Abstract
Objective
A state-of-the-art centrifugal pump combined with hollow-fiber oxygenator for extracorporeal membrane oxygenation has potential advantages such as smaller priming volumes and decreased potential to cause tubing rupture as compared with the traditional roller head/silicone membrane systems. Adoption of these state-of-the-art systems has been slow in neonates as a result of past evidence of severe hemolysis that may lead to renal failure and increased mortality. Extracorporeal systems have also been linked to platelet dysfunction, a contributing factor toward intracranial hemorrhage, a leading cause of infant morbidity. Little data exist comparing the centrifugal systems with the roller systems in terms of hemolysis and platelet aggregation at low flow rates commonly used in neonatal extracorporeal membrane oxygenation.
Design
Prospective, comparative laboratory study.
Setting
University research laboratory.
Subjects
Centrifugal pump, roller pump, hollow-fiber oxygenator, and silicone membrane oxygenator.
Interventions
Comparative study using two pumps, the centrifugal Jostra Rotaflow (Maquet, Wayne, NJ) and the roller-head (Jostra, Maquet, Wayne, NJ), and two oxygenators, polymethly-pentene Quadrox-D (Maquet) and silicone membrane (Medtronic, Minneapolis, MN). Five test runs of four circuit combinations were examined for hemolysis and platelet aggregation during 6 hrs of continuous use in a simulated in vitro extracorporeal membrane oxygenation circuit circulating whole swine blood at 300 mL/min.
Measurements and Main Results
Hemolysis was assessed by spectrophometric measurement of plasma-free hemoglobin. Platelet aggregation was evaluated using monoclonal CD61 antibody fluorescent flow cytometry profiles. All of the extracorporeal membrane oxygenation systems created plasma-free hemoglobin at a similar rate compared with static blood control. There was no difference in the mean normalized index of hemolysis of the centrifugal/hollow-fiber oxygenator system as compared with the roller-head/silicone membrane systems (0.0032 g/100 L vs. 0.0058 g/100 L, p ≥ .7). None of the extracorporeal membrane oxygenation systems had a significant increase in platelet aggregation above baseline.
Conclusions
In a low-flow neonatal environment, a state-of-the-art centrifugal pump combined with new fiber-type oxygenators appear to be safe in regard to hemolysis and platelet aggregation.
Keywords: cardiopulmonary bypass, centrifugation, extracorporeal membrane oxygenation, flow cytometry, hemolysis, platelet aggregates
Use of extracorporeal membrane oxygenation (ECMO) in the treatment of acute respiratory and cardiac failure has become an accepted modality in many institutions for patients failing conventional therapy. It has been shown to significantly reduce mortality and morbidity in critically ill infants and adults (1-6). The success of ECMO in providing gas exchange and circulatory support has led to an increase in its use in increasingly complex medical and surgical patients (4, 7). Recently, new technologies for ECMO in terms of centrifugal pumps and hollow-fiber oxygenators have become available in the marketplace. Although practitioners have been slow to adopt these newer ECMO components as a result of past concerns of severe hemolysis, many centers are now adopting these newer ECMO components. According to a 2008 survey of North American active ECMO centers, >80% routinely used roller pumps for neonatal ECMO (8). This study also showed that these centers primarily used the classic silicone membrane oxygenators (67%) in comparison to the centers using microporous hollow-fiber oxygenators (19%) and polymethylpentene hollow-fiber oxygenators (14%). A follow-up survey in 2010 found that the majority of centers had switched to hollow-fiber oxygenators, although centrifugal pump use remains less than roller pumps. The impact of these new systems on hemolysis and safety has not been thoroughly addressed.
Studies have shown that the release of cell-free plasma hemoglobin, a marker of hemolysis; generated during ECMO is associated with renal dysfunction, increased blood transfusions, clot formation in the circuit, and death in children on ECMO after cardiac surgery (9, 10). A 2004 Extracorporeal Life Support Organization registry analysis revealed that hemolysis occurred in 13.6% of all ECMO procedures with a higher incidence of dangerous hemolysis in centrifugal pumps (11). Prediction of severe hemorrhage during pediatric ECMO is challenging, because there is no currently acceptable specific clinical or laboratory marker (12, 13). A recent study showed that ECMO components can induce platelet microaggregates associated with impaired neurophysiological function (14) and can trigger microhemorrhage and inflammatory reactions.
Previous published data comparing ECMO components at low flow rates simulating neonatal ECMO conditions is limited. In 1996, a study by Moon et al (15) showed in a low-flow environment that there was no significant difference in hemolysis between previously used pumps. Our hypothesis is that at low flow rates, differences in hemolysis and platelet aggregation are negligible when comparing a centrifugal pump/hollow-fiber oxygenator system with a traditional roller-pump/silicone membrane system.
MATERIALS AND METHODS
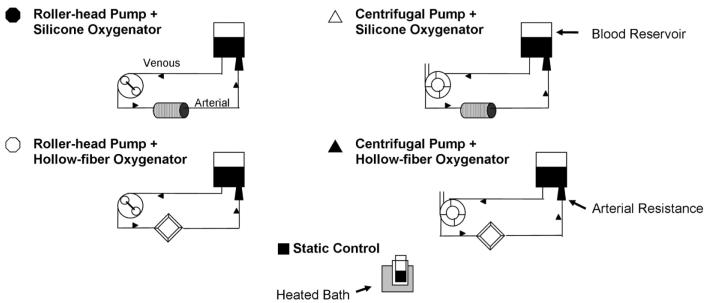
A total of four ECMO circuits were tested using the Jostra Rotaflow centrifugal pump (Maquet, Wayne, NJ), the Jostra HL-20 roller pump (Maquet), the Quadrox-D hollow-fiber oxygenator (Maquet), and the 0800 ECMO silicone membrane oxygenator (Medtronic, Minneapolis, MN). Figure 1 demonstrates the four ECMO system test circuits. All four ECMO systems were tested five times using heparinized porcine whole blood. The same blood from one donor animal was used for each experimental run of the four different ECMO systems. Static blood controls were also maintained in a similar test environment only without extracorporeal circuitry. Porcine whole blood was used as a result of the species’ lack of hemolytic impact in the setting of prolonged perfusion (16) and as a result of similarities between human and porcine platelets (17). Blood age was consistent for each system. Institutional Animal Care and Use Committees approval was obtained for the use of whole porcine blood used in this study. During 6 hrs of continuous use, hemolysis and platelet aggregation were assessed by plasma-free hemoglobin measurements and whole blood flow cytometric analysis, respectively.
Figure 1.
Diagram of the four different test circuits.
Experimental Procedures
Each ECMO system was ventilated with a mixture of oxygen and room air in such a manner to keep the pH, PCO2, and PO2 within normal ranges. Each roller pump was properly occluded before each experimental run as per our hospital perfusionist protocol (18). Quarter-inch heparin-coated tubing (Medtronic) was used primarily for connections between each of the different components. Three-eighths to quarter-inch connectors were used at the inlet and outlet of the centrifugal pumps and the inlet and outlet of the hollow-fiber oxygenator. Quarterinch tubing was used in the HL-20 pump boot raceway and for the inlet and outlet of the O800 silicone membrane oxygenator. Several 1/4 × 1/4-inch straight lured connectors with stopcock (also known as pigtails) were placed along the circuit for blood sample access. A Gish reservoir bag (Medtronic) was used as a venous reservoir. An arterial catheter was used on the outflow tubing from the oxygenator to achieve the 230 mm Hg pressure restriction on each of the test circuits. In our neonatal extracorporeal simulated circuit, we chose to not use a venous catheter from the blood reservoir to the pump inlet because the amount of shear stress, turbulence, and velocity generated in larger venous cannulae at low flows has not been shown to be a major contributor to hemolysis (19, 20).
The circuits were primed with porcine whole blood including 4 IU/mL of porcine heparin (Baxter Healthcare, Deerfield, IL). The blood was maintained at a temperature of 38°C at flow rates of 300 mL/min against a pressure gradient caused by the resistance of the arterial cannula. Flow rate of 300 mL/min was determined as standard support for a 3-kg child (150 mL/kg/min at roughly two-thirds cardiac index). The flow rate on all the test pumps was measured with a Transonic Flow Meter (Transonic Systems, Ithaca, NY). The test circuits and the static blood control were then sampled at the 30-min mark for baseline plasma-free hemoglobin (fHb) values and then at 60, 90, 120, 180, 240, 300, and 360 mins, respectively. The blood samples were then spun and run in triplicate for each data collection point. The test circuits and static blood control were also sampled at the 0-min mark for baseline flow cytometry and then at 120, 240, and 360 mins.
Hemolysis Analysis
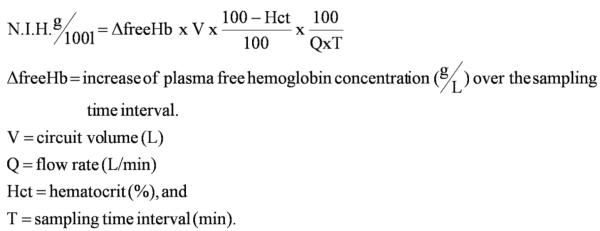
Plasma-free Hb was determined by using a spectrophotometer (Molecular Devices, Sunnyvale, CA) as described previously (21). The plasma-free Hb and measured hematocrit were used to calculate a normalized index of hemolysis to standardize the method of performing this in vitro evaluation (22) (Fig. 2).
Figure 2.
Normalized index of hemolysis (N.I.H.). Hb, hemoglobin.
Platelet Count
Platelets were isolated from whole blood samples using the BD Unopette Platelet/WBC system (BD, Franklin Lakes, NJ). The samples were then counted manually on an Improved Neubauer Hematocytometer (Hausser Scientific, Horsham, PA).
Flow Cytometry
Whole blood was also evaluated by a flow cytometric method described previously (23). Aliquots (5 μL) of heparinized whole blood were placed in flow cytometry staining buffer solution (eBioscience, San Diego, CA) and then incubated for 20 mins in the dark at room temperature with saturating concentrations of appropriate fluorescein isothiocyanate-labeled anti-porcine CD61 antibody (Clone: JM2E5; AbD Serotec, Kidlington, UK). This antibody is able to recognize and immunoprecipitate the gpIIb/IIIa surface glycoprotein from porcine platelets (24). The samples were washed to remove nonspecific antibody and then analyzed on a FACSCalibur flow cytometer (BD Bioscience, San Jose, CA).
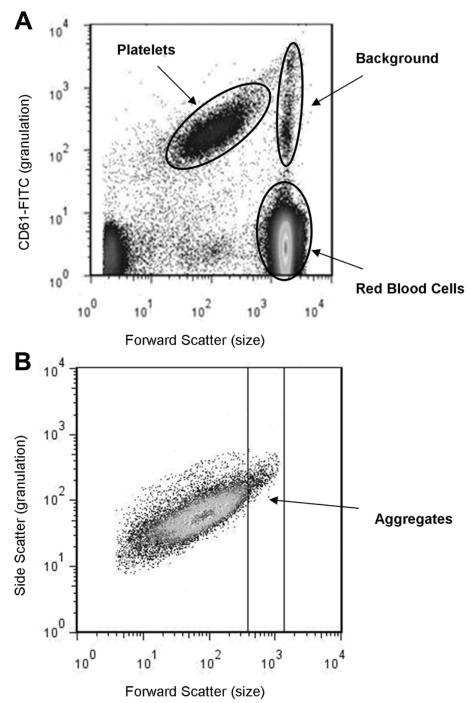
Platelets and platelet aggregates were distinguished from other blood cells and small particulate debris on the basis of platelet specific CD61-fluorescein isothiocyanate antibody fluorescence (see Fig. 3A). Nonspecific binding was evaluated using a control irrelevant monoclonal antibody, fluorescein isothiocyanate-mouse IgG1 Isotype Control (BD Bioscience). A flow cytometric gate was set for acquiring 35,000 cell events in the platelet population (see Fig. 3B). Based on previous methods (14), platelet aggregates were determined as fluorescein isothiocyanate-positive events larger than single platelets and quantified by depletion of fluorescein isothiocynate-positive events from the intact platelet population (single platelets):
Where Nafter = single platelet population at each time point collected after exposure to extracorporeal circuitry and Nbefore is the single platelet population at each time point collected before exposure to extracorporeal circuitry.
Figure 3.
A, An explanatory guide to interpreting a whole blood flow cytometry profile using CD61-fluorescein isothiocyanate (FITC) antibody. B, After gating on the platelet specific events, one can quantify the amount of aggregation created over time.
Statistical Methods
Data were collected electronically for analysis and reported as mean and SE for each experiment number. The hemolysis between pump combinations over time were analyzed using multivariate regression by xtgee (generalized estimating equation) in Stata 8.0 (College Station, TX) to account for the longitudinal measurements of free Hb by pump combination (roller + silicone, roller + Quadrox, centrifugal + silicone, centrifugal + Quadrox) over time (30, 60, 90, 120, 180, 240, 300, and 360 mins). In this regression model, the free Hb was regressed on: experiment number, elapsed time, pump combination, and the interaction of pump combination and elapsed time. The effect of interest was the pump combination and elapsed time (interaction term). A similar regression model was used for platelet and normalized index of hemolysis analysis and also ran using xtgee in Stata. The model fit for platelet and normalized index of hemolysis was, e.g., platelets = experiment number + time + pump combo + pump/time.
RESULTS
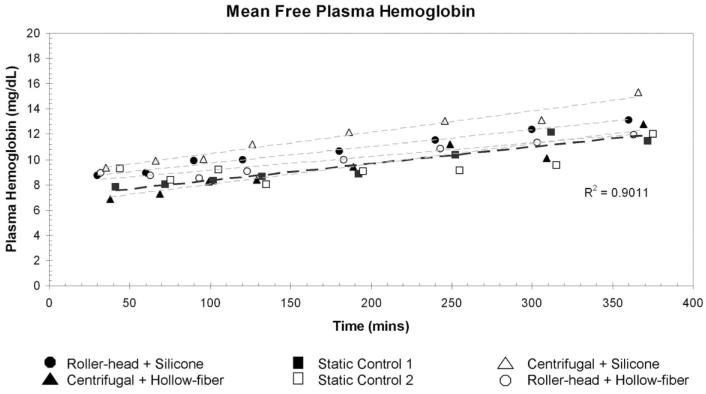
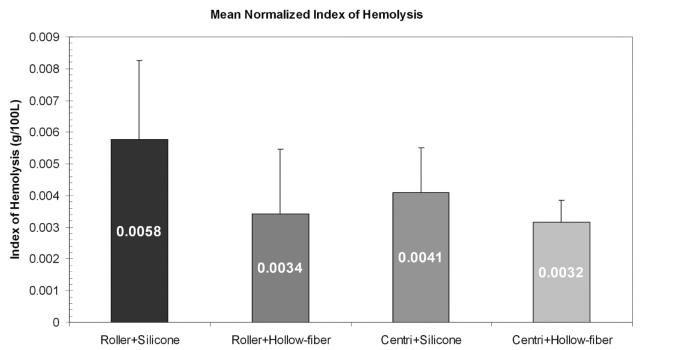
All four ECMO systems and the static blood samples had increases in the amount of plasma-free Hb (fPH) over time. None of the systems created fPH at a greater rate than the other systems or the static samples. The roller pump in combination with the Medtronic silicone membrane oxygenator or with the Quadrox D oxygenator had similar increases in fPH over time: 0.79 mg/dL/hr and 0.65 mg/dL/hr, respectively. The Rotaflow centrifugal pump in combination with the Medtronic silicone membrane or with the Quadrox D oxygenator had similar increases in fPH over time, 1.00 mg/dL/hr and 0.96 mg/dL/hr, respectively. The rate of fPH created over time was not statistically significantly different between the static blood samples or each of the pump/oxygenator combinations. Mean fPH values, hematocrit, and ses for each ECMO system and statics are listed in Table 1. Figure 4 shows a plot of the free fPH values over time. Using multivariate regression analysis, we found no difference between ECMO systems (p = .49). The normalized index of hemolysis showed similar results with no statistically significant difference between any of the ECMO systems (p = .73). Figure 5 is a graphic representation of the normalized index of hemolysis for each of the ECMO systems.
Table 1.
Free plasma hemoglobin, hematocrit, and platelet data
| Roller-Head + Silicone |
Centrifugal + Hollow-Fiber |
Static Sample Day 1 |
Roller-Head + Hollow-Fiber |
Centrifugal + Silicone |
Static Sample Day 2 |
|
|---|---|---|---|---|---|---|
| Hematocrit | 40 ± 1 | 40 ± 1 | 40 ± 1 | 43 ± 3 | 41 ± 1 | 43 ± 2 |
| Free plasma hemoglobin | ||||||
| 30 mins | 8.71 ± 1.3 | 6.88 ± 0.9 | 7.81 ± 1.0 | 8.93 ± 1.7 | 9.36 ± 0.9 | 9.28 ± 1.0 |
| 60 mins | 8.92 ± 0.9 | 7.30 ± 0.9 | 8.02 ± 0.5 | 8.71 ± 1.5 | 9.88 ± 1.0 | 8.40 ± 0.8 |
| 90 mins | 9.89 ± 1.0 | 8.26 ± 1.1 | 8.34 ± 0.6 | 8.51 ± 1.2 | 10.07 ± 1.1 | 9.24 ± 1.4 |
| 120 mins | 9.98 ± 0.6 | 8.37 ± 0.9 | 8.66 ± 0.5 | 9.05 ± 1.3 | 11.24 ± 1.5 | 8.01 ± 0.6 |
| 180 mins | 10.62 ± 0.6 | 9.42 ± 0.9 | 8.85 ± 0.6 | 10.00 ± 1.7 | 12.17 ± 1.9 | 9.07 ± 0.8 |
| 240 mins | 11.53 ± 0.9 | 11.17 ± 1.0 | 10.35 ± 0.8 | 10.88 ± 1.9 | 13.04 ± 2.7 | 9.17 ± 1.4 |
| 300 mins | 12.38 ± 1.1 | 10.10 ± 0.9 | 12.18 ± 1.8 | 11.31 ± 2.3 | 13.12 ± 3.1 | 9.57 ± 1.6 |
| 360 mins | 13.12 ± 1.2 | 12.75 ± 1.3 | 11.45 ± 1.0 | 11.97 ± 1.3 | 15.35 ± 4.0 | 11.99 ± 1.9 |
| Platelet counta | ||||||
| Baseline | 219 ± 12 | 249 ± 13 | 240 ± 20 | 129 ± 14 | 148 ± 17 | 139 ± 12 |
| 120 mins | 174 ± 19 | 171 ± 16 | 233 ± 13 | 99 ± 8 | 96 ± 11 | 129 ± 14 |
| 240 mins | 151 ± 15 | 149 ± 6 | 243 ± 15 | 89 ± 8 | 98 ± 7 | 140 ± 11 |
| 360 mins | 91 ± 20 | 140 ± 4 | 197 ± 12 | 70 ± 8 | 78 ± 3 | 118 ± 6 |
| Platelet aggregation | ||||||
| Baseline | 1.65 ± 0.3 | 1.44 ± 0.4 | 1.86 ± 0.5 | 1.69 ± 0.5 | 1.86 ± 0.3 | 1.58 ± 0.5 |
| 120 mins | 1.76 ± 0.3 | 1.28 ± 0.2 | 1.94 ± 0.2 | 1.62 ± 0.3 | 1.68 ± 0.2 | 1.66 ± 0.3 |
| 240 mins | 1.64 ± 0.3 | 1.37 ± 0.3 | 1.99 ± 0.4 | 1.79 ± 0.2 | 1.65 ± 0.3 | 1.99 ± 0.2 |
| 360 mins | 1.82 ± 0.4 | 1.41 ± 0.3 | 1.97 ± 0.3 | 1.86 ± 0.3 | 2.08 ± 0.3 | 1.88 ± 0.2 |
Platelet count data was collected only on two runs.
Figure 4.
Mean free hemoglobin plotted as a function of time. All four extracorporeal membrane oxygenation systems created free hemoglobin at a similar rate compared with the static control. Using multivariate regression, we found no difference between pump combinations (p = .49).
Figure 5.
Mean normalized index of hemolysis values in g/100 L. The difference in normalized index of hemolysis between each extracorporeal membrane oxygenation system was not large enough to reach statistical significance (p = .73).
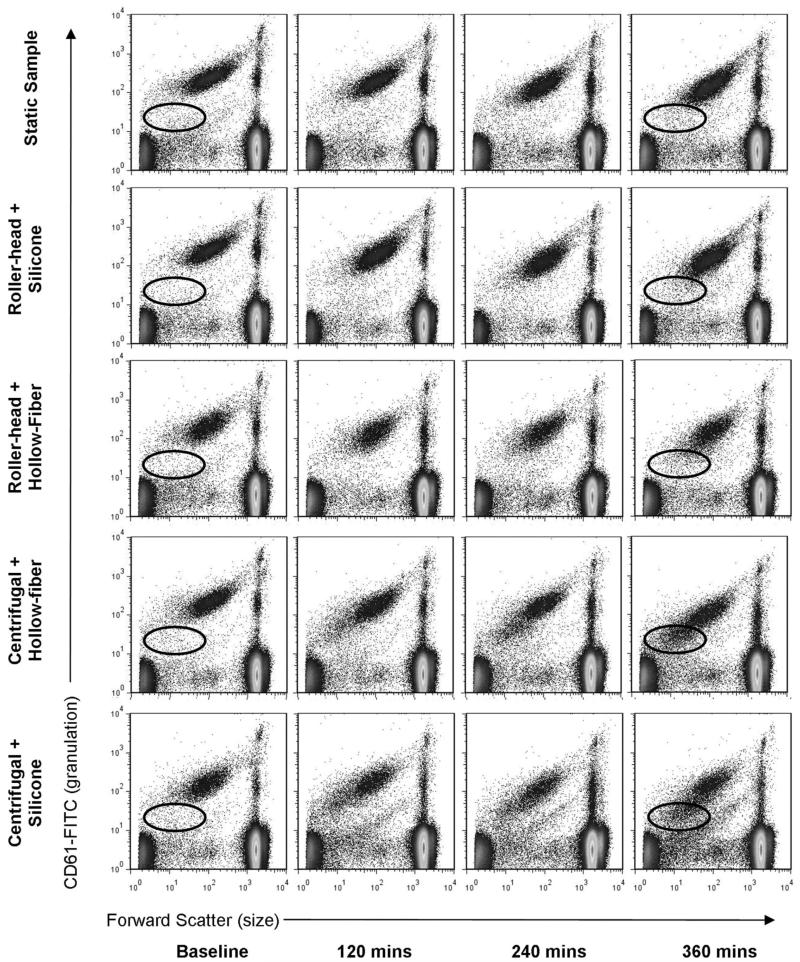
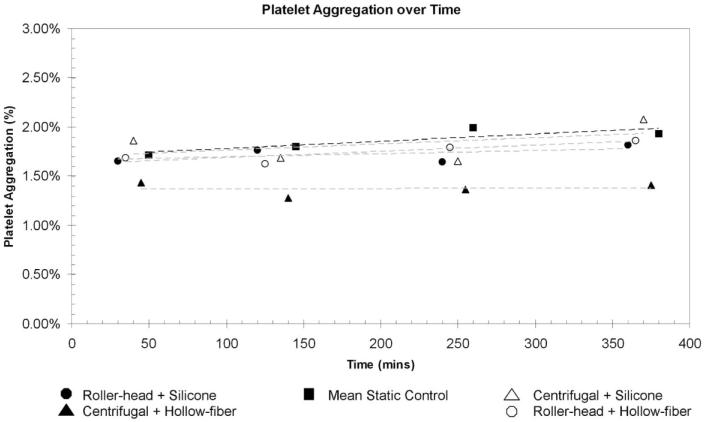
Figure 6 shows the whole blood flow cytometry profile of each ECMO system and static blood controls after 0 (baseline), 120, 240, and 360 mins of perfusion. Baseline represents blood samples that were drawn immediately after priming the artificial circulatory loop. The ellipse on the baseline and 360-min plots show a change over time in the amount of CD61 binding to the platelets during perfusion. Mean platelet aggregation percentages for all times are shown in Table 1. Figure 7 shows a plot of platelet aggregation over time. Using multivariate regression analysis, we found no difference in the amount of platelet aggregation between the ECMO systems as compared with the static control (p = .74). Platelet counts fell over time in all ECMO systems as compared with the static control.
Figure 6.
Whole blood flow cytometry profile of all four extracorporeal membrane oxygenation systems over time. The elliptical circle shows a change over time in the amount of CD61 binding to the platelets during perfusion in the centrifugal pumps. This could correlate to a noticeable change in platelets size and shape in the centrifugal pumps. This figure is a representation of four experiments. FITC, fluorescein isothiocyanate.
Figure 7.
Mean platelet aggregation percentage plotted as function of time. The percentage of platelet aggregation in all extracorporeal membrane oxygenation systems is similar to static control throughout 6 hrs of continuous use (p = .74).
DISCUSSION
Although ECMO has been in regular use since 1979, ECMO complications such as hemolysis and hemorrhage are still leading causes of mortality and morbidity (9, 25). Intravascular hemolysis can be generated in the ECMO circuit as a result of the change in pressure across either of its main components, the blood pump, or the membrane oxygenator (26, 27). Hemolysis leads to the creation of cell-free plasma Hb, a potent nitric oxide scavenger associated with adverse clinical signs and symptoms, including gastrointestinal, cardiovascular, pulmonary, urogenital, hematologic, and renal abnormalities (28). Elevated free plasma Hb has been implicated in thrombus formation, worsening renal function, and mortality on ECMO (9, 10).
Newer designed centrifugal pumps have less stagnant areas and less heat generation at the rotor/pump head interface primarily as a result of novel suspension techniques, which reduce friction, thus reducing heat generation, which can contribute to a longer pump head lifespan. Smaller blood volumes within the pump head may also reduce hemolysis seen in prior centrifugal pump setups by reducing stagnation, heating, and thrombosis. In our comparative study at low flow rates, the amount of hemolysis generated in each of the four ECMO circuits and the static control was minimal. This minimal amount of hemolysis over time and the longevity of both new pump heads and circuit components may reduce the need to replace ECMO circuits in prolonged ECMO runs, therefore reducing risks related to clinical instability, donor exposure, air embolism, bacterial contamination, and inflammatory response to a new nonendothelialized surface, all of which may occur when ECMO circuits are changed out.
Hemorrhage during ECMO is common, with >35% of cardiac patients on ECMO reported to have significant bleeding (29). Intracranial hemorrhage is a major predictor of morbidity and occurs in 3% to 12% of patients with the greatest risk in neonates (29, 30). Previous literature proposed that platelet levels were important in predicting intracranial hemorrhage when platelet counts were <200,000/mm3 (31), when platelet counts were difficult to maintain (32), or with increasing platelet transfusion requirements (33). Further research found a significant correlation between the total number of platelet transfusions and the length of the ECMO run (34), attributing the large need for platelet transfusions during ECMO because of impairment of platelet function (35-37). Although the mechanisms of platelet dysfunction are not completely understood, several researchers believe it is the result of deficiency of platelet agonists. These agonists are reduced in ECMO by hemodilution and the universal use of heparin that inhibits thrombin, the preeminent platelet activator (35, 36).
A study by Linneweber et al (14) used sensitive flow cytometric analysis to detect increased circulating platelet aggregates and increased platelet consumption in the roller pump when compared with the centrifugal pump. Under low-flow conditions, we performed a similar analysis and found no difference in the amount of aggregation generated over time for any of the systems. It is interesting to note that the centrifugal/hollow-fiber system had the lowest amount of platelet aggregation, although this combination was not statistically significant from the other systems. During the course of experimental runs, a change in the whole blood flow cytometry profile occurred demonstrating generation of smaller platelet fragments over time when compared with static blood control. This is most pronounced in the centrifugal pump/silicone membrane circuit, an uncommonly used clinical system as a result of the need to generate high revolutions per minute (approximately 3000 revolutions/min) to push blood forward through the high resistance of the silicone membrane lung. We hypothesize that this clinical combination of a higher negative pressure and high resistance creates subsequent shear stress, resulting in the platelets to break apart over time. The effects that the centrifugal pumps and/or the hollow-fiber oxygenators have on platelet degradation over time and how this may affect patient outcome will need further investigation.
Centrifugal pumps have been shown to have significant benefits of less priming volume, inability to cause tubing rupture, and similar generation of free hemoglobin as compared with roller pumps (38, 39). Additionally, hollow-fiber membrane oxygenators have demonstrated little to no plasma leakage, excellent blood compatibility, and low blood flow resistance (40). A recent retrospective clinical evaluation comparing the Medtronic centrifugal pump/hollow-fiber system with the Maquet centrifugal pump/hollow-fiber system found the latter system to have less hemolysis, lower transmembrane pressures, and less thrombus formation (41). This research correlates with previous published reports showing this latter system is ideal for long-term support such as ECMO and could be used in various sizes of patients (42). We contribute to this knowledge by presenting a comparative in vitro evaluation of each neonatal ECMO system for hemolysis and platelet aggregation, finding newer components to be possibly as safe, if not safer, than more traditional components.
Limitations of the Study
There are several limitations of our study including a limited time period that data were collected, the use of a mock in vitro study, which may not replicate the real neonatal ECMO condition, and the use of porcine blood instead of human blood. Clinically ECMO systems are used up to several weeks; therefore, future studies will need a longer time period than 6 hrs. The use of whole porcine blood was accounted for in this study by using porcine specific antibodies, maintaining a normal temperature during perfusion, and maintaining a static blood control at the same blood age for comparison. Our study did not address the issue of fibrinolysis, a major component of ECMO hemorrhage; therefore, future studies are needed examining this in more detail. Further studies are needed using human blood components in a multiple-day ECMO experiment to confirm current study findings.
CONCLUSIONS
The centrifugal pump/hollow-fiber oxygenator systems generate the same amount of fPH and platelet aggregation as the roller-head/silicone membrane system over 6 hrs of continuous use at low flow rates. Our study suggests that under similar in vitro conditions that newer components will generate no more hemolysis or platelet aggregation than previously used components. These results are encouraging as more hospitals begin using state-of-the-art ECMO components for infants and small children. Increasing use of similar systems is also occurring in rapid extracorporeal life support systems with cardiopulmonary resuscitation, low-prime cardiopulmonary bypass systems, and during interhospital transport of patients.
ACKNOWLEDGMENTS
The authors thank Lisa Williams, RN, Gerald Mikesel, CCP, Children’s National ECMO Core Team, and the Children’s National Animal Research Facility for their invaluable efforts on this project. The authors also thank Pamela Garcia-Filion for her statistical support. Lastly, this project would not have been possible without a generous donation from the Teh Shah Memorial ECMO research fund and a grant from the Children’s National Board of Lady Visitors.
Dr. Dalton received grant support from a private family donation used to support this project.
Footnotes
See also p.492.
The remaining authors have not disclosed any potential conflicts of interest.
Contributor Information
Andrew D. Meyer, Division of Pediatric Critical Care, Department of Pediatrics, University of Texas Health Science Center, San Antonio, TX.
Andrew A. Wiles, Research Center for Genetic Medicine, Children’s National Research Institute, Washington, DC.
Oswaldo Rivera, Department of Biomedical Engineering, Children’s National Medical Center, Washington, DC.
Edward C. Wong, Division of Laboratory Medicine, Center for Clinical and Community Research, Children’s National Medical Center, Washington, DC.
Robert J. Freishtat, Research Center for Genetic Medicine, Children’s National Research Institute, Washington, DC.
Khoydar Rais-Bahrami, Division of Neonatology, Center for Hospital Based Subspecialities, Children’s National Medical Center, Washington, DC.
Heidi J. Dalton, Division of Pediatric Critical Care, Phoenix Children’s Hospital, Phoenix, AZ..
REFERENCES
- 1.Conrad SA, Rycus PT, Dalton H. Extracorporeal Life Support registry report 2004. ASAIO J. 2005;51:4–10. doi: 10.1097/01.mat.0000151922.67540.e9. [DOI] [PubMed] [Google Scholar]
- 2.Lequier L. Extracorporeal life support in pediatric and neonatal critical care: A review. J Intensive Care Med. 2004;19:243–258. doi: 10.1177/0885066604267650. [DOI] [PubMed] [Google Scholar]
- 3.Rais-Bahrami K, Short BL. The current status of neonatal extracorporeal membrane oxygenation. Semin Perinatol. 2000;24:406–417. doi: 10.1053/sper.2000.20086. [DOI] [PubMed] [Google Scholar]
- 4.Zabrocki LA, Brogan TV, Statler KD, et al. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. 39:364–370. doi: 10.1097/CCM.0b013e3181fb7b35. [DOI] [PubMed] [Google Scholar]
- 5.McNally H, Bennett CC, Elbourne D, et al. United Kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: Followup to age 7 years. Pediatrics. 2006;117:e845–e854. doi: 10.1542/peds.2005-1167. [DOI] [PubMed] [Google Scholar]
- 6.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 7.Thiagarajan RR, Laussen PC, Rycus PT, et al. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116:1693–1700. doi: 10.1161/CIRCULATIONAHA.106.680678. [DOI] [PubMed] [Google Scholar]
- 8.Lawson DS, Lawson AF, Walczak R, et al. North American neonatal extracorporeal membrane oxygenation (ECMO) devices and team roles: 2008 survey results of Extracorporeal Life Support Organization (ELSO) centers. J Extra Corpor Technol. 2008;40:166–174. [PMC free article] [PubMed] [Google Scholar]
- 9.Gbadegesin R, Zhao S, Charpie J, et al. Significance of hemolysis on extracorporeal life support after cardiac surgery in children. Pediatr Nephrol. 2009;24:589–595. doi: 10.1007/s00467-008-1047-z. [DOI] [PubMed] [Google Scholar]
- 10.Steinhorn RH, Isham-Schopf B, Smith C, et al. Hemolysis during longterm extracorporeal membrane oxygenation. J Pediatr. 1989;115:625–630. doi: 10.1016/s0022-3476(89)80299-9. [DOI] [PubMed] [Google Scholar]
- 11.Masalunga C, Cruz M, Porter B, et al. Increased hemolysis from saline pre-washing RBCs or centrifugal pumps in neonatal ECMO. J Perinatol. 2007;27:380–384. doi: 10.1038/sj.jp.7211748. [DOI] [PubMed] [Google Scholar]
- 12.De Sanctis JT, Bramson RT, Blickman JG. Can clinical parameters help reliably predict the onset of acute intracranial hemorrhage in infants receiving extracorporeal membrane oxygenation? Radiology. 1996;199:429–432. doi: 10.1148/radiology.199.2.8668789. [DOI] [PubMed] [Google Scholar]
- 13.Reed RC, Rutledge JC. Laboratory and clinical predictors of thrombosis and hemorrhage in 29 pediatric extracorporeal membrane oxygenation nonsurvivors. Pediatr Dev Pathol. 2010;13:385–392. doi: 10.2350/09-09-0704-OA.1. [DOI] [PubMed] [Google Scholar]
- 14.Linneweber J, Chow TW, Kawamura M, et al. In vitro comparison of blood pump induced platelet microaggregates between a centrifugal and roller pump during cardiopulmonary bypass. Int J Artif Organs. 2002;25:549–555. doi: 10.1177/039139880202500610. [DOI] [PubMed] [Google Scholar]
- 15.Moon YS, Ohtsubo S, Gomez MR, et al. Comparison of centrifugal and roller pump hemolysis rates at low flow. Artif Organs. 1996;20:579–581. [PubMed] [Google Scholar]
- 16.Mueller XM, Tevaearai HT, Jegger D, et al. Hemolysis and hematology profile during perfusion: Interspecies comparison. Int J Artif Organs. 2001;24:89–94. [PubMed] [Google Scholar]
- 17.Delgado AV, Alexander SL, McManus AT, et al. Antibodies against human cell receptors, CD36, CD41a, and CD62P crossreact with porcine platelets. Cytometry B Clin Cytom. 2003;56:62–67. doi: 10.1002/cyto.b.10042. [DOI] [PubMed] [Google Scholar]
- 18.Tamari Y, Lee-Sensiba K, Leonard EF, et al. A dynamic method for setting roller pumps nonocclusively reduces hemolysis and predicts retrograde flow. ASAIO J. 1997;43:39–52. [PubMed] [Google Scholar]
- 19.Van Meurs KP, Mikesell GT, Seale WR, et al. Maximum blood flow rates for arterial cannulae used in neonatal ECMO. ASAIO Transs. 1990;36:M679–M681. [PubMed] [Google Scholar]
- 20.Yarborough KA, Mockros LF, Lewis FJ. Hydrodynamic hemolysis in extracorporeal machines. J Thorac Cardiovasc Surg. 1966;52:550–557. [PubMed] [Google Scholar]
- 21.Malinauskas RA. Plasma hemoglobin measurement techniques for the in vitro evaluation of blood damage caused by medical devices. Artif Organs. 1997;21:1255–1267. doi: 10.1111/j.1525-1594.1997.tb00486.x. [DOI] [PubMed] [Google Scholar]
- 22.American Society for Testing and Materials . Standard Practice for Assessment of Hemolysis in Continuous Flow Blood Pumps. American Society for Testing and Materials; West Conshohocken, PA: 1997. pp. 1–5. [Google Scholar]
- 23.Konstantopoulos K, Wu KK, Udden MM, et al. Flow cytometric studies of platelet responses to shear stress in whole blood. Biorheology. 1995;32:73–93. doi: 10.3233/bir-1995-32106. [DOI] [PubMed] [Google Scholar]
- 24.Perez de la Lastra JM, Moreno A, Perez J, et al. Characterization of the porcine homologue to human platelet glycoprotein IIb-IIIa (CD41/CD61) by a monoclonal antibody. Tissue Antigens. 1997;49:588–594. doi: 10.1111/j.1399-0039.1997.tb02806.x. [DOI] [PubMed] [Google Scholar]
- 25.Barrett CS, Bratton SL, Salvin JW, et al. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10:445–451. doi: 10.1097/PCC.0b013e318198bd85. [DOI] [PubMed] [Google Scholar]
- 26.Green TP, Kriesmer P, Steinhorn RH, et al. Comparison of pressure-volume-flow relationships in centrifugal and roller pump extracorporeal membrane oxygenation systems for neonates. ASAIO Trans. 1991;37:572–576. [PubMed] [Google Scholar]
- 27.Kawahito S, Maeda T, Motomura T, et al. Hemolytic characteristics of oxygenators during clinical extracorporeal membrane oxygenation. ASAIO J. 2002;48:636–639. doi: 10.1097/00002480-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Rother RP, Bell L, Hillmen P, et al. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 29.Haines NM, Rycus PT, Zwischenberger JB, et al. Extracorporeal Life Support registry report 2008: Neonatal and pediatric cardiac cases. ASAIO J. 2009;55:111–116. doi: 10.1097/MAT.0b013e318190b6f7. [DOI] [PubMed] [Google Scholar]
- 30.Zwischenberger JB, Nguyen TT, Upp JR, Jr, et al. Complications of neonatal extracorporeal membrane oxygenation. Collective experience from the Extracorporeal Life Support Organization. J Thorac Cardiovasc Surg. 1994;107:838–848. discussion 848–849. [PubMed] [Google Scholar]
- 31.Stallion A, Cofer BR, Rafferty JA, et al. The significant relationship between platelet count and haemorrhagic complications on ECMO. Perfusion. 1994;9:265–269. doi: 10.1177/026765919400900404. [DOI] [PubMed] [Google Scholar]
- 32.Hirthler MA, Blackwell E, Abbe D, et al. Coagulation parameter instability as an early predictor of intracranial hemorrhage during extracorporeal membrane oxygenation. J Pediatr Surg. 1992;27:40–43. doi: 10.1016/0022-3468(92)90101-c. [DOI] [PubMed] [Google Scholar]
- 33.Dela Cruz TV, Stewart DL, Winston SJ, et al. Risk factors for intracranial hemorrhage in the extracorporeal membrane oxygenation patient. J Perinatol. 1997;17:18–23. [PubMed] [Google Scholar]
- 34.Chevuru SC, Sola MC, Theriaque DW, et al. Multicenter analysis of platelet transfusion usage among neonates on extracorporeal membrane oxygenation. Pediatrics. 2002;109:e89. doi: 10.1542/peds.109.6.e89. [DOI] [PubMed] [Google Scholar]
- 35.Cheung PY, Sawicki G, Salas E, et al. The mechanisms of platelet dysfunction during extracorporeal membrane oxygenation in critically ill neonates. Crit Care Med. 2000;28:2584–2590. doi: 10.1097/00003246-200007000-00067. [DOI] [PubMed] [Google Scholar]
- 36.Kestin AS, Valeri CR, Khuri SF, et al. The platelet function defect of cardiopulmonary bypass. Blood. 1993;82:107–117. [PubMed] [Google Scholar]
- 37.Weerasinghe A, Taylor KM. The platelet in cardiopulmonary bypass. Ann Thorac Surg. 1998;66:2145–2152. doi: 10.1016/s0003-4975(98)00749-8. [DOI] [PubMed] [Google Scholar]
- 38.Thiara AP, Hoel TN, Kristiansen F, et al. Evaluation of oxygenators and centrifugal pumps for long-term pediatric extracorporeal membrane oxygenation. Perfusion. 2007;22:323–326. doi: 10.1177/0267659107086270. [DOI] [PubMed] [Google Scholar]
- 39.Lawson DS, Ing R, Cheifetz IM, et al. Hemolytic characteristics of three commercially available centrifugal blood pumps. Pediatr Crit Care Med. 2005;6:573–577. doi: 10.1097/01.pcc.0000163282.63992.13. [DOI] [PubMed] [Google Scholar]
- 40.Motomura T, Maeda T, Kawahito S, et al. Extracorporeal membrane oxygenator compatible with centrifugal blood pumps. Artif Organs. 2002;26:952–958. doi: 10.1046/j.1525-1594.2002.07117.x. [DOI] [PubMed] [Google Scholar]
- 41.Yu K, Long C, Hei F, et al. Clinical evaluation of two different extracorporeal membrane oxygenation systems: A single center report. Artif Organs. 2011;35:733–737. doi: 10.1111/j.1525-1594.2010.01173.x. [DOI] [PubMed] [Google Scholar]
- 42.Horton S, Thuys C, Bennett M, et al. Experience with the Jostra Rotaflow and QuadroxD oxygenator for ECMO. Perfusion. 2004;19:17–23. doi: 10.1191/0267659104pf702oa. [DOI] [PubMed] [Google Scholar]