Abstract
c-Jun NH2-terminal kinase (JNK) activation plays a major role in acetaminophen (APAP)-induced hepatotoxicity. However, the exact mechanism of APAP-induced JNK activation is incompletely understood. It has been established that apoptosis signal-regulating kinase 1 (ASK1) regulates the late phase of APAP-induced JNK activation, but the mitogen-activated protein kinase kinase kinase that mediates the initial phase of APAP-induced JNK activation has not been identified. Oxidative stress produced during APAP metabolism causes JNK activation, which promotes mitochondrial dysfunction and results in the amplification of oxidative stress. Therefore, inhibition of the initial phase of JNK activation may be key to protection against APAP-induced liver injury. The goal of this study was to determine whether mixed-lineage kinase 3 (MLK3) mediates the initial, ASK1-independent phase of APAP-induced JNK activation and thus promotes drug-induced hepatotoxicity. We found that MLK3 was activated by oxidative stress and was required for JNK activation in response to oxidative stress. Loss of MLK3 attenuated APAP-induced JNK activation and hepatocyte death in vitro, independent of receptor-interacting protein 1. Moreover, JNK and glycogen synthase kinase 3β activation was significantly attenuated, and Mcl-1 degradation was inhibited in APAP-treated MLK3-knockout mice. Furthermore, we showed that loss of MLK3 increased expression of glutamate cysteine ligase, accelerated hepatic GSH recovery, and decreased production of reactive oxygen species after APAP treatment. MLK3-deficient mice were significantly protected from APAP-induced liver injury, compared with wild-type mice. Together, these studies establish a novel role for MLK3 in APAP-induced JNK activation and hepatotoxicity, and they suggest MLK3 as a possible target in the treatment of APAP-induced liver injury.
Introduction
Acetaminophen (APAP) overdose is the leading cause of drug-induced liver injury in the United States (Larson et al., 2005). The underlying mechanism of APAP hepatotoxicity is thought to be conversion of APAP to N-acetyl-p-benzoquinoneimine (NAPQI), which is mediated by members of the cytochrome P450 family, especially CYP2E1 (Nelson, 1990). NAPQI is detoxified through conjugation with GSH (Mitchell et al., 1973). Once the intracellular pool of GSH is depleted, NAPQI covalently modifies cellular proteins, which results in mitochondrial dysfunction and oxidative stress (Hinson et al., 2004; Jaeschke and Bajt, 2006). Oxidative stress induces c-Jun NH2-terminal kinase (JNK) activation, which is thought to promote mitochondrial dysfunction and mitochondrial permeability transition and thereby to amplify oxidative stress and to lead to sustained JNK activation, which ultimately causes cell death (Saito et al., 2010; Chambers and LoGrasso, 2011). Genetic deletion and pharmacological inhibition studies suggested that JNK activation plays a major role in APAP-induced hepatotoxicity (Gunawan et al., 2006; Henderson et al., 2007; Latchoumycandane et al., 2007; Hanawa et al., 2008). APAP-induced liver injury results from mainly necrotic rather than apoptotic cell death (Gujral et al., 2002). This finding suggests that JNK may mediate both necrotic and apoptotic signaling pathways in hepatocytes. JNK activity is also required for hepatocyte proliferation and liver regeneration (Schwabe et al., 2003), however, and direct targeting of JNK may ultimately inhibit recovery from APAP-induced liver injury. A detailed understanding of the exact mechanisms of APAP-induced JNK activation is critical for therapeutic intervention.
Several groups of mitogen-activated protein kinase kinase kinases (MAP3Ks), including MEK kinases 1 to 4, MLKs 1 to 4, transforming growth factor β-activated kinase 1, ASK1, and tumor progression locus 2 (Weston and Davis, 2007), have been implicated in the regulation of JNK. MAP3Ks can provide selectivity for the activation of JNK by upstream stimuli. ASK1 is required for endoplasmic reticulum stress- and oxidative stress-induced JNK activation (Matsuzawa et al., 2002), whereas MLK3 mediates JNK activation in response to saturated free fatty acids (Jaeschke and Davis, 2007; Sharma et al., 2012). In addition, MAP3Ks can function either cooperatively or sequentially. For example, transforming growth factor β-activated kinase 1 is essential for the early phase of tumor necrosis factor α-induced JNK activation, whereas ASK1 plays an important role in the late phase (Tobiume et al., 2001; Sato et al., 2005). Studies using ASK1-KO mice established a late, ASK1-dependent phase and an early, ASK1-independent phase of JNK activation after APAP treatment (Nakagawa et al., 2008). However, the MAP3K that mediates the early phase of APAP-induced JNK activation is not known. It was demonstrated that glycogen synthase kinase 3β (GSK3β) is required for the initial phase of APAP-induced JNK activation (Shinohara et al., 2010), and several studies suggested that GSK3β mediates JNK activation through MLK3 (Mishra et al., 2007; Wang et al., 2010). Moreover, it was demonstrated that both MLK3 and GSK3β are required for saturated fatty acid-induced JNK activation and lipoapoptosis (Jaeschke and Davis, 2007; Ibrahim et al., 2011; Sharma et al., 2012); saturated free fatty acids are thought to promote mitochondrial dysfunction, to increase production of reactive oxygen species (ROS), and thus to induce oxidative stress (Srivastava and Chan, 2007; Schönfeld and Wojtczak, 2008). The goal of this study was to determine whether MLK3 mediates the initial, ASK1-independent phase of APAP-induced JNK activation and thus promotes drug-induced hepatotoxicity. Loss of MLK3 attenuated APAP-induced JNK activation, accelerated hepatic GSH recovery, decreased ROS production, and limited liver injury. Together, these studies establish an important role for MLK3 in APAP-induced liver injury.
Materials and Methods
Antibodies and Reagents.
Antibodies were purchased as follows: anti-MLK3 antibody from Santa Cruz Biotechnology (Santa Cruz, CA), anti-GLCc antibody from Novus Biologicals (Littleton, CO), anti-tubulin antibody from Sigma-Aldrich (St. Louis, MO), anti-actin, anti-phospho-JNK, anti-phospho-MLK3, anti-Mcl-1, anti-GSK3, anti-GS, anti-phospho-GS, and anti-phospho-Src (Tyr416) antibodies from Cell Signaling Technology (Danvers, MA), anti-cyclooxygenase IV antibody from Invitrogen (Carlsbad, CA), anti-Rac1 antibody from Millipore (Billerica, MA), anti-RIP1 antibodies from BD Biosciences (San Jose, CA) and BioVision (Mountain View, CA), and anti-JNK antibody from BD Biosciences Pharmingen (San Jose, CA). Acetaminophen was obtained from Sigma-Aldrich, and necrostatin-1 and 6,7-dimethoxy-N-(4-phenoxyphenyl)-4-quinazolinamine (Src I1) were obtained from Tocris Bioscience (Ellisville, MO).
Cell Lines and Cell Culture.
Hepa-1c1c7 cells were purchased from American Type Culture Collection (Manassas, VA) and were maintained in α minimal essential medium supplemented with 10% NuSerum (BD Biosciences). Primary hepatocytes were isolated from C57BL/6 and MLK3-KO mice as described previously (Seglen, 1976). Under anesthesia, livers were perfused with Ca2+-free Krebs-Ringer-Henseleit buffer (115 mM NaCl, 20 mM Hepes, pH 7.4, 5 mM KCl, 1 mM KH2PO4, 0.5 mM EGTA), followed by perfusion with Krebs-Ringer-Henseleit buffer containing CaCl2 and collagenase B (Roche Diagnostics, Indianapolis, IN). Hepatocytes were purified through Percoll centrifugation and were cultured in Dulbecco's modified Eagle's medium supplemented with 10% NuSerum (BD Biosciences). Cell viability, as judged through Trypan blue exclusion, was greater than 90%. After 4 h, the medium was changed to control medium or medium containing different concentrations of APAP.
Sytox Green Staining.
After 20 h of APAP treatment, the cells were stained with 1 μM Sytox Green (Invitrogen) for 15 min. Cells stained with Sytox Green were counted as necrotic cells. Cells were imaged by using an Olympus IX71 inverted epifluorescence microscope (Olympus, Tokyo, Japan) with a 20×/0.40 objective. Hepatocytes in five random fields were counted, the Sytox-positive counts were divided by the total number of cells, as determined from corresponding phase-contrast images, and results were plotted as the proportion of Sytox-positive cells.
Animals.
After an overnight fast, male mice (10–12 weeks of age) received intraperitoneal injections of 300 mg/kg APAP dissolved in warm saline solution or saline solution alone. At various time points, mice were sacrificed and blood and livers were harvested. For histological analyses, tissue samples were fixed in 10% formalin, dehydrated, and embedded in paraffin. Sections were stained with hematoxylin and eosin. The animal studies were approved by the institutional animal care and use committee of the University of Cincinnati.
Biochemical Assays.
Cell extracts were prepared by using Triton lysis buffer, as described previously (Jaeschke and Davis, 2007). Mitochondria were isolated as described previously (Shinohara et al., 2010). Livers were excised and homogenized in H-buffer (210 mM mannitol, 70 mM sucrose, 20 mM Hepes, 0.05% bovine serum albumin). The homogenate was centrifuged for 10 min at 800g, and the resulting supernatant was centrifuged for 15 min at 8500g. The pellet (mitochondrial fraction) was washed once and resuspended in H-buffer. Rac1 activation was analyzed by using a Rac1 activation assay kit from Millipore, according to the manufacturer's instructions. For measurement of RIP1 activity, hepatocytes were lysed in Triton lysis buffer. Immunoprecipitation was performed overnight at 4°C with a polyclonal anti-RIP1 antibody (BioVision). Protein A-Sepharose was added the following morning for 1 h, followed by two washes with Triton lysis buffer and one wash with kinase buffer (20 mM Hepes, pH 7.4, 5 mM MnCl2, 5 mM MgCl2). Kinase reactions were performed for 30 min at 37°C in kinase buffer with 5 μM ATP, 1 μCi of [γ-32P]ATP, and 1 μg of myelin basic protein.
ALT Measurements.
Plasma ALT levels were measured by using an alanine transaminase activity assay kit (Cayman Chemical, Ann Arbor, MI), according to the manufacturer's instructions.
GSH Measurements.
Total liver homogenates were mixed with metaphosphoric acid to prevent GSH auto-oxidation. GSH levels were measured by using a glutathione kit (Cayman Chemical), according to the manufacturer's instructions.
ROS Measurements.
Two probes, i.e., 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) and dihydroethidium (DHE), were used for measurement of ROS levels. Hepatic ROS contents were measured by using DCFH-DA (Sigma-Aldrich), as described previously (Abubakar et al., 2003). Liver homogenates were diluted 100-fold with phosphate-buffered saline (pH 7.4). The homogenates were then loaded with 5 μmol/ml DCFH-DA and were incubated for 30 min at 37°C in the dark. Fluorescence was measured by using a fluorescence spectrophotometer (Synergy HT; BioTek, Winooski, VT), with wavelengths of 485 nm for excitation and 528 nm for emission. Hepatic ROS levels were determined from DCFH-DA measurements and were normalized to the protein concentrations in the homogenates, as quantified with a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA). ROS contents in hepatocytes were measured by using DHE assays. After 16 h of APAP treatment, hepatocytes were stained with 5 μM DHE (Invitrogen) for 15 min. DHE fluorescence was detected with an Olympus IX71 inverted epifluorescence microscope with a 20×/0.40 objective.
Quantitative PCR Assays.
RNA was prepared by using a RNeasy kit from QIAGEN (Valencia, CA), according to the manufacturer's instructions. RNA was reverse-transcribed by using an iScript cDNA synthesis kit from Bio-Rad Laboratories, interleukin 1 (Mm00434228_m1) and interleukin 6 (Mm00446190_m1) gene expression was determined through quantitative PCR analyses with a Bio-Rad iCycler iQ real-time PCR detection system, and results were normalized to the expression of actin (Mm00607939_s1) by using TaqMan assays (Applied Biosystems, Foster City, CA).
Statistical Analyses.
Statistical analyses were performed by using Student's t test or analysis of variance; p values of <0.05 were considered statistically significant.
Results
Role of MLK3 in APAP-Induced JNK Activation.
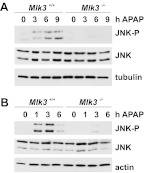
MLK3 was shown to mediate ROS-dependent JNK activation in a variety of cell types, such as neurons (Lotharius et al., 2005; Pan et al., 2006) and prostate cancer cells (Hong and Kim, 2007). We found that MLK3 and its upstream activator Rac1 were activated by H2O2 in hepatic cells and were required for oxidative stress-induced JNK activation in primary mouse hepatocytes (data not shown). Because JNK activation is thought to be a result of oxidative stress produced during APAP metabolism and we established that MLK3 plays a major role in oxidative stress-induced JNK activation, we hypothesized that MLK3 mediates APAP-induced JNK activation in hepatocytes. Treatment of primary mouse hepatocytes with APAP induced JNK phosphorylation in WT cells but not in MLK3-deficient cells (Fig. 1A). We investigated whether MLK3 was required for APAP-induced JNK activation in vivo. We found that JNK phosphorylation 1, 3, and 6 h after APAP treatment was significantly attenuated in MLK3-KO mice (Fig. 1B), which indicates that MLK3 mediates APAP-stimulated JNK activation in vivo. Together, these data suggest that MLK3 is required for APAP-induced JNK activation in vitro and in vivo.
Fig. 1.
Role of MLK3 in APAP-induced JNK activation. A, primary hepatocytes from wild-type and Mlk3(−/−) mice were treated with 5 mM APAP for the indicated times. A representative immunoblot for expression and phosphorylation of JNK, from three independent experiments, is shown. B, wild-type and Mlk3(−/−) mice were treated with APAP (300 mg/kg i.p.) for the indicated times. Livers from three mice per time point and genotype were excised, and total liver lysates were analyzed. A representative immunoblot for expression and phosphorylation of JNK is shown.
Role of MLK3 in APAP-Induced Hepatotoxicity.
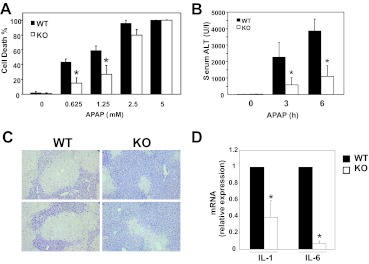
Because JNK activation mediates APAP-induced hepatotoxicity (Gunawan et al., 2006; Henderson et al., 2007; Latchoumycandane et al., 2007; Hanawa et al., 2008), we analyzed cell death of primary mouse hepatocytes. We observed that cells isolated from MLK3-KO mice were partially protected from necrosis caused by treatment with APAP, compared with hepatocytes isolated from WT mice (Fig. 2A). This protection was lost with high doses of APAP (≥5 mM), which suggests a threshold of protection. The protective effect of MLK3 deficiency in vitro suggests that MLK3 may serve an important role in APAP-induced liver injury in vivo. Loss of MLK3 resulted in significantly lower ALT levels 3 and 6 h after APAP treatment (Fig. 2B). Liver histological analyses showed significant decreases in necrosis in response to APAP treatment in the absence of MLK3 (Fig. 2C), consistent with reduced serum ALT levels. Inflammatory cytokine levels are increased in APAP-induced liver injury and seem to be correlated with the severity of injury (James et al., 2005). In agreement with decreased liver injury, we observed decreased inflammatory cytokine expression in Mlk(−/−) mice treated with APAP, compared with WT mice (Fig. 2D). Together, these data suggest that MLK3 mediates, in part, APAP-induced cell death of hepatocytes and plays an important role in APAP-induced liver injury.
Fig. 2.
Role of MLK3 in APAP-induced hepatotoxicity. A, necrosis was determined through Sytox green staining after 20 h of treatment of primary hepatocytes with increasing amounts of APAP. Results are mean ± S.D. from three independent experiments. *, p < 0.05. B and C, hepatotoxicity was analyzed through measurements of serum ALT levels 3 and 6 h (B) and histological analyses of hematoxylin- and eosin-stained samples from WT (left) and MLK3-KO (right) mice 6 h (C) after APAP treatment (300 mg/kg i.p.); top and bottom panels show data for different mice. D, relative interleukin 1β (IL-1) and interleukin 6 (IL-6) mRNA levels 6 h after APAP treatment were determined through quantitative PCR analyses. Results are mean ± S.D. (n = 6 or 7). *, p < 0.05.
Role of RIP1 in APAP-Induced Hepatotoxicity.
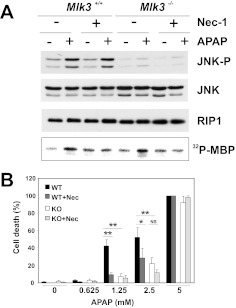
APAP-induced liver injury is thought to involve primarily hepatocyte necrosis, and RIP1 is emerging as a key regulator of necrotic cell death (for review, see Vanlangenakker et al., 2012). Because RIP1 is thought to contribute to JNK activation in response to distinct stimuli (Devin et al., 2003; Zhang et al., 2007; Seok et al., 2008), we examined the effects of APAP on RIP1 activity in WT and MLK3-KO hepatocytes. We found that loss of MLK3 did not affect APAP-induced RIP1 activity, which suggests that APAP induces RIP1 activation independent of MLK3 (Fig. 3A). Likewise, inhibition of RIP1 by necrostatin-1 had no effect on APAP-induced JNK activation (Fig. 3A). Treatment of hepatocytes with necrostatin-1, a specific RIP1 inhibitor, significantly decreased APAP-induced necrosis in WT hepatocytes and increased the protective effect of MLK3 deficiency in KO hepatocytes (Fig. 3B). Together, these data suggest that MLK3 and RIP1 act independently in promoting APAP-induced hepatotoxicity.
Fig. 3.
Role of RIP1 in APAP-induced hepatotoxicity. A, primary hepatocytes from WT and KO mice were treated with 5 mM APAP for 6 h, in the presence or absence of 30 μM necrostatin-1 (Nec-1). Expression and phosphorylation of JNK and expression of RIP1 were examined through immunoblot analyses. RIP1 activity was determined through in vitro kinase assays with ATP and myelin basic protein (MBP) as substrates. Representative data from two independent experiments are shown. B, necrosis was determined through Sytox green staining after 20 h of treatment of WT and KO hepatocytes with increasing amounts of APAP, in the presence or absence of 30 μM necrostatin-1. Results are mean ± S.D. from two independent experiments. *, p < 0.01; **, p < 0.001. NS, not significant.
Role of MLK3 in GSH Recovery.
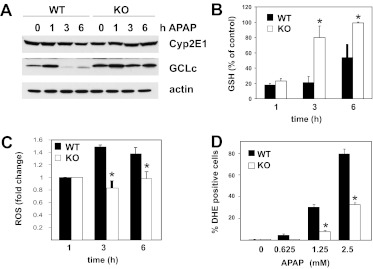
To determine whether attenuated liver injury is a result of altered APAP metabolism, we examined the levels of expression of CYP2E1, a protein involved in the conversion of APAP to NAPQI (Nelson, 1990). CYP2E1 expression levels were similar in WT and MLK3-KO mice (Fig. 4A), which indicates that the loss of MLK3 does not affect NAPQI production. This is consistent with the results of previous studies that suggested that inhibition of JNK does not affect APAP metabolism (Henderson et al., 2007). Because NAPQI is detoxified mainly by GSH in hepatocytes (Mitchell et al., 1973) and modulation of GSH levels significantly affects APAP hepatotoxicity, we analyzed hepatic GSH levels. Basal GSH levels were comparable (WT, 33 ± 2.2 nmol/mg protein; KO, 29 ± 1.9 nmol/mg protein) and the kinetic characteristics of APAP-induced GSH depletion were similar in mice with the two genotypes (Fig. 4B). These data are consistent with unaltered APAP metabolism in MLK3-deficient mice, compared with WT mice. However, recovery of hepatic GSH levels at later time points was accelerated in MLK3-deficient mice (Fig. 4B). To determine the mechanism through which MLK3 deficiency promoted GSH recovery, we analyzed the expression of GCLc, the catalytic subunit of the rate-limiting enzyme in GSH synthesis, which is known to be regulated by oxidative stress (Franklin et al., 2009). We found that basal GCLc levels were increased in MLK3-deficient mice and APAP treatment caused significant decreases in GCLc levels in WT but not MLK3-KO mice (Fig. 4A). These data suggest that MLK3 regulates GCLc expression and thus modulates the capacity for GSH biosynthesis. Because APAP-induced GSH depletion is known to lead to mitochondrial dysfunction, increased ROS production, and oxidative stress, we measured 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) and DHE fluorescence as indicators of ROS formation. APAP treatment caused significant increases in DCFH-DA fluorescence in WT but not MLK3-KO mice (Fig. 4C), which indicates decreased oxidative stress in Mlk3(−/−) livers. In addition, we observed significantly fewer DHE-positive cells after 16 h of treatment with the indicated amounts of APAP in MLK3-deficient hepatocytes, compared with WT hepatocytes (Fig. 4D), which indicates decreased superoxide production in MLK3-KO hepatocytes. Together, these data suggest that MLK3 regulates the capacity for GSH biosynthesis and mediates ROS formation.
Fig. 4.
Role of MLK3 in GSH recovery. Wild-type and MLK3-KO mice were treated with APAP (300 mg/kg i.p.) for the indicated times. A, levels of expression of hepatic CYP2E1 and GCLc were examined through immunoblot analyses. B, GSH levels in total liver lysates were determined 1, 3, and 6 h after APAP treatment. Results are mean ± S.D. (n = 6 or 7). *, p < 0.05. C, oxidative stress in total liver lysates was determined through measurements of DCFH-DA fluorescence 0, 3, and 6 h after APAP treatment. Results are mean ± S.D. from three experiments. *, p < 0.05. D, superoxide production was determined through DHE staining after 16 h of treatment of primary hepatocytes with the indicated amounts of APAP. Results are mean ± S.D. from two experiments. *, p < 0.05.
Role of MLK3 in Mcl-1 Expression.
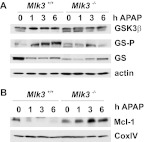
It was demonstrated that GSK3β promotes APAP-induced JNK activation (Shinohara et al., 2010), and several studies suggested that GSK3β induces JNK activation through MLK3 (Mishra et al., 2007; Wang et al., 2010). Therefore, we analyzed APAP-induced GSK3β activation in WT and MLK3-deficient mice. We found that phosphorylation of the GSK3 substrate GS was attenuated in MLK3-KO mice (Fig. 5A), which indicates that MLK3 and subsequent JNK activation are required for APAP-induced activation of GSK3β. It was shown that pharmacological inhibition of JNK prevented APAP-induced GSK3β activation, possibly through attenuation of oxidative stress (Shinohara et al., 2010). Therefore, it is possible that loss of MLK3 affects APAP-induced GSK3β activation indirectly, through inhibition of ROS production.
Fig. 5.
Role of MLK3 in Mcl-1 expression. Wild-type and Mlk3(−/−) mice were treated with APAP (300 mg/kg i.p.) for the indicated times, and livers were excised. A, livers from three mice per time point and genotype were excised, and total liver lysates were analyzed. A representative immunoblot for expression of GSK3β and expression and phosphorylation of GS is shown. B, livers from three mice per time point and genotype were excised, and mitochondria were isolated and analyzed. A representative immunoblot for expression of Mcl-1 and cyclooxygenase IV (COXIV) is shown.
The exact mechanism through which JNK activation promotes hepatotoxicity is unclear. It was shown that JNK regulates proapoptotic and antiapoptotic members of the Bcl-2 family that modulate mitochondrial death pathways (Dhanasekaran and Reddy, 2008). It was demonstrated that APAP induces Bim expression in a JNK-dependent manner and loss of Bim results in substantial protection from APAP-induced liver damage (Badmann et al., 2011). However, Bim expression levels were similar in wild-type and MLK3-KO mice (data not shown). It was shown that Mcl-1 phosphorylation by JNK in response to oxidative stress may be one of the mechanisms through which oxidative stress induces cellular damage (Inoshita et al., 2002). In addition, it was demonstrated that phosphorylation of Mcl-1 by JNK is required for phosphorylation of Mcl-1 by GSK3β and subsequent degradation of the antiapoptotic protein (Morel et al., 2009). Mcl-1 was degraded rapidly in WT but not MLK3-deficient livers (Fig. 5B), consistent with attenuated JNK and GSK3β activation.
Discussion
The main goal of this study was to evaluate the role of MLK3 in APAP-induced JNK activation and hepatotoxicity. By using WT and MLK3-deficient primary hepatocytes and hepatic cell lines, we found that MLK3 and its upstream activator Rac1 were activated by oxidative stress and were critically involved in H2O2-dependent JNK activation in hepatocytes (data not shown). APAP-induced JNK activation is thought to be a result of oxidative stress produced during APAP metabolism and, consistent with our finding that MLK3 is involved in H2O2-induced JNK activation in hepatocytes, we found that loss of MLK3 attenuated APAP-induced JNK activation in vivo (Fig. 1B) and in vitro (Fig. 1A), which indicates that MLK3 plays a critical role in APAP-induced JNK activation in hepatocytes. It was demonstrated that oxidative stress causes JNK activation and translocation to the mitochondria (Chambers and LoGrasso, 2011). However, it is not known which cellular compartment MLK3 signals for JNK activation or whether activated MLK3 also is translocated to the mitochondria. More studies will be required to address MLK3 compartmentation in response to oxidative stress.
It was proposed that saturated fatty acids induce MLK3 and JNK activation through c-Src (Holzer et al., 2011) and that H2O2-induced JNK activation involves c-Src (Chen et al., 2001). Here we observed that pharmacological inhibition of Src prevented APAP-induced activation of JNK in primary hepatocytes (Supplemental Fig. 1), which indicates an important role for c-Src in APAP-induced activation of the MLK3/JNK axis.
APAP largely induces necrotic cell death, and the serine/threonine kinase RIP1 was shown to be involved in the regulation of necrosis and in JNK activation in response to distinct stimuli (Devin et al., 2003; Zhang et al., 2007; Seok et al., 2008). To test the relationship between RIP1 and MLK3, we performed epistatic analysis of the APAP-induced pathway. Loss of MLK3 did not inhibit RIP1 activation, and inhibition of RIP1 did not affect MLK3 activity (Fig. 3A). Moreover, inhibition of both pathways led to increased protection (Fig. 3B), which suggests that MLK3 and RIP1 act through independent mechanisms to promote APAP-induced hepatotoxicity.
We showed that loss of MLK3 significantly attenuated APAP-induced liver injury, as indicated by decreased necrosis and reduced levels of ALT and inflammatory cytokines (Fig. 2, B–D). However, the exact mechanism through which MLK3 promotes hepatotoxicity is unclear. We found that GCLc expression levels were increased and GSH recovery was accelerated in MLK3-deficient mice (Fig. 4, A and B). Although we did not observe higher basal GSH levels, which is probably attributable to GSH negative feedback regulation of GCLc activity (Franklin et al., 2009), it is possible that increased GCLc expression enhances GSH synthesis in MLK3-KO mice, which might account for the decreased APAP-induced liver injury in Mlk3(−/−) mice. It was demonstrated that overexpression of GCLc does not affect basal GSH levels but enhances GSH resynthesis after APAP-induced GSH depletion, thereby attenuating drug-induced liver injury (Botta et al., 2006). GCLc levels are regulated transcriptionally, post-translationally, and through degradation, and additional studies will be required to address the mechanisms through which MLK3 modulates GCLc levels.
It was shown that GSK3β promotes APAP-induced JNK activation (Shinohara et al., 2010), and several studies suggested that GSK3β is required for MLK3-mediated JNK activation, which places MLK3 downstream of GSK3β (Mishra et al., 2007; Wang et al., 2010). We found that APAP-induced GSK3β activation was inhibited in MLK3-deficient mice (Fig. 5A); however, the underlying mechanism is unclear. It was suggested that positive feedback phosphorylation of MLK3 by JNK may lead to sustained JNK activation (Schachter et al., 2006), which may result in amplification of oxidative stress. We observed decreased ROS production in APAP-treated MLK3-KO mice, compared with WT mice (Fig. 4C), and APAP-treated KO hepatocytes (Fig. 4D). In addition, it was shown that pharmacological inhibition of JNK activation resulted in reduced oxidative stress and peroxynitrite formation (Saito et al., 2010) and decreased APAP-induced GSK3β activation (Shinohara et al., 2010). Together, these data suggest that MLK3 deficiency may regulate GSK3β indirectly through modulation of ROS production.
Another mechanism through which MLK3 may promote hepatotoxicity is JNK-mediated regulation of proapoptotic and antiapoptotic Bcl-2 family members. It was demonstrated that Bim-deficient mice are protected from APAP-induced liver damage and APAP strongly induces Bim expression in a JNK-dependent manner (Badmann et al., 2011). Because we did not observe any differences in Bim expression in MLK3-KO mice, compared with WT mice (data not shown), regulation of Bim expression does not seem to account for MLK3-mediated hepatotoxicity. Another possibility is that loss of MLK3 prevents JNK-mediated phosphorylation and inactivation of Bcl-2 and Bcl-XL, thereby preventing mitochondrial dysfunction (Latchoumycandane et al., 2007). However, Bcl-2-overexpressing mice showed greater drug-induced liver injury, compared with wild-type mice, which calls into question the protective role of Bcl-2 in APAP-induced hepatotoxicity (Adams et al., 2001). It was suggested that JNK-mediated Mcl-1 phosphorylation might be one of the mechanisms through which oxidative stress induces cellular damage (Inoshita et al., 2002), although the role of Mcl-1 in APAP-induced hepatotoxicity has not been established. JNK phosphorylation results in the priming of Mcl-1 for phosphorylation by GSK3β and subsequent degradation of the antiapoptotic protein (Morel et al., 2009). We observed that Mcl-1 was rapidly degraded in WT livers but not MLK3-deficient livers (Fig. 5B). Therefore, stabilization of Mcl-1 may be an important mechanism through which MLK3 deficiency protects against APAP-induced hepatocyte death, although additional studies will be required to evaluate the role of Mcl-1 in APAP-induced liver injury.
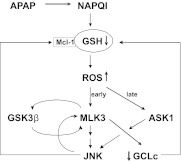
In summary, we show that MLK3 is activated by oxidative stress in hepatocytes, and we identify MLK3 as the MAP3K that mediates the initial phase of APAP-induced JNK activation (Fig. 6). Because APAP-induced JNK activation results in amplification of oxidative stress and oxidative stress plays a critical role in the propagation of cell death and liver injury, our findings suggest that MLK3 may be an important target in APAP-induced liver injury. In addition, we provide evidence that MLK3 regulates GCLc expression and thus modulates the capacity for GSH biosynthesis. Together, these studies establish a novel role for MLK3 in APAP-induced liver injury.
Fig. 6.
Model for the role of MLK3 in APAP-induced hepatotoxicity. The metabolism of APAP results in NAPQI formation, GSH depletion, mitochondrial dysfunction, and ROS production. Oxidative stress-induced MLK3 activation amplifies ROS formation by modulating the capacity for GSH biosynthesis through regulation of GCLc expression and by targeting JNK- and GSK3β-dependent Mcl-1 degradation, which ultimately leads to cell death.
Supplementary Material
Acknowledgments
We thank Dr. Roger Davis for providing Mlk3(−/−) mice. We thank Drs. Patrick Dennis and Carol Mercer for critical reading of the manuscript.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK082583].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- APAP
- acetaminophen
- MLK
- mixed-lineage kinase
- JNK
- c-Jun NH2-terminal kinase
- ASK
- apoptosis-regulating kinase
- MAP3K
- mitogen-activated protein kinase kinase kinase
- ROS
- reactive oxygen species
- NAPQI
- N-acetyl-p-benzoquinoneimine
- GSK
- glycogen synthase kinase
- ALT
- alanine transaminase
- GS
- glycogen synthase
- RIP
- receptor-interacting protein
- DCFH-DA
- 2′,7′-dichlorodihydrofluorescein diacetate
- DCF
- 2′,7′-dichlorofluorescein
- Src I1
- 6,7-dimethoxy-N-(4-phenoxyphenyl)-4-quinazolinamine
- DHE
- dihydroethidium
- WT
- wild-type
- KO
- knockout
- GCLc
- glutamate-cysteine ligase catalytic subunit.
Authorship Contributions
Participated in research design: Sharma, Gadang, and Jaeschke.
Conducted experiments: Sharma, Gadang, and Jaeschke.
Performed data analysis: Sharma, Gadang, and Jaeschke.
Wrote or contributed to the writing of the manuscript: Gadang and Jaeschke.
References
- Abubakar MG, Taylor A, Ferns GA. (2003) Aluminium administration is associated with enhanced hepatic oxidant stress that may be offset by dietary vitamin E in the rat. Int J Exp Pathol 84:49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ML, Pierce RH, Vail ME, White CC, Tonge RP, Kavanagh TJ, Fausto N, Nelson SD, Bruschi SA. (2001) Enhanced acetaminophen hepatotoxicity in transgenic mice overexpressing BCL-2. Mol Pharmacol 60:907–915 [DOI] [PubMed] [Google Scholar]
- Badmann A, Keough A, Kaufmann T, Bouillet P, Brunner T, Corazza N. (2011) Role of TRAIL and the pro-apoptotic Bcl-2 homolog Bim in acetaminophen-induced liver damage. Cell Death Dis 2:e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta D, Shi S, White CC, Dabrowski MJ, Keener CL, Srinouanprachanh SL, Farin FM, Ware CB, Ladiges WC, Pierce RH, et al. (2006) Acetaminophen-induced liver injury is attenuated in male glutamate-cysteine ligase transgenic mice. J Biol Chem 281:28865–28875 [DOI] [PubMed] [Google Scholar]
- Chambers JW, LoGrasso PV. (2011) Mitochondrial c-Jun N-terminal kinase (JNK) signaling initiates physiological changes resulting in amplification of reactive oxygen species generation. J Biol Chem 286:16052–16062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Vita JA, Berk BC, Keaney JF., Jr (2001) c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves SRC-dependent epidermal growth factor receptor transactivation. J Biol Chem 276:16045–16050 [DOI] [PubMed] [Google Scholar]
- Devin A, Lin Y, Liu ZG. (2003) The role of the death-domain kinase RIP in tumour-necrosis-factor-induced activation of mitogen-activated protein kinases. EMBO Rep 4:623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. (2008) JNK signaling in apoptosis. Oncogene 27:6245–6251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. (2009) Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med 30:86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. (2002) Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci 67:322–328 [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. (2006) c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 131:165–178 [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. (2008) Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem 283:13565–13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson NC, Pollock KJ, Frew J, Mackinnon AC, Flavell RA, Davis RJ, Sethi T, Simpson KJ. (2007) Critical role of c-jun (NH2) terminal kinase in paracetamol- induced acute liver failure. Gut 56:982–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinson JA, Reid AB, McCullough SS, James LP. (2004) Acetaminophen-induced hepatotoxicity: role of metabolic activation, reactive oxygen/nitrogen species, and mitochondrial permeability transition. Drug Metab Rev 36:805–822 [DOI] [PubMed] [Google Scholar]
- Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, Solinas G, Karin M. (2011) Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 147:173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong HY, Kim BC. (2007) Mixed lineage kinase 3 connects reactive oxygen species to c-Jun NH2-terminal kinase-induced mitochondrial apoptosis in genipin-treated PC3 human prostate cancer cells. Biochem Biophys Res Commun 362:307–312 [DOI] [PubMed] [Google Scholar]
- Ibrahim SH, Akazawa Y, Cazanave SC, Bronk SF, Elmi NA, Werneburg NW, Billadeau DD, Gores GJ. (2011) Glycogen synthase kinase-3 (GSK-3) inhibition attenuates hepatocyte lipoapoptosis. J Hepatol 54:765–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoshita S, Takeda K, Hatai T, Terada Y, Sano M, Hata J, Umezawa A, Ichijo H. (2002) Phosphorylation and inactivation of myeloid cell leukemia 1 by JNK in response to oxidative stress. J Biol Chem 277:43730–43734 [DOI] [PubMed] [Google Scholar]
- Jaeschke A, Davis RJ. (2007) Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell 27:498–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. (2006) Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol Sci 89:31–41 [DOI] [PubMed] [Google Scholar]
- James LP, Simpson PM, Farrar HC, Kearns GL, Wasserman GS, Blumer JL, Reed MD, Sullivan JE, Hinson JA. (2005) Cytokines and toxicity in acetaminophen overdose. J Clin Pharmacol 45:1165–1171 [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO, et al. (2005) Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology 42:1364–1372 [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. (2007) Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology 45:412–421 [DOI] [PubMed] [Google Scholar]
- Lotharius J, Falsig J, van Beek J, Payne S, Dringen R, Brundin P, Leist M. (2005) Progressive degeneration of human mesencephalic neuron-derived cells triggered by dopamine-dependent oxidative stress is dependent on the mixed-lineage kinase pathway. J Neurosci 25:6329–6342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, Nishitoh H, Tobiume K, Takeda K, Ichijo H. (2002) Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal 4:415–425 [DOI] [PubMed] [Google Scholar]
- Mishra R, Barthwal MK, Sondarva G, Rana B, Wong L, Chatterjee M, Woodgett JR, Rana A. (2007) Glycogen synthase kinase-3β induces neuronal cell death via direct phosphorylation of mixed lineage kinase 3. J Biol Chem 282:30393–30405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. (1973) Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther 187:211–217 [PubMed] [Google Scholar]
- Morel C, Carlson SM, White FM, Davis RJ. (2009) Mcl-1 integrates the opposing actions of signaling pathways that mediate survival and apoptosis. Mol Cell Biol 29:3845–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, et al. (2008) Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology 135:1311–1321 [DOI] [PubMed] [Google Scholar]
- Nelson SD. (1990) Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin Liver Dis 10:267–278 [DOI] [PubMed] [Google Scholar]
- Pan J, Pei DS, Yin XH, Hui L, Zhang GY. (2006) Involvement of oxidative stress in the rapid Akt1 regulating a JNK scaffold during ischemia in rat hippocampus. Neurosci Lett 392:47–51 [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. (2010) c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol 246:8–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. (2005) Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol 6:1087–1095 [DOI] [PubMed] [Google Scholar]
- Schachter KA, Du Y, Lin A, Gallo KA. (2006) Dynamic positive feedback phosphorylation of mixed lineage kinase 3 by JNK reversibly regulates its distribution to Triton-soluble domains. J Biol Chem 281:19134–19144 [DOI] [PubMed] [Google Scholar]
- Schönfeld P, Wojtczak L. (2008) Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic Biol Med 45:231–241 [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. (2003) c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology 37:824–832 [DOI] [PubMed] [Google Scholar]
- Seglen PO. (1976) Preparation of isolated rat liver cells. Methods Cell Biol 13:29–83 [DOI] [PubMed] [Google Scholar]
- Seok JH, Park KA, Byun HS, Won M, Shin S, Choi BL, Lee H, Kim YR, Hong JH, Park J, et al. (2008) Long-term activation of c-Jun N-terminal kinase through receptor interacting protein is associated with DNA damage-induced cell death. Korean J Physiol Pharmacol 12:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Urano F, Jaeschke A. (2012) Cdc42 and Rac1 are major contributors to the saturated fatty acid-stimulated JNK pathway in hepatocytes. J Hepatol 56:192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Ybanez MD, Win S, Than TA, Jain S, Gaarde WA, Han D, Kaplowitz N. (2010) Silencing glycogen synthase kinase-3β inhibits acetaminophen hepatotoxicity and attenuates JNK activation and loss of glutamate cysteine ligase and myeloid cell leukemia sequence 1. J Biol Chem 285:8244–8255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Chan C. (2007) Hydrogen peroxide and hydroxyl radicals mediate palmitate-induced cytotoxicity to hepatoma cells: relation to mitochondrial permeability transition. Free Radic Res 41:38–49 [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. (2001) ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep 2:222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlangenakker N, Vanden Berghe T, Vandenabeele P. (2012) Many stimuli pull the necrotic trigger: an overview. Cell Death Differ 19:75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MJ, Huang HY, Chen WF, Chang HF, Kuo JS. (2010) Glycogen synthase kinase-3β inactivation inhibits tumor necrosis factor-α production in microglia by modulating nuclear factor κB and MLK3/JNK signaling cascades. J Neuroinflammation 7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. (2007) The JNK signal transduction pathway. Curr Opin Cell Biol 19:142–149 [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang R, Zhang H, Lin Y, Li J, Pober JS, Min W. (2007) RIP1-mediated AIP1 phosphorylation at a 14–3-3-binding site is critical for tumor necrosis factor-induced ASK1-JNK/p38 activation. J Biol Chem 282:14788–14796 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.