Abstract
μ-Opioid receptor desensitization is considered an initial step in the development of tolerance. Curiously, the commonly used opioid morphine produces robust tolerance but minimal acute desensitization. This study was designed to test the hypothesis that desensitization is indeed present in morphine-treated animals and is distinguished from cellular tolerance by time course of recovery and mechanism. To induce tolerance, rats were treated with continuously released morphine for 1 week. Morphine-mediated activation of G protein-coupled inwardly rectifying potassium conductance was measured using voltage-clamp recordings from locus ceruleus neurons in brain slices from naive or morphine-treated rats. Cellular tolerance was observed as a decrease in morphine efficacy in slices from morphine-treated rats. This tolerance persisted for at least 6 h. An additional reduction in morphine-mediated current was observed when slices from morphine-treated rats were continuously maintained in morphine at approximately the circulating plasma concentration. This additional reduction recovered within 1 h after removal of morphine from the slice and represents desensitization that developed in the tolerant animal. Recovery from desensitization, but not long-lasting tolerance, was facilitated by protein phosphatase 1 (PP1) activity. Furthermore, desensitization, but not tolerance, was reversed by protein kinase C (PKC) inhibitor but not by an inhibitor of c-Jun N-terminal kinase. Therefore, morphine treatment leads to both long-lasting cellular tolerance and readily reversible desensitization, which are differentially dependent on PP1 and PKC activity and combine to result in a substantial decrease in morphine effectiveness. This PKC-mediated desensitization may contribute to the previously reported PKC-dependent reversal of behavioral tolerance.
Introduction
Morphine is one of the most commonly used opioids for treatment of acute and chronic pain. Unfortunately, long-term use of morphine results in tolerance requiring dose escalation. The mechanisms underlying opioid tolerance are not well understood, although many have been proposed (Dang and Christie, 2012). μ-Opioid receptor (MOR) desensitization is considered an initial step in the development of opioid tolerance. Many opioid agonists, such as [Met5]-enkephalin (ME), [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin (DAMGO), etorphine, methadone, and fentanyl cause robust acute desensitization (Bailey et al., 2003; Arttamangkul et al., 2008; Virk and Williams, 2008). However, morphine is relatively inefficient at producing acute desensitization in live neurons (Alvarez et al., 2002; Bailey et al., 2003, 2009b; Dang and Williams, 2005; Arttamangkul et al., 2008). Therefore, the role of desensitization in tolerance to morphine has been questioned (Finn and Whistler, 2001; Enquist et al., 2011).
The terms tolerance and desensitization can both be used accurately to describe a loss of receptor function due to prolonged agonist exposure. Multiple mechanisms probably contribute to the loss of receptor function. As a result, cellular tolerance and desensitization may be separable processes. In this study, tolerance and desensitization were examined on the cellular level in locus ceruleus (LC) neurons contained in brain slices from naive or morphine-treated rats. Cellular tolerance will be defined as a long-lasting decrease in efficacy found in slices from animals that have been treated for 1 week with morphine, which persists in the absence of morphine. Desensitization will be defined by a process that recovers over a period of approximately 1 h after the removal of morphine. Using these definitions, cellular tolerance has been observed previously in neurons from LC (Christie et al., 1987; Connor et al., 1999) and periaqueductal gray (Bagley et al., 2005) taken from morphine-treated animals. More recently, morphine desensitization was identified in LC neurons from morphine-treated animals, which was dependent on ongoing protein kinase C (PKC) activity (Bailey et al., 2009a). However, in that study cellular tolerance was not observed, leaving open the question of the role of PKC in cellular tolerance versus desensitization. Distinguishing these processes will help unravel the relative contributions of cellular tolerance and desensitization to antinociceptive tolerance, especially given the reversal of antinociceptive tolerance by PKC inhibitors that has been reported previously (Bohn et al., 2002; Hull et al., 2010).
This study tests the hypothesis that both desensitization and cellular tolerance are present in morphine-treated animals and that these processes can be distinguished by the time course of recovery and mechanism. Using whole-cell voltage-clamp recordings from LC neurons contained in rat brain slices, cellular tolerance was observed when slices from morphine-treated rats were incubated in morphine-free physiological buffer for at least 2 h to wash out any circulating morphine. This effect lasted at least 6 h and therefore represents long-lasting cellular tolerance. An additional reduction in morphine-mediated current was observed in slices from morphine-treated rats that were continuously maintained in morphine. This additional reduction represents desensitization that was induced in vivo and recovered within 1 h after removal of morphine from the slice. Recovery from desensitization, but not long-lasting tolerance, was facilitated by protein phosphatase 1 (PP1) activity. Furthermore, desensitization could be reversed by addition of PKC inhibitors but not by an inhibitor of c-Jun N-terminal kinase (JNK). Therefore, morphine treatment leads to both long-lasting cellular tolerance and desensitization, which are differentially dependent on PP1 and PKC activity and combine to result in a substantial decrease in morphine effectiveness. The rapid reversal of desensitization, but not cellular tolerance, by PKC inhibitors combined with the reversal of antinociceptive tolerance by PKC inhibitors reported previously (Bohn et al., 2002; Hull et al., 2010) suggests that desensitization contributes to behavioral tolerance.
Materials and Methods
Drugs.
Morphine sulfate, morphine alkaloid, and cocaine were obtained from the National Institute on Drug Abuse Neuroscience Center (Bethesda, MD). Naloxone, dizocilpine maleate (MK801), and anthra[1–9-cd]pyrazol-6(2H)-one (SP600125) were from Abcam (Cambridge, MA). 5-Bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine tartrate (UK14304 tartrate) and 5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile (Go6976) were from Tocris Bioscience (Ellisville, MO). Potassium methanesulfonate was from Alfa Aesar (Ward Hill, MA). Okadaic acid and calyculin A were from LC Laboratories (Woburn, MA). Staurosporine aglycone, ME, bestatin, and idazoxan were from Sigma-Aldrich (St. Louis, MO).
Morphine, naloxone, UK14304 tartrate, and idazoxan were dissolved in water, diluted in artificial cerebrospinal fluid (ACSF) and applied by bath superfusion. Phosphatase inhibitors, okadaic acid and calyculin A, and kinase inhibitors, Go6976 and staurosporine, were dissolved in dimethyl sulfoxide (final concentration <0.01%) and applied during incubation and superfusion. SP600125 was dissolved in dimethyl sulfoxide (final concentration 0.1%). Bath perfusion of ME was with bestatin (10 μM) and thiorphan (1 μM) to limit breakdown of ME.
Animal Treatment Protocols.
All animal experiments were conducted in accordance with the National Institutes of Health guidelines (Institute of Laboratory Animal Resources, 1996) and with approval from the Institutional Animal Care and Use Committee of the Oregon Health & Science University (Portland, OR). Adult (180–300 g) male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were used for all experiments. Rats were treated with morphine sulfate continuously released from osmotic pumps as described previously (Quillinan et al., 2011). Osmotic pumps (2ML1; Alzet, Cupertino, CA) were filled with the required concentration of morphine sulfate in water to deliver 50 mg · kg−1 · day−1. Each pump has a 2-ml reservoir that releases 10 μl/h for up to 7 days. Rats were anesthetized with isoflurane, and an incision was made in the midscapular region for subcutaneous implantation of osmotic pumps. Pumps remained until animals were used for experiments 6 or 7 days later.
Tissue Preparation.
Horizontal slices containing LC neurons were prepared as described previously (Williams and North, 1984). In brief, rats were killed, and the brain was removed, blocked, and mounted in a Vibratome chamber (VT 1200S; Leica, Wetzlar, Germany). Horizontal slices (230–240 μm) were prepared in ice-cold cutting solution containing 75 mM NaCl, 50 mM sucrose, 2.5 mM KCl, 6 mM MgCl2, 0.1 mM CaCl2, 1.2 mM NaH2PO4, 2.5 mM d-glucose, 25 mM NaHCO3, and 0.01 mM MK801 (equilibrated with 95% O2/5% CO2). Slices were stored at 34°C in glass vials with oxygenated (95% O2/5% CO2) ACSF containing 126 mM NaCl, 2.5 mM KCl, 1.2 mM MgCl2, 2.6 mM CaCl2, 1.2 mM NaH2PO4, 11 mM d-glucose, and 21.4 mM NaHCO3. In some experiments, morphine (1 μM) was included in the cutting solution and ACSF.
Recordings.
After an incubation period of at least 30 min, slices were hemisected and transferred to the recording chamber, which was superfused with 34°C ACSF at a rate of 1.5 to 2 ml/min. Whole-cell recordings were made from LC neurons with an Axopatch 1D amplifier in voltage-clamp mode (Vhold = −60 mV). Recording pipettes (1.7–2.1 MΩ) were filled with internal solution containing 115 mM potassium methanesulfonate or potassium methyl sulfate, 20 mM KCl, 1.5 mM MgCl2, 5 mM HEPES (potassium salt), 10 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, 2 mM Mg-ATP, and 0.2 mM Na-GTP, pH 7.4, 275 to 280 mOsM. Series resistance was monitored without compensation and remained <15 MΩ for inclusion. Data were collected at 400 Hz with PowerLab (Chart version 5.4.2; AD Instruments, Colorado Springs, CO). Most drugs were applied by bath superfusion. In some experiments, ME was applied by iontophoresis with 7 to 15 nA positive current for 2 to 3 s from thin-walled glass pipettes (50–70 MΩ resistance) filled with 10 mM ME. A backing current of −2 nA was applied to prevent passive leakage.
Data Analysis.
Analysis was performed using GraphPad Prism 4 software (GraphPad Software Inc., San Diego, CA). Summary data were presented as the morphine-induced current (I − morphine) as a percentage of the UK-mediated current, unless otherwise indicated. Values are presented as mean ± S.E.M. Statistical comparisons were made using one-way or two-way ANOVA, as appropriate, with Bonferroni post hoc tests. Comparisons with p < 0.05 were considered significant.
Results
Long-Lasting Cellular Tolerance to Morphine.
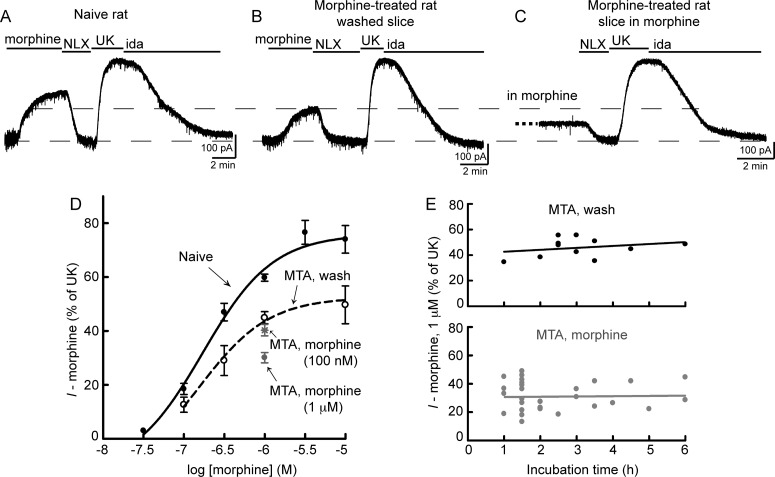
Morphine responses were assessed by whole-cell voltage-clamp recordings from LC neurons contained in acute brain slices from opioid naive or morphine-treated rats. The morphine concentration-response relationship was determined by measuring the outward current produced by various concentrations of morphine. Only one concentration of morphine was tested per slice. Because MORs and α2-adrenergic receptors activate the same G protein-coupled inwardly rectifying potassium (GIRK) channels (North and Williams, 1985), morphine current was normalized to the current induced by a saturating concentration of the α2-adrenergic agonist UK (3 μM). In neurons from naive rats, saturating concentrations of morphine caused an outward current that was 76 ± 3% of the current produced by UK. The EC50 of morphine was 171 nM (95% confidence interval 92–317 nM) (Fig. 1, A and D).
Fig. 1.
Morphine tolerance and desensitization induced in vivo. A, B, and C, examples of whole-cell voltage-clamp recordings from LC neurons in slices from opioid-naive rats (A) or slices from morphine-treated rats (MTA) that were washed for at least 2 h (B) or maintained in morphine (1 μM) (C). The outward potassium current induced by morphine (1 μM) was reversed by opioid antagonist naloxone (NLX) (1 μM) and normalized to the current produced by the α2-adrenergic receptor agonist UK (3 μM), which was reversed by the α2-adrenergic antagonist idazoxan (ida) (1 μM). Data are presented as the morphine-induced current (I − morphine) as a percentage of the UK-mediated current. D, concentration-response curves for morphine in slices from opioid-naive rats (Naive) or washed slices from morphine-treated rats (MTA, wash) reveals long-lasting tolerance (two-way ANOVA: treatment F1, 66 = 26.7; p < 0.0001; n = 3–15). In addition, the current produced by morphine (1 μM) was significantly desensitized in slices from morphine-treated rats that were maintained in morphine (1 μM) [MTA, morphine (1 μM)] (p < 0.001 versus MTA, wash by one-way ANOVA and Bonferroni post-test; n = 32). In contrast, the morphine (1 μM) current was not desensitized in slices from morphine-treated rats that were incubated in morphine (100 nM) [MTA, morphine (100 nM)], but instead was similar to the current in washed slices (p > 0.05 versus MTA, wash by one-way ANOVA and Bonferroni post-test; n = 14). E, effect of wash or morphine (1 μM) incubation time. Each data point represents a single experiment. Top, slices from morphine-treated rats were washed for 1 to 6 h before the current produced by morphine (1 μM) was recorded. The slope of linear regression is not different from 0, indicating no effect of long wash times (slope = 1.5 ± 1.8% of UK/h; p = 0.41). Bottom, slices from morphine-treated rats were incubated in morphine (1 μM) for several hours. Recordings were initiated in morphine (1 μM) and reversed with naloxone (1 μM) to reveal the morphine-mediated current. The slope of linear regression is not different from 0, suggesting no effect of longer morphine (1 μM) incubation times (slope = 0.8 ± 1.4% of UK/h; p = 0.58).
To induce tolerance, rats were implanted with osmotic pumps that continuously released morphine (50 mg · kg−1 · day−1). After 6 or 7 days, slices were prepared from treated animals and incubated in morphine-free ACSF for at least 2 h to remove any residual morphine. In washed slices from morphine-treated animals (MTA, wash), morphine efficacy was reduced with a decrease in the maximum morphine-mediated current to 53 ± 5% of UK (p < 0.01 compared with naive by two-way ANOVA and Bonferroni post-test) (Fig. 1, B and D). This decrease in efficacy was not accompanied by a shift in the EC50 (154 nM; 95% confidence interval 22–1091 nM), which indicates a lack of spare receptors and is consistent with previous findings (Christie et al., 1987). Slices were washed for at least 2 h but could be washed for up to 6 h without affecting the morphine (1 μM)-mediated current, as indicated by the shallow slope of linear regression when the current data were plotted versus wash time (slope of linear regression = 1.5 ± 1.8% of UK/h, p = 0.41) (Fig. 1E, top). Therefore, the decrease in morphine effectiveness in slices from morphine-treated animals represents long-lasting tolerance, which persists for at least 6 h after removal of morphine. This long-lasting cellular tolerance was not observed by Bailey et al. (2009a) probably because of a shorter morphine treatment time (3 days versus 6–7 days).
Morphine Desensitization Also Develops In Vivo.
Previous studies recording from LC neurons in slices from morphine-treated rats have identified a reversible decrease in morphine efficacy that represents desensitization (Bailey et al., 2009a). The next experiments were designed to test the hypothesis that during long-term morphine treatment both desensitization and cellular tolerance are induced. During treatment of rats with morphine (50 mg · kg−1 · day−1) released from osmotic pumps, neurons are continuously exposed to morphine with a circulating concentration of approximately 1 μM (Quillinan et al., 2011). To maintain the morphine equilibrium, slices from morphine-treated rats were prepared and incubated in ACSF containing morphine (1 μM). Recordings were initiated in the presence of morphine and the morphine-mediated current was revealed upon application of the opioid antagonist naloxone (1 μM) (Fig. 1C). This morphine-mediated current was much smaller than the current produced by the same concentration of morphine (1 μM) in washed slices from morphine-treated rats or in slices from naive rats (MTA, morphine = 30.1 ± 1.9% of UK; MTA, wash = 45.0 ± 2.2% of UK; naive = 61.6 ± 1.5% of UK) (summarized in Fig. 1D). The morphine desensitization remained consistent regardless of the amount of time the slices were incubated in morphine (1 μM) (slope of linear regression = 0.8 ± 1.4% of UK/h, p = 0.58) (Fig. 1E, bottom), indicating that this concentration of morphine effectively mimics the in vivo situation and does not lead to further desensitization. Therefore, the continuous presence of morphine in vivo induces both desensitization and long-lasting tolerance, which combine to result in a significant reduction of morphine effectiveness.
To determine the concentration dependence of the desensitization that developed in vivo, slices from morphine-treated rats were incubated in morphine (100 nM) for at least 1.5 h. This concentration of morphine activates only a small percentage of MORs in slices from naive or morphine-treated animals (Fig. 1D). Recordings were initiated in the presence of morphine (100 nM). Perfusion of morphine (1 μM) resulted in an additional increase in the outward current, and naloxone reversed the morphine (1 μM)-mediated current to baseline. The current induced by morphine (1 μM) in these slices was similar to the current in washed slices (Fig. 1D). Therefore, morphine (100 nM) was not sufficient to prevent recovery from desensitization. Furthermore, there was no time dependence to morphine (100 nM) incubation from 1.5 to 5 h (slope of linear regression = 0.5 ± 1.9% of UK/h, p = 0.78), indicating that desensitization recovered to its full extent within 1.5 h.
Morphine Desensitization Was Homologous.
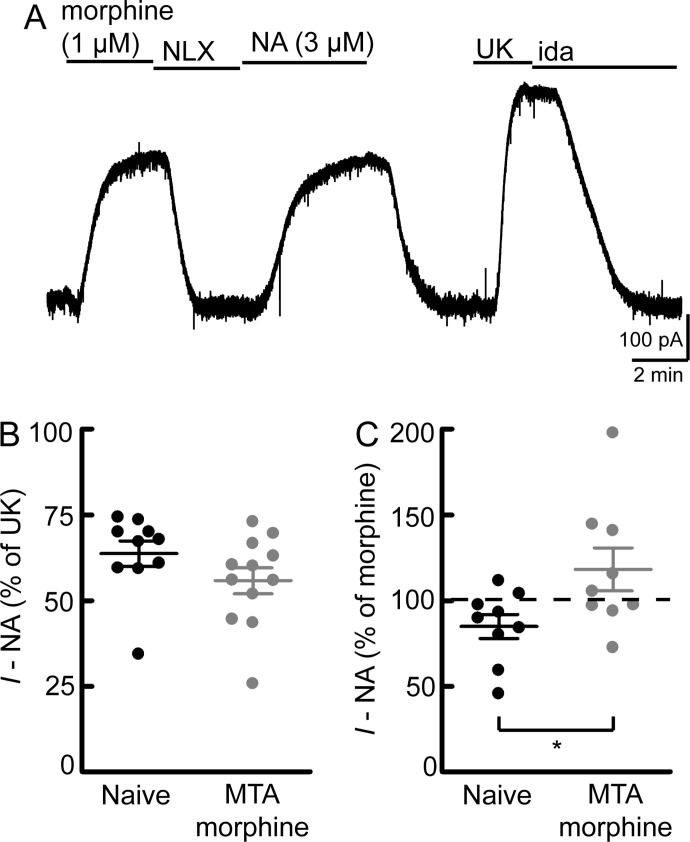
The decrease in morphine effectiveness could be due to effects on MORs (homologous) or downstream signaling components that are shared by multiple receptors (heterologous). Heterologous desensitization of α2-adrenergic receptor-mediated currents in LC neurons was recently shown to develop after prolonged (>10 min) application of ME (Dang et al., 2012). Therefore, currents mediated by α2-adrenergic receptors were used to examine the presence of heterologous desensitization in slices from morphine-treated rats. The current induced by a saturating concentration of the α2-adrenergic agonist UK (3 μM) was similar in slices from naive rats and slices from morphine-treated rats that were left in morphine or washed (naive = 247 ± 15 pA; MTA, wash = 279 ± 19 pA; MTA, morphine = 234 ± 12 pA; p > 0.05 for all comparisons by one-way ANOVA and Bonferroni posttest; n = 26–64). Because the UK-mediated current was unchanged, it is a reliable method for normalization of other currents for comparisons between cells. However, at saturating concentrations, it is not a sensitive measure of heterologous desensitization. To test for heterologous desensitization, a subsaturating concentration of noradrenaline (NA) (3 μM) was used. Noradrenaline was perfused with cocaine (3 μM) to prevent uptake and prazosin (100 nM) to block α1-adrenergic receptors. The current induced by morphine (1 μM), noradrenaline (3 μM), and UK (3 μM) was measured in slices from naive rats or in slices from morphine-treated rats that were maintained in morphine (Fig. 2). In slices from naive animals, noradrenaline (3 μM) produced a current that was 64 ± 4% of the UK-mediated current. This was not different from the noradrenaline-mediated current in slices from morphine-treated animals (56 ± 4% of UK; p > 0.05 by unpaired t test) (Fig. 2B), indicating a lack of heterologous desensitization between these receptors.
Fig. 2.
Lack of heterologous desensitization. The current mediated by a subsaturating concentration of noradrenaline (3 μM) was measured in slices from naive rats or slices from morphine-treated rats that were maintained in morphine (1 μM; MTA morphine). A, example of a whole-cell voltage-clamp recording in a slice from a naive rat. Holding current changes were monitored after bath perfusion of drugs in the following order: morphine (1 μM), naloxone (NLX) (1 μM), noradrenaline (3 μM), a saturating concentration of UK (3 μM), and idazoxan (ida) (1 μM). Noradrenaline perfusion included cocaine (3 μM) and the α1-adrenergic antagonist prazosin (100 nM). The NA-mediated current was compared with the current induced by UK (B) or morphine (C). Each data point represents an individual experiment. Line and error bars represent mean ± S.E.M. B, compared with UK, the current produced by NA was not different in slices from naive or morphine-treated rats, indicating a lack of heterologous desensitization (p = 0.16, unpaired t test). C, compared with morphine, the NA-mediated current was larger in slices from morphine-treated rats (p = 0.03, unpaired t test), which is due to reduction of the morphine-mediated current.
In slices from naive animals, the noradrenaline-mediated current was 85 ± 7% of the morphine-mediated current (Fig. 2C). If desensitization was mostly heterologous, the morphine- and noradrenaline-mediated currents would be expected to decline similarly, and the ratio between the two should remain constant. Instead, the ratio was increased in slices from morphine-treated animals, and noradrenaline produced a current that was 118 ± 13% of the morphine-mediated current (p = 0.03 by unpaired t test). Therefore, the desensitization induced by long-term morphine treatment was largely homologous.
Recovery from Morphine Desensitization.
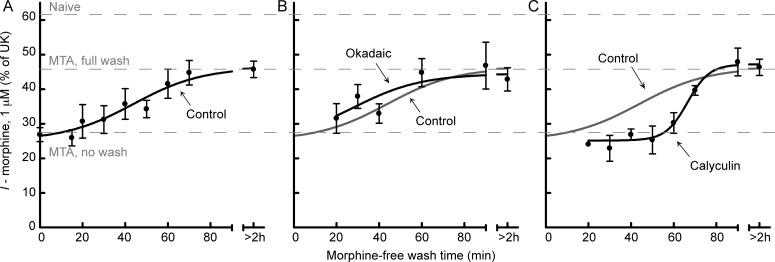
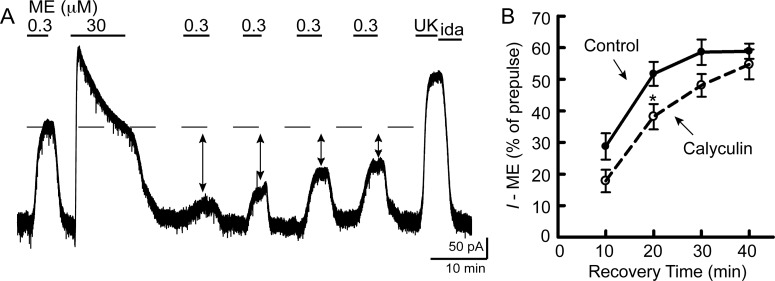
Recovery from ME-induced desensitization occurs in less than 1 h (Quillinan et al., 2011). The time course of recovery from morphine desensitization was determined by washing slices from morphine-treated rats in morphine-free ACSF for various times (15–90 min) before initiation of recordings. Subsequent perfusion of morphine (1 μM) produced an outward current that was normalized to the current produced by UK (3 μM). The effect of morphine began to recover after 20 min and completely recovered by 70 min (Fig. 3A). The half-time of recovery was 44 ± 7 min.
Fig. 3.
Recovery from morphine desensitization is facilitated by PP1. Slices from morphine-treated rats were maintained in morphine (1 μM) and then were washed in morphine-free ACSF without (A) or with the phosphatase inhibitors okadaic acid (100 nM) (B) or calyculin A (30 nM) (C) for various times (15–90 min) to monitor recovery from desensitization. Recordings were initiated in the absence of morphine. Morphine (1 μM) perfusion caused an outward current (I − morphine) that was normalized to UK (3 μM)-mediated current. In slices washed without phosphatase inhibitors (control; A), morphine (1 μM)-mediated current recovered from desensitization with a half-time of 44 ± 7 min (n = 3–6/time point). When okadaic acid (B) was included in the morphine-free wash there was no effect on recovery (two-way ANOVA: F1, 54 = 0.94, p = 0.34; n = 3–8/time point). In contrast, calyculin A (C) significantly delayed recovery from desensitization (two-way ANOVA: F1, 39 = 5.8, p = 0.02; n = 3–6/time point).
PP1 Inhibition Slows Recovery from Morphine Desensitization.
Dephosphorylation has been proposed to allow resensitization of GPCRs, including MORs after ME-induced desensitization (Osborne and Williams, 1995; Dang et al., 2011). However, phosphorylation of MORs follows a different pattern depending on the agonist used with morphine producing weaker phosphorylation in general (Doll et al., 2011; Lau et al., 2011). Two Ser/Thr phosphatase inhibitors were used to determine the role of dephosphorylation in recovery from morphine desensitization. Each phosphatase inhibitor was included in morphine-free ACSF wash for 20 to 90 min. The phosphatase inhibitor okadaic acid (100 nM) did not change the time course of recovery (two-way ANOVA: F1, 54 = 0.94, p = 0.34) (Fig. 3B). In contrast, the phosphatase inhibitor calyculin A (30 nM) significantly delayed the time course of recovery (two-way ANOVA: F1, 39 = 5.8, p = 0.02) (Fig. 3C). In slices that were washed with calyculin A, desensitization did not begin to recover until 60 min of wash (compared with 20 min in the control), and the half-time of recovery was 66 ± 3 min. Okadaic acid and calyculin A both inhibit protein phosphatase 2A. However, calyculin A is a more potent inhibitor of PP1, which has recently been shown to be required for dephosphorylation of MORs (Doll et al., 2012).
Although calyculin A delayed the onset of recovery, desensitization was still able to recover completely by 90 min. The steepness of the recovery curve was not due to breakdown of calyculin A. Slices that were washed in calyculin A that was refreshed every 40 min still recovered completely by 90 min (56% of UK; n = 2). In okadaic acid and calyculin A, the morphine-mediated current never reached the level of that of a slice from a naive animal. Thus, dephosphorylation cannot account for the cellular tolerance observed in slices from morphine-treated animals that were washed in morphine-free ACSF.
At the concentrations used in these experiments, neither okadaic acid nor calyculin A preincubation alone changed morphine- or UK-mediated currents in slices from naive rats. The current produced by morphine (300 nM) was similar between control slices and slices incubated in okadaic acid (100 nM) for 1 to 4 h (control = 89 ± 20 pA, okadaic acid = 90 ± 9 pA; n = 8; p = 0.94 by unpaired t test). The current produced by morphine (1 μM) was also similar between control slices and slices incubated in calyculin A (30 nM) for 0.5 to 2 h (control = 118 ± 11 pA, calyculin A = 138 ± 19 pA; n = 8–18; p = 0.35 by unpaired t test). The UK (3 μM)-mediated current was unchanged by preincubation with okadaic acid or calyculin A (control = 218 ± 9 pA, okadaic acid = 221 ± 13 pA, calyculin A = 224 ± 15 pA; n = 37–79; p > 0.05 for all comparisons by one-way ANOVA and Bonferroni posttest). The lack of effect on current amplitude suggests that there are no changes in basal MORs, α2-adrenergic receptors, or GIRK function due to the phosphatase inhibitors at these concentrations. At higher concentrations, both okadaic acid (1 μM) and calyculin A (100 nM) significantly reduced the current induced by UK (3 μM) [control = 218 ± 9 pA (n = 79), okadaic acid = 175 ± 11 pA (n = 28; p < 0.05), calyculin A = 61 ± 10 pA (n = 3; p < 0.05)]. Therefore, these higher concentrations were not used in any other experiments.
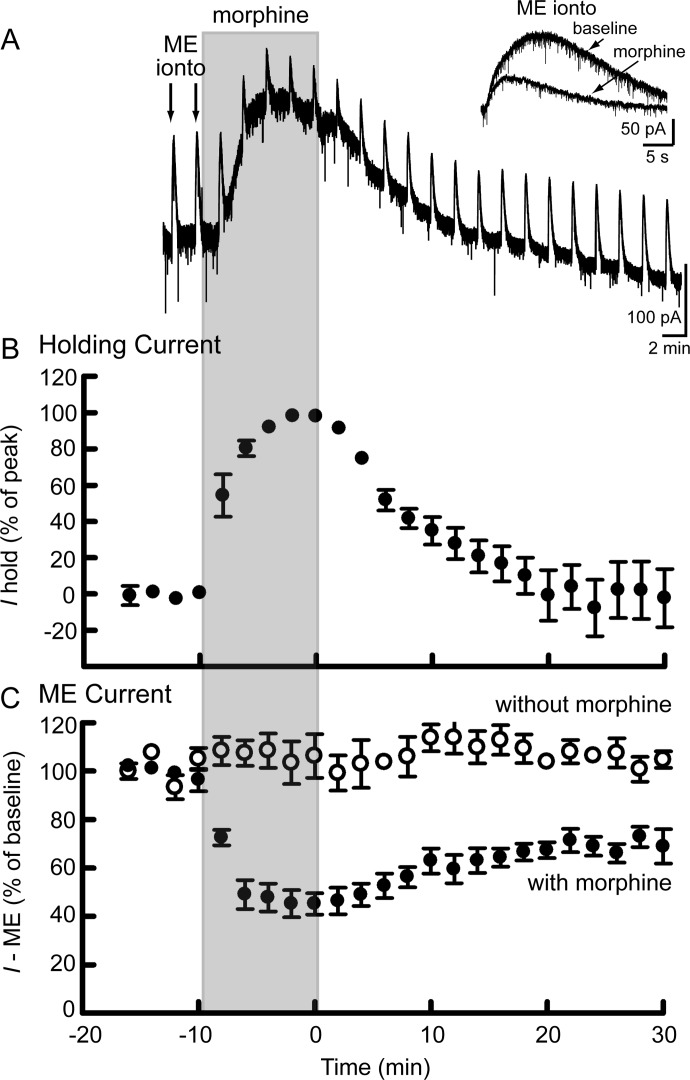
Morphine Washes Out of Slices in 20 Min.
Morphine does not quickly wash out of acute slice preparations, which could contribute to the time course of recovery from desensitization. The washout of morphine was tested in slices from naive rats by combining bath perfusion of morphine (1 μM) with iontophoresis of ME (Fig. 4). Bath perfusion of morphine increased the holding current (Fig. 4, A and B) and simultaneously decreased the current induced by iontophoresis of ME (Fig. 4, A and C) because of competition for MORs. Morphine reduced both the amplitude and the duration of the ME-induced current (Fig. 4A, inset). When perfusion was switched to a morphine-free solution, the holding current gradually returned to baseline. By 20 min, the current induced by iontophoresis of ME had also recovered but to a new baseline, which may indicate some desensitization, because the ME-induced current in the absence of morphine did not decline (Fig. 4C). Perfusion of the opioid antagonist naloxone (1 μM) at the end of these experiments abolished the ME-induced current to 3 ± 1% of baseline (n = 7) but did not change the holding current, suggesting that there was no residual morphine in the slice.
Fig. 4.
Morphine washed out of slices in 20 min. A, recording in a slice from a naive rat. Bath perfusion of morphine (1 μM) (shaded region) caused an outward change in the holding current (Ihold) and simultaneously reduced the current induced by iontophoretic application of ME (ME ionto, inset). B, summary of the holding current (Ihold) at 2-min intervals (measured just before ME iontophoresis) during the wash in and out of morphine (1 μM), normalized to the maximum increase in holding current produced by morphine (1 μM) (n = 9). C, summary of the amplitude of the ME current (as a percentage of the average amplitude of the first three ME currents) with (●; n = 9) or without (○; n = 4) perfusion of morphine (1 μM).
These data indicate that morphine (1 μM) washes out of slices in 20 min. In comparison, at 20 min the recovery from morphine desensitization described previously (Fig. 3) had just begun. Taken together, these results suggest that the time course of recovery from morphine desensitization measured in slices occurs in a stepwise process. First, the concentration of morphine in the slice is reduced during washout to a level that is insufficient to maintain desensitization (<100 nM). Then, MOR is resensitized in a PP1-facilitated manner.
Recovery from Acute ME-Induced Desensitization.
Morphine- and ME-induced desensitization may occur through different kinase-dependent mechanisms in LC neurons (Bailey et al., 2009b). The effect of calyculin A on recovery from ME-induced desensitization was tested in slices from naive rats using a protocol described previously (Quillinan et al., 2011). In brief, an EC50 concentration of ME (300 nM) was tested just before prolonged (10 min) application of a saturating concentration of ME (30 μM). The EC50 concentration of ME was then perfused every 10 min to monitor recovery from desensitization (Fig. 5A). Control experiments were compared with experiments in which slices were incubated in calyculin A (30 nM) for 30 to 60 min before the recording. Calyculin A was also included in wash and ME (30 μM) perfusions. Calyculin A did not change the decline in peak current observed during a 10-min application of saturating ME (control = 40 ± 2% decline, calyculin A = 38 ± 3% decline; n = 10; p = 0.73 by unpaired t test). However, calyculin A did slow the recovery from desensitization (two-way ANOVA: F2, 46 = 7.08, p = 0.002) (Fig. 5B). Although this effect was qualitatively smaller than the effect of calyculin A on recovery from morphine desensitization, it indicates that recoveries from morphine- and ME-induced desensitization share mechanistic components.
Fig. 5.
Recovery from acute ME desensitization is facilitated by PP1. Slices from naive rats were used for acute ME-induced desensitization experiments. A, example of a recording in an untreated slice from a naive rat. An EC50 concentration of ME (0.3 μM) was perfused initially. Desensitization was induced using a saturating concentration of ME (30 μM) for 10 min. ME (0.3 μM) was perfused every 10 min to monitor recovery from desensitization. The amplitude of the ME (0.3 μM) test pulse was compared with that of the initial ME (0.3 μM) prepulse and plotted as a percentage of prepulse in the summary graph (B). Treatment with of slices with calyculin A (30 nM) significantly delayed recovery from acute ME desensitization (two-way ANOVA: F2, 46 = 7.08, p = 0.002; n = 8–9). *, p < 0.05 by Bonferroni post-test.
PKC, but Not JNK, Inhibitors Reverse Desensitization.
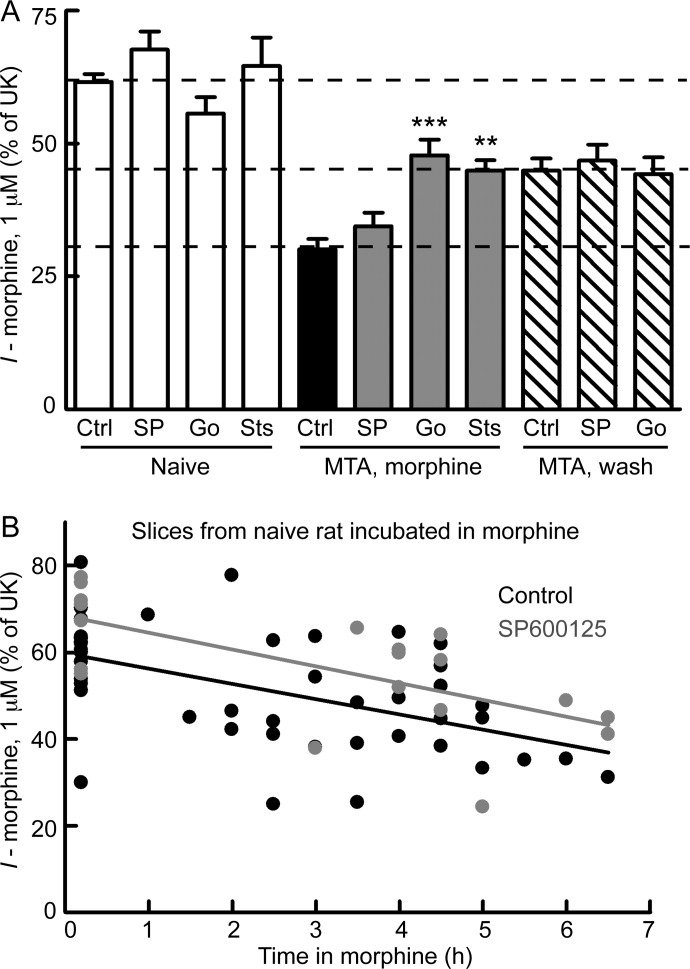
Because desensitization and cellular tolerance can be separated by time course of recovery, differences in mechanism can be further examined. Morphine desensitization in LC neurons has been shown to be dependent on ongoing PKC activity (Bailey et al., 2009a). However, it is not clear whether long-lasting cellular tolerance has a similar requirement. Because both cellular tolerance and desensitization were observed in this study, the role of PKC in these two processes could be assessed. Slices from morphine-treated rats were maintained in morphine, and a PKC inhibitor, Go6976 (1 μM) or staurosporine (1 μM), was added with morphine for 30 to 90 min. Recordings were initiated in the presence of morphine and the PKC inhibitor, and the morphine-mediated current was revealed upon application of naloxone. When slices were incubated with either Go6976 or staurosporine, morphine desensitization was no longer observed (MTA, morphine + Go6976 or staurosporine) (Fig. 6A), consistent with previous findings (Bailey et al., 2009a). The morphine-mediated current after either PKC inhibitor was similar to that in washed slices from morphine-treated animals (p > 0.05 versus MTA, wash), but still smaller than that in slices from naive rats (Go6976, p < 0.05 versus naive/control; staurosporine, p < 0.01 versus naive/control). These results suggest that the PKC inhibitors did not reverse long-lasting cellular tolerance. To directly test the role of PKC in cellular tolerance, Go6976 (1 μM) was added to washed slices from morphine-treated rats. Go6976 was either added during the entire wash (2–4.5 h) or was added for 1 to 2 h after an initial wash (>2 h). Similar results were obtained with both protocols, so the data were pooled. Go6976 did not change the degree of long-lasting cellular tolerance observed in washed slices from morphine-treated rats (p > 0.05 versus MTA, wash/control) (Fig. 6) and the morphine-mediated current remained smaller than that in slices from naive rats (p < 0.01 versus naive/control). This lack of effect of Go6976 on long-lasting tolerance persisted even after prolonged incubation times of up to 4.5 h. Therefore, PKC inhibitors were able to reverse morphine desensitization but not long-lasting cellular tolerance.
Fig. 6.
PKC (not JNK) inhibitors reverse morphine desensitization, but not tolerance. A, slices from naive rats (open bars) were incubated without [control (Ctrl)] or with inhibitors of JNK [SP600125 (SP, 10 μM)] or PKC [Go6976 (Go; 1 μM) or staurosporine (Sts; 1 μM)] for 30 to 90 min. Inhibitors had no effect on morphine-mediated current in slices from naive rats (p > 0.05 versus Naive/Ctrl; n = 7–16). Slices from morphine-treated rats were maintained in morphine (1 μM) (MTA, morphine; filled bars), and kinase inhibitor (same as above) was added with morphine for 30 to 90 min before morphine current was recorded. The JNK inhibitor SP600125 did not change morphine desensitization (p > 0.05 versus MTA, morphine/Ctrl; n = 15). The PKC inhibitors Go6976 and staurosporine reversed desensitization to a level similar to that for tolerant slices (p > 0.05 versus MTA, wash/Ctrl; n = 11). Some slices from morphine-treated rats were washed (MTA, wash; striped bars) and the PKC inhibitor Go6976 or the JNK inhibitor SP600125 was added to the wash for 1 to 4.5 h. Neither inhibitor changed long-lasting cellular tolerance (p > 0.05 versus MTA, wash/Ctrl; n = 8). All statistical comparisons were by one-way ANOVA and Bonferroni post-test. **, p < 0.01; ***, p < 0.001 compared with MTA, morphine/Ctrl. B, slices from naive rats were incubated in morphine (1 μM) for several hours with or without the JNK inhibitor SP600125. The negative slope of linear regression indicates gradual development of desensitization in control slices (slope = −3.5 ± 0.8% of UK/h; significantly nonzero p < 0.0001) and in slices with SP600125 added (slope = −3.8 ± 1.1% of UK/h; significantly nonzero p = 0.002). Thus, there was no effect of JNK inhibitor on the development of desensitization in slices from naive rats (two-way ANOVA: F1, 41 = 2.1, p = 0.15). Each data point represents an individual experiment.
Activity of JNK has been implicated in morphine tolerance (Melief et al., 2010). The role of JNK in morphine desensitization and cellular tolerance in LC neurons was examined using a protocol similar to that for PKC inhibitor experiments. The JNK inhibitor SP600125 (10 μM) was added for 30 to 120 min with morphine to slices from morphine-treated animals. In contrast to PKC inhibitors, incubation with the JNK inhibitor was unable to reverse morphine desensitization (p > 0.05 versus MTA, morphine) (Fig. 6A). The JNK inhibitor was also unable to reverse long-lasting cellular tolerance when added during the entire morphine-free wash (2–3.5 h) of slices from morphine-treated rats (p < 0.01 versus naive/control).
To address the possibility that JNK is involved in the development rather than the maintenance of desensitization, slices from naive animals were incubated in morphine (1 μM) with or without the JNK inhibitor SP600125 for several hours. Morphine (1 μM) incubation did result in some desensitization in control slices as indicated by the negative slope of linear regression (slope = −3.5 ± 0.8% of UK/h, significantly nonzero p < 0.0001) (Fig. 6B). The desensitization developed slowly and did not reach significance until more than 5 h of incubation with morphine (p < 0.01 at 5 h compared with no morphine incubation, one-way ANOVA and Bonferroni post-test). After 5 to 6 h in morphine, the morphine-mediated current was 37.8 ± 2.7% of the current induced by UK. This was desensitized compared with that of slices incubated in morphine-free ACSF for 4 to 6 h (52.1 ± 5.7% of UK; n = 6; p < 0.05, unpaired t test). Slices incubated in morphine and the JNK inhibitor SP600125 desensitized with a slope of linear regression similar to that of control slices (slope = −3.8 ± 1.1% of UK/h; significantly nonzero p = 0.002). Thus, there was no effect of JNK inhibitor on the development of desensitization in slices from naive rats (two-way ANOVA: F1,41 = 2.1, p = 0.15).
None of the kinase inhibitors altered the morphine-mediated current in slices from naive animals (p > 0.05 compared with naive in the absence of inhibitor) (Fig. 6A). The amplitude of the UK-mediated current was also unchanged by the kinase inhibitors (control = 250 ± 15 pA; Go6976 = 215 ± 11 pA; staurosporine = 257 ± 23 pA; SP600125 = 227 ± 21 pA; p > 0.05 for all inhibitors compared with controls by one-way ANOVA and Bonferroni post-test). Taken together, these results indicate that ongoing PKC, but not JNK, activity is essential for morphine desensitization. Furthermore, cellular tolerance cannot be readily reversed by either PKC or JNK inhibitors.
Discussion
Although morphine causes significant behavioral tolerance, it has been thought to cause comparatively little desensitization, raising doubts about the link between desensitization and tolerance. This study distinguished desensitization and cellular tolerance as separate processes; this separation is necessary to determine the relative role of these processes in behavioral tolerance and identify the mechanisms regulating both. Long-term treatment of rats with continuously released morphine caused desensitization, consistent with previous findings (Bailey et al., 2009a). In addition, morphine-treated animals developed long-lasting cellular tolerance, which did not recover even 6 h after removal of morphine. Desensitization required the continuous presence of morphine and ongoing PKC activity and recovered in a PP1-facilitated manner. Unlike desensitization, long-lasting cellular tolerance was not affected by phosphatase or kinase inhibition. Therefore, long-term morphine treatment causes both long-lasting cellular tolerance and desensitization that combine to result in a significant decrease in morphine effectiveness. Previously, antinociceptive tolerance has been shown to be reversed by PKC inhibitors (Hull et al., 2010). Given that PKC inhibition would only reverse desensitization in this amount of time (not cellular tolerance), the PKC inhibitor-mediated reversal of behavioral tolerance can be attributed to reversal of desensitization. Thus, desensitization does contribute to behavioral tolerance.
Morphine Desensitization and Efficacy.
Morphine causes very little acute desensitization in neurons when applied for relatively short times (Alvarez et al., 2002; Bailey et al., 2003; Dang and Williams, 2005; Arttamangkul et al., 2008). However, in this study, morphine induced desensitization during continuous exposure either for several hours in slices from naive rats or for several days in morphine-treated rats. This is consistent with findings that a saturating concentration of morphine produces a degree of functional receptor loss (80%) similar to that of DAMGO after 8 h (Bailey et al., 2009a). DAMGO or ME can induce this high level of receptor loss (90%) in just 5 to 10 min (Osborne and Williams, 1995; Bailey et al., 2009a). Lower concentrations of DAMGO or ME take longer to reach a similar degree of receptor loss and produce little to no desensitization in 5 to 10 min (Fiorillo and Williams, 1996; Virk and Williams, 2008; Bailey et al., 2009a). Therefore, differences in desensitization induced by morphine and highly efficacious agonists, such as ME or DAMGO, may be rate-dependent because of differences in efficacy. Indeed, relative agonist efficacy and degree of desensitization are often positively correlated, although some exceptions exist (Borgland et al., 2003; Virk and Williams, 2008).
Phosphorylation and Recovery from Desensitization.
Given the vast difference in the time required to induce desensitization by morphine and ME (hours or days versus minutes), the recovery from desensitization for these two agonists was surprisingly similar. Both morphine- and ME-induced desensitization recovered in less than 1 h after the removal of agonist. Dephosphorylation is thought to allow resensitization of GPCRs, including MORs (Osborne and Williams, 1995; Dang et al., 2011). Dephosphorylation of MORs (at Ser375) expressed in human embryonic kidney 293 cells occurs slightly faster than the recovery from desensitization presented here but is similar for both morphine- and DAMGO-induced phosphorylation (Doll et al., 2011). In the present study, the Ser/Thr phosphatase inhibitor calyculin A, but not okadaic acid, delayed recovery from morphine-induced desensitization. Calyculin A, but not okadaic acid, has been shown to prevent dephosphorylation of MORs, which was due to activity of PP1 (Doll et al., 2012). Recovery from acute ME-induced desensitization was also slowed by calyculin A. In a previous study, recovery from ME-induced desensitization was not changed by okadaic acid but was delayed by microcystin (Osborne and Williams, 1995). Similar to calyculin A, microcystin inhibits both PP1 and protein phosphatase 2A equally (MacKintosh et al., 1990). Thus, morphine and ME both induced desensitization that recovered in 1 h and was facilitated by PP1. These similarities suggest that even though morphine and ME induce different trafficking patterns (Arttamangkul et al., 2008), resensitization occurs through a common process regardless of the agonist used to induce desensitization.
Kinases in Morphine Desensitization.
In a previous study, morphine desensitization in LC neurons from morphine-tolerant rats was shown to be dependent on ongoing PKC activity that was mostly attributed to PKCα (Bailey et al., 2009a). These results have been replicated here using two PKC inhibitors with slightly different selectivity patterns. Staurosporine is notoriously broad-spectrum. The main overlapping enzymes, besides PKC, inhibited by both Go6976 and staurosporine are 3-phosphoinositide-dependent protein kinase 1, phosphorylase kinase, and checkpoint kinase 1 (Davies et al., 2000; Karaman et al., 2008). The JNK inhibitor SP600125 also inhibits phosphorylase kinase and checkpoint kinase 1 to a significant degree and slightly inhibits PDK1 (Bain et al., 2003). Because SP600125 did not affect morphine desensitization, these three enzymes are probably not involved in the effect of staurosporine and Go6976. Therefore, PKC is the most likely enzyme responsible for the effect of staurosporine and Go6976.
The site of action of PKC cannot be determined from these studies, but a possible site is phosphorylation of MOR itself. MOR is phosphorylated at multiple Ser/Thr residues on the C-terminal tail, both constitutively and in response to agonist (El Kouhen et al., 2001; Wang et al., 2002; Doll et al., 2011; Lau et al., 2011). PKC can phosphorylate Ser363 and Thr370, but neither of these sites is phosphorylated in response to morphine (Doll et al., 2011; Feng et al., 2011). In fact, bulk phosphorylation of MORs induced by morphine was not blocked by staurosporine (Zhang et al., 1996). Rather, morphine desensitization may require multiple phosphorylation sites including those phosphorylated by constitutive PKC activity (i.e., Thr370) and those phosphorylated in response to morphine (i.e., Ser375) (Doll et al., 2011). DAMGO, which does not require PKC for desensitization (Bailey et al., 2009b), effectively phosphorylates both Thr370 and Ser375 (Doll et al., 2011).
In a recent study, PKC was shown to cause heterologous desensitization in human embryonic kidney 293 cells by phosphorylation of Gαi2 (Chu et al., 2010). Heterologous desensitization between MORs and α2-adrenergic receptors was not observed here or in previous studies using LC neurons (Dang and Williams, 2004; Bailey et al., 2009a). Thus, prolonged morphine treatment did not alter the signaling proteins shared with α2-adrenergic receptors. However, heterologous desensitization of other GPCRs cannot be ruled out.
JNK was recently shown to be required for morphine tolerance in a ligand-specific manner (Melief et al., 2010). The role of JNK in morphine desensitization was previously untested. Unlike addition of PKC inhibitors, addition of the JNK inhibitor SP600125 to slices from morphine-treated animals was unable to reverse morphine desensitization. Furthermore, the JNK inhibitor did not alter the development of desensitization in slices that were incubated in morphine for several hours. Therefore, JNK does not appear to be involved in the maintenance or development of morphine desensitization in LC neurons. The process reported by Melief et al. (2010) is more representative of cellular tolerance (as defined here) because morphine was washed away before cellular assays were performed. Thus, cellular tolerance rather than desensitization may be the mechanism by which JNK regulates morphine tolerance. Alternatively, regulation of desensitization can be different, depending on the cell or synaptic context (Blanchet and Lüscher, 2002; Fyfe et al., 2010; Pennock et al., 2012). JNK has been shown to mediate desensitization of voltage-gated calcium channels in dorsal root ganglia neurons (Mittal et al., 2012).
Cellular Tolerance.
None of the manipulations performed in this study (PKC, JNK, or phosphatase inhibitors) could reverse cellular tolerance. Because JNK inhibition and knockdown have been shown to prevent the development of cellular tolerance (Melief et al., 2010), the negative result presented here probably indicates that cellular tolerance is a long-lasting consequence resulting from adaptive changes that are not readily reversible. There are other manipulations that prevent the development of cellular tolerance, most notably the loss of β-arrestin 2 (Bohn et al., 2000; Dang et al., 2011). However, β-arrestin 2 is not required for acute ME-induced desensitization (Dang et al., 2011; Quillinan et al., 2011). Instead, β-arrestin 2 may link desensitization and tolerance by slowing recovery from desensitization. Long-term treatment of animals with morphine produces β-arrestin 2- and G protein receptor-coupled kinase 2-dependent adaptive changes that reduce receptor recycling and reduce recovery from ME-induced desensitization (Dang et al., 2011; Quillinan et al., 2011).
Desensitization Can Contribute to Behavioral Tolerance.
The morphine desensitization described in this study required the continuous presence of a significant concentration of morphine and recovered in less than 1 h in the absence of morphine. This rapid recovery from desensitization could contribute to differences observed in the tolerance produced by intermittent versus continuous administration of morphine. In slices from rats that were treated intermittently with morphine, cellular tolerance in periaqueductal gray neurons was not only lost, but ME was actually more potent in inducing GIRK current (Ingram et al., 2008). Furthermore, intermittent morphine treatment causes less antinociceptive tolerance than continuous treatment (Dighe et al., 2009). Often, desensitization and tolerance are considered interchangeable. This study argues that careful investigation of desensitization as a process separate from cellular tolerance is critical to understanding the decrease in MOR function during long-term opioid exposure.
Acknowledgments
We thank Drs. Seksiri Arttamangkul and William Birdsong for comments on the article.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01-DA08163, F32-DA33036]; and the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant T32-NS07381].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- MOR
- μ-opioid receptor
- MTA
- morphine-treated animal
- LC
- locus coeruleus
- ME
- [Met5]-enkephalin
- DAMGO
- [d-Ala2,N-Me-Phe4,Gly5-ol]-enkephalin
- PKC
- protein kinase C
- PP1
- protein phosphatase 1
- JNK
- c-Jun N-terminal kinase
- MK-801
- dizocilpine maleate
- SP600125
- anthra[1–9-cd]pyrazol-6(2H)-one
- UK
- UK14304 tartrate, 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)-6-quinoxalinamine tartrate
- Gö6976
- 5,6,7,13-tetrahydro-13-methyl-5-oxo-12H-indolo[2,3-a]pyrrolo[3,4-c]carbazole-12-propanenitrile
- ACSF
- artificial cerebrospinal fluid
- ANOVA
- analysis of variance
- GIRK
- G protein-coupled inwardly rectifying potassium
- NA
- noradrenaline.
Authorship Contributions
Participated in research design: Levitt and Williams.
Conducted experiments: Levitt and Williams.
Performed data analysis: Levitt.
Wrote or contributed to the writing of the manuscript: Levitt and Williams.
References
- Alvarez VA, Arttamangkul S, Dang V, Salem A, Whistler JL, Von Zastrow M, Grandy DK, Williams JT. (2002) mu-Opioid receptors: ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci 22:5769–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. (2008) Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol 74:972–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley EE, Chieng BC, Christie MJ, Connor M. (2005) Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br J Pharmacol 146:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Couch D, Johnson E, Griffiths K, Kelly E, Henderson G. (2003) μ-Opioid receptor desensitization in mature rat neurons: lack of interaction between DAMGO and morphine. J Neurosci 23:10515–10520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Llorente J, Gabra BH, Smith FL, Dewey WL, Kelly E, Henderson G. (2009a) Role of protein kinase C and μ-opioid receptor (MOPr) desensitization in tolerance to morphine in rat locus coeruleus neurons. Eur J Neurosci 29:307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Oldfield S, Llorente J, Caunt CJ, Teschemacher AG, Roberts L, McArdle CA, Smith FL, Dewey WL, Kelly E, et al. (2009b) Involvement of PKCα and G-protein-coupled receptor kinase 2 in agonist-selective desensitization of μ-opioid receptors in mature brain neurons. Br J Pharmacol 158:157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain J, McLauchlan H, Elliott M, Cohen P. (2003) The specificities of protein kinase inhibitors: an update. Biochem J 371 (Pt 1):199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet C, Lüscher C. (2002) Desensitization of μ-opioid receptor-evoked potassium currents: initiation at the receptor, expression at the effector. Proc Natl Acad Sci USA 99:4674–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. (2000) μ-Opioid receptor desensitization by β-arrestin-2 determines morphine tolerance but not dependence. Nature 408:720–723 [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. (2002) Differential mechanisms of morphine antinociceptive tolerance revealed in (β)arrestin-2 knock-out mice. J Neurosci 22:10494–10500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. (2003) Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem 278:18776–18784 [DOI] [PubMed] [Google Scholar]
- Christie MJ, Williams JT, North RA. (1987) Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol Pharmacol 32:633–638 [PubMed] [Google Scholar]
- Chu J, Zheng H, Zhang Y, Loh HH, Law PY. (2010) Agonist-dependent μ-opioid receptor signaling can lead to heterologous desensitization. Cell Signal 22:684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor M, Borgland SL, Christie MJ. (1999) Continued morphine modulation of calcium channel currents in acutely isolated locus coeruleus neurons from morphine-dependent rats. Br J Pharmacol 128:1561–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Chieng B, Azriel Y, Christie MJ. (2011) Cellular morphine tolerance produced by βarrestin-2-dependent impairment of μ-opioid receptor resensitization. J Neurosci 31:7122–7130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Chieng BC, Christie MJ. (2012) Prolonged stimulation of μ-opioid receptors produces β-arrestin-2 mediated heterologous desensitization of α2-adrenoceptor function in locus ceruleus neurons. Mol Pharmacol 82:473–480 [DOI] [PubMed] [Google Scholar]
- Dang VC, Christie MJ. (2012) Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol 165:1704–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. (2004) Chronic morphine treatment reduces recovery from opioid desensitization. J Neurosci 24:7699–7706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang VC, Williams JT. (2005) Morphine-induced μ-opioid receptor desensitization. Mol Pharmacol 68:1127–1132 [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. (2000) Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J 351 (Pt 1):95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dighe SV, Madia PA, Sirohi S, Yoburn BC. (2009) Continuous morphine produces more tolerance than intermittent or acute treatment. Pharmacol Biochem Behav 92:537–542 [DOI] [PubMed] [Google Scholar]
- Doll C, Konietzko J, Pöll F, Koch T, Höllt V, Schulz S. (2011) Agonist-selective patterns of μ-opioid receptor phosphorylation revealed by phosphosite-specific antibodies. Br J Pharmacol 164:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll C, Poll F, Peuker K, Loktev A, Gluck L, Schulz S. (2012) Deciphering mu-opioid receptor phosphorylation and dephosphorylation in HEK293 cells. Br J Pharmacol http://dx.doi.org/10.1111/j.1476-5381.2012.02080.x [DOI] [PMC free article] [PubMed]
- El Kouhen R, Burd AL, Erickson-Herbrandson LJ, Chang CY, Law PY, Loh HH. (2001) Phosphorylation of Ser363, Thr370, and Ser375 residues within the carboxyl tail differentially regulates μ-opioid receptor internalization. J Biol Chem 276:12774–12780 [DOI] [PubMed] [Google Scholar]
- Enquist J, Kim JA, Bartlett S, Ferwerda M, Whistler JL. (2011) A novel knock-in mouse reveals mechanistically distinct forms of morphine tolerance. J Pharmacol Exp Ther 338:633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B, Li Z, Wang JB. (2011) Protein kinase C-mediated phosphorylation of the μ-opioid receptor and its effects on receptor signaling. Mol Pharmacol 79:768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. (2001) Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron 32:829–839 [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Williams JT. (1996) Opioid desensitization: interactions with G-protein-coupled receptors in the locus coeruleus. J Neurosci 16:1479–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe LW, Cleary DR, Macey TA, Morgan MM, Ingram SL. (2010) Tolerance to the antinociceptive effect of morphine in the absence of short-term presynaptic desensitization in rat periaqueductal gray neurons. J Pharmacol Exp Ther 335:674–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull LC, Llorente J, Gabra BH, Smith FL, Kelly E, Bailey C, Henderson G, Dewey WL. (2010) The effect of protein kinase C and G protein-coupled receptor kinase inhibition on tolerance induced by μ-opioid agonists of different efficacy. J Pharmacol Exp Ther 332:1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram SL, Macey TA, Fossum EN, Morgan MM. (2008) Tolerance to repeated morphine administration is associated with increased potency of opioid agonists. Neuropsychopharmacology 33:2494–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed., Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. (2006) Agonist-selective mechanisms of μ-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol 70:676–685 [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, Gallant P, Atteridge CE, Campbell BT, Chan KW, Ciceri P, Davis MI, Edeen PT, et al. (2008) A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol 26:127–132 [DOI] [PubMed] [Google Scholar]
- Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, Burlingame AL, von Zastrow M. (2011) Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Sci Signal 4:ra52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA. (1990) Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett 264:187–192 [DOI] [PubMed] [Google Scholar]
- Melief EJ, Miyatake M, Bruchas MR, Chavkin C. (2010) Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci USA 107:11608–11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal N, Tan M, Egbuta O, Desai N, Crawford C, Xie CW, Evans C, Walwyn W. (2012) Evidence that behavioral phenotypes of morphine in β-arr2−/− mice are due to the unmasking of JNK signaling. Neuropsychopharmacology 37:1953–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Williams JT. (1985) On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol 364:265–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne PB, Williams JT. (1995) Characterization of acute homologous desensitization of mu-opioid receptor-induced currents in locus coeruleus neurones. Br J Pharmacol 115:925–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock RL, Dicken MS, Hentges ST. (2012) Multiple inhibitory G-protein-coupled receptors resist acute desensitization in the presynaptic but not postsynaptic compartments of neurons. J Neurosci 32:10192–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillinan N, Lau EK, Virk M, von Zastrow M, Williams JT. (2011) Recovery from mu-opioid receptor desensitization after chronic treatment with morphine and methadone. J Neurosci 31:4434–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virk MS, Williams JT. (2008) Agonist-specific regulation of μ-opioid receptor desensitization and recovery from desensitization. Mol Pharmacol 73:1301–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Chang WT, Hsu CY, Huang PC, Chow YW, Li AH. (2002) Identification of two C-terminal amino acids, Ser355 and Thr357, required for short-term homologous desensitization of μ-opioid receptors. Biochem Pharmacol 64:257–266 [DOI] [PubMed] [Google Scholar]
- Williams JT, North RA. (1984) Opiate-receptor interactions on single locus coeruleus neurones. Mol Pharmacol 26:489–497 [PubMed] [Google Scholar]
- Zhang L, Yu Y, Mackin S, Weight FF, Uhl GR, Wang JB. (1996) Differential μ opiate receptor phosphorylation and desensitization induced by agonists and phorbol esters. J Biol Chem 271:11449–11454 [DOI] [PubMed] [Google Scholar]