] [PubMed] [Google Scholar]
- Whiteside GT, Adedoyin A, Leventhal L. (2008) Predictive validity of animal pain models? A comparison of the pharmacokinetic-pharmacodynamic relationship for pain drugs in rats and humans. Neuropharmacology 54:767–775 [DOI] [PubMed] [Google Scholar]
- Wiley JL, O'connell MM, Tokarz ME, Wright MJ., Jr (2007) Pharmacological effects of acute and repeated administration of Δ9-tetrahydrocannabinol in adolescent and adult rats. J Pharmacol Exp Ther 320:1097–1105 [DOI] [PubMed] [Google Scholar]
- Williams CM, Rogers PJ, Kirkham TC. (1998) Hyperphagia in pre-fed rats following oral Δ9-THC. Physiol Behav 65:343–346 [DOI] [PubMed] [Google Scholar]
- Willis WD., Jr (2009) The role of TRPV1 receptors in pain evoked by noxious thermal and chemical stimuli. Exp Brain Res 196:5–11 [DOI] [PubMed] [Google Scholar]
- Zimmermann M. (1983) Ethical guidelines for investigations of experimental pain in conscious animals. Pain 16:109–110 [DOI] [PubMed] [Google Scholar]