Abstract
The adhesion G protein-coupled receptors (GPCRs) are a distinct family of more than 30 receptors in vertebrate genomes. These receptors have been shown to play pivotal roles in a diverse range of biological functions and are characterized by extremely large N termini featuring various adhesion domains capable of mediating cell-cell and cell-matrix interactions. The adhesion GPCR N termini also contain GPCR proteolytic site motifs that undergo autocatalytic cleavage during receptor processing to create mature GPCRs existing as noncovalently attached complexes between the N terminus and transmembrane regions. There is mounting evidence that adhesion GPCRs can couple to G proteins to activate a variety of different downstream signaling pathways. Furthermore, recent studies have demonstrated that adhesion GPCR N termini can bind to multiple ligands, which may differentially activate receptor signaling and/or mediate cell adhesion. In addition, studies on several distinct adhesion GPCRs have revealed that truncations of the N termini result in constitutively active receptors, suggesting a model of receptor activation in which removal of the N terminus may be a key event in stimulating receptor signaling. Because mutations to certain adhesion GPCRs cause human disease and because many members of this receptor family exhibit highly discrete distribution patterns in different tissues, the adhesion GPCRs represent a class of potentially important drug targets that have not yet been exploited. For this reason, understanding the mechanisms of activation for these receptors and elucidating their downstream signaling pathways can provide insights with the potential to lead to novel therapeutic agents.
Introduction
G protein-coupled receptors (GPCRs) are a superfamily of cell surface receptors that allow cells to sense a variety of extracellular signals, including neurotransmitters, hormones, odorants, tastants, and light. GPCRs share a conserved seven-transmembrane (7TM) structure and communicate through heterotrimeric G proteins and other signaling pathways to transduce extracellular signals into intracellular changes in cellular physiology (Rosenbaum et al., 2009). The diversity of ligands that GPCRs are able to detect and the multitude of downstream signaling pathways make GPCRs important drug targets, with approximately 30% of all current therapeutic agents acting directly on GPCRs (Overington et al., 2006). More than 100 GPCRs are still orphan receptors, meaning that they do not have identified ligands, and the largest family of orphan receptors is the adhesion GPCRs.
Adhesion GPCRs are characterized by extremely long N-terminal regions that contain various modular adhesion domains, such as epidermal growth factor-like repeats, thrombospondin-like repeats, and cadherin-like repeats, among others (Fredriksson et al., 2003; Bjarnadóttir et al., 2007). Vertebrate genomes encode several dozen members of this family, including 31 members in mice and 33 members in humans (Bjarnadóttir et al., 2004). Of interest, certain invertebrates exhibit a dramatic expansion of this family, notably sea urchins, which express nearly 100 different adhesion GPCRs (Whittaker et al., 2006). The adhesion GPCR family can be subdivided into several subfamilies, based on sequence similarity (Table 1).
TABLE 1.
Comprehensive list of adhesion GPCRs with reported G protein coupling and extracellular ligands
The members of the adhesion GPCR family are shown grouped by sequence similarity, according to the scheme proposed by Bjarnadóttir et al. (2007). In addition, for receptors that have been reported to couple to G proteins, the coupling preference is listed. Question marks indicate cases in which G protein coupling has been suggested on the basis of second messenger production but not definitively proven. Reported ligands for each receptor are also listed. It is important to note that the ligands listed here are not necessarily agonists, because some ligands may mediate adhesive and/or regulatory functions without inducing receptor activation.
| Subfamily | Receptor | G protein | Ligands | Reference |
|---|---|---|---|---|
| 1 | BAI1 | TBD | Phosphatidylserine on apoptotic cells | Park et al., 2007 |
| 1 | BAI2 | TBD | TBD | |
| 1 | BAI3 | TBD | C1q-like proteins | Bolliger et al., 2011 |
| 2 | GPR56 | G12/13 | Transglutaminase 2, CD9, CD81, GPR56 N terminus, collagen III | Little et al., 2004; Xu et al., 2006; Iguchi et al., 2008; Luo et al., 2011; Paavola et al., 2011 |
| 2 | GPR97 | Go | Beclomethasone dipropionate | Gupte et al., 2012 |
| 2 | GPR112 | TBD | TBD | |
| 2 | GPR114 | Gs | TBD | Gupte et al., 2012 |
| 2 | GPR126 | Gs? | TBD | Monk et al., 2009 |
| 2 | GPR128 | TBD | TBD | |
| 2 | HE6 | TBD | TBD | |
| 2 | VLGR1 | TBD | TBD | |
| 3 | CD97 | G12/13 | Chondroitin sulfates, CD55, CD90 | Hamann et al., 1996; Stacey et al., 2003; Ward et al., 2011; Wandel et al., 2012 |
| 3 | EMR1 | TBD | TBD | |
| 3 | EMR2 | TBD | Chondroitin sulfates | Stacey et al., 2003 |
| 3 | EMR3 | TBD | TBD | |
| 3 | EMR4 | TBD | TBD | |
| 3 | ETL | TBD | TBD | |
| 3 | LEC1 (latrophilin-1; CIRL-1) | Gq, Go | LTX, teneurin-2, neurexin, FLRT proteins | Lelianova et al., 1997; Rahman et al., 1999; Silva et al., 2011; Boucard et al., 2012; O'Sullivan et al., 2012 |
| 3 | LEC2 (latrophilin-2; CIRL-2) | TBD | LTX | Ichtchenko et al., 1999 |
| 3 | LEC3 (latrophilin-3; CIRL-3) | TBD | FLRT proteins | O'Sullivan et al., 2012 |
| 4 | GPR123 | TBD | TBD | |
| 4 | GPR124 | TBD | Integrins, glycosaminoglycans | Vallon and Essler, 2006 |
| 4 | GPR125 | TBD | TBD | |
| 5 | CELSR1 | TBD | TBD | |
| 5 | CELSR2 | Gq? | Celsr2-N terminus | Shima et al., 2007 |
| 5 | CELSR3 | Gq? | Celsr3-N terminus | Shima et al., 2007 |
| 6 | GPR133 | Gs | TBD | Bohnekamp and Schöneberg, 2011 |
| 6 | GPR144 | TBD | TBD | |
| 7 | GPR110 | TBD | TBD | |
| 7 | GPR111 | TBD | TBD | |
| 7 | GPR113 | TBD | TBD | |
| 7 | GPR115 | TBD | TBD | |
| 7 | GPR116 | TBD | TBD |
TBD, to be determined.
Almost all members of the adhesion GPCR family also feature an N-terminal GPCR proteolytic site (GPS) motif. These GPS motifs exhibit structural similarity to the self-cleaving domains of inteins (Paulus, 2000), and there is evidence that the GPS motifs of adhesion GPCRs do in fact undergo autoproteolysis as part of normal receptor processing (Lin et al., 2010). Furthermore, it has been shown for several different adhesion GPCRs that the receptors' N-terminal and 7TM regions (sometimes referred to as the receptors' α and β subunits, respectively) remain noncovalently associated for some period of time after autoproteolysis at the GPS motif (Gray et al., 1996; Krasnoperov et al., 1997, 2002; Kwakkenbos et al., 2002; Zhang et al., 2004; Lin et al., 2010; Paavola et al., 2011). Recent structural studies have shown that the GPS motif may in fact be part of a larger domain, and the term “GPCR autoproteolysis-inducing” (GAIN) domain has been suggested to describe this larger conserved region (Araç et al., 2012). The physiological importance of GPS motif/GAIN domain cleavage is mysterious, but mutations in this domain can cause receptor misfolding and human disease in some cases (Krasnoperov et al., 2002; Piao et al., 2004; Zhang et al., 2004; Jin et al., 2007; Ke et al., 2008; Chiang et al., 2011).
Genetic studies, including analyses of gene deletions in mice and zebrafish and studies on inherited mutations in humans, have provided striking evidence regarding the physiological importance of various adhesion GPCRs. For example, mutations to GPR56 have been shown to cause the inherited human developmental disorder known as bilateral frontal parietal polymicrogyria, which is characterized by a malformed cerebral cortex due to the overmigration of neuronal progenitors (Piao et al., 2004). Furthermore, knockout of Gpr56 in mice results in similar aberrations in the development of the cerebral cortex, as well as perturbations in the development of other brain regions such as the cerebellum (Li et al., 2008; Koirala et al., 2009). Mutations to the very large G protein-coupled receptor (VLGR1) lead to Usher's syndrome, a genetic disorder characterized by blindness and deafness (Weston et al., 2004). Knockout studies on Gpr126 have revealed a pivotal role of this receptor in the myelination of Schwann cells (Monk et al., 2009, 2011), and knockout studies on He6 have demonstrated an essential role of this adhesion GPCR in spermatogenesis and fertility (Davies et al., 2004). Given the importance of adhesion GPCRs in so many diverse systems and the potential of these receptors as drug targets, there has been tremendous interest in understanding how these receptors are activated and how they might induce changes in cellular physiology. Until recently, very little was known about this topic. However, there have been significant advances in this area over the past few years, and these recent advances in our understanding of the activation and signaling of adhesion GPCRs are the subject of this minireview.
Adhesion GPCR Signaling through G Proteins
Studies on several different adhesion GPCRs have provided evidence that these receptors are in fact authentic G protein-coupled receptors (Table 1). For example, overexpression of GPR56 in various cell types can lead to Rho activation through Gα12/13 (Iguchi et al., 2008; Paavola et al., 2011). Moreover, GPR56 has been shown via coimmunoprecipitation to interact with Gαq/11 (Little et al., 2004), which is consistent with work on other receptor types demonstrating that receptors coupling to Gα12/13 can also typically couple to Gαq/11 (Takashima et al., 2008). In a similar vein, overexpression of GPR133 in various cell types has been shown to stimulate Gαs and promote cAMP generation (Bohnekamp and Schöneberg, 2011; Gupte et al., 2012). Gpr126 has also been shown to exert actions on Schwann cells consistent with a cAMP- and Gαs-dependent mechanism (Monk et al., 2009), and GPR114 has been shown to constitutively increase cAMP levels when overexpressed in HEK293 cells (Gupte et al., 2012). GPR97 has also been shown to be constitutively active upon overexpression in HEK293 cells, but only when coexpressed with a chimeric version of Gαo (Gupte et al., 2012).
Other studies on adhesion GPCR signaling have made use of activating antibodies or toxins. There is precedent from work on certain classic GPCRs, including adrenergic, muscarinic, and angiotensin receptors, demonstrating that antibodies or other large proteins associating with the receptors' extracellular regions can sometimes cause conformational changes to stimulate receptor signaling (Lebesgue et al., 1998; Peter et al., 2004; Dragun et al., 2005; Dragun, 2007). Along these same lines, the aforementioned Gα12/13-mediated signaling by GPR56 has been shown to be robustly promoted by treatment with antibodies directed against the receptor's N terminus (Iguchi et al., 2008). Moreover, regulation of neutrophil signaling by the adhesion GPCR EMR2 has been shown to be modulated by anti-EMR2-N-terminal antibodies in a manner that probably involves receptor coupling to G proteins (Yona et al., 2008; Huang et al., 2012). The adhesion GPCR latrophilin-1 has been intensively studied because it is a key target of latrotoxin (LTX), which is derived from the venom of the black widow spider (Krasnoperov et al., 1997; Lelianova et al., 1997). LTX binds to the latrophilin-1 N terminus, as well as to the related latrophilin-2 N terminus (Ichtchenko et al., 1999), and has been demonstrated to promote latrophilin-1 coupling to Gαq and Gαo (Lelianova et al., 1997; Rahman et al., 1999). The pathological effects of LTX are complicated by the fact that the toxin can integrate into membranes to form pores, but the specific ability of LTX to bind latrophilin-1 and promote the receptor's G protein coupling has been established using a mutant version of the toxin that does not form pores but still binds to latrophilin-1 (Ichtchenko et al., 1998; Capogna et al., 2003; Volynski et al., 2003).
Importance of the N Terminus for Adhesion GPCR Signaling
If the binding of antibodies or toxins to the N termini of adhesion GPCRs can stimulate receptor signaling, critical importance of the N termini in controlling receptor activity is suggested. For this reason, several groups have created truncated adhesion GPCR mutants with shortened N termini, the prediction being that such truncations might impair receptor activity by removing N-terminal regions where large adhesive ligands might bind. Surprisingly, however, truncation studies of this type have revealed that removal of the N-terminal regions from adhesion GPCRs actually activates receptor signaling. For example, a truncated version of GPR56 that lacks nearly the entire N-terminal region exhibits greatly enhanced coupling to Gα12/13 and activation of downstream Rho relative to the wild-type receptor (Paavola et al., 2011). Moreover, the truncated GPR56 mutant also exhibits profoundly enhanced ubiquitination and binding to arrestins, which are hallmarks of constitutively active GPCRs (Paavola et al., 2011). Likewise, it has been shown that naturally occurring splice variants of GPR56, which have shorter N-terminal regions than the more widely expressed longer form of the receptor, exhibit enhanced constitutive activation of an SRE reporter when overexpressed in heterologous cells (Kim et al., 2010).
Similar results, demonstrating that N-terminal truncations can induce enhanced constitutive activity of adhesion GPCRs, have been found for several other receptors beyond GPR56. For example, the brain-specific angiogenesis inhibitor 2 (BAI2) was shown to activate NFAT signaling upon overexpression in HEK293 cells, possibly via a G protein-dependent pathway, whereas overexpression of an N-terminal-truncated mutant resulted in dramatically increased NFAT activation compared with that of the wild-type receptor (Okajima et al., 2010). Furthermore, transfection of the adhesion GPCR CD97 into COS-7 cells was shown to stimulate Rho and SRE through a Gα12/13-dependent mechanism, and transfection of an N-terminal-truncated mutant version of CD97 resulted in stimulation of signaling to SRE that was 10-fold stronger than that induced by the wild-type receptor (Ward et al., 2011). Taken together, these data from work on GPR56, BAI2, and CD97 paint a picture of a potentially general mechanism of activation for adhesion GPCRs, in which the N-terminal regions are cleaved by autoproteolysis but remain associated with the receptors' 7TM regions to exert an inhibitory influence on receptor signaling. In this model, engagement of the N terminus by a large protein, whether an antibody, toxin, or endogenous adhesive ligand, can result in either the removal of the N terminus or a gross conformational rearrangement that alleviates the inhibitory constraint of the N terminus on signaling by the 7TM region, thereby allowing for the initiation of G protein-mediated signaling.
Potential Ligands for Adhesion GPCRs
If it is true that adhesion GPCR signaling can be initiated by engagement of the receptors' large N-terminal regions by extracellular adhesive ligands, then it is a mission of clear importance to identify the ligands for the various adhesion GPCRs. Although all members of the adhesion GPCR family are still considered to be orphan receptors, over the past few years extracellular binding partners have been identified for a number of different members of the family (Table 1). It should be noted that not every adhesion GPCR binding partner must necessarily be an agonist that activates the receptors' coupling to G proteins; some of the interactions may be purely adhesive in nature, consistent with the general view of adhesion GPCRs as both adhesion molecules and cell surface receptors. For example, chondroitin sulfates have been reported to be ligands for both EMR2 and CD97 (Stacey et al., 2003). These interactions have been characterized as low-affinity, calcium-dependent associations that are mediated through the receptors' epidermal growth factor-like repeats, resulting in changes in cell attachment and motility. However, there is no evidence at present that these interactions with chondroitin sulfates can activate signaling by EMR2 or CD97. Likewise, CD97 was first identified as a counter-receptor on immune cells for CD55, also known as the decay-accelerating factor (Hamann et al., 1996). This interaction has been extensively studied and shown to have a variety of effects on cell adhesion, cell motility, and carcinoma invasiveness but at present there is no evidence that this interaction can activate G protein-coupled signaling by CD97 (Mustafa et al., 2004; Liu et al., 2005). Moreover, the N terminus of GPR124 has been shown to facilitate adhesion by binding to both glycosaminoglycans and integrins (Vallon and Essler, 2006), and Thy-1 (CD90) has recently been shown to interact with CD97 to regulate polymorphonuclear cell adhesion (Wandel et al., 2012), but no corresponding signaling effects have been reported for these interactions.
As mentioned earlier, the adhesion GPCR latrophilin-1 has been shown to initiate G protein-dependent signaling when bound by latrotoxin, an exogenous toxin that is a component of black widow spider venom (Lelianova et al., 1997; Rahman et al., 1999). Recent studies have revealed three distinct potential endogenous ligands for latrophilin-1. One of these reported ligands is the single-transmembrane glycoprotein teneurin-2 (also called Oz, tenascin-m, neurestin, and DOC4), which has been shown to bind to the latrophilin-1 N terminus with nanomolar affinity and form heterophilic complexes with latrophilin-1 at points of cell-cell contact (Silva et al., 2011). Moreover, treatment of cells expressing latrophilin-1 with a soluble fragment of teneurin-2 was found to induce increases in intracellular calcium, probably reflecting activation of G protein-dependent signaling (Silva et al., 2011). A second reported ligand for latrophilin-1 is the presynaptic transmembrane protein neurexin (Boucard et al., 2012). Of interest, neurexin, like latrophilin-1, is a cellular target of latrotoxin (Davletov et al., 1995). Like teneurin-2, neurexin was shown to interact with latrophilin-1 with nanomolar affinity to form heterophilic complexes at cell-cell junctions (Boucard et al., 2012). However, it remains to be explored whether this interaction can stimulate latrophilin-1 signaling. A third identified family of ligands for latrophilin-1 is the fibronectin leucine-rich repeat transmembrane (FLRT) proteins (O'Sullivan et al., 2012). Latrophilin-1 and the related latrophilin-3 were shown to interact with FLRT proteins in a heterophilic cell-cell manner with nanomolar affinity, and a transsynaptic complex between FLRT3 and latrophilin-3 was found to regulate synaptic density and dendritic spine number in cultured neurons (O'Sullivan et al., 2012). It is not yet clear whether FLRT interactions with latrophilin N-terminal regions can activate latrophilin signaling, but this point will probably be clarified by future work in this area.
GPR56 is another adhesion GPCR that has been reported to bind to multiple extracellular ligands. The first identified binding partners of GPR56 were the tetraspanins CD9 and CD81, although the region of GPR56 required for these interactions and the significance for GPR56 signaling have not been fully defined (Little et al., 2004). A second ligand that has been identified for GPR56 is transglutaminase 2 (TG2), an extracellular matrix protein that enzymatically cross-links proteins together to help form adhesive complexes (Xu et al., 2006). TG2 was shown to bind to a specific domain on the GPR56 N terminus, and deletion of this domain was shown to lead to increased GPR56-promoted tumor growth in vivo (Yang et al., 2011). However, it is not yet clear whether TG2 binding to the GPR56 N terminus can stimulate GPR56-mediated signaling. A third ligand that has been found for GPR56 is collagen III, which binds to the GPR56 N terminus and can stimulate GPR56-mediated signaling to Rho in NIH 3T3 cells (Luo et al., 2011). Of interest, knockout of the gene for collagen III (Col3a1) has been shown to result in a cobblestone-like malformation of the cerebral cortex due to neuronal overmigration during brain development (Jeong et al., 2012), which is a phenotype strikingly similar to that observed upon knockout of Gpr56 (Li et al., 2008).
The brain-specific angiogenesis inhibitors 1 to 3 (BAI1–3) are a subfamily of adhesion GPCRs that have been shown to associate with both lipids and proteins via the multiple thrombospondin-like repeats on their large N-terminal regions. For example, BAI1 was shown to bind to externalized phosphatidylserine on apoptotic cells to promote apoptotic cell engulfment, in a manner that involves ELMO, a protein that associates with the cytoplasmic regions of BAI1, acting as a guanine nucleotide exchange factor for Rac (Park et al., 2007). However, it remains to be determined whether BAI1-mediated engulfment of apoptotic cells involves G protein-dependent signaling by BAI1 or whether any such signaling is initiated by the binding of the BAI1-N terminus to phosphatidylserine-rich membranes. In separate studies, the BAI3-N terminus has been shown to be a high-affinity binding partner for a family of complement-like secreted proteins called the C1q-like proteins (Bolliger et al., 2011). Upon addition of C1ql to cultured hippocampal neurons, a significant decrease in synaptic density was observed in a manner that could be blocked by interfering with the ability of C1ql to bind to thrombospondin-like repeats (Bolliger et al., 2011). The specificity of the C1ql proteins for different members of the BAI family and the importance of these interactions for stimulating BAI-mediated signaling are likely to be topics of significant future research interest.
Several adhesion GPCRs have been shown to undergo homophilic trans-trans interactions, meaning that they can interact with other versions of themselves on neighboring cells. Of interest, these homophilic associations have been shown in several cases to promote adhesion GPCR signaling. For example, the adhesion GPCRs Celsr2 and Celsr3 have been shown to undergo receptor-specific N terminus-N terminus interactions that induce increases in intracellular calcium in a phospholipase-dependent (and probably G protein-dependent) manner (Shima et al., 2007). The homophilic trans-trans interactions of Celsr2 and Celsr3 were demonstrated to be physiologically important in the regulation of neurite outgrowth in cultured neurons (Shima et al., 2007). Likewise, GPR56 has been shown to be capable of N terminus-N terminus interactions that promote the receptor's signaling through Gα12/13 to activate Rho (Paavola et al., 2011). In addition, the Drosophila adhesion GPCR known as Flamingo has been shown to be capable of homophilic trans-trans associations, although it is not yet clear whether these associations promote receptor signaling (Chen and Clandinin, 2008). It should be pointed out that important roles for N terminus-N terminus interactions in adhesion GPCR activation are not mutually exclusive with crucial roles for other large adhesive ligands, because N terminus-N terminus interactions might be required to create binding sites for certain ligands. Conversely, or perhaps concurrently, association with large adhesive ligands might stabilize N terminus-N terminus interactions in a manner that promotes receptor signaling.
Conclusions and Future Directions
The various advances in the area of adhesion GPCR signaling described here suggest several general conclusions. First, it seems to be generally true that truncation or removal of the N-terminal regions of these receptors leads to activation of receptor signaling. This phenomenon has been demonstrated for GPR56, BAI2, and CD97 (Okajima et al., 2010; Paavola et al., 2011; Ward et al., 2011) and might well be a conserved feature for all members of the adhesion GPCR family. Second, adhesion GPCRs have extremely large extracellular N-terminal regions that are likely to bind to multiple ligands per receptor. Latrophilin-1 is a good example of this phenomenon, with its recently reported N-terminal interactions with teneurin-2, neurexin, and FLRT proteins (Silva et al., 2011; Boucard et al., 2012; O'Sullivan et al., 2012), and it seems highly probable that other adhesion GPCRs (perhaps all members of the family) will eventually be found to have a number of different binding partners for their massive N-terminal regions. Third, certain binding partners of the N-terminal regions of adhesion GPCRs (antibodies, toxins, and endogenous ligands) can stimulate receptor signaling through G proteins.
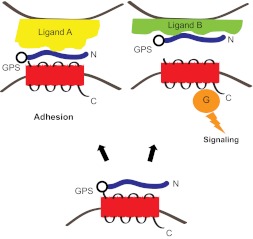
Taken together, these observations suggest a model of adhesion GPCR activation in which the receptors' N-terminal regions are cleaved at the GPS motif/GAIN domain but remain associated with the receptors' 7TM regions to exert an inhibitory constraint on receptor signaling (Fig. 1). Engagement of a receptor's N-terminal region by a ligand can induce conformational changes, leading to either removal of the N terminus from the 7TM region or a rearrangement of the N-terminal and 7TM regions that alleviates the inhibitory constraint imposed by the N terminus, thereby activating receptor signaling. In considering such a model of adhesion GPCR activation, it is easy to conceptualize how some adhesion GPCR ligands might activate the receptor (by inducing separation of the N-terminal and 7TM regions), whereas other ligands might serve an adhesive function yet have no effect on signaling by the receptor's 7TM region if they fail to induce changes in the association between the N-terminal and 7TM regions. In addition, it is even conceivable that certain endogenous ligands might stabilize the N terminus-7TM complex to act as natural antagonists for the signaling activity of certain adhesion GPCRs. This possibility has not yet been explored but might be worth examining for adhesion GPCR ligands that are not found to activate receptor signaling.
Fig. 1.
Differential ligand binding to adhesion GPCRs can result in distinct physiological responses. An unliganded adhesion GPCR is shown in the lower portion of the figure, with its large N-terminal region cleaved at the GPS motif but remaining associated with the receptor's seven-transmembrane region. Ligands for adhesion GPCRs are often large secreted glycoproteins and/or components of the extracellular matrix. Some ligands (illustrated here by “Ligand A”) can interact with adhesion GPCRs to facilitate cell adhesion without stimulating downstream receptor signaling. Conversely, other ligands (illustrated here by “Ligand B”) induce either removal of the receptor's N terminus or large-scale N-terminal conformational changes to promote receptor coupling to intracellular G proteins and activation of G protein-mediated signaling pathways.
By way of comparison with other GPCR subfamilies, it should be pointed out that removal of N-terminal regions does not typically lead to activation of GPCRs. In fact, the only examples of this phenomenon beyond the adhesion GPCRs are the members of the protease-activated receptor family (PAR1–4) (Macfarlane et al., 2001) and the thyrotropin receptor (Van Sande et al., 1996; Zhang et al., 2000). In the case of the well studied PAR family, cleavage by an exogenous protease (such as thrombin) is required for receptor activation, and the PAR N-terminal regions do not seem to remain associated with the receptors' 7TM regions for any period of time after cleavage (Traynelis and Trejo, 2007). Thus, this mechanism of activation for the PAR family is quite distinct from that proposed here for adhesion GPCR activation, which, as discussed above, seems to involve autoproteolysis followed by sustained association between the cleaved portions of the receptor, until engagement of the N terminus by a ligand results in a conformational rearrangement to the N terminus-7TM complex, allowing for signaling by the 7TM region.
Further complexity in the realm of adhesion GPCR signaling comes from the fact that the N-terminal regions of these receptors can exert physiological effects that may be independent of the 7TM regions. For example, a fragment of the BAI1 N terminus has been shown to suppress tumor growth in vivo, independent of the BAI1 7TM, in a manner that is dependent on association of the released BAI1-N terminus with CD36 and integrins (Koh et al., 2004; Kaur et al., 2009). Thus, the N-terminal regions of adhesion GPCRs may serve multiple biological functions, including 1) inhibiting receptor signaling activity for as long as they are in complex with the receptors' 7TM regions, 2) mediating cell adhesion, 3) allowing signaling by the 7TM regions to occur after engagement by particular endogenous ligands, and 4) exerting additional effects as extracellular secreted proteins after their disengagement from the 7TM regions.
The study of adhesion GPCR signaling is an emerging area that is highly relevant to drug development. GPCRs are outstanding drug targets in general, and adhesion GPCRs are particularly intriguing targets for therapeutic agents because several members of the adhesion GPCR family are human disease genes. Moreover, almost all members of the adhesion GPCR family exhibit very discrete patterns of distribution (Bjarnadóttir et al., 2007; Schiöth et al., 2010), which is appealing in terms of the possibilities for developing therapeutic agents with tissue-specific and cell-specific actions. As proof of principle that adhesion GPCRs can be activated by small molecules, recent high-throughput screening studies have identified beclomethasone dipropionate as a small-molecule activator of the adhesion GPCR GPR97 (Gupte et al., 2012). It seems likely that small-molecule agonists, antagonists, and allosteric modulators of other members of the adhesion GPCR family can be developed in the near future. Thus, understanding the mechanisms of activation, diversity of potential ligands, and multifaceted physiological functions of adhesion GPCRs may offer tremendous future opportunities for pharmacological intervention in a number of different disease states.
Acknowledgments
We thank Jason Stephenson, Stephanie Ritter, and Ayush Kishore (Emory University, Atlanta, GA) for constructive comments on this manuscript.
The work is supported in part by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS072394].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- GPCR
- G protein-coupled receptor
- 7TM
- seven-transmembrane
- GPS
- GPCR proteolytic site
- GAIN
- GPCR autoproteolysis-inducing
- HEK
- human embryonic kidney
- EMR2
- EGF-like module-containing mucin-like hormone receptor-like 2
- LTX
- latrotoxin
- SRE
- serum response element
- BAI
- brain-specific angiogenesis inhibitor
- NFAT
- nuclear factor of activated T-cells
- FLRT
- fibronectin leucine-rich repeat transmembrane
- TG2
- transglutaminase 2
- PAR
- protease-activated receptor.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Paavola and Hall.
References
- Araç D, Boucard AA, Bolliger MF, Nguyen J, Soltis SM, Südhof TC, Brunger AT. (2012) A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J 31:1364–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnadóttir TK, Fredriksson R, Höglund PJ, Gloriam DE, Lagerström MC, Schiöth HB. (2004) The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics 84:23–33 [DOI] [PubMed] [Google Scholar]
- Bjarnadóttir TK, Fredriksson R, Schiöth HB. (2007) The adhesion GPCRs: a unique family of G protein-coupled receptors with important roles in both central and peripheral tissues. Cell Mol Life Sci 64:2104–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnekamp J, Schöneberg T. (2011) Cell adhesion receptor GPR133 couples to Gs protein. J Biol Chem 286:41912–41916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolliger MF, Martinelli DC, Südhof TC. (2011) The cell-adhesion G protein-coupled receptor BAI3 is a high-affinity receptor for C1q-like proteins. Proc Natl Acad Sci USA 108:2534–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard AA, Ko J, Südhof TC.(2012) High affinity neurexin binding to cell adhesion G-protein-coupled receptor CIRL1/latrophilin-1 produces an intercellular adhesion complex. J Biol Chem 287:9399–9413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capogna M, Volynski KE, Emptage NJ, Ushkaryov YA.(2003) The alpha-latrotoxin mutant LTXN4C enhances spontaneous and evoked transmitter release in CA3 pyramidal neurons. J Neurosci 23:4044–4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PL, Clandinin TR. (2008) The cadherin Flamingo mediates level-dependent interactions that guide photoreceptor target choice in Drosophila. Neuron 58:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang NY, Hsiao CC, Huang YS, Chen HY, Hsieh IJ, Chang GW, Lin HH.(2011) Disease-associated GPR56 mutations cause bilateral frontoparietal polymicrogyria via multiple mechanisms. J Biol Chem 286:14215–14225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B, Baumann C, Kirchhoff C, Ivell R, Nubbemeyer R, Habenicht UF, Theuring F, Gottwald U.(2004) Targeted deletion of the epididymal receptor HE6 results in fluid dysregulation and male infertility. Mol Cell Biol 24:8642–8648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletov BA, Krasnoperov V, Hata Y, Petrenko AG, Südhof TC.(1995) High affinity binding of α-latrotoxin to recombinant neurexin Iα. J Biol Chem 270:23903–23905 [DOI] [PubMed] [Google Scholar]
- Dragun D. (2007) Agonistic antibody-triggered stimulation of angiotensin II type 1 receptor and renal allograft vascular pathology. Nephrol Dial Transplant 22:1819–1822 [DOI] [PubMed] [Google Scholar]
- Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, et al. (2005) Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 352:558–569 [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. (2003) The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63:1256–1272 [DOI] [PubMed] [Google Scholar]
- Gray JX, Haino M, Roth MJ, Maguire JE, Jensen PN, Yarme A, Stetler-Stevenson MA, Siebenlist U, Kelly K. (1996) CD97 is a processed, seven-transmembrane, heterodimeric receptor associated with inflammation. J Immunol 157:5438–5447 [PubMed] [Google Scholar]
- Gupte J, Swaminath G, Danao J, Tian H, Li Y, Wu X. (2012) Signaling property study of adhesion G-protein-coupled receptors. FEBS Lett 586:1214–1219 [DOI] [PubMed] [Google Scholar]
- Hamann J, Hartmann E, van Lier RA. (1996) Structure of the human CD97 gene: exon shuffling has generated a new type of seven-span transmembrane molecule related to the secretin receptor superfamily. Genomics 32:144–147 [DOI] [PubMed] [Google Scholar]
- Hamann J, Vogel B, van Schijndel GM, van Lier RA. (1996) The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J Exp Med 184:1185–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YS, Chiang NY, Hu CH, Hsiao CC, Cheng KF, Tsai WP, Yona S, Stacey M, Gordon S, Chang GW, et al. (2012) Activation of myeloid cell-specific adhesion class G protein-coupled receptor EMR2 via ligation-induced translocation and interaction of receptor subunits in lipid raft microdomains. Mol Cell Biol 32:1408–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Bittner MA, Krasnoperov V, Little AR, Chepurny O, Holz RW, Petrenko AG. (1999) A novel ubiquitously expressed alpha-latrotoxin receptor is a member of the CIRL family of G-protein-coupled receptors. J Biol Chem 274:5491–5498 [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Khvotchev M, Kiyatkin N, Simpson L, Sugita S, Südhof TC. (1998) α-Latrotoxin action probed with recombinant toxin: receptors recruit alpha-latrotoxin but do not transduce an exocytotic signal. EMBO J 17:6188–6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi T, Sakata K, Yoshizaki K, Tago K, Mizuno N, Itoh H. (2008) Orphan G protein-coupled receptor GPR56 regulates neural progenitor cell migration via a Gα12/13 and Rho pathway. J Biol Chem 283:14469–14478 [DOI] [PubMed] [Google Scholar]
- Jeong SJ, Li S, Luo R, Strokes N, Piao X. (2012) Loss of Col3a1, the gene for Ehlers-Danlos syndrome type IV, results in neocortical dyslamination. PLoS One 7:e29767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Tietjen I, Bu L, Liu-Yesucevitz L, Gaur SK, Walsh CA, Piao X. (2007) Disease-associated mutations affect GPR56 protein trafficking and cell surface expression. Hum Mol Genet 16:1972–1985 [DOI] [PubMed] [Google Scholar]
- Kaur B, Cork SM, Sandberg EM, Devi NS, Zhang Z, Klenotic PA, Febbraio M, Shim H, Mao H, Tucker-Burden C, et al. (2009) Vasculostatin inhibits intracranial glioma growth and negatively regulates in vivo angiogenesis through a CD36-dependent mechanism. Cancer Res 69:1212–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke N, Ma H, Diedrich G, Chionis J, Liu G, Yu DH, Wong-Staal F, Li QX. (2008) Biochemical characterization of genetic mutations of GPR56 in patients with bilateral frontoparietal polymicrogyria (BFPP). Biochem Biophys Res Commun 366:314–320 [DOI] [PubMed] [Google Scholar]
- Kim JE, Han JM, Park CR, Shin KJ, Ahn C, Seong JY, Hwang JI. (2010) Splicing variants of the orphan G-protein-coupled receptor GPR56 regulate the activity of transcription factors associated with tumorigenesis. J Cancer Res Clin Oncol 136:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JT, Kook H, Kee HJ, Seo YW, Jeong BC, Lee JH, Kim MY, Yoon KC, Jung S, Kim KK. (2004) Extracellular fragment of brain-specific angiogenesis inhibitor 1 suppresses endothelial cell proliferation by blocking αvβ5 integrin. Exp Cell Res 294:172–184 [DOI] [PubMed] [Google Scholar]
- Koirala S, Jin Z, Piao X, Corfas G. (2009) GPR56-regulated granule cell adhesion is essential for rostral cerebellar development. J Neurosci 29:7439–7449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnoperov V, Lu Y, Buryanovsky L, Neubert TA, Ichtchenko K, Petrenko AG. (2002) Post-translational proteolytic processing of the calcium-independent receptor of α-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J Biol Chem 277:46518–46526 [DOI] [PubMed] [Google Scholar]
- Krasnoperov VG, Bittner MA, Beavis R, Kuang Y, Salnikow KV, Chepurny OG, Little AR, Plotnikov AN, Wu D, Holz RW, et al. (1997) α-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron 18:925–937 [DOI] [PubMed] [Google Scholar]
- Kwakkenbos MJ, Chang GW, Lin HH, Pouwels W, de Jong EC, van Lier RA, Gordon S, Hamann J. (2002) The human EGF-TM7 family member EMR2 is a heterodimeric receptor expressed on myeloid cells. J Leukoc Biol 71:854–862 [PubMed] [Google Scholar]
- Lebesgue D, Wallukat G, Mijares A, Granier C, Argibay J, Hoebeke J. (1998) An agonist-like monoclonal antibody against the human β2-adrenoceptor. Eur J Pharmacol 348:123–133 [DOI] [PubMed] [Google Scholar]
- Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, Ushkaryov YA. (1997) α-Latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem 272:21504–21508 [DOI] [PubMed] [Google Scholar]
- Li S, Jin Z, Koirala S, Bu L, Xu L, Hynes RO, Walsh CA, Corfas G, Piao X. (2008) GPR56 regulates pial basement membrane integrity and cortical lamination. J Neurosci 28:5817–5826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Stacey M, Yona S, Chang GW. (2010) GPS proteolytic cleavage of adhesion-GPCRs. Adv Exp Med Biol 706:49–58 [DOI] [PubMed] [Google Scholar]
- Little KD, Hemler ME, Stipp CS. (2004) Dynamic regulation of a GPCR-tetraspanin-G protein complex on intact cells: central role of CD81 in facilitating GPR56-Gαq/11 association. Mol Biol Cell 15:2375–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chen L, Peng S, Chen Z, Gimm O, Finke R, Hoang-Vu C. (2005) The expression of CD97EGF and its ligand CD55 on marginal epithelium is related to higher stage and depth of tumor invasion of gastric carcinomas. Oncol Rep 14:1413–1420 [PubMed] [Google Scholar]
- Luo R, Jeong SJ, Jin Z, Strokes N, Li S, Piao X. (2011) G protein-coupled receptor 56 and collagen III, a receptor-ligand pair, regulates cortical development and lamination. Proc Natl Acad Sci USA 108:12925–12930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. (2001) Proteinase-activated receptors. Pharmacol Rev 53:245–282 [PubMed] [Google Scholar]
- Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. (2009) A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science 325:1402–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Oshima K, Jörs S, Heller S, Talbot WS. (2011) Gpr126 is essential for peripheral nerve development and myelination in mammals. Development 138:2673–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa T, Klonisch T, Hombach-Klonisch S, Kehlen A, Schmutzler C, Koehrle J, Gimm O, Dralle H, Hoang-Vu C. (2004) Expression of CD97 and CD55 in human medullary thyroid carcinomas. Int J Oncol 24:285–294 [PubMed] [Google Scholar]
- O'Sullivan ML, de Wit J, Savas JN, Comoletti D, Otto-Hitt S, Yates JR, 3rd, Ghosh A. (2012) FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron 73:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okajima D, Kudo G, Yokota H. (2010) Brain-specific angiogenesis inhibitor 2 (BAI2) may be activated by proteolytic processing. J Recept Signal Transduct Res 30:143–153 [DOI] [PubMed] [Google Scholar]
- Overington JP, Al-Lazikani B, Hopkins AL. (2006) How many drug targets are there? Nat Rev Drug Discov 5:993–996 [DOI] [PubMed] [Google Scholar]
- Paavola KJ, Stephenson JR, Ritter SL, Alter SP, Hall RA. (2011) The N terminus of the adhesion G protein-coupled receptor GPR56 controls receptor signaling activity. J Biol Chem 286:28914–28921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. (2007) BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450:430–434 [DOI] [PubMed] [Google Scholar]
- Paulus H. (2000) Protein splicing and related forms of protein autoprocessing. Annu Rev Biochem 69:447–496 [DOI] [PubMed] [Google Scholar]
- Peter JC, Wallukat G, Tugler J, Maurice D, Roegel JC, Briand JP, Hoebeke J. (2004) Modulation of the M2 muscarinic acetylcholine receptor activity with monoclonal anti-M2 receptor antibody fragments. J Biol Chem 279:55697–55706 [DOI] [PubMed] [Google Scholar]
- Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, Dobyns WB, Qasrawi B, Winter RM, Innes AM, et al. (2004) G protein-coupled receptor-dependent development of human frontal cortex. Science 303:2033–2036 [DOI] [PubMed] [Google Scholar]
- Rahman MA, Ashton AC, Meunier FA, Davletov BA, Dolly JO, Ushkaryov YA. (1999) Norepinephrine exocytosis stimulated by α-latrotoxin requires both external and stored Ca2+ and is mediated by latrophilin, G proteins and phospholipase C. Philos Trans R Soc Lond B Biol Sci 354:379–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. (2009) The structure and function of G-protein-coupled receptors. Nature 459:356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiöth HB, Nordström KJ, Fredriksson R. (2010) The adhesion GPCRs; gene repertoire, phylogeny and evolution. Adv Exp Med Biol 706:1–13 [DOI] [PubMed] [Google Scholar]
- Shima Y, Kawaguchi SY, Kosaka K, Nakayama M, Hoshino M, Nabeshima Y, Hirano T, Uemura T. (2007) Opposing roles in neurite growth control by two seven-pass transmembrane cadherins. Nat Neurosci 10:963–969 [DOI] [PubMed] [Google Scholar]
- Silva JP, Lelianova VG, Ermolyuk YS, Vysokov N, Hitchen PG, Berninghausen O, Rahman MA, Zangrandi A, Fidalgo S, Tonevitsky AG, et al. (2011) Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc Natl Acad Sci USA 108:12113–12118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey M, Chang GW, Davies JQ, Kwakkenbos MJ, Sanderson RD, Hamann J, Gordon S, Lin HH. (2003) The epidermal growth factor-like domains of the human EMR2 receptor mediate cell attachment through chondroitin sulfate glycosaminoglycans. Blood 102:2916–2924 [DOI] [PubMed] [Google Scholar]
- Takashima S, Sugimoto N, Takuwa N, Okamoto Y, Yoshioka K, Takamura M, Takata S, Kaneko S, Takuwa Y. (2008) G12/13 and Gq mediate S1P2-induced inhibition of Rac and migration in vascular smooth muscle in a manner dependent on Rho but not Rho kinase. Cardiovasc Res 79:689–697 [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Trejo J. (2007) Protease-activated receptor signaling: new roles and regulatory mechanisms. Curr Opin Hematol 14:230–235 [DOI] [PubMed] [Google Scholar]
- Vallon M, Essler M. (2006) Proteolytically processed soluble tumor endothelial marker (TEM) 5 mediates endothelial cell survival during angiogenesis by linking integrin αvβ3 to glycosaminoglycans. J Biol Chem 281:34179–34188 [DOI] [PubMed] [Google Scholar]
- Van Sande J, Massart C, Costagliola S, Allgeier A, Cetani F, Vassart G, Dumont JE. (1996) Specific activation of the thyrotropin receptor by trypsin. Mol Cell Endocrinol 119:161–168 [DOI] [PubMed] [Google Scholar]
- Volynski KE, Capogna M, Ashton AC, Thomson D, Orlova EV, Manser CF, Ribchester RR, Ushkaryov YA. (2003) Mutant α-latrotoxin (LTXN4C) does not form pores and causes secretion by receptor stimulation: this action does not require neurexins. J Biol Chem 278:31058–31066 [DOI] [PubMed] [Google Scholar]
- Wandel E, Saalbach A, Sittig D, Gebhardt C, Aust G. (2012) Thy-1 (CD90) is an interacting partner for CD97 on activated endothelial cells. J Immunol 188:1442–1450 [DOI] [PubMed] [Google Scholar]
- Ward Y, Lake R, Yin JJ, Heger CD, Raffeld M, Goldsmith PK, Merino M, Kelly K. (2011) LPA receptor heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate cancer cells. Cancer Res 71:7301–7311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston MD, Luijendijk MW, Humphrey KD, Möller C, Kimberling WJ. (2004) Mutations in the VLGR1 gene implicate G-protein signaling in the pathogenesis of Usher syndrome type II. Am J Hum Genet 74:357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker CA, Bergeron KF, Whittle J, Brandhorst BP, Burke RD, Hynes RO. (2006) The echinoderm adhesome. Dev Biol 300:252–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Begum S, Hearn JD, Hynes RO. (2006) GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc Natl Acad Sci USA 103:9023–9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Chen G, Mohanty S, Scott G, Fazal F, Rahman A, Begum S, Hynes RO, Xu L. (2011) GPR56 regulates VEGF production and angiogenesis during melanoma progression. Cancer Res 71:5558–5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Lin HH, Dri P, Davies JQ, Hayhoe RP, Lewis SM, Heinsbroek SE, Brown KA, Perretti M, Hamann J, et al. (2008) Ligation of the adhesion-GPCR EMR2 regulates human neutrophil function. FASEB J 22:741–751 [DOI] [PubMed] [Google Scholar]
- Zhang L, Lin J, Ji G. (2004) Membrane anchoring of the AgrD N-terminal amphipathic region is required for its processing to produce a quorum-sensing pheromone in Staphylococcus aureus. J Biol Chem 279:19448–19456 [DOI] [PubMed] [Google Scholar]
- Zhang M, Tong KP, Fremont V, Chen J, Narayan P, Puett D, Weintraub BD, Szkudlinski MW. (2000) The extracellular domain suppresses constitutive activity of the transmembrane domain of the human TSH receptor: implications for hormone-receptor interaction and antagonist design. Endocrinology 141:3514–3517 [DOI] [PubMed] [Google Scholar]