Abstract
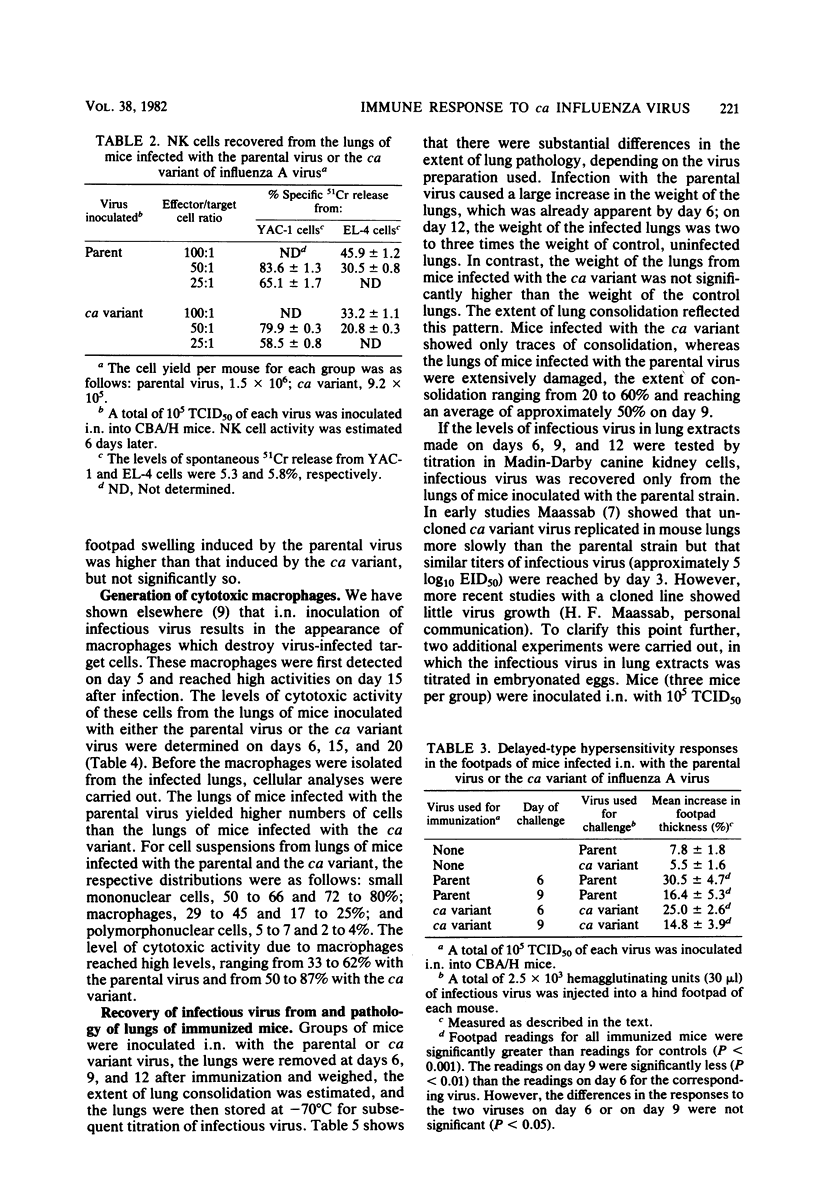
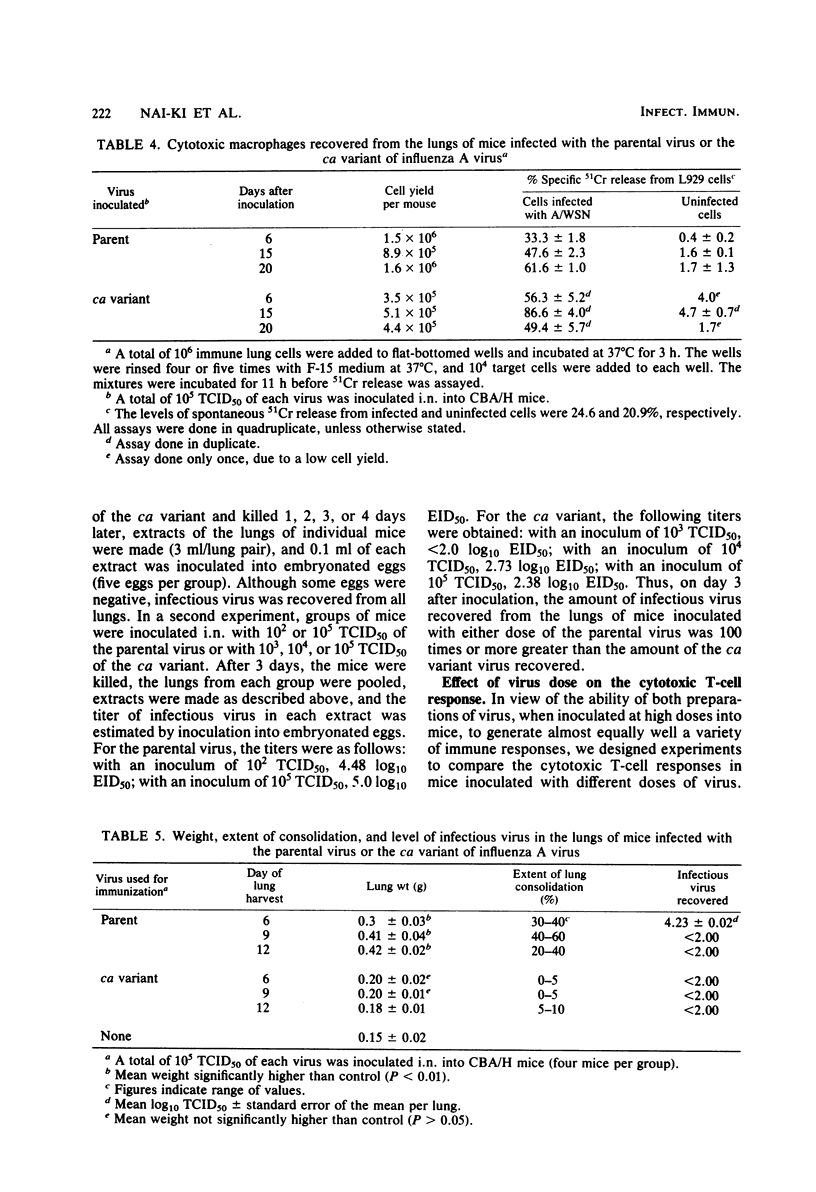
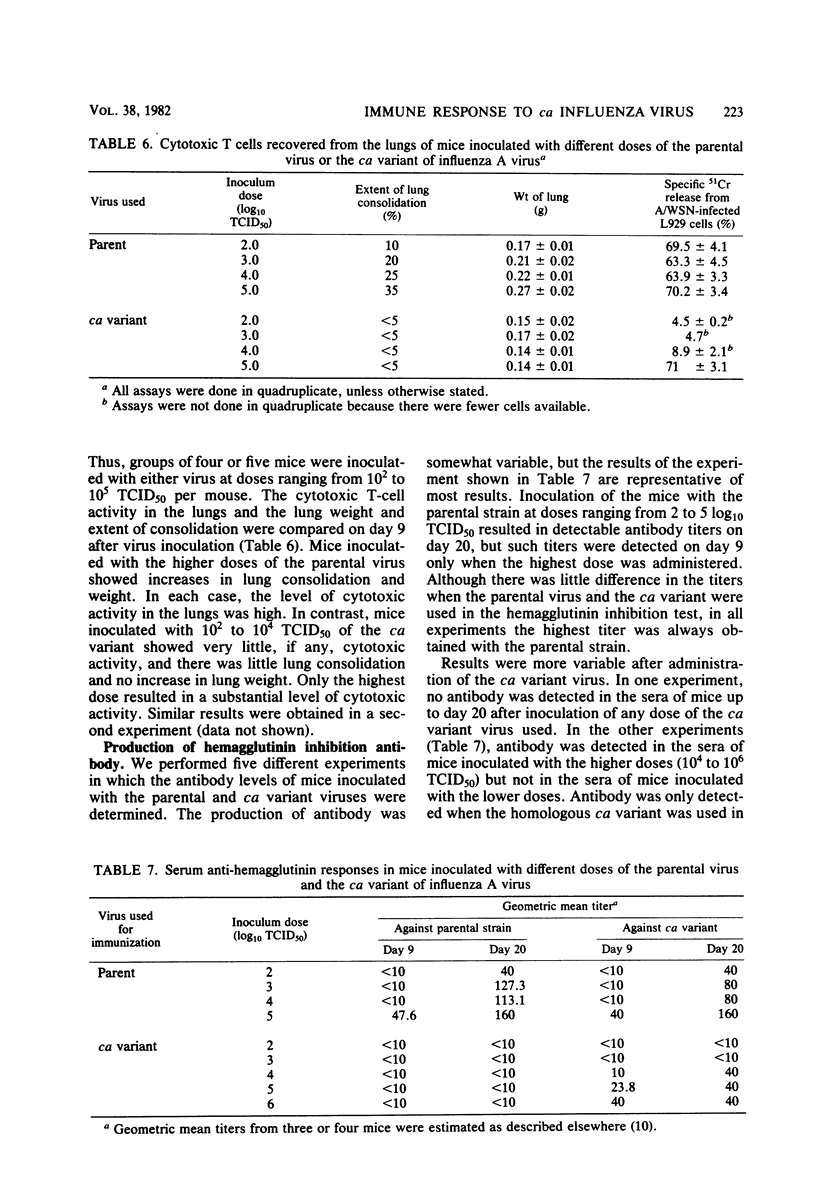
The serum antibody response and four different cellular immune responses (cytotoxic T cells, delayed-type hypersensitivity T cells, natural killer cells, and cytotoxic macrophage levels) induced in CBA/H mice were measured at different times after intranasal inoculation of a cold-adapted (ca) variant of influenza A virus, influenza virus A/Ann Arbor/6/60-ca, or the parental virus, influenza virus A/Ann Arbor/6/60. At the highest dose of virus inoculated (5 log10 50% tissue culture infective doses), all four cellular responses reached high levels in the lungs of both groups of mice, and serum antibody titers were detected on day 20 after inoculation of either virus. However, whereas extensive replication of the parental virus occurred in the mouse lungs, very limited replication of the ca variant was observed. Macroscopically, infection with the parental virus caused gross lung damage, whereas such damage was almost absent in mice inoculated with the ca variant. Inoculation of 2 to 5 log10 50% tissue culture infective doses of the parental virus induced high cytotoxic T-cell responses, whereas only the highest dose of the ca variant caused a clearly significant cytotoxic T-cell response. As an inoculum of 5 log10 50% tissue culture infective doses of the ca variant caused a substantial primary immune response without appreciable lung damage, the avirulence of the ca variant may be primarily related to its limited ability to replicate productively in mouse lungs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ada G. L., Leung K. N., Ertl H. An analysis of effector T cell generation and function in mice exposed to influenza A or Sendai viruses. Immunol Rev. 1981;58:5–24. doi: 10.1111/j.1600-065x.1981.tb00347.x. [DOI] [PubMed] [Google Scholar]
- Davidson W. F., Parish C. R. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975 Jun;7(2-3):291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- Hoskins T. W., Davies J. R., Smith A. J., Miller C. L., Allchin A. Assessment of inactivated influenza-A vaccine after three outbreaks of influenza A at Christ's Hospital. Lancet. 1979 Jan 6;1(8106):33–35. doi: 10.1016/s0140-6736(79)90468-9. [DOI] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Different functions of subsets of effector T cells in murine influenza virus infection. Cell Immunol. 1982 Mar 1;67(2):312–324. doi: 10.1016/0008-8749(82)90223-4. [DOI] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Induction of natural killer cells during murine influenza virus infection. Immunobiology. 1981;160(3-4):352–366. doi: 10.1016/s0171-2985(81)80061-7. [DOI] [PubMed] [Google Scholar]
- Leung K. N., Ada G. L. Production of DTH in the mouse to influenza virus: comparison with conditions for stimulation of cytotoxic T cells. Scand J Immunol. 1980;12(2):129–139. doi: 10.1111/j.1365-3083.1980.tb00049.x. [DOI] [PubMed] [Google Scholar]
- Maassab H. F. Adaptation and growth characteristics of influenza virus at 25 degrees c. Nature. 1967 Feb 11;213(5076):612–614. doi: 10.1038/213612a0. [DOI] [PubMed] [Google Scholar]
- Mahmud M. I., Maassab H. F., Jennings R., Potter C. W. Influenza virus infection of a newborn rats: virulence of recombinant strains prepared from a cold-adapted, attenuated parent. Arch Virol. 1979;61(3):207–216. doi: 10.1007/BF01318055. [DOI] [PubMed] [Google Scholar]
- Mak N. K., Leung K. N., Ada G. L. The generation of 'cytotoxic' macrophages in mice during infection with influenza A or Sendai virus. Scand J Immunol. 1982 Jun;15(6):553–561. doi: 10.1111/j.1365-3083.1982.tb00683.x. [DOI] [PubMed] [Google Scholar]
- Smith J. W., Pollard R. Vaccination against influenza: a five-year study in the Post Office. J Hyg (Lond) 1979 Aug;83(1):157–170. doi: 10.1017/s0022172400025936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring S. B., Maassab H. F., Kendal A. P., Murphy B. R., Chanock R. M. Cold adapted variants of influenza A. II. Comparison of the genetic and biological properties of ts mutants and recombinants of the cold adapted A/AA/6/60 strain. Arch Virol. 1977;55(3):233–246. doi: 10.1007/BF01319909. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R. Studies on the pathogenesis of influenzal pneumonitis; intranasal vs. intravenous infection of mice. Yale J Biol Med. 1956 Jun;28(6):598–614. [PMC free article] [PubMed] [Google Scholar]
- Wark M. C., Tannock G. A., Sutherland M. M., Smith L. E. A murine model for assessment of living attenuated influenza A vaccines. Aust J Exp Biol Med Sci. 1980 Dec;58(6):615–626. doi: 10.1038/icb.1980.64. [DOI] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L. Cytotoxic T cells specific for influenza virus-infected target cells. Immunology. 1977 Feb;32(2):151–159. [PMC free article] [PubMed] [Google Scholar]



