Abstract
Several basic leucine zipper (B-ZIP) transcription factors have been implicated in cancer, substance abuse, and other pathological conditions. We previously identified arylstibonic acids that bind to B-ZIP proteins and inhibit their interaction with DNA. In this study, we used electrophoretic mobility shift assay to analyze 46 arylstibonic acids for their activity to disrupt the DNA binding of three B-ZIP [CCAAT/enhancer-binding protein α, cyclic AMP-response element-binding protein (CREB), and vitellogenin gene-binding protein (VBP)] and two basic helix-loop-helix leucine zipper (B-HLH-ZIP) [USF (upstream stimulating factor) and Mitf] proteins. Twenty-five arylstibonic acids showed activity at micromolar concentrations. The most active compound, P6981 [2-(3-stibonophenyl)malonic acid], had half-maximal inhibition at ∼5 nM for CREB. Circular dichroism thermal denaturation studies indicated that P6981 binds both the B-ZIP domain and the leucine zipper. The crystal structure of an arylstibonic acid, NSC13778, bound to the VBP leucine zipper identified electrostatic interactions between both the stibonic and carboxylic acid groups of NSC13778 [(E)-3-(3-stibonophenyl)acrylic acid] and arginine side chains of VBP, which is also involved in interhelical salt bridges in the leucine zipper. P6981 induced GFP-B-ZIP chimeric proteins to partially localize to the cytoplasm, demonstrating that it is active in cells. P6981 inhibited the growth of a patient-derived clear cell sarcoma cell line whose oncogenic potential is driven by a chimeric protein EWS-ATF1 (Ewing's sarcoma protein-activating transcription factor 1), which contains the DNA binding domain of ATF1, a B-ZIP protein. NSC13778 inhibited the growth of xenografted clear cell sarcoma, and no toxicity was observed. These experiments suggest that antimony containing arylstibonic acids are promising leads for suppression of DNA binding activities of B-ZIP and B-HLH-ZIP transcription factors.
Introduction
B-ZIP and B-HLH-ZIP proteins are two structurally related families of dimeric transcription factors that contain an α-helical basic region critical for DNA binding and a leucine zipper (LZ) domain essential for dimerization (Vinson et al., 1989, 2002; Vinson and Garcia, 1992; Massari and Murre, 2000). Members of these two protein families are required in a wide range of biological processes, such as immune response, metabolism, cell differentiation, and proliferation (Mayr and Montminy, 2001; Widlund and Fisher, 2003; Corre and Galibert, 2005; Nerlov, 2008; Xiao et al., 2010). Elevated DNA-binding activities of some B-ZIP and B-HLH-ZIP proteins, such as CREB and Mitf, through dysregulation of upstream signaling pathways, have been implicated in human cancers, substance abuse, and other pathologies (Bonci and Carlezon, 2005; Shankar et al., 2005; Shukla et al., 2009; Giuliano et al., 2010). The inhibition of their DNA binding activities by dominant-negatives has been shown to prevent and reverse some of these pathological states (Walton et al., 1992; Xie et al., 1997; Gerdes et al., 2006; Oh et al., 2007; Rozenberg et al., 2009).
The results obtained by genetically inhibiting the DNA binding of unique families of B-ZIP proteins suggest that they are potentially attractive drug targets. However, B-ZIP proteins are devoid of a catalytic pocket, complicating the identification of small molecules that inhibit B-ZIP/DNA interactions. A small molecule that disrupts the B-ZIP protein/DNA complex has recently been identified from a screen of the National Cancer Institute Diversity Library that contains 1990 small molecules (Rishi et al., 2005). NSC13778, an arylstibonic acid, binds to and stabilizes the C/EBPα dimer and inhibits the binding of C/EBPα to DNA. A subsequent study using 14 additional arylstibonic acid derivatives of NSC13778 identified that NSC13746 inhibits the DNA binding of C/EBPβ at submicromolar concentrations in vitro and specifically suppresses the transcriptional activation of B-ZIP proteins in a luciferase reporter assay (Rishi et al., 2010). NSC13746 also decreases the proliferation of a clear cell sarcoma cell line, CCS-1, which expresses EWS-ATF1 chimeric protein containing the B-ZIP domain of ATF1, a CREB family member (Rishi et al., 2010). These results suggest that arylstibonic acids could be promising lead compounds to treat cancers or other diseases driven by constitutively activated B-ZIP proteins.
In this study, 46 arylstibonic acids, all derivatives of NSC13778 and NSC13746, were screened using EMSA for their inhibitory activities against the DNA binding of three B-ZIP (CREB, C/EBPα, and VBP) and two B-HLH-ZIP proteins (USF and Mitf). P6981, the most potent inhibitor identified, was further characterized by assessing the mechanism of inhibition and by evaluating its effect on tumor cell proliferation.
Materials and Methods
Arylstibonic Acid Library.
The 46-member arylstibonic acid compound library was provided by the Developmental Therapeutics Program, Frederick National Laboratory for Cancer Research. The arylstibonic acids were obtained as powders and dissolved in 50 mM NH4OAc, pH 9.0, measured in aliquots, and stored at −20°C. Seventeen compounds had very low solubility in the experimental conditions, or they contained insoluble impurities.
Twelve arylstibonic acids used in this study have been previously characterized for their inhibitory activities against activator protein AP1, VBP, C/EBPα, C/EBPβ, and CREB (Rishi et al., 2010). They were included in this study as controls and also to investigate their inhibitory activities against Mitf and USF. The results from the two studies are mostly consistent, with the exception of NSC13740 and NSC13743, which in the previous study were shown to be active at 10 and 1 μM, respectively, but were inactive here (data not shown). The discrepancy probably resulted from variable solubility in the experimental conditions or from variable purity of the samples used in the two studies. However, the most active compounds identified in this and a previous study, P6981 and NSC13746, were purified to ≥95% purity (Supplemental Fig. 1).
Proteins.
Two synthesized VBP-LZs, VBP47 and VBP39 (Biopeptide Co. Inc., San Diego, CA), were kindly provided by David Waugh, National Cancer Institute-Frederick. The amino acid sequence corresponding to the VBP-LZ is underlined as follows: VBP47, RDPDLEIRAAFLEKENTALRTEVAELRKEVGRCKNIVSKYETRYGPL, and VBP39: RDPDLEIRAAFLEKENTALRTEVAELRKEVGRCKNIVSK.
The H2A-H2B chimera protein was a gift from Yawen Bai, National Cancer Institute. All other proteins used in this study have been described previously, including C/EBPα (Krylov et al., 1995), C/EBPβ (Rishi et al., 2005), CREB (Ahn et al., 1998), CREB-LZ (Ahn et al., 1998), VBP (Moll et al., 2000), VBP-LZ (Moll et al., 2000), Mitf (Rishi et al., 2004), and USF (Rishi et al., 2004). The recombinant proteins were overexpressed in the Escherichia coli BL21 (LysE) strain and purified as described previously (Krylov et al., 1995; Olive et al., 1997; Rishi et al., 2010).
DNA Oligonucleotide.
The DNA oligonucleotides were polyacrylamide gel electrophoresis purified (Sigma-Aldrich, St. Louis, MO). The sense strand sequences of the 28-mer oligonucleotides for the EMSAs are as follows: CRE, GTCAGTCAGATGACGTCATATCGGTCAG; C/EBP, GTCAGTCAGATTGCGCAATATCGGTCAG; VBP: GTCAGTCAGATTACGTAATATCGGTCAG; E-box: GTCAGTCAGGCCACGTGAGATCGGTCAG.
The consensus binding sites for the B-ZIP and B-HLH-ZIP proteins are underlined. The sense strand was end-labeled with [γ-32P] ATP (specific activity 5000 Ci/mmol; MP Biomedicals, Solon, OH) using T4 polynucleotide kinase (New England Biolabs, Ipswich, MA) and was purified by the ProbeQuant G-50 microcolumn (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). The double-stranded DNA probes were generated by annealing the labeled sense strand and unlabeled antisense strand.
Cell Lines.
The suspension cells K562, a BCR/ABL-transformed cell line, was obtained from American Type Culture Collection (Manassas, VA). The clear cell sarcoma of soft tissue cell line, SU-CCS-1, bearing a transforming EWS-ATF1 translocation, was a gift from Alan Epstein, University of Southern California. Both cell lines were maintained in RPMI 1640 media supplemented with 10% fetal calf serum (HyClone Laboratories, Logan, UT) and glutamine (Invitrogen, Carlsbad, CA).
EMSA.
EMSA was carried out to test the inhibitory effects of the arylstibonic acids on DNA binding of B-ZIP and B-HLH-ZIP proteins. Protein stock solutions (5 μM dimer) were heated at 65°C for 15 min in the presence of 1 mM DTT, followed by cooling at room temperature for 5 min. Protein dimers were then added to the gel shift reaction buffer (0.5 mg/ml bovine serum albumin, 10% glycerol, 2.5 mM DTT, 12.5 mM K2HPO4-KH2PO4, pH 7.4, and 0.25 mM EDTA) in the presence or absence of the arylstibonic acids, and the mixture was incubated at 37°C for 5 min. 32P-Labeled 28-mer double-stranded oligonucleotide (7 pM) was then added, and the final volume of the reaction was adjusted to 20 μl. The reaction was incubated at 37°C for 15 min, followed by cooling at room temperature for 5 min before being loaded to a 7.5% polyacrylamide gel. The protein/DNA complexes were separated by electrophoresis at 150 V for 90 min in 1× Tris borate-EDTA buffer (25 mM Tris boric acid, 0.5 mM EDTA). The gel was dried, and the autoradiograph was generated by exposure to a Kodak MR X-ray film or by phosphorimaging (GE Healthcare).
EMSAs with the nuclear extracts were conducted similarly. In brief, 5-μg mouse liver nuclear extracts, purified as described previously (Rishi et al., 2010), were added to the gel shift reaction buffer (10 mM HEPES, pH 8.0, 6% glycerol, 80 mM KCl, 0.05 mM EDTA, 1 mM MgCl2 and 1 mM DTT) in the presence or absence of the arylstibonic acids, and the mixture was incubated at 37°C for 15 min, followed by cooling at room temperature for 5 min. 32P-Labeled DNA probes (7 pM) were added, and the reaction was incubated at 37°C for 15 min. After incubation at room temperature for 5 min, the reaction was resolved by a 6% polyacrylamide gel at 150 V for 90 min in the 1× Tris borate-EDTA buffer. The reaction also contained 1 μg of poly(dI·dC) to prevent nonspecific protein/DNA interactions.
Inhibition Potency Determination.
The half-maximal inhibition concentrations (IC50) were estimated by determining the arylstibonic acid concentrations at which equal intensities of the bands corresponding to the protein/DNA complex and the free DNA were produced on the EMSA gel.
Circular Dichroism Spectroscopy.
Circular dichroism (CD) spectroscopy was performed using a Jasco J-720 spectropolarimeter (Jasco, Tokyo, Japan). All samples were prepared in the CD buffer containing 12.5 mM K2HPO4-KH2PO4, pH 7.4, 150 mM KCl, 0.25 mM EDTA, and 1 mM DTT, with the exception that KCl concentrations were varied from 15 to 2000 mM in the salt-dependent experiments. Before CD spectroscopy measurement, the proteins were heated in the CD buffer at 65°C for 15 min, followed by cooling at room temperature for 5 min. Arylstibonic acid was then added, and the reaction was incubated at 37°C for 30 min before CD analyses. For the thermal stability studies, the samples were heated from 6 to 85°C, with a heating rate of 1°C/min. Changes in ellipticity (θ, mdeg) for the proteins were recorded at 222 nm by the spectropolarimeter. The thermal denaturation curves were fitted as previously described (Krylov et al., 1994) according to eq. 1.
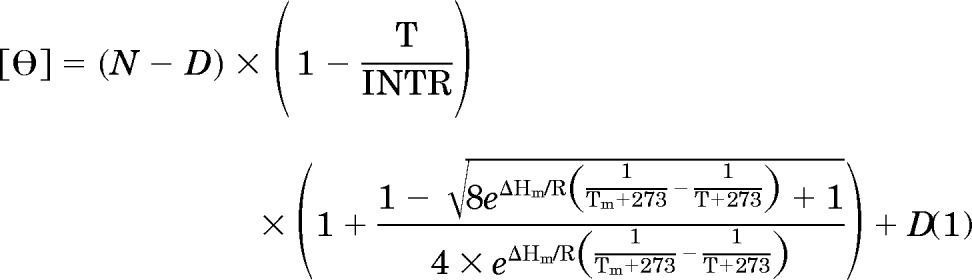 |
where T is temperature (°C); N and D are the values of θ222 at 0°C of monomer and dimer, respectively; INTR is the temperature at which linear temperature dependencies of dimer and monomer intercept; Tm is the midpoint of the thermal denaturation (°C); ΔHm is the enthalpy change (cal/mol) at Tm, and R is the universal gas constant (2.0 cal · °C−1 · mol−1).
VBP39-NSC13778 Complex Formation, Crystallization, and Structure Solution.
NSC13778 (1.25 μmol) was dissolved in 10 ml of 25 mM HEPES, pH 7.5, 50 mM KCl, and 0.25 mM EDTA. The synthetic VBP39 peptide (1 μmol) was added, and the solution was incubated in a 65°C water bath for 15 min, followed by overnight rocking at room temperature. The peptide-inhibitor complex was concentrated to a protein concentration of 10 mg/ml on the next day. Crystallization was carried out at room temperature using sitting-drop vapor diffusion. Very fine needle-like crystals of the complex were grown from crystallization buffer containing 0.2 M ammonium phosphate, 20% PEG 3350, and 3% trimethylamine N-oxide and cryoprotected in crystallization buffer supplemented with 20% ethylene glycol. X-ray diffraction data were collected at Stanford Synchrotron Radiation Lightsource (SSRL, Menlo Park, CA) beamline 7-1 and processed with the software package XDS (MPI for Medical Research, Heidelberg, Germany) (Kabsch, 2010) to 3.3-Å resolution. The structure was solved by molecular replacement using Protein Data Bank entry 1CE9 (Lu et al., 1999) as a search model and refined using PHENIX (Adams et al., 2002) with noncrystallographic symmetry restraints applied to backbone atoms. The final model was validated using MolProbity (Chen et al., 2010). Structural illustrations were generated using PyMol (DeLano Scientific, Palo Alto, CA). Data collection and refinement statistics are summarized in Supplemental Table 3.
Transfection and Confocal Microscopy.
Murine NIH-3T3 cells were maintained in the Dulbecco's modified Eagle's medium with 4.5 g/liter d-glucose and 110 mg/liter sodium pyruvate (Invitrogen), supplemented with 10% bovine calf serum and 1 mg/ml penicillin/streptomycin in a 5% CO2 incubator at 37°C. Cells (6 × 104) were plated on the Lab-Tek II chamber glass coverslips (Nalge Nunc International, Rochester, NY) and grown overnight to ∼80% confluence. NIH-3T3 cells were transiently transfected with 0.5 μg of plasmid DNA using Lipofectamine 2000 according to the manufacturer's instructions (Invitrogen). The arylstibonic acid compound or equal volume of 50 mM NH4OAc, pH 9.0, was added 5 h after the transfection, and the cells were grown overnight.
For fluorescence imaging, transfected cells were fixed with 10% neutral buffered formalin at room temperature for 10 min. The images were captured using a Zeiss LSM 510 confocal laser scanning microscope (Carl Zeiss MicroImaging Inc., Jena, Germany).
Cell Survival Assay.
K562 and CCS-1 cells were seeded at 4 × 104 per well in RPMI 1640 media in 96-well plates. Serial dilutions of arylstibonic acid compounds in the same media were added to each well to a final 100-μl volume. Cytotoxicity was measured using the Cell Titer 96 Aqueous One Solution cell proliferation assay (Promega, Madison, WI) according to the manufacturer's protocol. In brief, after 24 to 96 h of exposure, 20 μl of MTS reagent was added to each well, and the plates were incubated in a humidified 37°C incubator with 5% CO2 for 2 to 4 h until the OD600 of untreated cells was equal to 1.0. Absorbance at 490 nM was recorded using a BioTek Power Wave XS/XS2 microplate spectrophotometer (BioTek Instruments, Winooski, VT). Data were normalized against the untreated cells to generate the dose-response curves. All experiments were performed at least three times, and each sample was treated in six replicates. Statistical analysis was performed using the Student's t test.
Cell Cycle Analysis.
Cells were expanded in fresh media 48 h before the experiment and treated for 24, 48, or 72 h with 25 μM arylstibonic acids as indicated. At the end of each time point, cells were pulsed with bromodeoxyuridine for 4 h, fixed, and permeabilized as per manufacturer's instructions (BD Biosciences, Franklin Lakes, NJ). Cells were then stained for caspase 3 using anti-active caspase 3 antibody (BD Biosciences), washed, and subjected to flow cytometry using a FACSCalibur cytometer (BD Biosciences). Live-dead discrimination was performed by staining the DNA with 7-aminoactinomycin D, and cell aggregates were discarded from the analysis by doublet discrimination. The cell cycle analysis was performed on 50,000 cells and normalized against the untreated cells. Each arylstibonic acid compound was analyzed in biological replicates.
Mouse Xenograft of a Clear Cell Sarcoma of Soft Tissue Cell Line.
All experiments were conducted in an Association for Assessment and Accreditation of Laboratory Animal Care accredited facility in accordance with the Public Health Service Guidelines for the Care and Use of Animals in Research. The xenograft of the CCS-1 cell line was generated by subcutaneously injecting 1 × 106 cells into SCID/NCr mice (BALB/c background) in a 1:1 mixture of Matrigel (BD Biosciences)/ Dulbecco's modified Eagle's medium. The drug treatment began 28 days after the cell implantation, designated as time 0, when tumor mass reached between 75 and 150 mg. The arylstibonic acids were dissolved in dimethyl sulfoxide, diluted in phosphate-buffered saline, and stored in aliquots at −20°C. Treatment was performed by injecting drugs at 60 mg/kg i.p. twice a day for 4 weeks. The tumor volume was determined biweekly using caliper measurements in two perpendicular dimensions (length and width). Tumor weights were calculated using the formula for a prolate ellipsoid and assuming a specific gravity of 1.0 g/cm3. The experiment was terminated at day 37 when the larger tumors were determined to represent a significant burden to the mice.
Results
Arylstibonic Acids (25) Inhibit the DNA Binding of B-ZIP and B-HLH-ZIP Proteins.
We previously identified that NSC13778, an arylstibonic acid, binds to several B-ZIP proteins and inhibits their DNA binding (Rishi et al., 2005, 2010). To identify additional compounds with potentially more potent properties, we screened a library of 46 arylstibonic acids obtained from the Developmental Therapeutics Program, Frederick National Laboratory for Cancer Research, for the inhibition of DNA binding of five proteins: three B-ZIPs (CREB, C/EBPα, and VBP) and two B-HLH-ZIPs (USF and Mitf) (Fig. 1). The chemical structures and DNA binding inhibition activities (IC50) of these 46 compounds for each of the five proteins are summarized in Fig. 1 and Supplemental Table 1. EMSA using different concentrations of the compound (0.1, 0.3, 1.0, 3.0, and 10 μM) identified P6981 as the most potent arylstibonic acid in this library (Fig. 2). P6981 abolished the DNA binding of CREB and C/EBPα at 0.1 μM (Fig. 2A). At 1.0 μM, P6981 inhibited the DNA binding of all five proteins examined (Fig. 2C).
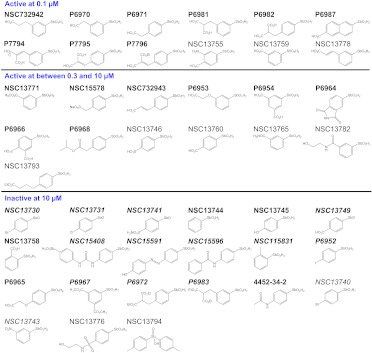
Fig. 1.
Chemical structures of 46 arylstibonic acids. A summary of inhibitory activities of arylstibonic acids determined by EMSA. The 34 new compounds that were not present in our previous study (Rishi et al., 2010) are labeled in boldface. The 21 arylstibonic acids listed in the bottom section were not soluble in the experimental conditions (italics) or did not inhibit the DNA binding of B-ZIP and B-HLH-ZIP proteins.
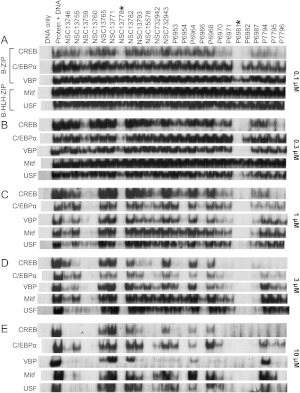
Fig. 2.
Inhibitory effects of the 25 most active arylstibonic acids on DNA binding of three B-ZIP (CREB, C/EBPα, and VBP) and two B-HLH-ZIP (Mitf and USF) proteins. EMSA showing the protein/DNA bands at five arylstibonic acid concentrations: 0.1 (A), 0.3 (B), 1 (C), 3 (D), and 10 (E) μM. Each sample included 10 nM protein dimer and 7 pM 32P-labeled 28-base pair DNA probe containing the consensus binding site of each protein, with the exception of the “DNA only” lane. The protein/DNA complexes were resolved on 7.5% native gels.
The structure-activity relationship indicates that the 12 most active compounds contain one or two carboxylic acid groups either para or meta to the stibonic acid group on the benzene ring, suggesting that the carboxylic acid group is important for inhibitory activity. Specific arylstibonic acids were found to inhibit particular B-ZIP motifs. At 0.1 μM, P6981 inhibited both CREB and C/EBPα, whereas P6982, containing two carboxylic groups para to the stibonic acid group, inhibited CREB but not C/EBPα binding to DNA (Figs. 1 and 2A). At 0.3 μM, P7796 was active against only CREB, P6982 was active against both CREB and VBP, and P6981 was active against all three B-ZIP proteins examined and one of the B-HLH-ZIP proteins, USF (Fig. 2B). At 10 μM, all 25 compounds were active against at least one of the five proteins (Fig. 2E). In general, the library was more active against the B-ZIPs than the B-HLH-ZIPs, and CREB/DNA complex was the easiest to be disrupted.
P6981 Inhibits CREB Binding to DNA with an IC50 of ∼5 nM.
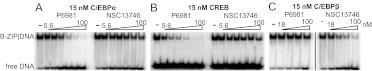
To evaluate P6981 activity at the nanomolar range, EMSAs were performed with P6981 against three B-ZIP proteins, CREB, C/EBPα, and C/EBPβ, a protein not included in Fig. 2 but inhibited by NSC13746 (Rishi et al., 2010). P6981 disrupted CREB at lower concentrations (IC50 = ∼5 nM) than C/EBPα (IC50 = ∼20 nM) and had little activity for C/EBPβ at 100 nM (Fig. 3, A and B; Supplemental Table 1). In contrast, NSC13746 was much more potent in the disruption of C/EBPβ/DNA complex (IC50 = ∼30 nM) (Fig. 3C and Supplemental Table 1). The results demonstrate that arylstibonic acids have distinct inhibition specificities.
Fig. 3.
Specificity of NSC13746 and P6981 in the inhibition of the DNA binding of unique B-ZIP domains. The dimer (15 nM) of C/EBPα (A), CREB (B), or C/EBPβ (C) B-ZIP protein was incubated with increasing concentrations of P6981 or NSC13746 (18, 32, 56, and 100 nM for C/EBPβ; 5.6, 10, 18, 32, 56, and 100 nM for CREB and C/EBPα) and assayed by EMSA. IC50 values were estimated and shown in Supplemental Table 1.
CD Spectroscopy of P6981 Binding to Both B-ZIP and Leucine Zipper Domains.
Thermal denaturation curves have been extensively used to identify ligand-protein interactions (Rishi et al., 2005; Crowther et al., 2009; Guiguemde et al., 2010). Previously, we used thermal denaturations monitored by CD spectroscopy to demonstrate that NSC13778 stabilizes B-ZIP proteins (Rishi et al., 2010). The B-ZIP domain is composed of a bipartite α-helix: the N-terminal basic region that becomes α-helical when it interacts with DNA, and the C-terminal leucine zipper that homo- or heterodimerizes to form a parallel coiled coil (Krylov et al., 1994, 1995, 1998; Moitra et al., 1997; Moll et al., 2000; Vinson et al., 2002).
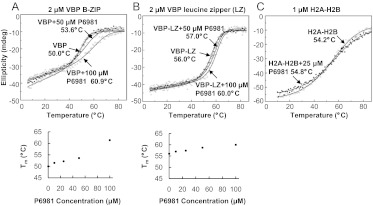
To further investigate the inhibitory mechanisms of P6981, thermal denaturations were performed using B-ZIP and leucine zipper domains (Fig. 4, A and B). Increasing concentrations of P6981 were added to 2 μM VBP dimer and thermally denatured. The increasing melting temperatures of the VBP B-ZIP domain showed that P6981 increased the stability of the VBP B-ZIP domain. The leucine zipper domain is more stable than the B-ZIP domain, which indicates that the basic region destabilizes the dimer, as observed previously (Fig. 4, A and B) (Moll et al., 2000). P6981 also increased the stability of VBP and CREB leucine zippers (Fig. 4B and Supplemental Fig. 2), suggesting that there is additional binding site(s) in the leucine zipper. P6981 did not change the thermal stability of a chimeric histone protein H2A-H2B, demonstrating that the interaction is specific between P6981 and the B-ZIP domains (Fig. 4C).
Fig. 4.
Circular dichroism at 222 nm of the thermal stabilization of the VBP B-ZIP domain dimer by P6981. A and B, top, thermal denaturations of 2 μM VBP B-ZIP domain dimer or leucine zipper-only dimer either alone (○) or with 10 μM (●), 25 μM (□), 50 μM (+), or 100 μM (▵) P6981. Bottom, Tm derived from the thermal denaturations was plotted against the P6981 concentrations. C, thermal denaturations of 1 μM chimeric histone protein H2A-H2B either alone ○ or with 25 μM P6981 ●.
To evaluate possible electrostatic interactions between arylstibonic acids and B-ZIP proteins, thermal denaturation experiments using either the VBP B-ZIP or leucine zipper domain were performed at three salt concentrations (15, 150, and 2000 mM KCl). P6981 increased the Tms of the VBP B-ZIP and leucine zipper domains only at the two lower salt concentrations (Supplemental Fig. 3, right panels, and Supplemental Table 2), indicating that there is an electrostatic component for the interactions between P6981 and the B-ZIP domain, as observed in the X-ray structure (see below).
Crystal Structure of NSC13778 Bound to the VBP Leucine Zipper.
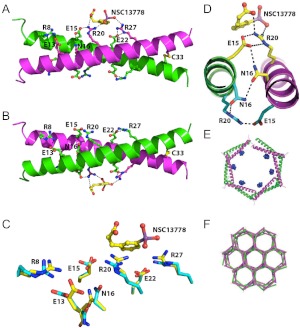
To better understand the molecular basis of the interaction between arylstibonic acids and B-ZIP proteins, we tried to crystallize both the VBP B-ZIP domain and leucine zipper bound to NSC13778, an arylstibonic acid, which was used because of compound availability. Crystals were only obtained with the leucine zipper. Although the two monomers of the leucine zipper dimer appear to be structurally equivalent, one NSC13778 molecule binds to only one of the two interfaces in the VBP dimer (Fig. 5). Both interfaces, with or without the arylstibonic acid, contain interhelical salt bridges between arginine and glutamic acid side chains as suggested previously (Krylov et al., 1994). The stibonate group is coordinated between two arginine side chains, Arg20 and Arg27, of one peptide, which form interhelical salt bridges with glutamic acid side chains, Glu15 and Glu22, respectively, of the other peptide of the dimer (Fig. 5, A and B). The conformational differences between the two interfaces are shown in Fig. 5C. The guanidinium plane of Arg20 is oriented for favorable interactions with both the stibonate and carboxylate groups of NSC13778. The asymmetry is generated from the “one up, one down” rotamer conformations of Asn16 required for their interhelical interaction, as was observed in the first coiled coil structure (O'Shea et al., 1991). The result is a polarizing effect whereby only one asparagine conformer provides the necessary side chain network of interactions for ligand binding (Asn16 to Glu15 to Arg20, yellow), whereas the same set of residues on the opposing face results in an alternate network of interactions (Asn16 to Arg20 to Glu15, cyan), which precludes binding of the arylstibonic acid (Fig. 5D). The VBP/NSC13778 complex is further stabilized by simultaneous coordination of the stibonate group by both Arg20 and Arg27 (Fig. 5A). The guanidinium plane of Arg27 is also oriented for binding (Fig. 5C), probably by an induced fit mechanism.
Fig. 5.
Crystal structure of the VBP leucine zipper (VBP39) in complex with NSC13778, color-coded by chain (chain A, magenta; chain B, green). A, the overall structure showing six salt bridges (three per peptide), Arg8/Glu13, Glu15/Arg20, Glu22/Arg27; asparagine bridge (Asn16); disulfide bridge (C33); and NSC13778 bound to one face of the coiled coil. B, same as A but rotated 180° along the horizontal axis. The asparagine-asparagine interaction is asymmetric. C, superposition of bound (yellow) and unbound (cyan) interfaces, showing the differences in side-chain conformations. D, interhelical N16 interaction creates a polarizing effect whereby only one conformer provides the necessary side-chain network of interactions for ligand binding (Asn16 to Glu15 to Arg20, yellow). The same set of residues on the opposing face results in an alternate network of interactions (Asn16 to Arg20 to Glu15, cyan), which precludes binding of the compound. E, the VBP leucine zippers arrange head to tail to form a hexagonal staircase, which defines the crystallographic six-fold screw axis of symmetry. The bound molecules of NSC13778 are shown in blue. F, the crystal lattice. The hexagonal coils of the leucine zippers interlock to form a continuous geometric structure that resembles a beehive honeycomb when viewed along the six-fold screw axis of symmetry. Each vertex is the interlocking interface of three adjacent hexagonal coils.
The VBP leucine zipper in complex with NSC13778 crystallized in space group P61, containing one leucine zipper in the asymmetric unit. It is noteworthy that six leucine zippers orient head to tail in the form a hexagonal staircase structure, thereby generating the crystallographic 6-fold screw axis of symmetry (Fig. 5E). The compound is centrally located along the long axis of the leucine zipper and is therefore farthest away from neighboring leucine zippers in the crystal lattice. The hexagonal coils of the leucine zippers interlock to form a continuous geometric structure that depicts that of a beehive honeycomb (Fig. 5F) when viewed along the 6-fold screw axis.
Inhibition of the DNA Binding of Nuclear Extracts by Arylstibonic Acids.
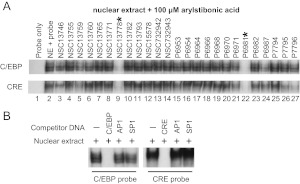
The disruption of DNA binding of recombinant B-ZIP domains by the arylstibonic acids prompted us to assess their inhibitory activities in nuclear extracts containing native proteins. EMSAs using mouse liver nuclear extracts with the same CRE and C/EBP probes used in Fig. 2 and in 25 active arylstibonic acids produced identical patterns of activity as those observed with recombinant CREB and C/EBPα B-ZIP domains (compare Figs. 2 and 6). P6981 was again identified as the most active compound. The protein/DNA complexes were disrupted with 100-fold excess of unlabeled probe, but not with 100-fold excess of DNA containing unrelated AP1 and SP1 sequences, showing that nuclear extracts binding to the C/EBP or CRE sequences were specific (Fig. 6B). These results demonstrate that P6981 can inhibit biologically relevant nuclear extracts from binding to DNA containing C/EBP and CRE sequences.
Fig. 6.
EMSAs using mouse liver nuclear extracts show that specific arylstibonic acids inhibit the binding of C/EBP or CRE-containing DNA. A, the 32P-labeled C/EBP (top) or CRE (bottom) DNA probe (lane 1) was incubated with mouse liver nuclear extracts in the absence (lane 2) or presence (lanes 3–27) of 100 μM arylstibonic acids. B, competition assay using nuclear extracts and three unlabeled DNA probes at 100-fold molar excess.
P6981 Alters the Subcellular Localization of B-ZIP and B-HLH-ZIP Proteins.
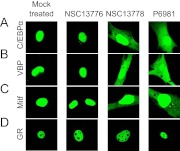
Previously, NSC13746 was shown to cause GFP-B-ZIP proteins, which reside in the nucleus under normal physiological conditions, to both move faster in the nucleus and to partially localize in the cytoplasm, demonstrating activity in cells (Heyerdahl et al., 2010). To investigate whether P6981 was also active in cells, the subcellular localization of GFP-tagged B-ZIP or B-HLH-ZIP proteins was determined by confocal microscopy. NIH-3T3 cells were transiently transfected with one of the four GFP-tagged transcription factors, including two B-ZIP proteins (C/EBPα and VBP), one B-HLH-ZIP protein (Mitf), and one hormone receptor (glucocorticoid receptor, GR) that served as a negative control. When the NIH-3T3 cells were mock-treated or treated with NSC13776, an inactive arylstibonic acid (Fig. 1), all four chimeric proteins localized to the nucleus (Fig. 7, two left panels). When the cells were incubated with 100 μM NSC13778 or P6981, cytoplasmic localization was observed for GFP-tagged C/EBPα, VBP, and Mitf (Fig. 7, two right panels), consistent with the results obtained by cotransfection of GFP-B-ZIPs and their respective dominant-negative proteins that disrupt the DNA binding of specific B-ZIP proteins (Heyerdahl et al., 2010). The subcellular localization of GFP-GR was not changed, demonstrating specificity of the arylstibonic acids. Cytoplasmic localization of GFP-tagged B-ZIP proteins in the presence of P6981 and NSC13778 suggests that these compounds inhibited the DNA binding of B-ZIP proteins in cells. Neither NSC13778 nor P6981 at 100 μM altered the morphology of the transfected cells, suggesting that there is no overt toxicity.
Fig. 7.
P6981 causes cytoplasmic localization of C/EBPα, VBP, and Mitf, but not GR. NIH-3T3 cells were transfected with GFP-tagged C/EBPα (A), VBP (B), Mitf (C), or GR (D), and were mock treated (50 mM NH4OAc, pH 9.0) or treated with 100 μM NSC13776, NSC13778, or P6981. GR served as a negative control. The confocal images are representative of three independent experiments.
P6981 Inhibits the Growth of EWS-ATF1 Driven Clear Cell Sarcoma Cells.
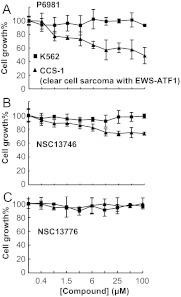
Previously, we showed that NSC13746 inhibits the growth of a cell line derived from a patient with CCS-1, which contains an oncogenic chimeric protein, EWS-ATF1 (Heyerdahl et al., 2010). Fusion of the B-ZIP domain of ATF1, a CREB family member (Vinson et al., 2002; Newman and Keating, 2003), with the N-terminal region of EWS, leads to the constitutive binding of the chimeric protein to some CRE sequences that drives tumorigenesis (Zucman et al., 1993; Fujimura et al., 1996; Schaefer et al., 2004). Polymerase chain reaction analysis confirmed that the CCS-1 cells contained the in-frame fusion of EWS and ATF1 (Supplemental Fig. 4) (Panagopoulos et al., 2002). CCS-1 and the control K562 cells were incubated with increasing concentrations of three arylstibonic acids: the most active compound (P6981), a compound with moderate activity (NSC13746), and an inactive compound (NSC13776). Cell growth was measured after 4 days (Fig. 8). P6981 was more effective than NSC13746 in suppressing CCS-1 growth (Fig. 8, A and B), consistent with the EMSA results (Fig. 3). An ∼50% decrease in the proliferation of CCS-1 by P6981 was observed at 25 to 100 μM, whereas NSC13746 decreased the proliferation of CCS-1 only by ∼25% at 100 μM. NCS13776 was inactive at all tested concentrations, suggesting that inhibition of the B-ZIP domain of the EWS-ATF1 chimera drives the observed growth inhibition effect and that it is not a nonspecific effect of the arylstibonic acids (Figs. 2 and 8). The arylstibonic acids did not decrease the growth of K562 leukemia cells, which bears the Bcr-Abl (breakpoint cluster region-Abelson murine leukemia oncogene) fusion protein, a constitutively active tyrosine kinase unrelated to B-ZIP transcription factors (Sawyers, 1999), arguing for the specificity of function.
Fig. 8.
P6981 inhibits the growth of CCS-1, a clear cell sarcoma cell line containing the EWS-ATF1 chimera. CCS-1 and the control K562 cells were incubated with P6981 (A), NSC13746 (B), or NSC13776 (C) at 1:2 serially diluted concentrations up to 100 μM. The cell growth was monitored by MTS assay at the end of 96 h. Data shown are the average of triplicates. Error bars indicate mean ± S.D.
Cell Cycle Arrest of CCS-1 by P6981.
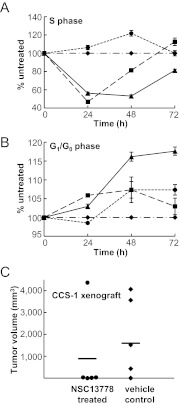
We next examined the cell cycle profile of CCS-1 cells at 24, 48, and 72 h after a single dose of 25 μM P6981, NSC13778, or NSC13776 (Fig. 9, A and B). We used the bromodeoxyuridine pulse exposure to identify cells in S phase and used 7-aminoactinomycin D to label the total DNA. Cell cycle analysis showed that P6981 produced a 50% reduction in S phase at 24 h of treatment that persisted until 48 h with a concomitant increase in G1/G0 phase (Fig. 9, A and B). In contrast, NSC13778 produced a more modest and transient S-phase suppression that lasted for only 24 h without the G1/G0 arrest. The inactive NSC13776 did not cause any changes in cell cycle. To study the effect of the arylstibonic acid compounds on apoptosis, the active caspase 3 was quantified (Supplemental Fig. 5). There were no detectable changes in the active caspase staining, in agreement with a cytostatic mode of action for these compounds. However, caspase 3 deficiency in CCS-1 cannot be ruled out, as is well documented for other human cancer cell lines, such as MCF-7 breast carcinoma cells (Jänicke et al., 1998). In conclusion, P6981 has a cytostatic effect on cell cycle progression.
Fig. 9.
P6981 induced CCS-1 growth arrest and inhibited xenografted tumor growth in vivo. A, changes in S phase when CCS-1 cells were untreated (♦) or treated with three compounds at 25 μM for 24, 48, and 72 h: P6981 (▴), NSC13778 (■) or NSC13776 (●). Each sample was analyzed in biological replicates. B, changes in G1/G0 phase after treatment described in A. C, CCS-1 cells were implanted subcutaneously into immunocompromised SCID/NCr mice, and the tumor sizes of NSC13778 (●) and vehicle control (♦)-treated mice were measured on day 37 after treatment. The bars indicate the average tumor volume.
Growth Inhibition of Xenografted CCS-1 by NSC13778.
To evaluate whether the arylstibonic acids can decrease the growth of EWS-ATF1 containing CCS-1 cells in vivo, 1 × 106 cells were injected subcutaneously into immunocompromised SCID/NCr mice to generate a xenograft model. Because of availability, NSC13778 was administered intraperitoneally twice a day at 60 mg/kg to randomly selected mice, and the control group was treated with vehicle buffer. Tumor volumes were measured on day 37 after the beginning of the treatment, and control and treated groups were compared (Fig. 9C). NSC13778 treatment did not result in loss of body weight (Supplemental Table 4), and no signs of toxicity were apparent. The tumor growth was inhibited in four of the five mice treated with NSC13778, and the average tumor volume of the control group was 80% higher than that of the treatment group. Only one of five tumors in the NSC13778-treated group grew larger, and that tumor was already the largest (∼150 mg) at the beginning of the therapy. These results suggest that the arylstibonic acid NSC13778 decreased the growth of CCS-1-derived xenograft tumors, consistent with what was observed in both cell survival and cell cycle experiments (Figs. 8 and 9).
Discussion
Using EMSA to measure inhibition of the DNA binding to three B-ZIP (CREB, C/EBPα, and VBP) and two B-HLH-ZIP (USF and Mitf) proteins, we report that 25 of the 46 arylstibonic acids screened were active at a concentration of 10 μM. P6981, the most potent compound in this arylstibonic acid library, inhibited DNA binding of CREB and C/EBPα, with IC50 values of ∼5 and 20 nM, respectively. P6981 bound to both the B-ZIP and the leucine zipper domains. P6981 does not inhibit the growth of K562 cells but does inhibit the growth of a patient derived clear cell sarcoma cell line driven by a chimeric protein containing the B-ZIP domain of ATF1. Presently, there are no compounds in clinical use that inhibit the DNA binding of B-ZIP proteins. The data presented here suggest that arylstibonic acids could potentially address this therapeutic void.
To gain insight into the inhibitory effects of arylstibonic acids on DNA binding, we analyzed the structure-activity relationship. Two carboxylic acid groups attached to the phenyl ring via an ethyl group yields the most active arylstibonic acids, P6981 and P6982. The ethyl linkage seems to be important for their inhibitory activities, given that P6954 and P6966, which have two carboxylic acid groups directly attached to the phenyl ring, are much less active. Similar effects were observed by the Stivers group when arylstibonic acids were screened for apurinic/apyrimidinic endonuclease (Ape1) inhibitors (Seiple et al., 2008), although a different set of compounds was found to be active (see below). In addition, the compounds with carboxylic acid groups on the phenyl ring meta to the stibonic acid group are more active than those with carboxylic acid groups at the para positions (compare NSC13778 and NSC732942 with NSC732943; compare P6981 with P6982; compare NSC13759 with NSC13760).
Arylstibonic acids have been recently identified in several small-molecule screens. NSC13778 was reported to have anti-human immunodeficiency virus activity, mediated by binding to CD4 on cell surfaces and thus interfering with viral gp120 binding and virus entry (Yang et al., 2005). Stivers and colleagues recently showed that arylstibonic acids inhibit type I DNA topoisomerases (human and poxvirus Topo) and Ape1 (Kim et al., 2008; Seiple et al., 2008). Different arylstibonic acids showed different activities in these screens. In the screens for Topo inhibitors, P6981 was one of the least active arylstibonic acids to inhibit poxvirus Topo in a supercoil relaxation assay. P6981 even showed a slightly stimulatory effect on human Topo (Kim et al., 2008). In a molecular beacon high-throughput assay where the abasic DNA cleavage activity of Ape1 was assessed, P6981 also failed to inhibit Ape1 activity by ≥50% at a concentration as high as 10 μM (Seiple et al., 2008). In contrast, P6954, P6970, NSC13755, and NSC13759, the most potent inhibitors of Topo or Ape1, are relatively weak inhibitors of the B-ZIP and B-HLH-ZIP proteins examined in our study (Figs. 1 and 2). These data suggest that arylstibonic acids have specific activities for distinct molecular targets.
CD thermal denaturations suggest that P6981 may have binding sites on both the basic region and leucine zipper of the VBP B-ZIP domain (Fig. 4). Moreover, Kim et al. (2008) proposed that the structural similarity between arylstibonic acid and phosphate of B-DNA could explain the multiple binding sites of an arylstibonic acid on a DNA-binding protein. This is further supported in the VBP39-NSC13778 crystal structure, showing that the stibonate group of NSC13778 is bound between two arginines in a phosphate-like coordination. Previous work has mapped the binding of NSC13778 to the region between the basic region and the leucine zipper (Rishi et al., 2005). A structure of the B-ZIP domain bound to NSC13778 is being pursued.
Both USF and CREB activate the Helicobacter pylori induced COX-2 gene expression, contributing to ulcers and gastric cancers (Jüttner et al., 2003). Furthermore, in the clear cell sarcoma cells, the constitutively active EWS-ATF1 chimera induces Mitf expression via the B-ZIP domain of ATF1, and Mitf in turn maintains proliferation of the tumor cells (Davis et al., 2006). The pleiotropic effect, or polypharmacology (Knight et al., 2010; Dar et al., 2012), of P6981 on B-ZIPs and B-HLH-ZIPs may be desirable for therapeutic intervention of cancer, given that two or more of these transcription factors often regulate the same gene expression pathways implicated in pathogenic conditions.
It is worth noting that P6981 was less potent in cells than in vitro, which could result from its possible low stability in cells and/or off-target effects that could counteract its B-ZIP inhibition activity. Nonetheless, the xenograft mouse data suggest that NSC13778, a P6981 analog, was able to decrease the growth of clear cell sarcoma in vivo without toxicity.
In summary, we have characterized the inhibitory effects of 46 arylstibonic acids and identified P6981 as a potent inhibitor that disrupted CREB and C/EBPα/DNA complexes at nanomolar concentrations. This compound also inhibited VBP, USF, and Mitf binding to their target DNA sequences. To our knowledge, this is the first report that identifies a small-molecule inhibitor of USF and Mitf, the B-HLH-ZIP proteins. More importantly, P6981 demonstrated substantially improved efficacy in suppressing the growth of a clear cell sarcoma cell line compared with the previously reported NSC13746 compound. Although still rare, a diverse group of neoplasms containing EWS-ATF1 or EWS-CREB is being detected at an increasing frequency, and therefore requires attention to development of molecularly targeted therapies (Thway and Fisher, 2012). The combination of P6981 that inhibits the DNA binding of B-ZIP transcription factors, including CREB and ATF1, with other anticancer compounds may be a promising approach to increasing treatment efficacy.
Supplementary Material
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Institutes of Health National Cancer Institute.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- B-ZIP
- basic leucine zipper
- B-HLH-ZIP
- basic helix-loop-helix leucine zipper
- LZ
- leucine zipper
- CREB
- cyclic AMP-response element-binding protein
- EMSA
- electrophoretic mobility shift assay
- C/EBP
- CCAAT/enhancer-binding protein
- VBP
- vitellogenin gene-binding protein
- CCS-1
- clear cell sarcoma cell line
- Mitf
- microphthalamia-associated transcription factor
- USF
- upstream stimulating factor
- DTT
- dithiothreitol
- CD
- circular dichroism
- GFP
- green fluorescent protein
- GR
- glucocorticoid receptor
- Ape1
- apurinic/apyrimidinic endonuclease 1
- EWS
- Ewing's sarcoma protein
- P6981
- 2-(3-stibonophenyl)malonic acid
- NSC13778
- (E)-3-(3-stibonophenyl)acrylic acid
- ATF
- activating transcription factor.
Authorship Contributions
Participated in research design: Zhao, Stagno, Rishi, Shoemaker, Ji, and Vinson.
Conducted experiments: Zhao, Stagno, Varticovski, Nimako, McKinnon, and Akee.
Performed data analysis: Zhao, Stagno, Varticovski, McKinnon, and Akee.
Wrote or contributed to the writing of the manuscript: Zhao, Stagno, Varticovski, Akee, McKinnon, Shoemaker, Ji, and Vinson.
References
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58:1948–1954 [DOI] [PubMed] [Google Scholar]
- Ahn S, Olive M, Aggarwal S, Krylov D, Ginty DD, Vinson C. (1998) A dominant-negative inhibitor of CREB reveals that it is a general mediator of stimulus-dependent transcription of c-fos. Mol Cell Biol 18:967–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, Carlezon WA., Jr (2005) Ion channels and intracellular signaling proteins as potential targets for novel therapeutics for addictive and depressive disorders. Pharmacol Ther 108:65–75 [DOI] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corre S, Galibert MD. (2005) Upstream stimulating factors: highly versatile stress-responsive transcription factors. Pigment Cell Res 18:337–348 [DOI] [PubMed] [Google Scholar]
- Crowther GJ, Napuli AJ, Thomas AP, Chung DJ, Kovzun KV, Leibly DJ, Castaneda LJ, Bhandari J, Damman CJ, Hui R, et al. (2009) Buffer optimization of thermal melt assays of Plasmodium proteins for detection of small-molecule ligands. J Biomol Screen 14:700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar AC, Das TK, Shokat KM, Cagan RL. (2012) Chemical genetic discovery of targets and anti-targets for cancer polypharmacology. Nature 486:80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IJ, Kim JJ, Ozsolak F, Widlund HR, Rozenblatt-Rosen O, Granter SR, Du J, Fletcher JA, Denny CT, Lessnick SL, et al. (2006) Oncogenic MITF dysregulation in clear cell sarcoma: defining the MiT family of human cancers. Cancer Cell 9:473–484 [DOI] [PubMed] [Google Scholar]
- Fujimura Y, Ohno T, Siddique H, Lee L, Rao VN, Reddy ES. (1996) The EWS-ATF-1 gene involved in malignant melanoma of soft parts with t(12;22) chromosome translocation, encodes a constitutive transcriptional activator. Oncogene 12:159–167 [PubMed] [Google Scholar]
- Gerdes MJ, Myakishev M, Frost NA, Rishi V, Moitra J, Acharya A, Levy MR, Park SW, Glick A, Yuspa SH, et al. (2006) Activator protein-1 activity regulates epithelial tumor cell identity. Cancer Res 66:7578–7588 [DOI] [PubMed] [Google Scholar]
- Giuliano S, Cheli Y, Ohanna M, Bonet C, Beuret L, Bille K, Loubat A, Hofman V, Hofman P, Ponzio G, et al. (2010) Microphthalmia-associated transcription factor controls the DNA damage response and a lineage-specific senescence program in melanomas. Cancer Res 70:3813–3822 [DOI] [PubMed] [Google Scholar]
- Guiguemde WA, Shelat AA, Bouck D, Duffy S, Crowther GJ, Davis PH, Smithson DC, Connelly M, Clark J, Zhu F, et al. (2010) Chemical genetics of Plasmodium falciparum. Nature 465:311–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyerdahl SL, Rozenberg J, Jamtgaard L, Rishi V, Varticovski L, Akah K, Scudiero D, Shoemaker RH, Karpova TS, Day RN, et al. (2010) The arylstibonic acid compound NSC13746 disrupts B-ZIP binding to DNA in living cells. Eur J Cell Biol 89:564–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänicke RU, Sprengart ML, Wati MR, Porter AG. (1998) Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem 273:9357–9360 [DOI] [PubMed] [Google Scholar]
- Jüttner S, Cramer T, Wessler S, Walduck A, Gao F, Schmitz F, Wunder C, Weber M, Fischer SM, Schmidt WE, et al. (2003) Helicobacter pylori stimulates host cyclooxygenase-2 gene transcription: critical importance of MEK/ERK-dependent activation of USF1/-2 and CREB transcription factors. Cell Microbiol 5:821–834 [DOI] [PubMed] [Google Scholar]
- Kabsch W. (2010) XDS. Acta Crystallogr D Biol Crystallogr 66:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Cardellina JH, 2nd, Akee R, Champoux JJ, Stivers JT. (2008) Arylstibonic acids: novel inhibitors and activators of human topoisomerase IB. Bioorg Chem 36:190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight ZA, Lin H, Shokat KM. (2010) Targeting the cancer kinome through polypharmacology. Nat Rev Cancer 10:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylov D, Barchi J, Vinson C. (1998) Inter-helical interactions in the leucine zipper coiled coil dimer: pH and salt dependence of coupling energy between charged amino acids. J Mol Biol 279:959–972 [DOI] [PubMed] [Google Scholar]
- Krylov D, Mikhailenko I, Vinson C. (1994) A thermodynamic scale for leucine zipper stability and dimerization specificity: e and g interhelical interactions. EMBO J 13:2849–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krylov D, Olive M, Vinson C. (1995) Extending dimerization interfaces: the bZIP basic region can form a coiled coil. EMBO J 14:5329–5337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M, Shu W, Ji H, Spek E, Wang L, Kallenbach NR. (1999) Helix capping in the GCN4 leucine zipper. J Mol Biol 288:743–752 [DOI] [PubMed] [Google Scholar]
- Massari ME, Murre C. (2000) Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol Cell Biol 20:429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayr B, Montminy M. (2001) Transcriptional regulation by the phosphorylation-dependent factor CREB. Nat Rev Mol Cell Biol 2:599–609 [DOI] [PubMed] [Google Scholar]
- Moitra J, Szilák L, Krylov D, Vinson C. (1997) Leucine is the most stabilizing aliphatic amino acid in the d position of a dimeric leucine zipper coiled coil. Biochemistry 36:12567–12573 [DOI] [PubMed] [Google Scholar]
- Moll JR, Olive M, Vinson C. (2000) Attractive interhelical electrostatic interactions in the proline- and acidic-rich region (PAR) leucine zipper subfamily preclude heterodimerization with other basic leucine zipper subfamilies. J Biol Chem 275:34826–34832 [DOI] [PubMed] [Google Scholar]
- Nerlov C. (2008) C/EBPs: recipients of extracellular signals through proteome modulation. Curr Opin Cell Biol 20:180–185 [DOI] [PubMed] [Google Scholar]
- Newman JR, Keating AE. (2003) Comprehensive identification of human bZIP interactions with coiled-coil arrays. Science 300:2097–2101 [DOI] [PubMed] [Google Scholar]
- O'Shea EK, Klemm JD, Kim PS, Alber T. (1991) X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science 254:539–544 [DOI] [PubMed] [Google Scholar]
- Oh WJ, Rishi V, Orosz A, Gerdes MJ, Vinson C. (2007) Inhibition of CCAAT/enhancer binding protein family DNA binding in mouse epidermis prevents and regresses papillomas. Cancer Res 67:1867–1876 [DOI] [PubMed] [Google Scholar]
- Olive M, Krylov D, Echlin DR, Gardner K, Taparowsky E, Vinson C. (1997) A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J Biol Chem 272:18586–18594 [DOI] [PubMed] [Google Scholar]
- Panagopoulos I, Mertens F, Dêbiec-Rychter M, Isaksson M, Limon J, Kardas I, Domanski HA, Sciot R, Perek D, Crnalic S, et al. (2002) Molecular genetic characterization of the EWS/ATF1 fusion gene in clear cell sarcoma of tendons and aponeuroses. Int J Cancer 99:560–567 [DOI] [PubMed] [Google Scholar]
- Rishi V, Gal J, Krylov D, Fridriksson J, Boysen MS, Mandrup S, Vinson C. (2004) SREBP-1 dimerization specificity maps to both the helix-loop-helix and leucine zipper domains: use of a dominant negative. J Biol Chem 279:11863–11874 [DOI] [PubMed] [Google Scholar]
- Rishi V, Oh WJ, Heyerdahl SL, Zhao J, Scudiero D, Shoemaker RH, Vinson C. (2010) 12 Arylstibonic acids that inhibit the DNA binding of five B-ZIP dimers. J Struct Biol 170:216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rishi V, Potter T, Laudeman J, Reinhart R, Silvers T, Selby M, Stevenson T, Krosky P, Stephen AG, Acharya A, et al. (2005) A high-throughput fluorescence-anisotropy screen that identifies small molecule inhibitors of the DNA binding of B-ZIP transcription factors. Anal Biochem 340:259–271 [DOI] [PubMed] [Google Scholar]
- Rozenberg J, Rishi V, Orosz A, Moitra J, Glick A, Vinson C. (2009) Inhibition of CREB function in mouse epidermis reduces papilloma formation. Mol Cancer Res 7:654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers CL. (1999) Chronic myeloid leukemia. N Engl J Med 340:1330–1340 [DOI] [PubMed] [Google Scholar]
- Schaefer KL, Brachwitz K, Wai DH, Braun Y, Diallo R, Korsching E, Eisenacher M, Voss R, Van Valen F, Baer C, et al. (2004) Expression profiling of t(12;22) positive clear cell sarcoma of soft tissue cell lines reveals characteristic up-regulation of potential new marker genes including ERBB3. Cancer Res 64:3395–3405 [DOI] [PubMed] [Google Scholar]
- Seiple LA, Cardellina JH, 2nd, Akee R, Stivers JT. (2008) Potent inhibition of human apurinic/apyrimidinic endonuclease 1 by arylstibonic acids. Mol Pharmacol 73:669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar DB, Cheng JC, Kinjo K, Federman N, Moore TB, Gill A, Rao NP, Landaw EM, Sakamoto KM. (2005) The role of CREB as a proto-oncogene in hematopoiesis and in acute myeloid leukemia. Cancer Cell 7:351–362 [DOI] [PubMed] [Google Scholar]
- Shukla A, Bosenberg MW, MacPherson MB, Butnor KJ, Heintz NH, Pass HI, Carbone M, Testa JR, Mossman BT. (2009) Activated cAMP response element binding protein is overexpressed in human mesotheliomas and inhibits apoptosis. Am J Pathol 175:2197–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thway K, Fisher C. (2012) Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol 36:e1–e11 [DOI] [PubMed] [Google Scholar]
- Vinson C, Myakishev M, Acharya A, Mir AA, Moll JR, Bonovich M. (2002) Classification of human B-ZIP proteins based on dimerization properties. Mol Cell Biol 22:6321–6335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinson CR, Garcia KC. (1992) Molecular model for DNA recognition by the family of basic-helix-loop-helix-zipper proteins. New Biol 4:396–403 [PubMed] [Google Scholar]
- Vinson CR, Sigler PB, McKnight SL. (1989) Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science 246:911–916 [DOI] [PubMed] [Google Scholar]
- Walton KM, Rehfuss RP, Chrivia JC, Lochner JE, Goodman RH. (1992) A dominant repressor of cyclic adenosine 3′,5′-monophosphate (cAMP)-regulated enhancer-binding protein activity inhibits the cAMP-mediated induction of the somatostatin promoter in vivo. Mol Endocrinol 6:647–655 [DOI] [PubMed] [Google Scholar]
- Widlund HR, Fisher DE. (2003) Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene 22:3035–3041 [DOI] [PubMed] [Google Scholar]
- Xiao X, Li BX, Mitton B, Ikeda A, Sakamoto KM. (2010) Targeting CREB for cancer therapy: friend or foe. Curr Cancer Drug Targets 10:384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Price JE, Luca M, Jean D, Ronai Z, Bar-Eli M. (1997) Dominant-negative CREB inhibits tumor growth and metastasis of human melanoma cells. Oncogene 15:2069–2075 [DOI] [PubMed] [Google Scholar]
- Yang QE, Stephen AG, Adelsberger JW, Roberts PE, Zhu W, Currens MJ, Feng Y, Crise BJ, Gorelick RJ, Rein AR, et al. (2005) Discovery of small-molecule human immunodeficiency virus type 1 entry inhibitors that target the gp120-binding domain of CD4. J Virol 79: 6122–6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucman J, Delattre O, Desmaze C, Epstein AL, Stenman G, Speleman F, Fletchers CD, Aurias A, Thomas G. (1993) EWS and ATF-1 gene fusion induced by t(12;22) translocation in malignant melanoma of soft parts. Nat Genet 4:341–345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.