Abstract
Objective
To describe the design of a clinical trial testing the hypothesis that children randomized to tight glycemic control with intensive insulin therapy after cardiac surgery will have improved clinical outcomes compared to children randomized to conventional blood glucose management
Design
Two-center, randomized controlled trial
Setting
Cardiac intensive care units (CICUs) at two large academic pediatric centers
Patients
Children from birth-36 months recovering in the CICU after surgery with cardiopulmonary bypass
Interventions
Subjects in the tight glycemic control (intervention) group receive an intravenous insulin infusion titrated to achieve normoglycemia (target blood glucose range of 80–110 mg/dL; 4.4–6.1 mmol/L). The intervention begins upon admission to the CICU from the operating room and terminates when the patient is ready for discharge from the intensive care unit. Continuous glucose monitoring is performed for the duration of insulin infusion to minimize the risks of hypoglycemia. The standard care group has no target blood glucose range.
Measurements and Main Results
The primary outcome is the development of any nosocomial infection (bloodstream, urinary tract, and surgical site infection, or nosocomial pneumonia). Secondary outcomes include mortality, measures of cardiorespiratory function and recovery, laboratory indices of nutritional balance, immunologic, endocrinologic, and neurologic function, CICU and hospital length of stay, and neurodevelopmental outcome at 1 and 3 years of age. A total of 980 subjects will be enrolled (490 in each treatment arm) for sufficient power to show a 50% reduction in the incidence of the primary outcome.
Conclusions
Pediatric cardiac surgical patients may recognize great benefit from tight glycemic control in the postoperative period, particularly in regard to reduction of nosocomial infections. The SPECS trial is designed to provide an unbiased answer to the question of whether this therapy is indeed beneficial and to define the associated risks of therapy.
Keywords: Cardiac surgery, critical illness/therapy, insulin/therapeutic use, intensive care/methods, children, hyperglycemia/drug therapy
Introduction
Stress hyperglycemia, a state of abnormal metabolism with supra-normal blood glucose (BG) levels, occurs frequently in critically ill patients. Previously thought to be an adaptive response to illness as a means of increasing energy supply to metabolically stressed systems, stress hyperglycemia was infrequently treated in non-diabetic patients. Over the last two decades there has been a new appreciation for the negative outcomes associated with hyperglycemia in the intensive care unit. Up to 90% of pediatric cardiac surgical patients requiring cardiopulmonary bypass (CPB) are at risk for hyperglycemia in the perioperative period 90% [1–4] and several retrospective analyses suggest an association between hyperglycemia and perioperative morbidity and mortality in children recovering from cardiac surgery [2, 3, 5]. On the basis of several investigations [6–8] including the landmark study by Van den Berghe and colleagues [9], tight glycemic control (TGC) protocols with intensive insulin therapy were instituted in many adult critical care units to ameliorate the effects of stress hyperglycemia. Particularly in adult cardiac surgical patients, glycemic control led to improvements in mortality, intensive care length of stay, and benefits in multiple organ systems, most notably decreasing intravenous device, bloodstream, and surgical site infections by nearly 50% [7, 9]. However, in part because of subsequent trials challenging these single-center findings [10–13] and benefits in the original Van den Berghe study being limited to certain subgroups, pediatric intensivists have not widely adopted the practice of TGC [14–16].
Though there are now several reports of glycemic control protocols being used in critically ill pediatric patients [17–19], questions remain over the optimal BG target range for these patients, and the risks associated with treatment-induced hypoglycemia. In the only previous randomized trial evaluating the benefit of TGC in pediatric cardiac surgical patients [20], children in the intensive insulin therapy arm derived several benefits including reduced inflammation, vasoactive support, infections, length of intensive care stay and mortality. However, regular use of this therapeutic strategy in the pediatric cardiac intensive care unit is still rare because of generalizability concerns from the mixed critical care study population and the reported rate of hypoglycemia; the intervention group in the pediatric trial had a 25% incidence of severe hypoglycemia (<40 mg/dL; 2.2 mmol/L) with a 45% incidence rate in infants. Further study is necessary to devise a TGC protocol with an acceptable risk-benefit profile, and within that context to confirm the clinical benefits in the pediatric critically-ill population.
We designed a clinical trial to target the patient group most likely to benefit from TGC using the most highly advanced FDA-approved continuous glucose monitoring technology to minimize the risk of hypoglycemia associated with the intervention. This report describes the design and rationale for this active, National Institutes of Health (NIH)-funded (R01 HL088448), multisite, randomized controlled trial. The study, entitled Safe Pediatric Euglycemia after Cardiac Surgery (SPECS; NCT00443599), compares outcomes of infants and young children treated in the cardiac intensive care unit (CICU) after cardiac surgery with TGC and continuous glucose monitoring to those who receive conventional BG management.
Materials and Methods
Study overview
This trial tests the primary hypothesis that children randomized to TGC with intensive insulin therapy in the CICU post-operatively will have fewer nosocomial infections compared to children randomized to conventional BG management. The TGC group receives an intravenous insulin infusion titrated to achieve normoglycemia (target BG range of 80–110 mg/dL; 4.4–6.1 mmol/L). The standard care (STD) group has no target range for BG management and patients are treated according to the preference of the attending physician. Our choice of target range for TGC was higher than a recent large pediatric ICU trial that implemented TGC based on age-specific fasting BG values [20]. Hypoglycemia in association with fasting is accompanied by increased production of ketones and free fatty acids as available alternative fuels to the brain. However, TGC induced by insulin suppresses lipolysis resulting in reduced concentrations of these alternative fuels to the brain.
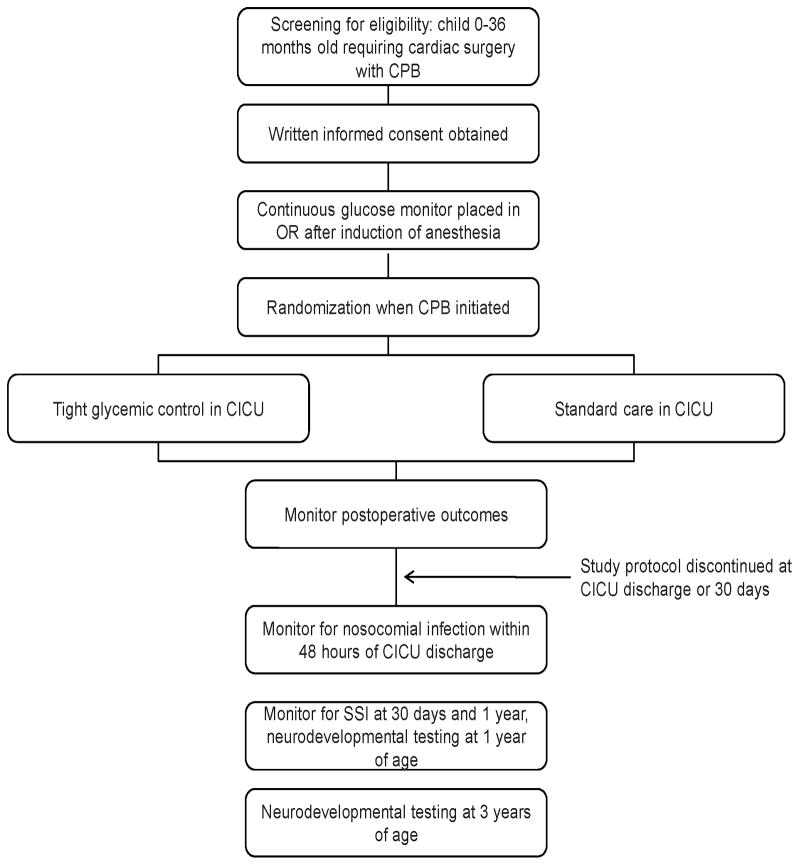
The concept for this study was developed by investigators at Children’s Hospital Boston (CHB) which serves as the coordinating center, with patients also being enrolled at the University of Michigan C.S. Mott Children’s Hospital (CSM). Study protocol modifications are developed collaboratively. We plan to enroll 980 patients randomly assigned to one of these two treatment groups. Study enrollment began in 2008 and will conclude in mid-2012. A study flow chart is shown in Figure 1. Approval from the two institutional review boards (IRB) was obtained prior to enrollment.
Figure 1.
Flow diagram for trial. CPB, cardiopulmonary bypass; OR, operating room; CICU, cardiac intensive care unit; SSI, surgical site infection.
Subject Selection
Subject selection criteria were defined to ensure a relatively homogeneous study population at highest risk for post-operative hyperglycemia and nosocomial infection, and in which the safety and functionality of study monitoring equipment was previously validated [21]. Inclusion and exclusion criteria are shown in Table 1. Potential subjects are approached by a member of the study team in the outpatient surgical pre-operative clinic or on an inpatient floor. Patients who do not consent to participate are treated according to usual practice by the clinical team, led by the attending CICU physician, occasionally including therapy for glycemic control.
Table 1.
SPECS Trial subject inclusion and exclusion criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
Study Protocol
Continuous glucose monitoring
The study protocol begins after induction of anesthesia and prior to surgical incision with placement of the subcutaneous sensor wirelessly paired with the continuous glucose monitor (CGM; Guardian® REAL-Time device, Medtronic Minimed, Northridge, CA). Prior to the study, the CGM was tested in a study of critically-ill pediatric cardiac surgical patients less than 3 years of age; it functioned well compared to glucose measurement with a blood gas analyzer in a wide range of clinical states (mean absolute relative difference of 18% and a Pearson correlation coefficient of 0.8) [21]. Other authors have reported similar results [22, 23]. The sensor is inserted with minimal discomfort into the lateral thigh of the subject and communicates wirelessly to a pager-sized monitor every five minutes. The monitor records and displays a BG estimate every five minutes, and alarms when the BG drops below manually set limits. This device is FDA-approved for patients seven years of age or older, and was approved by local IRBs for the age range targeted in this trial as a non-significant risk device. CGM values are confirmed when necessary with an arterial blood sample measured with bedside meters available at the respective institutions (SureStep®Flexx®, LifeScan, at CHB and ACCU-CHECK® Inform, Roche, at CSM). CGM calibration is performed every 6 hours using these glucometers.
The sensor alarm is set to notify the bedside nurse of actual or impending hypoglycemia (defined as a BG concentration < 60 mg/dL; 3.3 mmol/L) in both study groups. After an early review of sensor performance, the alarm threshold was raised from 60 mg/dL to 70 mg/dL (3.3 to 3.9 mmol/L) and a 15 minute prediction setting was activated [24]. Sensors are activated upon admission to the CICU and are replaced every 72 hours according to the manufacturer’s guidelines.
CICU therapy: Tight glycemic control (TGC) vs. Standard of Care (STD)
Patients are randomized after initiation of CPB using sealed opaque envelopes generated by the CHB Clinical Research Program containing the group assignment for each patient based on a serially numbered sequence using permuted blocks with random block sizes. Patients are block-randomized by center. For those patients randomized to TGC, the glycemic control protocol begins after admission to the CICU and assumption of care by the CICU attending physician and bedside nurse. The insulin infusion (0.2 units regular human insulin per 1 mL 0.9% NaCl) is left to dwell in dedicated IV tubing for ≥16 hours to saturate insulin binding sites prior to administration. An initial whole BG measurement is made within 60 minutes of CICU admission on a hospital-approved glucose meter and the CGM is calibrated at this time. All BG measurements are performed using 0.05 mL samples obtained from an indwelling arterial catheter placed for standard post-operative monitoring and blood sampling. The study protocol is suspended or discontinued when there is no indwelling arterial catheter. An attachment [VAMP Jr. (Edwards Lifesciences, LLC, Irvine, CA) or Neonatal SafeSet (ICU Medical Inc., San Clemente, CA)] to the arterial catheter tubing allows for a closed-line system, minimal blood waste, and standardized sampling. Insulin therapy is initiated and continued for TGC subjects if the glucose concentration exceeds or is expected to exceed 110 mg/dL (6.1 mmol/L) within 30 minutes as predicted by the treatment algorithm (see below).
Insulin is administered only when there is a functional CGM in place; in the case of a temporary CGM malfunction, BG is measured every hour until a new sensor can be placed. Insulin is always infused along with 0.9% NaCl carrier running at 1 mL/hr, preferentially through a dedicated peripheral intravenous catheter. The lumen of a central venous or intracardiac catheter can be used if there is compatibility with other infusions and the route is judged to be safe by the bedside clinicians. The protocol is suspended at any point if there is inadequate venous access to administer the insulin infusion; the protocol can be re-started if venous access becomes available, but catheters are never placed solely to administer the study protocol.
The dose of insulin is titrated according to a paper-based algorithm translated into a Microsoft Excel® spreadsheet displayed on a dedicated laptop computer at the bedside. BG concentration is monitored by the CGM and a hospital-approved glucose meter at a clinically-indicated frequency determined by the infusion algorithm. Glucose meter checks occur at least as frequent as every two hours during insulin infusion, and every six hours when insulin is not being infused. Meter checks are required 1 hour after any increase in the insulin infusion rate, 30 minutes after any algorithm-recommended dextrose bolus, and at any time the sensor alarms for hypoglycemia.
The goal of post-operative glycemic control in the TGC group is a BG concentration of 80–110 mg/dL (4.4–6.1 mmol/L). This target was determined from previous adult studies demonstrating the greatest benefit in patients with true normoglycemia as opposed to moderate hyperglycemia [25]. Literature published since the start of SPECS has suggested different optimal target ranges for BG management in adults [12]. However, as there are no equivalent data in children, we continued our strategy of comparing a normoglycemic BG target to STD.
The insulin infusion algorithm has been described previously [24, 26]. A summary of the proportional, integral, and derivative components is depicted in Box 1. The algorithm calculates a recommended intravenous insulin infusion rate as the sum of these components factoring the present BG level, the patient’s BG profile and insulin requirements over time, and the rate of change of the BG level. If the BG level is falling faster than desired, a dose reduction is recommended by the algorithm; if BG increases at a stable dose the infusion rate is increased slightly. When the BG level drops or is anticipated to drop below 60 mg/dL (3.3 mmol/L) the algorithm recommends suspension of insulin delivery and administration of an IV dextrose bolus. A minimum change in insulin delivery is set to prevent frequent minor adjustments and necessitating excessive blood draws.
Box 1. Summary of insulin infusion algorithm components.
| Proportional (P) | Present – the difference between measured and target glucose |
| Integral (I) | Past – area under the curve between measured and target glucose |
| Derivative (D) | Future – Rate of change in glucose level |
The dose of insulin is actively maintained or titrated hourly by the bedside nurse according to recommendations from the algorithm. Insulin dose changes are only made in response to a BG value obtained with a glucose meter; no changes are made in response to values reported by the CGM which are used primarily to demonstrate stable BG or to alert the bedside clinician to perform more frequent BG measurement (for instance if it senses impending or actual hypoglycemia). The protocol can be interrupted if it is deemed unsafe for any period of time by the investigators or the clinical team. For example, during sterile procedures where it would be difficult for the nurse to perform the necessary study functions, insulin infusions are typically suspended. The protocol is suspended when patients leave the CICU for any reason. All protocol deviations are recorded and tracked.
In patients randomized to the STD group, the CGM is placed to serve as a hypoglycemic alarm to give all subjects some benefit from participation. Audible alarms are enabled only for detection of hypoglycemia; sensor-measured glucose values are not reported to the bedside clinicians. Hyperglycemia in STD subjects is treated at the discretion of the attending intensivist. Typical practice at each institution for treatment of hyperglycemia is to initiate insulin if glucosuria is present, or if the BG concentration is persistently >200–300 mg/dL (11.1–16.7 mmol/L). There is no unit-based clinical protocol at either institution for glycemic control, and insulin therapy for this indication is rarely utilized. If the treating clinician chooses to initiate insulin therapy, CGM is not used to guide treatment decisions although the alarm function for hypoglycemia remains activated. The protocol is not altered in any way for either treatment arm if the patient is on extracorporeal circulatory support; CGMs are inserted and replaced according to the protocol.
All patients in both groups otherwise receive the institution’s standard anesthetic and surgical management [27], as well as standard postoperative care; the protocol does not restrict use of glucocorticoids, vasoactive support, nutritional support, or antibiotics peri-operatively, nor does it change infection monitoring, control, and treatment in the CICU. Neither study site has a protocol for therapeutic hypothermia after cardiac arrest. The study protocol ends in either group if one of the following occurs: removal of arterial catheter(s), transfer out of the CICU, or if the subject remains in the CICU beyond 30 days after the index procedure. Insulin infusions are discontinued if the patient starts ad lib feeds by mouth or bolus feeds via a nasogastric tube. In STD subjects, CGM occurs for at least the first three post-operative days. Monitoring is discontinued after 72 hours if the patient is judged to be clinically stable with no recorded hypoglycemia (BG <60 mg/dL; 3.3mmol/L) for at least 12 hours. The protocol is not resumed if study patients require re-admission to the CICU.
Study Measurements and Follow-up
Baseline, intraoperative, and postoperative clinical and laboratory data are collected daily by study personnel. Postoperative data collection is continued until either the subject is designated by the clinical team for transfer out of the CICU, or after 30 days from the index procedure. Subjects are monitored for infections attributable to the CICU for 48 hours after leaving the CICU. Families of subjects are contacted at 30 days and 1 year after the index procedure to monitor hospital readmission and development of surgical site infection. Families invited to return for neurodevelopmental testing when participants reach 1 and 3 years of age. This testing is completed within 8 weeks of the patient’s birthday, and occurs no less than 6 weeks after a major hospitalization or operative procedure.
Trial Outcomes
The primary outcome of the SPECS trial is the development of nosocomial infection (bloodstream infection, urinary tract infection, nosocomial/ventilator-associated pneumonia, or surgical site infection) according to Centers for Disease Control definitions as of 2005 [28]. The infection rate will be reported as infections per 1,000 patient days in the CICU. Infections are adjudicated by the local infection control clinicians at each institution who are not part of the study team and are blinded to the subjects’ treatment group assignment. Secondary outcomes are shown in Table 2 and include mortality, measurements of organ function, nutritional data, CICU length of stay, and neurodevelopmental outcomes. The standardized test battery used to measure neurodevelopmental outcomes is shown in Table 3.
Table 3.
Neurodevelopmental testing battery
| Developmental domain | 1-year assessment | 3-year assessment |
|---|---|---|
| Cognition | BSID-III: cognitive scale | BSID-III: cognitive scale |
| Communication | BSID-III: language scale | BSID-III: language scale |
| Motor | BSID-III: motor scale | BSID-III: motor scale |
| Growth parameters | Height, weight, head circumference | Height, weight, head circumference |
| Adaptive behavior | ABAS-II | ABAS-II |
| Socio-emotional | BITSEA | BITSEA |
| Attention/behavior | NA | BASC-II |
| Developmental history | ASQ | ASQ |
| Socio-demographic data | Family questionnaire | Family questionnaire |
BSID, Bayley Scales of Infant and Toddler Development (44); ABAS, Adaptive Behavior Assessment System (45); BITSEA, Brief Infant Toddler Social and Emotional Assessment (46); BASC, Behavior Assessment Scale for Children (47); ASQ, Ages & stages questionnaire (48)
Statistical Analysis
All primary analyses will be performed on an intention-to-treat basis. Based on adult data, we hypothesize that TGC will lead to a 50% decrease in nosocomial infection rate. Pre-trial nosocomial infection rate (February through August of 2005) in the population of interest at CHB was 11.4 infections per 1,000 CICU days, and 10.4 per 1,000 days at CSM. Using these values and a stratified Poisson-based power calculation with a 0.05 two-sided significance level, we estimate 80% power to detect a 50% difference in nosocomial infection rate between the TGC and STD groups when the sample size is 490 subjects in each treatment arm (980 total). There was no accounting for potential patient withdrawals in this calculation and all patients will be analyzed regardless of protocol violations.
Nosocomial infections in post-operative cardiac surgery patients may be influenced by factors not affected by the intervention of TGC. In adult medical ICU patients, prevention of morbidity was seen in all patients randomized to TGC, but mortality reduction attributable to TGC was seen only in those patients whose ICU stay lasted for more than 2 days [8]. Thus, an a priori sub-group analysis of patients admitted to the ICU for 3 or more days after surgery will be performed to explore the effect of TGC in patients with an extended ICU stay. In addition, we will examine the impact of procedure complexity on outcomes by adjusting for RACHS-I score (Risk Adjustment for Congenital Heart Surgery-I), a consensus-based method to adjust for case-mix differences [29].
Study Oversight
An independent data and safety monitoring board (DSMB) monitors the trial for adverse events, adherence to study protocol, and potential early stopping. A planned interim analysis will be performed at the midpoint of the study to determine whether the intervention is associated with increased mortality or increased adverse events, and whether a highly significant benefit of one group (efficacy) or extreme lack of difference (futility) emerges before the planned end of the study [30]. In addition, DSMB meetings occur at regular 6 month intervals to review emerging data on TGC in other populations. Changes to the study protocol will be recommended by the DSMB based on their independent review.
Discussion
Rationale for Choice of Outcome Measures
Mortality, the common primary outcome in adult ICU studies, was not a plausible outcome in this study. With an expected 3–5% mortality rate in our study population, demonstrating a significant reduction would require a much larger sample size for equivalent power. Furthermore, mortality in this population is largely driven by procedural complexity and not modifiable by TGC. For these reasons we, like many other pediatric critical care trialists, chose an alternative important outcome.
Pediatric cardiac surgical patients are at particular risk for nosocomial infections; in addition to hyperglycemia, children experience a state of immunoparalysis after CPB, incur significant tissue damage, are exposed to multiple invasive monitoring catheters, and undergo ischemia-reperfusion injury. Published [31] and unpublished local data demonstrate that the risk is highest among children under 3 years of age who undergo the most complex procedures necessitating longer recovery in the CICU, making this population ideal candidates for this trial. In addition to complicating and prolonging the CICU stay, nosocomial infections result in increased healthcare expenditures. The additional average direct cost of a nosocomial bloodstream infection for patients in an academic tertiary care center pediatric ICU has been estimated at $39,219 [32]. Thus, reducing the incidence of nosocomial infections will result in clinical and cost benefits.
Analysis of clinical trials involving TGC suggests that control of the BG level, rather than the insulin dose, leads to the clinical improvements seen in sepsis and multi-organ system failure [6, 33]. Several potential mechanisms explain the deleterious effects of hyperglycemia on normal immune function including upregulation of pro-inflammatory cytokines IL-8 and NF-κB, impairment of humoral and cellular immunity [34], and diminishing wound healing by protein glycosylation and stimulation of collagenase activity [35].
The theoretical advantages of perioperative insulin therapy after cardiac surgery may not be strictly limited to control of hyperglycemia and immunomodulation. Insulin inhibits pro-inflammatory mediators and enhances nitric oxide synthase [36]. Insulin favorably alters myocardial metabolism towards glycolysis under ischemic conditions [37], reduces oxidative stress after ischemia-reperfusion [38], and improves calcium handling in the sarcoplasmic reticulum [39]. These benefits may play an important role in myocardial recovery after CPB and shorten duration of vasoactive support, time to negative fluid balance, and duration of mechanical ventilation and intensive care stay.
As mortality after pediatric cardiac surgery has decreased substantially over the past three decades [40], neurodevelopmental outcome has become an increasingly important measure by which to compare perioperative practices. Neurodevelopmental outcomes are particularly germane to the study of TGC in pediatric patients given the concern about potential deleterious effects of hypoglycemia on the developing brain. Previous adult and pediatric studies of TGC have been plagued by high rates of hypoglycemia in the treatment group, and hypoglycemia has been associated with increased mortality [8, 20, 41, 42]. To date, neither intraoperative [43] nor postoperative [1] hyperglycemia has been associated with worse neurodevelopmental outcomes after pediatric cardiac surgery. Furthermore, experimental data exist suggesting that hyperglycemia may be protective to the immature brain after hypoxic-ischemic injury [44]. However, it is also possible to hypothesize that TGC could be neuroprotective: by improving post-operative recovery and shortening ICU length of stay, a known risk factor for later functional limitation [45–47], TGC may be beneficial. Comparing neurodevelopmental outcomes between the two treatment groups is essential to determine the ultimate risk-benefit profile of this therapy.
Protocol Considerations
Our rationale for choosing the PID algorithm involved several factors [48]. The algorithm is configured with a minimal number of parameters, had been specifically designed to work with CGM, and worked well in pediatric critical care environments when implemented as a paper-based protocol with Excel® spreadsheet verification [26]. This algorithm also holds the future promise of possible direct linkage to the CGM system, creating closed-loop monitoring and therapy, but such a system is yet to be developed.
This trial is the first to use CGM technology as an adjunct to intensive insulin therapy in any critical care patient population. CGM technology has the potential to reduce the incidence of hypoglycemia while targeting a normoglycemic BG range. Under ideal circumstances, predictive alarms on CGM devices would alert study staff to impending hypoglycemia with sufficient time to allow IV dextrose administration to prevent any fall in glucose below a critical level. However, questions remain regarding the reliability and accuracy of these devices to detect hypoglycemia, particularly under specific physiologic conditions [23, 24]. Though this technology requires improvement for optimal function, we believe that the potential safety benefits warrant strong consideration for using CGM in all future trials of TGC in pediatric patients.
Limitations
While it is typically desirable to achieve blinding of caregivers in a clinical trial, it would not be practical or ethical in this study to do so and utilize a placebo infusion. Excess placebo fluid delivery in the control group is possibly detrimental with no potential benefit. Additionally, physicians and nurses caring for patients in the CICU must be aware of the insulin infusion for safety reasons. The operating surgeon and anesthesiologist are blinded to the randomization during the surgical procedure.
We did not protocolize intra- or post-operative practices such as bypass techniques, or post-operative vasoactive and nutritional support that could impact the rate of hyperglycemia and the likelihood of developing a nosocomial infection. This was done to promote adoption of the study protocol by the surgical and CICU teams, and to increase generalizability of the study findings by implementing the study protocol in “real-life” CICUs. Given that patients were block randomized by center, between center practice differences will be equally distributed between treatment arms.
The CHB and CSM CICUs are two of the highest volume pediatric cardiac surgery programs in the United States, care for a high percentage of complex neonatal and infant cases, and have dedicated cardiac intensive care physician and nursing staffs. How the findings from these two CICUs will generalize to settings with different patient volumes, case-mix, and resource availability remains to be determined. Subgroup analysis may clarify some of these questions.
This study is powered to show a large difference between treatment groups for the primary outcome (50% reduction in the rate of nosocomial infection per 1000 patient days in the CICU). Concurrent strategies to lower nosocomial infection rates in both units may decrease the overall incidence of the outcome and reduce the study power. It is possible that smaller, but clinically important differences may not be statistically significant, particularly when analyzing secondary outcomes. In order to adequately power the study to detect smaller differences between groups, the study sample size would be prohibitively large to complete the trial within a reasonable period. There were no plans to readdress the power calculations during the study to account for changes in the overall infection rate.
This study used bedside glucose meters used clinically at the study institutions. These were considered state-of-the-art at the time the study was initiated, but have since been surpassed by superior technology. International Organization for Standardization (ISO) guidelines require an accuracy of +/−20%. Other more accurate options were available but not incorporated into this study design due to prohibitive cost.
Conclusion
The care of infants and children who require cardiac surgery has advanced considerably in the previous 30 years. As mortality rates have declined, CICU clinicians have become focused on prevention of postoperative morbidity and improvement of long-term clinical and functional outcomes. Previous literature suggests that pediatric cardiac surgical patients may recognize benefit from TGC in the postoperative period, particularly in regard to reduction of nosocomial infections. The SPECS study is designed to provide an unbiased answer to the question of whether TGC, when delivered with the maximal safety of CGM, is indeed beneficial to this high-risk population, and to define the associated risks of therapy. Furthermore, at its conclusion this will be the largest cohort of pediatric cardiac surgical patients ever prospectively enrolled in a clinical trial, and the knowledge acquired now and in the future promises to greatly advance our understanding of the care of critically ill children with cardiovascular disease.
Table 2.
Trial secondary outcomes
| Mortality |
|
| Cardiovascular/respiratory |
|
| Nutrition |
|
| Immune |
|
| Endocrine |
|
| Neurologic |
|
| Length of CICU and hospital admission |
| Neurodevelopment |
EEG, electroencephalogram
Acknowledgments
This project was funded in part by grant M01-RR02172 from the National Center for Research Resources, National Institutes of Health, to the Children’s Hospital Boston General Clinical Research Center, and by Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health. The SPECS Study Group is comprised of John M. Costello, M.D., Martha A. Curley R.N., Ph.D, Pedro J. Del Nido M.D., Tom Jaksic M.D., Ph.D, Anjali Sadhwani, Ph.D, Michael G. Gaies, M.D., M.P.H, Monica Langer, M.D., Jamin Alexander, B.A., Garry M. Steil, Ph.D, Janice Ware, Ph.D, David Wypij Ph.D, Peter C. Laussen, M.B.B.S., Jane W. Newburger, M.D, M.P.H, Frank A. Pigula, M.D., Avinash C. Shukla, M.B.B.S., Christopher P. Duggan M.D., M.P.H., and Michael S.D. Agus, M.D., and assumes full responsibility for the integrity of the data. The affiliations of the SPECS Study Group are listed in the Appendix.
Appendix
Institutional affiliations for the SPECS study group are Department of Medicine (J.A., G.M.S, C.P.D., M.S.D.A), Department of Nursing (M.A.Q.), Department of Cardiac Surgery (P.J.D.N., F.A.P.), Department of Surgery (T.J.), Department of Psychology (A.S., J.W.), Department of Anesthesia (A.C.S.), and Department of Cardiology (J.W.N., P.C.L.), Children’s Hospital Boston, Harvard Medical School, Boston, MA. Department of Biostatistics, Harvard University School of Public Health, Boston, MA (D.W.). Department of Pediatrics and Communicable Diseases, C.S. Mott Children’s Hospital, University of Michigan Medical School, Ann Arbor, MI (M.G.G., C.S.G.). Department of Surgery, Maine Medical Center, Portland, ME (M.L.).
Footnotes
All work was performed at Children’s Hospital Boston, Boston, MA and C.S. Mott Children’s Hospital, Ann Arbor, MI. No reprints will be requested.
Conflicts of interest: Michael S.D. Agus is a paid consultant to two glucose monitoring companies to aid in developing improved glucose measurement techniques in the ICU (Roche Diagnostics, Medtronic Diabetes).
References
- 1.Ballweg JA, Wernovsky G, Ittenbach RF, Bernbaum J, Gerdes M, Gallagher PR, Dominguez TE, Zackai E, Clancy RR, Nicolson SC, et al. Hyperglycemia after infant cardiac surgery does not adversely impact neurodevelopmental outcome. Ann Thorac Surg. 2007;84(6):2052–2058. doi: 10.1016/j.athoracsur.2007.06.099. [DOI] [PubMed] [Google Scholar]
- 2.Moga MA, Manlhiot C, Marwali EM, McCrindle BW, Van Arsdell GS, Schwartz SM. Hyperglycemia after pediatric cardiac surgery: Impact of age and residual lesions. Crit Care Med. 39(2):266–272. doi: 10.1097/CCM.0b013e3181fee88e. [DOI] [PubMed] [Google Scholar]
- 3.Polito A, Thiagarajan RR, Laussen PC, Gauvreau K, Agus MS, Scheurer MA, Pigula FA, Costello JM. Association between intraoperative and early postoperative glucose levels and adverse outcomes after complex congenital heart surgery. Circulation. 2008;118(22):2235–2242. doi: 10.1161/CIRCULATIONAHA.108.804286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ulate KP, Lima Falcao GC, Bielefeld MR, Morales JM, Rotta AT. Strict glycemic targets need not be so strict: a more permissive glycemic range for critically ill children. Pediatrics. 2008;122(4):e898–904. doi: 10.1542/peds.2008-0871. [DOI] [PubMed] [Google Scholar]
- 5.Yates AR, Dyke PC, 2nd, Taeed R, Hoffman TM, Hayes J, Feltes TF, Cua CL. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med. 2006;7(4):351–355. doi: 10.1097/01.PCC.0000227755.96700.98. [DOI] [PubMed] [Google Scholar]
- 6.Finney SJ, Zekveld C, Elia A, Evans TW. Glucose control and mortality in critically ill patients. Jama. 2003;290(15):2041–2047. doi: 10.1001/jama.290.15.2041. [DOI] [PubMed] [Google Scholar]
- 7.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67(2):352–360. doi: 10.1016/s0003-4975(99)00014-4. discussion 360-352. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354(5):449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 9.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345(19):1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 10.Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Syed SJ, Giridhar HR, Rishu AH, Al-Daker MO, et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36(12):3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 11.De La Rosa Gdel C, Donado JH, Restrepo AH, Quintero AM, Gonzalez LG, Saldarriaga NE, Bedoya M, Toro JM, Velasquez JB, Valencia JC, et al. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care. 2008;12(5):R120. doi: 10.1186/cc7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 13.Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35(10):1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 14.Hirshberg E, Lacroix J, Sward K, Willson D, Morris AH. Blood glucose control in critically ill adults and children: a survey on stated practice. Chest. 2008;133(6):1328–1335. doi: 10.1378/chest.07-2702. [DOI] [PubMed] [Google Scholar]
- 15.Preissig CM, Rigby MR. A disparity between physician attitudes and practice regarding hyperglycemia in pediatric intensive care units in the United States: a survey on actual practice habits. Crit Care. 2010;14(1):R11. doi: 10.1186/cc8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faraon-Pogaceanu C, Banasiak KJ, Hirshberg EL, Faustino EV. Comparison of the effectiveness and safety of two insulin infusion protocols in the management of hyperglycemia in critically ill children. Pediatr Crit Care Med. 2010;11(6):741–749. doi: 10.1097/PCC.0b013e3181e88cfb. [DOI] [PubMed] [Google Scholar]
- 17.Verhoeven JJ, Brand JB, van de Polder MM, Joosten KF. Management of hyperglycemia in the pediatric intensive care unit; implementation of a glucose control protocol. Pediatr Crit Care Med. 2009;10(6):648–652. doi: 10.1097/PCC.0b013e3181ae787b. [DOI] [PubMed] [Google Scholar]
- 18.Preissig CM, Hansen I, Roerig PL, Rigby MR. A protocolized approach to identify and manage hyperglycemia in a pediatric critical care unit. Pediatr Crit Care Med. 2008;9(6):581–588. doi: 10.1097/PCC.0b013e31818d36cb. [DOI] [PubMed] [Google Scholar]
- 19.Preissig CM, Rigby MR, Maher KO. Glycemic control for postoperative pediatric cardiac patients. Pediatr Cardiol. 2009;30(8):1098–1104. doi: 10.1007/s00246-009-9512-4. [DOI] [PubMed] [Google Scholar]
- 20.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373(9663):547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 21.Piper HG, Alexander JL, Shukla A, Pigula F, Costello JM, Laussen PC, Jaksic T, Agus MS. Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics. 2006;118(3):1176–1184. doi: 10.1542/peds.2006-0347. [DOI] [PubMed] [Google Scholar]
- 22.Bridges BC, Preissig CM, Maher KO, Rigby MR. Continuous glucose monitors prove highly accurate in critically ill children. Crit Care. 2010;14(5):R176. doi: 10.1186/cc9280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branco RG, Chavan A, Tasker RC. Pilot evaluation of continuous subcutaneous glucose monitoring in children with multiple organ dysfunction syndrome. Pediatr Crit Care Med. 2010;11(3):415–419. doi: 10.1097/PCC.0b013e3181c59144. [DOI] [PubMed] [Google Scholar]
- 24.Steil GM, Langer M, Jaeger K, Alexander J, Gaies M, Agus MS. Value of continuous glucose monitoring for minimizing severe hypoglycemia during tight glycemic control. Pediatr Crit Care Med. 2012;12(6):643–648. doi: 10.1097/PCC.0b013e31821926a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van den Berghe G. Tight blood glucose control with insulin in “real-life” intensive care. Mayo Clin Proc. 2004;79(8):977–978. doi: 10.4065/79.8.977. [DOI] [PubMed] [Google Scholar]
- 26.Wintergerst KA, Deiss D, Buckingham B, Cantwell M, Kache S, Agarwal S, Wilson DM, Steil G. Glucose control in pediatric intensive care unit patients using an insulin-glucose algorithm. Diabetes Technol Ther. 2007;9(3):211–222. doi: 10.1089/dia.2006.0031. [DOI] [PubMed] [Google Scholar]
- 27.Kussman BD, Wypij D, Laussen PC, Soul JS, Bellinger DC, DiNardo JA, Robertson R, Pigula FA, Jonas RA, Newburger JW. Relationship of intraoperative cerebral oxygen saturation to neurodevelopmental outcome and brain magnetic resonance imaging at 1 year of age in infants undergoing biventricular repair. Circulation. 122(3):245–254. doi: 10.1161/CIRCULATIONAHA.109.902338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.CDC. Nosocomial Infection Definitions. 2005 (Accessed at http://www.cdc.gov/ncidod/hip/NNIS/NosInfDefinitions.pdf.)
- 29.Jenkins KJ, Gauvreau K. Center-specific differences in mortality: preliminary analyses using the Risk Adjustment in Congenital Heart Surgery (RACHS-1) method. J Thorac Cardiovasc Surg. 2002;124(1):97–104. doi: 10.1067/mtc.2002.122311. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549–556. [PubMed] [Google Scholar]
- 31.Levy I, Ovadia B, Erez E, Rinat S, Ashkenazi S, Birk E, Konisberger H, Vidne B, Dagan O. Nosocomial infections after cardiac surgery in infants and children: incidence and risk factors. J Hosp Infect. 2003;53(2):111–116. doi: 10.1053/jhin.2002.1359. [DOI] [PubMed] [Google Scholar]
- 32.Elward AM, Hollenbeak CS, Warren DK, Fraser VJ. Attributable cost of nosocomial primary bloodstream infection in pediatric intensive care unit patients. Pediatrics. 2005;115(4):868–872. doi: 10.1542/peds.2004-0256. [DOI] [PubMed] [Google Scholar]
- 33.Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P. Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med. 2003;31(2):359–366. doi: 10.1097/01.CCM.0000045568.12881.10. [DOI] [PubMed] [Google Scholar]
- 34.Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30(5):748–756. doi: 10.1007/s00134-004-2167-y. [DOI] [PubMed] [Google Scholar]
- 35.Hennessey PJ, Ford EG, Black CT, Andrassy RJ. Wound collagenase activity correlates directly with collagen glycosylation in diabetic rats. J Pediatr Surg. 1990;25(1):75–78. doi: 10.1016/s0022-3468(05)80167-8. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch IB. Effect of insulin therapy on nonglycemic variables during acute illness. Endocr Pract. 2004;10 (Suppl 2):63–70. doi: 10.4158/EP.10.S2.63. [DOI] [PubMed] [Google Scholar]
- 37.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33(2):243–257. doi: 10.1016/s0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 38.Vlasselaers D, Mesotten D, Langouche L, Vanhorebeek I, van den Heuvel I, Milants I, Wouters P, Meyns B, Bjerre M, Hansen TK, et al. Tight glycemic control protects the myocardium and reduces inflammation in neonatal heart surgery. Ann Thorac Surg. 90(1):22–29. doi: 10.1016/j.athoracsur.2010.03.093. [DOI] [PubMed] [Google Scholar]
- 39.Yu J, Zhang HF, Wu F, Li QX, Ma H, Guo WY, Wang HC, Gao F. Insulin improves cardiomyocyte contractile function through enhancement of SERCA2a activity in simulated ischemia/reperfusion. Acta Pharmacol Sin. 2006;27(7):919–926. doi: 10.1111/j.1745-7254.2006.00388.x. [DOI] [PubMed] [Google Scholar]
- 40.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001;103(19):2376–2381. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 41.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 42.Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118(1):173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 43.de Ferranti S, Gauvreau K, Hickey PR, Jonas RA, Wypij D, du Plessis A, Bellinger DC, Kuban K, Newburger JW, Laussen PC. Intraoperative hyperglycemia during infant cardiac surgery is not associated with adverse neurodevelopmental outcomes at 1, 4, and 8 years. Anesthesiology. 2004;100(6):1345–1352. doi: 10.1097/00000542-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Callahan DJ, Engle MJ, Volpe JJ. Hypoxic injury to developing glial cells: protective effect of high glucose. Pediatr Res. 1990;27(2):186–190. doi: 10.1203/00006450-199002000-00020. [DOI] [PubMed] [Google Scholar]
- 45.Limperopoulos C, Majnemer A, Shevell MI, Rosenblatt B, Rohlicek C, Tchervenkov C, Darwish HZ. Functional limitations in young children with congenital heart defects after cardiac surgery. Pediatrics. 2001;108(6):1325–1331. doi: 10.1542/peds.108.6.1325. [DOI] [PubMed] [Google Scholar]
- 46.Limperopoulos C, Majnemer A, Shevell MI, Rohlicek C, Rosenblatt B, Tchervenkov C, Darwish HZ. Predictors of developmental disabilities after open heart surgery in young children with congenital heart defects. J Pediatr. 2002;141(1):51–58. doi: 10.1067/mpd.2002.125227. [DOI] [PubMed] [Google Scholar]
- 47.Fuller S, Nord AS, Gerdes M, Wernovsky G, Jarvik GP, Bernbaum J, Zackai E, Gaynor JW. Predictors of impaired neurodevelopmental outcomes at one year of age after infant cardiac surgery. Eur J Cardiothorac Surg. 2009;36(1):40–47. doi: 10.1016/j.ejcts.2009.02.047. [DOI] [PubMed] [Google Scholar]
- 48.Steil GMDD, Shih J, Buckingham B, Weinzimmer S, Agus MSD. Intensive care unit insulin delivery algorithms: Why so many? How to choose? Journal of Diabetes Science and Technology. 2009;3(1):125–140. doi: 10.1177/193229680900300114. [DOI] [PMC free article] [PubMed] [Google Scholar]