Abstract
Interleukin-2-inducible T-cell kinase (Itk) is a member of the Btk (Bruton's tyrosine kinase) family of tyrosine kinases. Itk plays an important role in normal T-cell functions and in the pathophysiology of both autoimmune diseases and T-cell malignancies. Here, we describe the initial characterization of a selective inhibitor, 7-benzyl-1-(3-(piperidin-1-yl)propyl)-2-(4-(pyridin-4-yl)phenyl)-1H-imidazo[4,5-g]quinoxalin-6(5H)-one (CTA056), that was developed through screening a 9600-compound combinatorial solution phase library, followed by molecular modeling, and extensive structure-activity relationship studies. CTA056 exhibits the highest inhibitory effects toward Itk, followed by Btk and endothelial and epithelial tyrosine kinase. Among the 41 cancer cell lines analyzed, CTA056 selectively targets acute lymphoblastic T-cell leukemia and cutaneous T-cell lymphoma. Normal T cells are minimally affected. Incubation of Jurkat and MOLT-4 cells with CTA056 resulted in the inhibition of the phosphorylation of Itk and its effectors including PLC-γ, Akt, and extracellular signal-regulated kinase, as well as the decreased secretion of targeted genes such as interleukin-2 and interferon-γ. Jurkat cells also underwent apoptosis in a dose-dependent manner when incubated with CTA056. The potent apoptosis-inducing potential of CTA056 is reflected by the significant modulation of microRNAs involved in survival pathways and oncogenesis. The in vitro cytotoxic effect on malignant T cells is further validated in a xenograft model. The selective expression and activation of Itk in malignant T cells, as well as the specificity of CTA056 for Itk, make this molecule a potential therapeutic agent for the treatment of T-cell leukemia and lymphoma.
Introduction
T-cell malignancies are a heterogeneous group of cancers that encompass several entities, including acute T-cell acute lymphoblastic leukemia (T-ALL), adult T-cell leukemia/lymphoma, and Sézary syndrome/cutaneous T-cell lymphoma. Although there are marked differences between these cancers, there is some overlap in their management, especially at advanced stages of the disease. In addition, in terms of survival, patients with relapsed T-cell malignancies uniformly have a poor outcome. Results from the large UKALL XII/Eastern Cooperative Oncology Group-2993 trial demonstrated that after therapy, 42% (95% confidence interval, 36–47%) of patients with T-ALL relapsed within 5 years (Marks et al., 2009). Similar to T-ALL, patients with Sézary/cutaneous T-cell lymphoma have a dismal 5-year survival rate of only 11% (Kim et al., 2003). Therefore, management of these patients is an important area of research. Even if a patient responds well to therapy, there are significant short-term and potentially long-term side effects from the intensive chemotherapy regimens needed to attain sustained remission. Thus, the identification of new targets for drug development is critical.
Protein tyrosine kinases play a central role in the regulation of signals originating from the cell surface (August et al., 1994). Interleukin-2-inducible T-cell kinase (Itk) is one of the five members of the Bruton tyrosine kinase (Btk) family of nonreceptor tyrosine kinases and is known to be essential for T-cell function (Siliciano et al., 1992; Berg et al., 2005). It is a critical modulator of T-cell receptor signaling, which connects receptor proximal signal molecules such as Lck, ZAP70, LAT, and SLP-76 to PLC-γ (Kim et al., 2003; Grasis et al., 2010), as well as other effectors in the cytosol (Andreotti et al., 2010). Phosphorylation and activation of PLC-γ by Itk mediates calcium flux, resulting in the activation of calcineurin and NFATc with consequent up-regulation of cytokines. Itk was also shown to engage G-protein-coupled receptor signaling mediated by CXCL14/SDF1 and induce actin polymerization and cellular migration (Berge et al., 2010). Though viable, Itk-deficient animals have profound defects in T-cell differentiation and development, especially with respect to CD4+ T cells (Fowell et al., 1999; Schaeffer et al., 2001). At the cellular level, Itk-deficient cells have impaired T-cell proliferation, adhesion, migration, and F-actin reorganization (Woods et al., 2001; Dombroski et al., 2005). Although Itk inhibitors are not commercially available, selective Itk inhibitors are currently under development (Lin et al., 2004; Cook et al., 2009). Such inhibitors have been tested in models of lung inflammation, skin dermatitis, asthma (Lin et al., 2004), and HIV infection models with promising results (Readinger et al., 2008), although none has entered clinical trials (Hao et al., 2006). In addition, because the prior emphasis of these inhibitors was on inflammation, the characterizations of these inhibitors were primarily limited to cytokine release and immune modulation, with a paucity of information regarding their effects on T-cell proliferation and apoptosis. We are interested in targeting Itk for T-cell malignancies due to Itk's heightened expression and aberrant activation in these cells (Kaukonen et al., 1999). Itk has also been shown to be up-regulated in skin lesions of patients with cutaneous T-cell lymphoma (Shin et al., 2007), and the Itk-dependent CD25 signaling pathway is known to be important in T-cell malignancies. Targeting CD25-expressing T-cell leukemias/lymphomas with anti-CD25 monoclonal antibodies has shown therapeutic efficacy (Zhang et al., 2003; Dancey et al., 2009). A t(5;9)(q33;q22) chromosomal translocation was identified in a subset of unspecified peripheral T-cell lymphomas. The translocation resulted in an Itk-Syk fusion gene (Streubel et al., 2006), which is oncogenic in animal models (Pechloff et al., 2010). In this case, Itk's pleckstrin homology, but not kinase, domain is involved. These findings point to Itk's involvement in the pathogenesis of T-cell malignancies and support Itk as a potential therapeutic target for anticancer drug development. Herein, we report the development of a new class of Itk inhibitors, which are structurally different from the other reported Itk inhibitors. We chose to characterize CTA056, which has selectivity toward Itk and preferentially targets T-cell malignancies expressing Itk. The signals diminished by CTA056 treatment are consistent with the inhibition of Itk kinase activity. MicroRNA (miRNA) profiling of malignant T cells treated with CTA056 display the up-regulation of miRNAs that suppress survival factors and the down-modulation of those that increase oncogenicity. Thus, CTA056 appears to be a promising new lead to target Itk in T-cell malignancy. To our knowledge, this is the first report of the application of an Itk selective inhibitor as an anticancer agent.
Materials and Methods
Materials.
Purified Itk, Btk, Etk, and Src kinases were obtained from Millipore, Inc. (Dundee, Scotland, UK). Propidium iodide (PI), N,N-diisopropylethylamine (DIEA), N,N-dimethylformamide (DMF), ethanol, acetonitrile, 1-(3-aminopropyl)piperidine, trifluoroacetic acid, palladium on carbon (Pd/C), ammonium formate, and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St. Louis, MO). l-Phenylalanine methyl ester hydrochloride and 4-(4-pyridyl)benzaldehyde were purchased from Chem-Impex International, Inc. (Wood Dale, IL). The Annexin-V-FITC apoptosis detection kit was obtained from Abcam, Inc. (Cambridge, MA). Reversed-phase high-performance liquid chromatography (RP-HPLC; C18 column, 5 μm, 19 × 150 mm and 4.6 × 150 mm for preparative and analytical high-performance liquid chromatography, respectively) from Waters (Milford, MA) was used for analysis and purification of CTA056.
One-Pot Synthesis of CTA056.
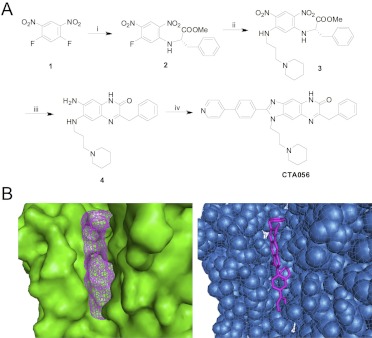
The synthetic approach of CTA056 is similar to that reported previously (Zhang et al., 2005), but with slight modification as shown in Fig. 1A. In brief, a solution of l-phenylalanine methyl ester hydrochloride (215.7 mg, 1.0 mmol) and DIEA (383.2 μl, 2.2 mmol) in DMF (1.5 ml) was added dropwise under vigorous stirring to a solution of 1,5-difluoro-2,4-dinitrobenzene (204.0 mg, 1.0 mmol) in DMF (0.5 ml). The reaction solution was stirred at room temperature for 30 min. This was followed by the addition of a solution of 1-(3-aminopropyl) piperidine (159 μl, 1.0 mmol) and DIEA (174.2 μl, 1.0 mmol) in DMF (1 ml). The resulting mixture was agitated at room temperature overnight. Ethanol (20 ml), Pd/C (10%, 200 mg), and ammonium formate (1.50 g, 23.8 mmol) were added to the solution. The solution was heated to reflux for 3 h and then cooled to room temperature. The Pd/C was filtered out, and the filtrate was concentrated with rotary evaporator. 4-(4-Pyridyl)benzaldehyde (183.2 mg, 1.0 mmol) in DMF was added to the solution. The resulting solution was stirred at room temperature for 1 day. The DMF solution was poured into 40 ml of ice. The precipitate was collected by filtration and washed with water, followed by RP-HPLC purification. The fraction was collected and lyophilized to give a yellow powder as the final product. The homogeneity of the compound was checked by analytical RP-HPLC. The purity was determined to be >95%. The identity of the compounds was confirmed by orbitrap high-resolution mass spectrometry: found, 555.2868 Da (calculated, 555.2866 Da for MH+).
Fig. 1.
Synthetic scheme of CTA056 (A) and molecular modeling studies of binding conformation of CTA056 to the kinase domain of human Itk (B). The docking pocket was created from the crystal structure of the kinase domain of Itk using AutoDock 4.0. Docking was performed using AutoDock Vina 1.0, and the docking results were analyzed using PyMOL 0.99. CTA056 was shown to bind to the ATP-binding pocket in the kinase domain of Itk.
Molecular Modeling.
Itk kinase domain in complex with staurosporine was used to create the docking pocket (Brown et al., 2004). Staurosporine was deleted, and the Itk crystal structure was optimized for docking using AutoDock 4.0 (Scripps Research Institute, La Jolla, CA). Docking was performed using AutoDock Vina 1.0 (Scripps Research Institute). The docking results were analyzed using PyMOL 0.99 (Schrödinger, Inc., Portland, OR) (Seeliger and de Groot, 2010; Trott and Olson, 2010).
Kinase Activity Assay.
For tyrosine kinases, kinase activity was measured using thin-layer chromatography (TLC). In brief, purified kinases (20 nM), the corresponding substrate (500 μM, TSFYGRH for Itk and YIYGSFK for the other kinases), and CTA056 (0–10 μM) were incubated in a kinase reaction [100 mM Hepes, pH 7.4, 10 mM MnCl2, 10 mM MgCl2, 1 mM dithiothreitol (DTT)] for 5 min, and the reaction was started by adding 5 μCi of 33P-labeled ATP. The reaction (10 μl) was incubated at room temperature for 1 h and was stopped by adding 10 μl of H3PO4. The radioactivity of the peptide substrate was analyzed using TLC as described previously (Lou et al., 1996). For Ser/Thr kinases, the kinase activity was measured using Kinase-Glo Assay (Promega, Madison, WI). Purified kinases (20 nM), the corresponding substrate (mitogen-activated protein kinase substrate peptide for Mkk1 and Erk1, PDKtide for PDK1, and Akt/SGK peptide substrate for Akt; Millipore, Inc.), and CTA056 (0–10 μM) were incubated in a kinase reaction (100 mM Hepes, pH 7.4, 10 mM MnCl2, 10 mM MgCl2, and 1 mM DTT) for 5 min, and the reaction was started by adding 50 μM ATP. The reaction (10 μl) was incubated at room temperature for 1 h, and the kinase activity was measured using Kinase-Glo Assay according to the manufacture's instruction (Promega).
Itk Autophosphorylation Assay.
Itk autophosphorylation activity was measured by an in vitro kinase assay. In brief, purified Itk (100 ng) was mixed with CTA056 inhibitor in the kinase assay buffer (20 mM Hepes, pH 7.55, 10 mM MgCl2, 10 mM MnCl2, 1 mM DTT, 500 μM Na3VO4). The unlabeled ATP (5 μM) and labeled [33P]ATP (5 μCi) were added to the mixture, and the kinase reaction was performed at 30°C for 30 min. The reactions were terminated with 4× SDS-polyacrylamide gel electrophoresis sample buffer and then loaded onto an 8% SDS-polyacrylamide gel for electrophoresis. The gel was vacuum-dried, and the ITK auto-kinase activity was analyzed with filmless autoradiographic analysis (Bio-Rad Laboratories, Hercules, CA).
Cell Culture.
Jurkat, MOLT-4, LNCaP, PC3, and RWPE1 cells were maintained in RPMI 1640 medium containing 10% fetal bovine serum and 1% penicillin-streptomycin-glutamine. BCBL-1 cells were in RPMI 1640 medium containing 15% fetal bovine serum and 1% penicillin-streptomycin-glutamine.
Western Blotting.
Western blotting was performed as described previously (Grasso et al., 1997). Proteins were detected by the following antibodies: β-actin (Sigma-Aldrich), Itk (Santa Cruz Biotechnology, Santa Cruz, CA), and phospho-Itk (Epitomics, Inc., Burlingame, CA). For phospho-Itk, cells were pretreated with 100 μM pervanadate for 15 min before harvest.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide Assay.
Cells were seeded in 96-well plates and cultured overnight, followed by treatment with 0.1% DMSO, as vehicle control, and CTA056 at the indicated concentrations for 72 h. Growth inhibition was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Roche Diagnostics, Mannheim, Germany).
Flow Cytometry.
Jurkat cells were treated with 0.1% DMSO (control) and CTA056 at the indicated concentrations for 24 h. Cell-cycle arrest was determined by the incorporation of PI (Sigma-Aldrich) into permeabilized cells. Cells undergoing apoptosis were identified using an Annexin-V-FITC kit (Abcam, Inc.) following the manufacturer's instructions. The cells were analyzed using a Coulter Epics XL flow cytometer (Beckman Coulter, Fullerton, CA).
Stimulation of Phospho-Itk Assay.
Jurkat cells were pretreated with CTA056 at 37°C for 30 min and then incubated with anti-CD3 and anti-CD28 monoclonal antibodies (eBioscience, San Diego, CA) at 10 μg/ml and protein G (BD Biosciences, San Jose, CA) at 10 μg/ml. At 30 s, 1 min, and 5 min, the stimulation was stopped by addition of Fix Buffer I (BD Biosciences), and then the cells were permeabilized with Perm Buffer III (BD Biosciences). A phospho-Itk-specific antibody (BD Biosciences) was then used for intracellular staining. Samples were analyzed on a BD FACSCalibur flow cytometer (BD Biosciences).
Cytokine Secretion Assay.
Jurkat cells were pretreated with 0.5 μM CTA056 for 30 min at 37°C and then stimulated with anti-CD3 and anti-CD28 antibodies (eBioscience) at 1 μg/ml for 8 h. Cells were harvested, RNA was isolated using TRIzol reagent (Ambion, Austin, TX), and mRNA levels of interleukin (IL)-2 and interferon (IFN)-γ were measured using real-time polymerase chain reaction (PCR) with primers 5′GTCTTGCACTTGTCACAAACAG3′ and 5′CAGTTCTGTGGCCTTCTTGG′ for IL-2 and 5′AATTGGAAAGAGGAGAGTGACAG3′ and 5′ATTCATGTCTTCCTTGATGGTCTC3′ for IFN-γ. For cytokine release, IL-2 was quantified using a sandwich enzyme-linked immunosorbent assay (ELISA) kit, and IFN-γ was quantified using the BD enzyme-linked immunosorbent spot assay (BD Biosciences).
Microarray Assay for MiRNAs.
Jurkat cells were pretreated with CTA056 (0.5 μM) for 30 min at 37°C and then stimulated with anti-CD3 and anti-CD28 antibodies (eBioscience) at 1 μg/ml for 8 h. Cells were harvested, and RNA was isolated using TRIzol reagent (Ambion). MiRNA profile was examined using miRNA array. MiRNAs with the most significant changes from miRNA array were further confirmed using real-time PCR with the corresponding primers (QIAGEN, Valencia, CA).
Inhibition of MOLT-4 Xenograft Tumor Growth by CTA056.
Ten million MOLT-4 cells were injected subcutaneously to nude mice. The tumors were grown to the indicated size, and the mice were randomly divided into two groups (eight mice per group). The control group was treated with vehicle. The treatment group was treated with CTA056 at 5 mg/kg twice a week with intratumoral injection. The tumor size and body weight were measured once a week.
Statistics.
One-way analysis of variance (ANOVA) was used in combination with a Tukey test for pairwise comparison. P values less than 0.05 were considered significant.
Results
CTA056 as a Selective Inhibitor Against Itk Tyrosine Kinase.
Through screening a 9600-diversity combinatorial solution-phase small-molecule library, compounds with inhibitory activities against Btk were discovered. Subsequent molecular modeling and extensive structure-activity relationship studies led to the identification of CTA056 (Fig. 1A). Molecular modeling studies based on the structure elucidated by Brown et al. (2004) indicate that this compound binds well to the kinase domain of Itk, where it fits into the ATP-binding pocket. The binding appears to be mediated through hydrogen bonds, as well as π-π hydrophobic interactions with residues in the ATP-binding pocket (Fig. 1B).
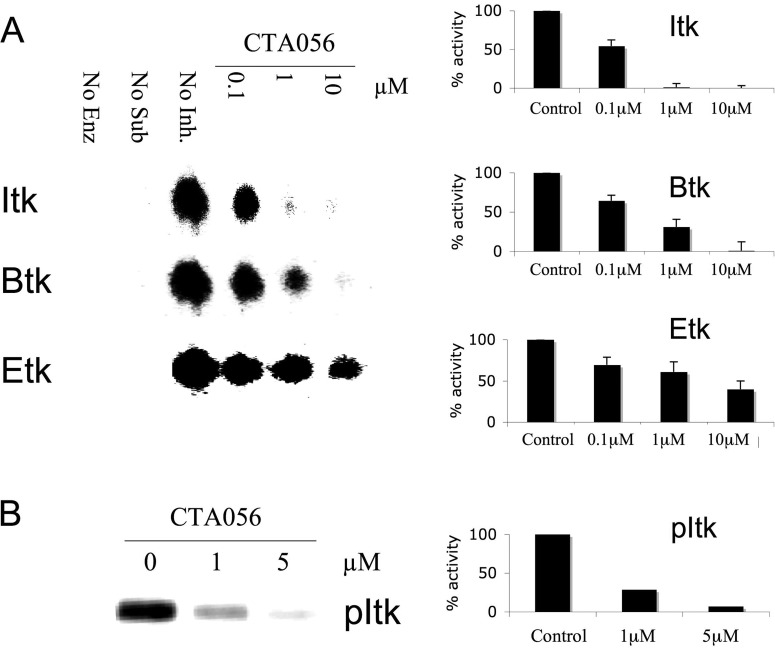
Having isolated a small molecule that fits well into the ATP-binding pocket of Itk, the next logical step was to determine the molecule's ability to inhibit the kinase function of Itk and to determine its specificity for this enzyme. To accomplish this, purified Itk, Btk, and Etk were incubated in a kinase reaction buffer with CTA056 (0–10 μM) in the presence of 33P-labeled ATP. TSFYGRH was used as substrates for Itk, and YIYGSFK was used for Btk and Etk. YIYGSFK was identified previously as an efficient substrate for Src through screening a one-bead–one-compound combinatorial peptide library (Lam et al., 1995). We subsequently found that YIYGSFK is also a very good substrate for both Btk and Etk, but not for Itk. We then screened XXXYXXX one-bead–one-compound library with Itk and identified TSFYGRH as an Itk substrate (data not shown). A kinase assay with TLC technique (Lou et al., 1996) revealed that CTA056 was a potent and selective inhibitor for Itk, with an IC50 of approximately 100 nM (Fig. 2A). Inhibition was observed in a concentration-dependent manner, and at higher concentrations, CTA056 could also inhibit Btk (IC50 ≈ 400 nM). However, Etk was significantly more resistant to CTA056 inhibition, with an IC50 of approximately 5 μM (Fig. 2A). In summary, CTA056 showed selectivity for Itk over other Tec family members.
Fig. 2.
Kinase inhibitory activity of CTA056 (A) and inhibition of Itk autophosphorylation by CTA056 (B). Kinase activity was measured using TLC assay. Purified kinase (20 nM), CTA056 (0–10 μM), and the peptide substrate were incubated with [33P]ATP in a kinase reaction. The resulting product was analyzed on a TLC plate. The relative kinase activity to control was calculated using densitometry. Itk autophosphorylation activity was measured by an in vitro kinase assay. Purified Itk (100 ng) was mixed with CTA056 inhibitor, the ice-cold ATP and hot r-[33P]ATP in the kinase assay buffer, and the Itk auto-kinase activity was analyzed by filmless autoradiographic analysis (Bio-Rad Laboratories). Columns, mean; bars, S.D., n = 3.
Btk family nonreceptor tyrosine kinases are characterized by the presence of an autophosphorylation site within their noncatalytic Src homology 3 domain. Thus, in addition to characterizing the ability of CTA056 to inhibit Itk's ability to phosphorylate cognate peptides, it was also important to determine the ability of CTA056 to inhibit Itk autophosphorylation. Therefore, an in vitro Itk autophosphorylation assay was established in which purified Itk was mixed with CTA056 in the presence of [33P]ATP. After 30 min, the reaction was terminated, and the samples were loaded onto an SDS-polyacrylamide gel for electrophoresis. After drying, the gel was analyzed with filmless autoradiographic analysis. Figure 2B reveals that CTA056 was able to inhibit Itk autophosphorylation in a concentration-dependent manner.
In addition to Btk family tyrosine kinases Itk, Btk, and Etk, the inhibitory activity of CTA056 to other kinases, including Src, Yes, Lyn, Axl, Mer, EGFR, Abl, was investigated using a TLC assay. As shown in Table 1, CTA056 appears to have reactivity toward Btk family kinases and Src, but not toward any of the other kinases tested.
TABLE 1.
Kinase inhibition profile of CTA056
| Kinase | IC50 |
|---|---|
| μM | |
| Itk | 0.1 |
| Btk | 0.4 |
| Etk | 3 |
| Src | 1.2 |
| Yes | >10 |
| Lyn | >10 |
| Axl | >10 |
| Mer | >10 |
| EGFR | >10 |
| Abl | >10 |
| Mkk1 | >10 |
| PDK1 | >10 |
| Akt | >10 |
| Erk1 | >10 |
CTA056 Preferentially Inhibits the Growth of Malignant Cells Harboring Itk.
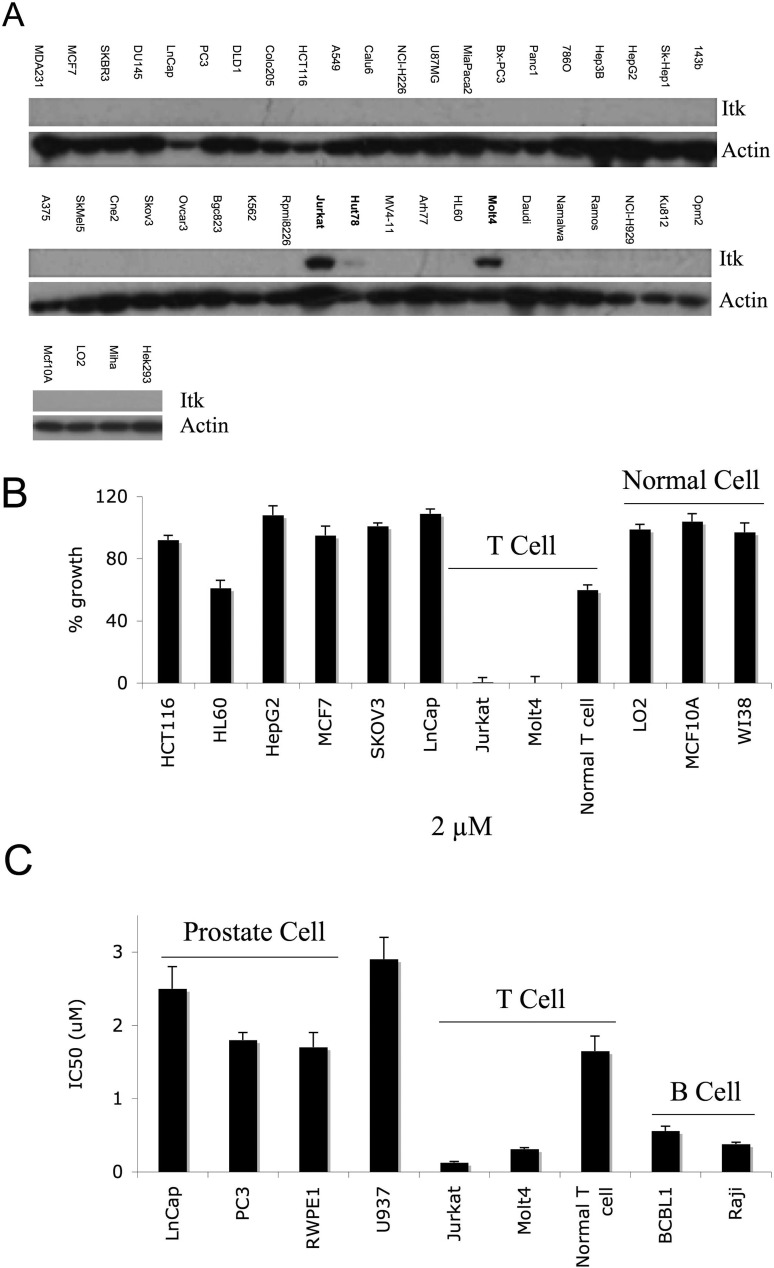
Because CTA056 selectively inhibits Itk, Itk expression in 41 different cancer cell lines was examined, and Itk was detected only in the T-ALL cell lines, Jurkat and MOLT-4, and in the cutaneous T-cell lymphoma cell line, Hut78 (Fig. 3A). To determine the effect of CTA056 on proliferation, a panel of cancer cell lines including Jurkat and MOLT-4 cells were incubated with CTA056, and proliferation was measured using the MTT assay. At 2 μM, CTA056 was very effective at inhibiting the growth of Itk-high cancer cells (Jurkat and MOLT-4), but not the Itk-null cancer cells (HCT116, HL60, HepG2, MCF7, and SKOV3). The three normal cell lines LO2, WI38, and MCF10A, as well as normal T cells, were resistant to CTA056 (Fig. 3B). Titration experiments further confirmed these findings and revealed that Jurkat cells were most sensitive to CTA056-induced growth inhibition (Fig. 3C). Because CTA056 also showed moderate inhibition to Btk (Fig. 2A), two B-cell leukemia/lymphoma cell lines, BCBL-1 and Raji, were examined. As expected, CTA056 showed moderate growth inhibition to these two cell lines (Fig. 3C). These results strongly suggest that the in vivo target of CTA056 is Itk. The inhibitory effect of CTA056 on BCBL-1 and Raji is likely due to its modest inhibitory activity against Btk (Fig. 2A).
Fig. 3.
Expression profile of Itk in 45 cell lines (41 cancer cell lines and 4 normal cell lines) and CTA056-induced growth inhibition of a panel of cancer cells. The expression profile of Itk was measured using Western blot (A). For growth inhibition, cells were seeded at 5000 cells/well in 96-well plate overnight and were treated with CTA056 (B, 0 and 2 μM; C, 0–10 μM). After 72 h, cell proliferation was measured using the MTT assay. Columns, mean; bars, S.D., n = 3.
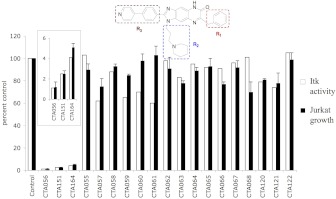
We then synthesized and tested a series of analogs with a fused three-ring core structure identical to CTA056, differing only in the side groups R1, R2, and R3 (Fig. 4). These compounds showed significant variability in their abilities to inhibit Itk (open bar), with CTA056, CTA151, and CTA164 being the most potent. These three compounds have the same R2 and very similar R1 and R3 groups. In addition, when their abilities to inhibit Jurkat growth were measured (solid bar), there was a strong correlation between Itk inhibition and Jurkat growth retardation (Fig. 4). These data further suggest that Itk is indeed the target responsible for the growth inhibition observed for Jurkat cells.
Fig. 4.
Itk inhibition and growth inhibition to Jurkat cells of a series of CTA compounds. Kinase activity was measured using TLC assay. Purified Itk (20 nM), CTA compounds (1 μM), and the peptide substrate TSFYGRH were incubated with [33P]ATP in a kinase reaction. The resulting product was analyzed on a TLC plate. For growth inhibition, cells were seeded at 5000 cells/well in 96-well plate overnight and were treated with CTA compounds (2 μM). After 72 h, cell proliferation was measured using the MTT assay. Columns, mean; bars, S.D., n = 3. CTA056 was shown to inhibit Itk and inhibit the growth of Jurkat cells.
CTA056 Induces Apoptosis in Target Cells.
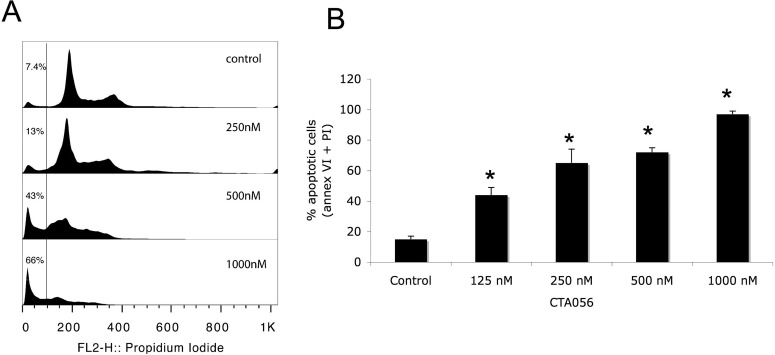
To determine whether the growth inhibition induced by CTA056 on Jurkat cells was due to apoptosis, flow cytometric analysis was performed. After treatment with CTA056 for 24 h, a dose-dependent accumulation of a “subG1” fraction was observed using PI staining (Fig. 5A). Data based on Annexin-V reactivity also indicated a dose-dependent increase of apoptosis of Jurkat cells after treatment with CTA056 (Fig. 5B).
Fig. 5.
Induction of apoptosis of Jurkat cells after treatment with CTA056. Jurkat cells were seeded at 106 cells/ml (2 ml) in a six-well plate and treated with CTA056 at the indicated concentrations for 24 h. Cell-cycle arrest was analyzed using PI staining (A). Apoptosis was analyzed using Annexin-V-FITC apoptosis detection kit (Abcam, Inc.) (B). Columns, mean; bars, S.D., n = 3. 125, 250, 500, and 1000 nM are significantly different from control (*, p < 0.05, one-way ANOVA with Tukey's test for pairwise comparison).
CTA056 Inhibits the Formation of Phospho-Itk in Jurkat cells and Its Downstream Signals.
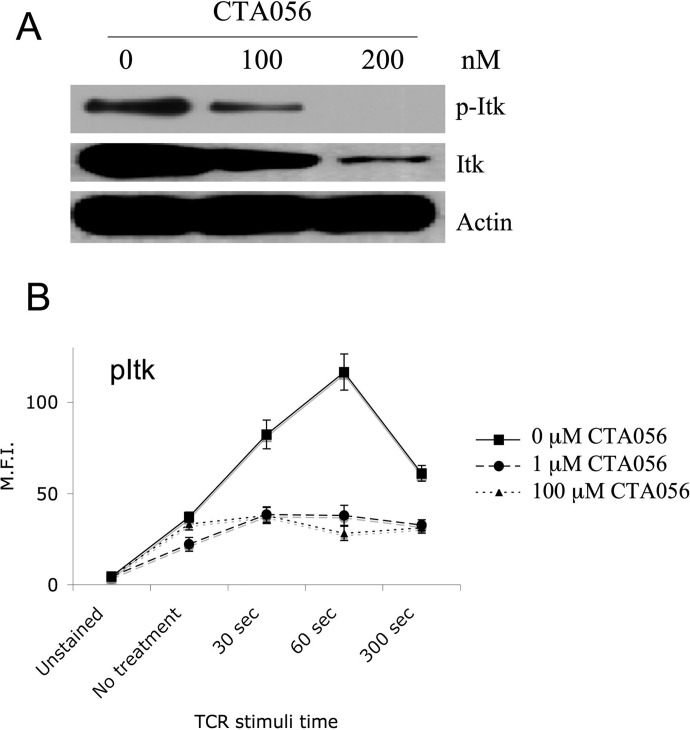
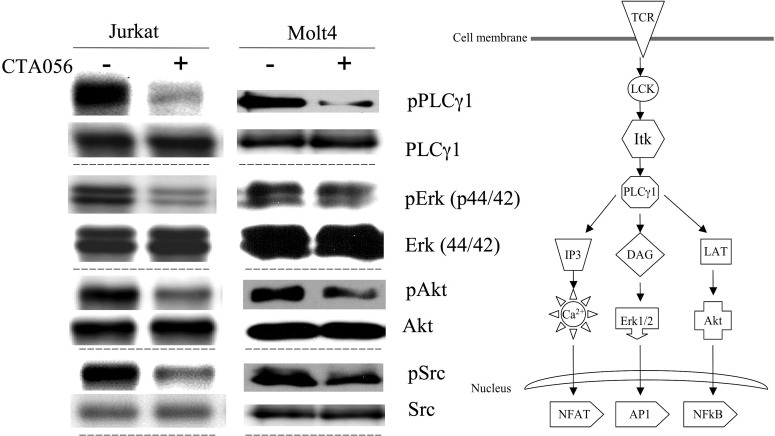
The inhibitory activity of CTA056 against autophosphorylation of Itk in intact cells was examined by Western blot. Itk phosphorylation in Jurkat cells was significantly inhibited at 100 nM and completely inhibited at 200 nM. This decrease in Itk phosphorylation was accompanied by a decrease in the total Itk levels in Jurkat cells (Fig. 6A). Moreover, the stimulation of Itk phosphorylation in Jurkat cells by anti-CD3 and anti-CD28 antibodies was also hampered by CTA056 (Fig. 6B). Itk inhibition in Jurkat and MOLT-4 cells leads to the suppression of phosphorylation of downstream effectors such as PLC-γ1, Erk, and Akt (Fig. 7). Treatment of Jurkat and MOLT-4 cells with CTA056 resulted in the inhibition of Src phosphorylation. This could be due to its moderate inhibitory effect on Src (Table 1), and the reported cross-activation of Src and Btk family kinases (Tsai et al., 2000).
Fig. 6.
Inhibition of Itk phosphorylation in Jurkat cells after treatment with CTA056. Itk phosphorylation in Jurkat cells was examined using Western blot (A). Cells were seeded at 106 cells/ml (5 ml) and treated with CTA056 at the indicated concentrations for 24 h. Before harvest, cells were pretreated with pervanadate for 15 min. pItk, Itk, and actin levels were measured using the corresponding antibodies through Western blot. B, Jurkat cells were pretreated with CTA056 30 min and then incubated with anti-CD3, anti-CD28 monoclonal antibodies (10 μg/ml), and protein G (10 μg/ml). At 30 s, 1 min, and 5 min, the stimulation was stopped. The cells were then permeabilized and stained with a phospho-Itk-specific antibody. One of three similar experiments is depicted. Values are averages of triplicate samples.
Fig. 7.
Inhibition of cell signaling in Jurkat and MOLT-4 cells after treatment with CTA056. Cells were seeded at 106 cells/ml (5 ml) and pretreated with 0.5 μM CTA056 for 30 min. Cells were then stimulated with anti-CD3, anti-CD28 monoclonal antibodies (10 μg/ml), and protein G (10 μg/ml) for 1 min. pPLC-γ1, PLC-γ1, pERK, ERK, pSrc, Src, pAkt, and Akt levels were measured using the corresponding antibodies through Western blot. One of three similar experiments is depicted.
CTA056 Inhibits IL-2 and IFN-γ Secretion.
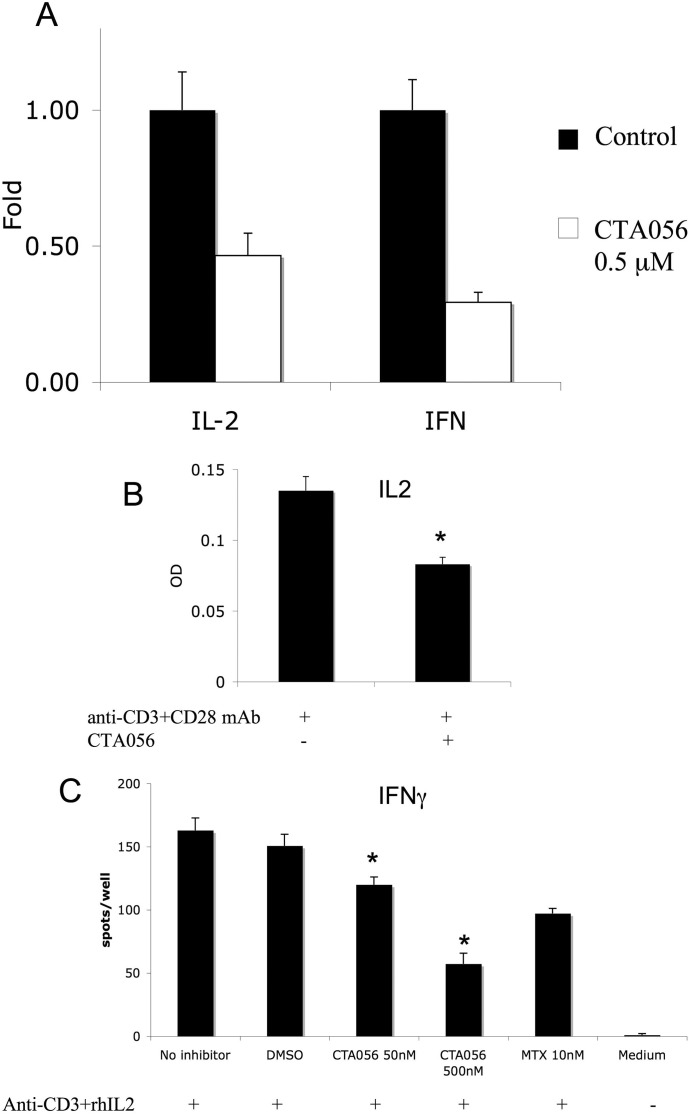
Itk plays an important role in transmitting signals at the immunological synapse, Itk(−/−) cells had severe reduction of IL-2 and IFN-γ secretion (Schaeffer et al., 1999; von Bonin et al., 2011). After incubation of Jurkat cells with 0.5 μM CTA056, the mRNA levels of IL-2 and IFN-γ were measured using real-time PCR, and the IL-2 and IFN-γ secretions were measured using an ELISA assay. As expected, the mRNA levels of IL-2 and IFN-γ, as well as the IL-2 and IFN-γ release by Jurkat cells, were significantly decreased after treatment with CTA056 (Fig. 8), indicating the effect of CTA056 as inhibiting T-cell activation and cytokine release.
Fig. 8.
Inhibition of IL-2 and IFN-γ mRNA level (A) and inhibition of IL-2 (B) and IFN-γ (C) release of Jurkat cells after treatment with CTA056. For mRNA levels, Jurkat cells were pretreated with CTA056 0.5 μM for 30 min and then stimulated with anti-CD3 and anti-CD28 antibodies (1 μg/ml) for 6 h. RNA was isolated and the mRNA levels were measured using real-time PCR. Supernatants were then harvested and IL-2 was quantified using a sandwich ELISA. For interferon-γ release assay, Jurkat cells were pretreated with CTA056 (0, 50, and 500 nM) or methotrexate (MTX) for 30 min then stimulated with recombinant human IL-2 and anti-CD3 antibody (1 μg/ml) for 6 h. IFN-γ was quantified using the enzyme-linked immunosorbent spot assay. Values are averages of triplicate samples (columns, mean; bars, S.D.). CTA056 treatment significantly decreased IL-2 and IFN-γ secretion from Jurkat cells compared with control. *, p < 0.05, one-way ANOVA with Tukey's test for pairwise comparison.
CTA056 Modulates Cancer-Related MiRNAs.
Many miRNAs have been reported to play a role in carcinogenesis. To explore whether CTA056 modulates miRNAs, 272 miRNA levels in Jurkat cells were examined using a microarray assay after treatment with CTA056. Among them, 97 exhibited alterations more than 2-fold, of which most of the functions to oncogenesis have not been fully established. However, a number of them are known to possess oncogenic and tumor suppressive properties. We found that those miRNAs with tumor-suppressive (Fig. 9, green bars) properties were generally up-regulated, and those with oncogenic (red bars) properties were down-regulated after treatment with CTA056. For instance, Mir-135a, which is up-regulated 285.3-fold after treatment with CTA056, has been reported to target JAK2 and decrease the expression of the antiapoptotic protein Bcl-xL (Navarro et al., 2009). Mir-365 (28.8-fold increase) and Mir-195 (27.0-fold increase) both down-regulate the expression of the antiapoptotic protein Bcl-2 (Chen et al., 2011; Nie et al., 2012). Likewise, Mir-1224-5p (112.9-fold increase) down-regulates tumor necrosis factor through SP1 (Niu et al., 2011). Mir-520b targets MEKK2 and IL-8 (Hu et al., 2011; Zhang et al., 2012) (Supplemental Fig. 1A). On the other hand, CTA056 down-regulates Mir-421, which has been reported to down-regulate ATM, a pro-apoptotic protein (Hu et al., 2010) (Supplemental Fig. 1A). The effects of CTA056 on these miRNAs could in part account for its ability to induce apoptosis and suppress cytokine release. To validate the microarray results, we have used RT-PCR to confirm the modulation of selected miRNAs by CTA056 (Supplemental Fig. 1B).
Fig. 9.
Induction of tumor suppressive miRNAs and decrease of oncogenic miRNAs in Jurkat cells after treatment of 0.5 μM CTA056 for 8 h. RNA was isolated and miRNA profile was examined using miRNA array. The fold change between treatment and control was indicated. Green columns, tumor suppressive miRNAs; red columns, oncogenic miRNAs.
CTA056 Inhibits MOLT-4 Xenograft Tumor Growth In Vivo.
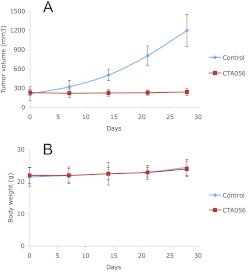
Given the in vitro activity of CTA056 against T-cell leukemia, it is important to validate these results in vivo. Because of the low take rate and weak oncogenicity of Jurkat in our experimental model system, we resorted to a MOLT-4 xenograft model. As shown in Fig. 10, CTA056 prevented MOLT-4 xenograft tumor growth at 5 mg/kg (twice a week, intratumoral injection) without significant toxicity.
Fig. 10.
Inhibition of MOLT-4 xenograft tumor growth by CTA056. Ten million MOLT-4 cells were injected subcutaneously to nude mice. The tumors were grown to the indicated size, and the mice were randomly divided into two groups (eight mice per group). The control group was treated with vehicle. The treatment group was treated with CTA056 at 5 mg/kg twice a week with intratumoral injection. The tumor size (A) and body weight (B) were measured once a week. Marks, mean; bars, S.D.; n = 8.
Discussion
Given the initial success of imatinib, tyrosine kinases have become appealing targets for drug development (Demetri et al., 2002; O'Brien et al., 2003). Excellent combinatorial chemistry approaches and high-throughput screening assays have led to successful identification of many selective enzyme inhibitors (Lam et al., 1991; McDonald et al., 1999; Su et al., 2000; Wilhelm et al., 2006). For drug development, the universal goal is to find specific kinase inhibitors with selectivity toward a particular malignant cell type. In theory, the more specific an inhibitor is, the less toxic it will be. On the other hand, a broader specificity may contribute to higher efficacy. Though effective clinically, the current kinase inhibitors have usually proven to be less specific than initially realized (Wilhelm et al., 2006). Specificity comes from both the selectivity of an inhibitor for a particular kinase and the expression of that kinase among different cell types. Our results indicated that among tumors, Itk expression was limited to malignant cell types of T-cell origin (Fig. 3A). This result is consistent with earlier reports of the Itk expression profile in nonmalignant cell types. The expression profile of Itk gives Itk inhibitors an advantage in targeting T-cell malignancies while not affecting other cell types. Our inhibitor, CTA056, binds to the ATP-binding pocket of Itk and blocks the kinase activity. This inhibitor showed specificity for Itk over other Tec family kinases. CTA056 has demonstrated selective cytotoxicity toward T-cell malignancies, such as Jurkat and MOLT-4 cells, and it has inhibited IL-2 and IFN-γ release from Jurkat cells. In this regard, we found CTA056 to be more effective than other Itk inhibitors currently under development at inducing apoptosis (data not shown). We attribute this to the differential specificities of these inhibitors toward other kinases or toward different conformational isoforms of Itk (e.g., monomer versus multimer, phosphorylated versus unphosphorylated, etc.), which may engage different substrates (Dombroski et al., 2005; Qi and August, 2009; Joseph et al., 2010). As discussed above, our inhibitor also inhibits Src kinase, albeit with less potency, which may contribute to the observed apoptosis-inducing effects. In addition to inhibition of Itk phosphorylation, CTA056 treatment of Jurkat cells also decreased total Itk levels. It has been reported that Itk could bind Cbl, a ubiquitin ligase, through the Src homology 3 domain, whereas phosphorylated Itk loses its ability to bind Cbl (Bunnell et al., 1996; Andreotti et al., 1997). Therefore, inhibition of Itk phosphorylation by CTA056 may lead to Itk degradation. This is important because Itk has been shown to possess kinase-independent signal potential (Dombroski et al., 2005; Hao et al., 2006). The selective expression of Itk, as well as the specificity of CTA056 toward Itk, coupled with its ability to induce apoptosis, makes CTA056 an appropriate inhibitor for T-cell malignancies. Treatment of Jurkat and MOLT-4 cells with CTA056 resulted in the decrease of phosphorylation of PLC-γ, AKT, and ERK, as well as the expression and release of IL-2 and IFN-γ, as expected from Itk inhibition. Perhaps the most striking consequence of CTA056 is its ability to modulate oncomirs, which regulate survival and apoptosis. Many up-regulated miRNAs are involved in suppressing survival factors (Bcl-XL, Bcl-2, nuclear factor κB, Jak2, etc.), and down-modulated miRNAs target oncogenes or cytokine genes involved in growth and metastasis. By contrast, CTA056 has little effect on the survival of normal T cells, suggesting that leukemic T cells exemplified by Jurkat and MOLT-4 are “addicted” to Itk signals for survival. Further investigation will be required to identify the Itk signals involved in the regulation of these oncomirs.
In summary, we have identified an Itk inhibitor, CTA056, with good selectivity toward Itk and T-cell malignancies. Further evaluation of its pharmacokinetics/pharmacodynamics and animal studies in new formulations are underway. Itk inhibition holds exceptional promise as a novel antileukemia and antilymphoma treatment strategy.
Supplementary Material
Acknowledgments
We thank Hsing-Hsien Kung, Sandy Chau, Yiyou Chen, and Chunping Xu for their enthusiastic supports and helpful discussions. We also thank William Jewell of the Campus Mass Spectrometry Facilities at University of California Davis for assistance with the mass spectrometry data.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK52659]; the National Institutes of Health National Cancer Institute [Grants CA150197, CA098116]; C-Tag Bioscience, Inc.; a U.S. Department of Defense postdoctoral training award [Grant PC080859] and Auburn Community Cancer Endowment Fund (to W.G.); and an early career award from the Howard Hughes Medical Institute, the Burroughs Wellcome Fund, and the Martin and Dorothy Spatz Charitable Foundation (to E.M.).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- T-ALL
- T-cell acute lymphoblastic leukemia
- Itk
- interleukin-2-inducible T-cell kinase
- Btk
- Bruton's tyrosine kinase
- Etk
- endothelial and epithelial tyrosine kinase
- miRNA
- microRNA
- PI
- propidium iodide
- DIEA
- N,N-diisopropylethylamine
- DMF
- N,N-dimethylformamide
- Pd/C
- palladium on carbon
- DMSO
- dimethyl sulfoxide
- RP-HPLC
- reversed-phase high-performance liquid chromatography
- TLC
- thin-layer chromatography
- DTT
- dithiothreitol
- CTA056
- 7-benzyl-1-(3-(piperidin-1-yl)propyl)-2-(4-(pyridin-4-yl)phenyl)-1H-imidazo[4,5-g]quinoxalin-6(5H)-one
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- IL
- interleukin
- IFN
- interferon
- PCR
- polymerase chain reaction
- ELISA
- enzyme-linked immunosorbent assay
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Guo, Liu, Maverakis, Lam, and Kung.
Conducted experiments: Guo, Liu, Ono, Ma, Martinez, Sanchez, Wang, Huang, Mazloom, Li, and Ning.
Performed data analysis: Guo, Liu, and Ono.
Wrote or contributed to the writing of the manuscript: Guo, Liu, Mazloom, Maverakis, Lam, and Kung.
References
- Andreotti AH, Bunnell SC, Feng S, Berg LJ, Schreiber SL. (1997) Regulatory intramolecular association in a tyrosine kinase of the Tec family. Nature 385:93–97 [DOI] [PubMed] [Google Scholar]
- Andreotti AH, Schwartzberg PL, Joseph RE, Berg LJ. (2010) T-cell signaling regulated by the Tec family kinase, Itk. Cold Spring Harb Perspect Biol 2:a002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- August A, Gibson S, Kawakami Y, Kawakami T, Mills GB, Dupont B. (1994) CD28 is associated with and induces the immediate tyrosine phosphorylation and activation of the Tec family kinase ITK/EMT in the human Jurkat leukemic T-cell line. Proc Natl Acad Sci USA 91:9347–9351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg LJ, Finkelstein LD, Lucas JA, Schwartzberg PL. (2005) Tec family kinases in T lymphocyte development and function. Annu Rev Immunol 23:549–600 [DOI] [PubMed] [Google Scholar]
- Berge T, Sundvold-Gjerstad V, Granum S, Andersen TC, Holthe GB, Claesson-Welsh L, Andreotti AH, Inngjerdingen M, Spurkland A. (2010) T cell specific adapter protein (TSAd) interacts with Tec kinase ITK to promote CXCL12 induced migration of human and murine T cells. PLoS One 5:e9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Long JM, Vial SC, Dedi N, Dunster NJ, Renwick SB, Tanner AJ, Frantz JD, Fleming MA, Cheetham GM. (2004) Crystal structures of interleukin-2 tyrosine kinase and their implications for the design of selective inhibitors. J Biol Chem 279:18727–19732 [DOI] [PubMed] [Google Scholar]
- Bunnell SC, Henry PA, Kolluri R, Kirchhausen T, Rickles RJ, Berg LJ. (1996) Identification of Itk/Tsk Src homology 3 domain ligands. J Biol Chem 271:25646–25656 [DOI] [PubMed] [Google Scholar]
- Chen YQ, Wang XX, Yao XM, Zhang DL, Yang XF, Tian SF, Wang NS. (2011) MicroRNA-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am J Nephrol 34:549–559 [DOI] [PubMed] [Google Scholar]
- Cook BN, Bentzien J, White A, Nemoto PA, Wang J, Man CC, Soleymanzadeh F, Khine HH, Kashem MA, Kugler SZ, Jr., Wolak JP, Roth GP, De Lombaert S, Pullen SS, Takahashi H. (2009) Discovery of potent inhibitors of interleukin-2 inducible T-cell kinase (ITK) through structure-based drug design. Bioorg Med Chem Lett 19:773–777 [DOI] [PubMed] [Google Scholar]
- Dancey G, Violet J, Malaroda A, Green AJ, Sharma SK, Francis R, Othman S, Parker S, Buscombe J, Griffin N, et al. (2009) A phase I clinical trial of CHT-25 a 131I-labeled chimeric anti-CD25 antibody showing efficacy in patients with refractory lymphoma. Clin Cancer Res 15:7701–7710 [DOI] [PubMed] [Google Scholar]
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, et al. (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347:472–480 [DOI] [PubMed] [Google Scholar]
- Dombroski D, Houghtling RA, Labno CM, Precht P, Takesono A, Caplen NJ, Billadeau DD, Wange RL, Burkhardt JK, Schwartzberg PL. (2005) Kinase-independent functions for Itk in TCR-induced regulation of Vav and the actin cytoskeleton. J Immunol 174:1385–1392 [DOI] [PubMed] [Google Scholar]
- Fowell DJ, Shinkai K, Liao XC, Beebe AM, Coffman RL, Littman DR, Locksley RM. (1999) Impaired NFATc translocation and failure of Th2 development in Itk-deficient CD4+ T cells. Immunity 11:399–409 [DOI] [PubMed] [Google Scholar]
- Grasis JA, Guimond DM, Cam NR, Herman K, Magotti P, Lambris JD, Tsoukas CD. (2010) In vivo significance of ITK-SLP-76 interaction in cytokine production. Mol Cell Biol 30:3596–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso AW, Wen D, Miller CM, Rhim JS, Pretlow TG, Kung HJ. (1997) ErbB kinases and NDF signaling in human prostate cancer cells. Oncogene 15:2705–2716 [DOI] [PubMed] [Google Scholar]
- Hao S, Qi Q, Hu J, August A. (2006) A kinase independent function for Tec kinase ITK in regulating antigen receptor induced serum response factor activation. FEBS Lett 580:2691–2697 [DOI] [PubMed] [Google Scholar]
- Hu H, Du L, Nagabayashi G, Seeger RC, Gatti RA. (2010) ATM is down-regulated by N-Myc-regulated microRNA-421. Proc Natl Acad Sci USA 107:1506–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Zhang J, Cui W, Kong G, Zhang S, Yue L, Bai X, Zhang Z, Zhang W, Zhang X, et al. (2011) miR-520b regulates migration of breast cancer cells by targeting hepatitis B X-interacting protein and interleukin-8. J Biol Chem 286:13714–13722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RE, Xie Q, Andreotti AH. (2010) Identification of an allosteric signaling network within Tec family kinases. J Mol Biol 403:231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukonen J, Savolainen ER, Palotie A. (1999) Human Emt tyrosine kinase is specifically expressed both in mature T-lymphocytes and T-cell associated hematopoietic malignancies. Leuk Lymphoma 32:513–522 [DOI] [PubMed] [Google Scholar]
- Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. (2003) Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol 139:857–866 [DOI] [PubMed] [Google Scholar]
- Lam KS, Salmon SE, Hersh EM, Hruby VJ, Kazmierski WM, Knapp RJ. (1991) A new type of synthetic peptide library for identifying ligand-binding activity. Nature 354:82–84 [DOI] [PubMed] [Google Scholar]
- Lam KS, Wu J, Lou Q. (1995) Identification and characterization of a novel synthetic peptide substrate specific for Src-family protein tyrosine kinases. Int J Pept Protein Res 45:587–592 [DOI] [PubMed] [Google Scholar]
- Lin TA, McIntyre KW, Das J, Liu C, O'Day KD, Penhallow B, Hung CY, Whitney GS, Shuster DJ, Yang X, et al. (2004) Selective Itk inhibitors block T-cell activation and murine lung inflammation. Biochemistry 43:11056–11062 [DOI] [PubMed] [Google Scholar]
- Lou Q, Wu J, Lam KS. (1996) A protein kinase assay system for both acidic and basic peptides. Anal Biochem 235:107–109 [DOI] [PubMed] [Google Scholar]
- Marks DI, Paietta EM, Moorman AV, Richards SM, Buck G, DeWald G, Ferrando A, Fielding AK, Goldstone AH, Ketterling RP, et al. (2009) T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993). Blood 114:5136–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Diaz T, Martinez A, Gaya A, Pons A, Gel B, Codony C, Ferrer G, Martinez C, Montserrat E, et al. (2009) Regulation of JAK2 by miR-135a: prognostic impact in classic Hodgkin lymphoma. Blood 114:2945–2951 [DOI] [PubMed] [Google Scholar]
- Nie J, Liu L, Zheng W, Chen L, Wu X, Xu Y, Du X, Han W. (2012) microRNA-365, down-regulated in colon cancer, inhibits cell cycle progression and promotes apoptosis of colon cancer cells by probably targeting cyclin D1 and Bcl-2. Carcinogenesis 33:220–225 [DOI] [PubMed] [Google Scholar]
- Niu Y, Mo D, Qin L, Wang C, Li A, Zhao X, Wang X, Xiao S, Wang Q, Xie Y, et al. (2011) Lipopolysaccharide-induced miR-1224 negatively regulates tumour necrosis factor-α gene expression by modulating Sp1. Immunology 133:8–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald OB, Chen WJ, Ellis B, Hoffman C, Overton L, Rink M, Smith A, Marshall CJ, Wood ER. (1999) A scintillation proximity assay for the Raf/MEK/ERK kinase cascade: high-throughput screening and identification of selective enzyme inhibitors. Anal Biochem 268:318–329 [DOI] [PubMed] [Google Scholar]
- O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, Cornelissen JJ, Fischer T, Hochhaus A, Hughes T, et al. (2003) Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 348:994–1004 [DOI] [PubMed] [Google Scholar]
- Pechloff K, Holch J, Ferch U, Schweneker M, Brunner K, Kremer M, Sparwasser T, Quintanilla-Martinez L, Zimber-Strobl U, Streubel B, et al. (2010) The fusion kinase ITK-SYK mimics a T cell receptor signal and drives oncogenesis in conditional mouse models of peripheral T cell lymphoma. J Exp Med 207:1031–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Q, August A. (2009) The Tec family kinase Itk exists as a folded monomer in vivo. J Biol Chem 284:29882–29892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readinger JA, Schiralli GM, Jiang JK, Thomas CJ, August A, Henderson AJ, Schwartzberg PL. (2008) Selective targeting of ITK blocks multiple steps of HIV replication. Proc Natl Acad Sci USA 105:6684–6689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL. (1999) Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science 284:638–641 [DOI] [PubMed] [Google Scholar]
- Schaeffer EM, Yap GS, Lewis CM, Czar MJ, McVicar DW, Cheever AW, Sher A, Schwartzberg PL. (2001) Mutation of Tec family kinases alters T helper cell differentiation. Nat Immunol 2:1183–1188 [DOI] [PubMed] [Google Scholar]
- Seeliger D, de Groot BL. (2010) Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J Comput Aided Mol Des 24:417–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Monti S, Aires DJ, Duvic M, Golub T, Jones DA, Kupper TS. (2007) Lesional gene expression profiling in cutaneous T-cell lymphoma reveals natural clusters associated with disease outcome. Blood 110:3015–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siliciano JD, Morrow TA, Desiderio SV. (1992) itk, a T-cell-specific tyrosine kinase gene inducible by interleukin 2. Proc Natl Acad Sci USA 89:11194–11198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel B, Vinatzer U, Willheim M, Raderer M, Chott A. (2006) Novel t(5;9)(q33;q22) fuses ITK to SYK in unspecified peripheral T-cell lymphoma. Leukemia 20:313–318 [DOI] [PubMed] [Google Scholar]
- Su GH, Sohn TA, Ryu B, Kern SE. (2000) A novel histone deacetylase inhibitor identified by high-throughput transcriptional screening of a compound library. Cancer Res 60:3137–3142 [PubMed] [Google Scholar]
- Trott O, Olson AJ. (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YT, Su YH, Fang SS, Huang TN, Qiu Y, Jou YS, Shih HM, Kung HJ, Chen RH. (2000) Etk, a Btk family tyrosine kinase, mediates cellular transformation by linking Src to STAT3 activation. Mol Cell Biol 20:2043–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonin A, Rausch A, Mengel A, Hitchcock M, Krüger M, von Ahsen O, Merz C, Röse L, Stock C, Martin SF, et al. (2011) Inhibition of the IL-2-inducible tyrosine kinase (Itk) activity: a new concept for the therapy of inflammatory skin diseases. Exp Dermatol 20:41–47 [DOI] [PubMed] [Google Scholar]
- Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. (2006) Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov 5:835–844 [DOI] [PubMed] [Google Scholar]
- Woods ML, Kivens WJ, Adelsman MA, Qiu Y, August A, Shimizu Y. (2001) A novel function for the Tec family tyrosine kinase Itk in activation of beta 1 integrins by the T-cell receptor. EMBO J 20:1232–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Zhang S, Wang Y, Liu G. (2005) Solution-phase parallel synthesis of a 1,2,7-trialkyl-1H-imidazo[4,5-g]quinoxalin-6-ol library scaffold. J Comb Chem 7:657–664 [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhang Z, Garmestani K, Schultz J, Axworthy DB, Goldman CK, Brechbiel MW, Carrasquillo JA, Waldmann TA. (2003) Pretarget radiotherapy with an anti-CD25 antibody-streptavidin fusion protein was effective in therapy of leukemia/lymphoma xenografts. Proc Natl Acad Sci USA 100:1891–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Kong G, Zhang J, Wang T, Ye L, Zhang X. (2012) MicroRNA-520b inhibits growth of hepatoma cells by targeting MEKK2 and cyclin D1. PLoS One 7:e31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.