Abstract
‘Old’ colistin and polymyxin B are increasingly used as last-line therapy against multidrug-resistant Gram-negative bacteria Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae. For intravenous administration, colistin is dosed as its inactive prodrug colistin methanesulfonate (sodium), while polymyxin B is used as its sulfate (active antibacterial). Over the last decade significant progress has been made in understanding their chemistry, pharmacokinetics (PK) and pharmacodynamics (PD). The first scientifically based dosing suggestions are now available for colistin methanesulfonate to generate a desired target steady-state plasma concentration of formed colistin in various categories of critically-ill patients. As simply increasing polymyxin dosage regimens is not an option for optimizing their PK/PD due to nephrotoxicity, combination therapy with other antibiotics has great potential to maximize the efficacy of polymyxins while minimizing emergence of resistance. We must pursue rational approaches to the use of polymyxins and other existing antibiotics through the application of PK/PD principles.
Keywords: Colistin, polymyxin B, pharmacokinetics, pharmacodynamics
1. Introduction
Rapidly increasing antibiotic resistance and lack of new antibiotics in the development pipeline present a major global medical challenge. This unmet medical need was highlighted by the Infectious Diseases Society of America (IDSA) in the ‘Bad Bugs, No Drugs’ report. As the world faces a growing threat from bacterial ‘superbugs’ resistant to almost all available antibiotics, the WHO has identified antibiotic resistance as one of the three greatest threats to human health. The situation is especially worrying with multidrug-resistant (MDR) Gram-negative bacteria, namely Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae, against which no new antibiotics will be available for many years to come. IDSA has placed these three very problematic pathogens on a hit-list of top-priority dangerous pathogens. In addition, the recent rapid global dissemination of New Delhi metallo-β-lactamase (NDM) producing Enterobacteriaceae is another major medical challenge as these pathogens are resistant to almost all current antibiotics except polymyxins. Without novel antibiotics in the development pipeline, polymyxins are increasingly used as the only therapeutic option. Polymyxins were discovered in the 1940s and never subjected to contemporary drug development procedures. Although clinical use of polymyxins waned in the 1970s due to the early experience of nephrotoxicity and neurotoxicity after intravenous administration, the rapid increase in resistance to all other antibiotics has necessitated their resurgence in the clinic. This paper will review the latest progress in polymyxin pharmacokinetics (PK), pharmacodynamics (PD) and integrated PK/PD, essential information to optimize use of these antibiotics in patients.
2. Chemistry
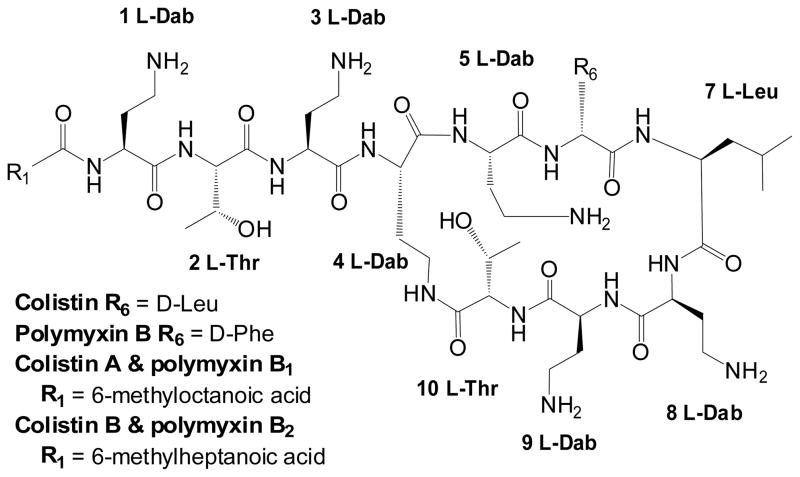
Valid interpretation of polymyxin PK and PD requires an understanding of the chemistry of these compounds. There are two polymyxins available for clinical use, colistin (i.e. polymyxin E) and polymyxin B (Figure 1). There is only one amino acid difference between colistin and polymyxin B and both are polycations at physiological pH owing to the five L-α,γ-diaminobutyric acid (Dab) residues. Polymyxins are amphipathic, with hydrophobicity mainly attributable to the fatty acyl moiety and hydrophilicity due to the five L-Dab γ-amino groups. Hence, polymyxins are ‘sticky’ and able to bind non-specifically to different surfaces (e.g. glass). Polymyxin B and colistin are a mixture of multiple components which differ slightly (e.g. fatty acyl tail). For colistin and polymyxin B, the two major components are colistin A and B, and polymyxin B1 and B2, respectively (Figure 1); however, the proportion of these two major components in commercial material differs between pharmaceutical suppliers and batches.
Figure 1.
Structures of colistin and polymyxin B. Dab: α,γ-diaminobutyric acid; Thr: threonine; Leu: leucine; Phe: phenylalanine.
For parenteral administration, colistin is used in the form of sodium colistin methanesulfonate (CMS), an inactive prodrug of colistin, while polymyxin B (active antibacterial) is used as its sulfate salt. CMS is prepared from colistin by reaction of the free γ-amino groups of the Dab residues with formaldehyde followed by sodium bisulfite. Therefore, at physiological pH, CMS is a polyanion. It is very important to appreciate that CMS is not a salt form of colistin but a different chemical entity. Both CMS and polymyxin B can be used by inhalation for treatment of respiratory tract infections. Topical formulations of colistin sulfate and polymyxin B sulfate are also available in many countries. CMS is much more commonly used internationally (e.g. North America, South America, Asia, Europe and Australia) whereas parenteral polymyxin B is mainly available in the USA, Brazil and Singapore.
To understand the PK and PD of colistin it is crucial to appreciate that CMS is not stable and converts to colistin at low, clinically relevant concentrations in plasma and urine in vivo and in buffer solutions and microbiological media (e.g. <100 μg/ml at 37°C). Such conversion forms a complex mixture of partially sulfomethylated intermediates and colistin, with the potential to produce up to 32 different products even for a single component (e.g. CMS A). A long-term stability study showed that CMS at 2 and 30 μg/ml in human plasma is stable for up to 4 months when stored at −80°C and −70°C, while at −20°C a substantial concentration of colistin (~0.4 μg/ml) was detected in the plasma spiked with 2 μg/ml CMS even within 2 months. The instability of CMS has significant implications for sample collection and handling in pre-clinical and clinical PK studies with CMS. If there is significant conversion of CMS to colistin after samples are collected from patients or animals, concentrations of colistin will be overestimated, thereby leading to inaccurate pharmacological analyses. Indeed, this is the major problem with use of microbiological assays which are not able to differentiate between the colistin present in a sample at the time of its collection from a patient and that formed by ongoing conversion from CMS during the incubation period of a microbiological assay.
3. Mechanisms of activity and resistance
The detailed mechanism of polymyxin activity is unclear. The ‘self-promoted’ uptake theory is widely accepted. Polymyxin activity involves an initial polar interaction of the cationic lipopeptide with lipid A of lipopolysaccharide (LPS) in the outer membrane (OM), displacing divalent cations (Ca2+ and Mg2+) from the negatively charged phosphate groups of lipid A, followed by uptake across the OM. The fatty acyl tail of polymyxins is crucial for activity as polymyxin B nonapeptide (derived by proteolytic cleavage of the fatty acyl-Dab from polymyxin B) is devoid of antibacterial activity. Our structure-activity relationship model for polymyxins indicates the hydrophobic interaction between the fatty acyl tail of polymyxins and lipid A also plays a very important role in this first step of polymyxin action. A recent study reported the inhibitory effect of polymyxin B against alternative NADH dehydrogenase (NDH-2) and malate:quinone oxidoreductase (MQO) from Mycobacterium smegmatis. No such enzymatic study has been conducted in any Gram-negative bacteria.
Cross-resistance exists between colistin and polymyxin B. As described above, a critical first step in the action of polymyxins against Gram-negative bacteria is the electrostatic interaction between the positively charged Dab residues of polymyxins and the negatively charged phosphate groups on lipid A. Thus, many of the bacterial mechanisms of resistance to polymyxins are based on modifications to lipid A that reduce or abolish this initial polar interaction. In E. coli, Salmonella enterica Serovar Typhimurium, K. pneumoniae, A. baumannii and P. aeruginosa, modifications of the phosphates of lipid A with positively charged moieties, such as 4-amino-4-deoxy-L-arabinose (L-Ara4N) and/or phosphoethanolamine (PEtn), reduce the net negative charge of lipid A, thereby increasing resistance to polymyxins. In addition, LPS loss and presence of capsule have been reported for polymyxin resistance in A. baumannii and K. pneumoniae, respectively.
4. Pharmacodynamics
As CMS is an inactive prodrug of colistin, PD studies using CMS are not valid and will not be reviewed here.
4.1 Susceptibility and minimal inhibitory concentrations (MICs)
Polymyxins are mainly active against Gram-negative bacteria while Gram-positive micro-organisms are usually resistant. Antimicrobial susceptibility testing for colistin and polymyxin B can be performed using disc diffusion, E-test, agar dilution and broth dilution, although the accuracy of the disc diffusion method compared to other methods has been questioned. Different susceptibility breakpoints have been employed by various organizations (Table 1). As CMS is an inactive pro-drug, it should not be employed for susceptibility testing. All currently available breakpoints for colistin susceptibility are for colistin sulfate. As the PK/PD information of colistin and polymyxin B only became available recently (see sections below), it is very likely that some of the current breakpoints (Table 1) may not be appropriate; for example, >4 μg/ml for colistin resistance against P. aeruginosa may be too high based upon the emerging data on achievable plasma concentrations in patients, as discussed below.
Table 1.
Susceptibility breakpoints (μg/ml) of colistin and polymyxin B
| Polymyxin | P. aeruginosa | A. baumannii | Enterobacteriaceae | Committee† (year) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| S* | I | R | S | I | R | S | I | R | ||
| Colistin | ≤2 | 4 | ≥8 | ≤2 | - | > 2 | - | - | - | CLSI (2012) |
| ≤ 4 | - | > 4 | ≤2 | - | > 2 | ≤2 | - | > 2 | EUCAST (2012) | |
| ≤ 4 | - | > 4 | ≤2 | - | > 2 | ≤2 | - | > 2 | BSAC (2011) | |
| S: ≤2; R: >2 (no specified species) | CA-SFM (2003) | |||||||||
|
| ||||||||||
| Polymyxin B | ≤ 2 | 4 | ≥8 | ≤2 | - | > 2 | - | - | - | CLSI (2012) |
S: susceptible; I: intermediate; R: resistant.
CLSI: Clinical and Laboratory Standards Institute; EUCAST: European Committee on Antimicrobial Susceptibility Testing; BSAC: British Society for Antimicrobial Chemotherapy; CA-SFM: CA-Comité de l’Antibiogramme de la Société Française de Microbiologie, France.
Although multidrug resistance is very challenging in Gram-negative pathogens, most isolates of P. aeruginosa, A. baumannii and K. pneumoniae remain susceptible to colistin and polymyxin B (Table 2). In general, colistin and polymyxin B demonstrated excellent in vitro activity against a large collection of clinical isolates of Acinetobacter spp. (MIC90 ≤ 2 μg/ml), K. pneumoniae (MIC90 ≤ 2 μg/ml), E. coli (MIC90 ≤ 1 μg/ml) and P. aeruginosa (MIC90 ≤ 2 μg/ml). Resistance rates to colistin and polymyxin B during 2006 to 2009 generally remained stable among the clinical isolates examined, except for Klebsiella spp. isolates collected from the Asia-Pacific and Latin American regions where a trend of slight increase in resistance was observed. This may be due to suboptimal use of polymyxins as a result of lack of PK/PD knowledge. Neither polymyxin B nor colistin is currently available for clinical use in China. In a national surveillance conducted between January 1 to December 31, 2010 in 129 Chinese hospitals, the susceptibility rates of P. aeruginosa (4925 isolates) and A. baumannii (3490 isolates) to polymyxin B were 96.4% and 97.2% respectively.
Table 2.
Susceptibility of polymyxins in common clinical Gram-negative bacteria
| Ref | Year | Polymyxin (MIC method) | Species (number of isolates) | MIC50 (μg/ml) | MIC90 (μg/ml) | Range (μg/ml) | Susceptible (%) |
|---|---|---|---|---|---|---|---|
| 2006–2009 | Colistin (broth microdilution) | Acinetobacter spp. (4686) | ≤0.5 | 1.0 | - | 98.6 | |
| E. coli (17035) | ≤0.5 | ≤0.5 | - | 99.8 | |||
| Klebsiella spp. (9774) | ≤0.5 | ≤0.5 | - | 98.5 | |||
| P. aeruginosa (9130) | 1 | 1 | - | 99.6 | |||
|
| |||||||
| Polymyxin B (broth microdilution) | Acinetobacter spp. (4686) | ≤0.5 | ≤0.5 | - | 99.2 | ||
| E. coli (17035) | ≤0.5 | ≤0.5 | - | 99.9 | |||
| Klebsiella spp. (9774) | ≤0.5 | ≤0.5 | - | 98.6 | |||
| P. aeruginosa (9130) | 1 | 1 | - | 99.8 | |||
|
| |||||||
| 2009 | Polymyxin B (agar dilution) | E. coli (3049) | 1 | 1 | ≤0.12 – >8 | 99.8 | |
| K. pneumoniae (1155) | 1 | 2 | 0.25 – >16 | 96 | |||
| Enterobacter spp. (199) | 1 | >8 | ≤0.12 – >16 | 76 | |||
| Acinetobacter spp. (407) | 1 | 2 | ≤0.25 – >16 | 97 | |||
| P. aeruginosa (679) | 1 | 2 | 0.25 – 4 | 99.5 | |||
|
| |||||||
| 2010 | Colistin (broth microdilution) | Acinetobacter spp. (514) | 1 | 2 | 0.12 – >32 | 94.7 | |
4.2 In vitro static and dynamic time-kill kinetics
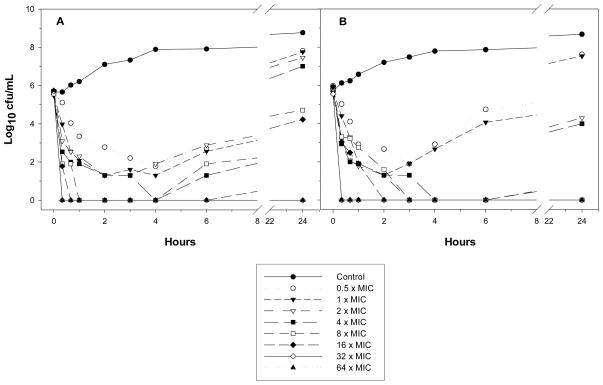
Colistin (sulfate) showed rapid concentration-dependent killing against MDR P. aeruginosa, A. baumannii and K. pneumoniae in in vitro static time-kill studies. However, regrowth can occur even at colistin concentrations up to 64 × MIC (Figure 2). It is very likely that the rapid emergence of resistance to colistin is due to colistin heteroresistance (defined as the presence of colistin-resistant subpopulations in an isolate that is susceptible based upon MIC). Population analysis profiles (PAPs) have revealed that a small proportion of colistin-resistant cells exists in many clinical isolates of P. aeruginosa, A. baumannii and K. pneumoniae; upon exposure to colistin, killing of the susceptible population plus amplification of the resistant subpopulations may result in up to 100% of the remaining bacterial cells being able to grow on PAPs agar plates containing 10 μg/ml colistin. These in vitro studies suggest that polymyxin monotherapy may lead to emergence of resistance and, as will be discussed below, highlight the importance of investigating combination therapy. Static time-kill studies also demonstrated that the rate and extent of killing by colistin are markedly decreased at high (i.e. 108 CFU/mL) compared to low inocula (i.e. 106 CFU/mL).
Figure 2.
Static time-kill curves for colistin against two A. baumannii isolates. Reproduced from Owen et al. [2007] with permission.
There have been only a small number of in vitro dynamic time-kill studies on polymyxins against Gram-negative pathogens. Bergen et al. examined the impact of three different colistin dosing regimens against P. aeruginosa in an in vitro dynamic model. The three treatment regimens simulated the plasma concentration - time profiles of formed colistin after intravenous administration of CMS once, twice or thrice daily (colistin Cmax of 9.0, 4.5 or 3.0 μg/ml, respectively); the regimens, which delivered essentially the same daily dose of colistin, were evaluated against two strains of P. aeruginosa. With all regimens against both strains, rapid initial concentration-dependent killing was followed by regrowth. Although no difference in overall bacterial killing was found, the 8-hourly regimen appeared most effective at delaying the onset of resistance development. In an in vitro hollow-fiber infection model, three regimens (8-, 12-, and 24-hourly administration of the same daily dose) were employed to simulate the steady-state polymyxin B PK in patients and assess activity against P. aeruginosa over 4 days. Emergence of resistance was less with the 8-hourly regimen of polymyxin B, similar to the finding with colistin above. In the third study, the same intermittent regimens of colistin examined by Bergen et al. were simulated in an in vitro PK/PD model against two colistin-heteroresistant strains of A. baumannii. Extensive initial killing was followed by regrowth as early as 6 h later, and bacterial density in the 24- to 72-h period was within 1 log10 CFU/mL of growth control. PAPs revealed extensive emergence of resistant subpopulations regardless of the colistin regimen. Thus, the propensity for emergence of colistin resistance being dependent upon the dosing interval selected appears to differ across bacterial species and may depend on the resistance mechanisms involved. None of these studies was designed to elucidate the PK/PD index correlating with antibacterial effect. However, the finding that the time course of total bacterial counts was not greatly different across the various dosing regimens within the given studies suggests that time-averaged exposure to polymyxins is important for bacterial killing; as discussed below, recent more extensive dose-fractionation studies in both in vitro dynamic and animal infection models have demonstrated that free AUC/MIC (fAUC/MIC) is the PK/PD index that correlates best with antibacterial activity.
4.3 Post-antibiotic effect (PAE)
Only very modest PAE was observed with colistin against P. aeruginosa and K. pneumoniae at high concentrations (e.g. 32 × MIC). With 1-h treatment at 1 and 4 × MIC, Özbek and Şentürk reported a 2.50 – 7.0 h PAE in 6 non-duplicate, nosocomially acquired, meropenem-resistant A. baumannii isolates. In a separate study, a modest (0.3 – 3.5 h) or negative PAE (i.e. where polymyxin-treated bacteria grew more quickly after removal of the drug compared to controls) was revealed in A. baumannii ATCC 19606 and 5 clinical isolates after 20-min exposure to colistin at 0.5 – 64 × MIC ; the mechanism of a negative PAE is unknown. After 5-h exposure to colistin with 19 clinical A. baumannii isolates, a PAE of 0.82 – 8.04 h was observed with 1 × MIC by Plachouras et al. . In colistin PAE studies, an appropriate exposure time is crucial since prolonged treatment may eradicate bacterial cells due to rapid bactericidal killing at high concentrations (e.g. 64 × MIC), and substantial regrowth can occur as early as 4 h after exposure due to colistin-resistant subpopulations. The role of PAE of colistin in relation to antibacterial activity in patients is unclear and may not be relevant because of the relatively long half-life of formed colistin and the small fluctuations in plasma colistin concentration that occur across a CMS dosage interval in critically-ill patients.
4.4 In vivo time-kill studies
There is only one in vivo colistin time-kill study against P. aeruginosa using a neutropenic mouse thigh infection model. Mice were infected by two isolates with 6.3 – 6.6 log CFU per thigh and treated with single subcutaneous doses of colistin 2.5 – 40 mg/kg. Colistin showed concentration-dependent killing with maximal kill of 2.8 – 3.3 log. Similar to in vitro static studies, regrowth was observed at 6 – 9 h with 10 mg/kg, at 12 h with 20 mg/kg and 12 – 24 h with 40 mg/kg of colistin. There is little information about the efficacy of polymyxin B in animal infection models. However, considering the single amino acid difference between polymyxin B and colistin (Figure 1), it is very likely that polymyxin B and colistin have very similar bacterial killing in vitro and in vivo.
5. In vitro and in vivo PK/PD of polymyxins
Considerable progress has been made over the past few years towards the elucidation of the PK/PD relationship of polymyxins with most studies focusing on colistin. Bergen et al. first employed an in vitro PK/PD model to demonstrate that the fAUC/MIC is the PK/PD index that best predicts colistin antibacterial activity against P. aeruginosa, being superior to fCmax/MIC and fT > MIC. Dudhani et al. used neutropenic murine thigh and lung infection models to identify the most predictive PK/PD index of the antibacterial activity of colistin against P. aeruginosa and A. baumannii. Consistent with the in vitro PK/PD study, fAUC/MIC correlated best with colistin activity in both lung and thigh infection models against both pathogens. The fAUC/MIC targets were generally similar in the two infection models for 1- and 2-log kill of P. aeruginosa. For example, in the thigh model the fAUC/MIC values for 1- and 2-log kill of three P. aeruginosa strains ranged from 15.6 – 22.8 and 27.6 – 36.1, respectively; for the lung infection model, the corresponding values were 12.2 – 16.7 and 36.9 – 45.9. These values are generally consistent with those obtained with an in vitro PK/PD model. Against A. baumannii, the fAUC/MIC values required to achieve stasis and 1-log kill were 1.57 – 6.52 and 8.18 – 42.1 in the lung infection model, respectively; the corresponding values were 1.89 – 7.41 and 6.98 – 13.6 in the thigh infection model. Amplification of colistin-resistant subpopulations was revealed for all three A. baumannii isolates in both models after 24-h colistin treatment. These PK/PD studies defined fAUC/MIC values for various magnitudes of kill and indicated the importance of achieving adequate time-averaged exposure to colistin. As discussed below, these pre-clinical PK/PD results have begun to be used translationally to facilitate efforts to optimize CMS/colistin dosing in humans.
More recently, Wang et al. determined the PK/PD indices for colistin that were the most predictive of activity against planktonic and biofilm cells of P. aeruginosa in a neutropenic murine lung biofilm infection model. In agreement with the results in the in vitro PK/PD model and the neutropenic lung and thigh mouse infection studies (see above), AUC/MIC was the PK/PD index best correlated with bacterial killing of colistin against planktonic cells; for biofilm cells in the lung, AUC to minimal biofilm inhibitory concentration (MBIC) ratio (AUC/MBIC) was the most predictive PK/PD index. Not surprisingly, the AUC/MBIC targets required for biofilm infections were much higher than for planktonic cells. For example, 1-log kill by colistin required an AUC/MIC of 297 for planktonic cells, while an AUC/MBIC of 185 (i.e. an AUC/MIC of 867) was required for cells in biofilm. In this study, plasma protein binding was not measured for colistin; therefore AUC/MIC and AUC/MBIC values were for total colistin.
6. Pharmacokinetics of polymyxins
6.1 Preclinical PK studies
In a rat model, very different PK of colistin and CMS were revealed using accurate HPLC methods that are capable of distinguishing between CMS and colistin. Following an intravenous bolus dose of colistin (sulfate, 1 mg/kg), the total body clearance (CL) of colistin was 5.2 ± 0.4 mL/min/kg, with a renal clearance (CLR) of 0.010 ± 0.008 mL/min/kg; this latter value was far lower than the anticipated clearance by glomerular filtration of 2.3 mL/min/kg, with only 0.18 ± 0.14% of the total colistin dose recovered in urine over 24 h. This result first indicated very extensive renal tubular reabsorption of colistin through a carrier-mediated process, and its clearance mainly via non-renal pathway(s); an isolated perfused kidney study in rats confirmed the extensive tubular reabsorption. The unbound fraction of colistin (ƒu) in healthy rat plasma was ~0.44 for colistin concentrations of 1.5 – 6.0 μg/ml.
Following a single intravenous bolus of CMS (15 mg/kg), conversion of CMS to colistin was observed and the CL of CMS was 11.7 mL/min/kg. In contrast to the low CLR observed previously for colistin, the CLR of CMS was greater than the glomerular filtration rate (7.2 ± 2.2 mL/min/kg versus ~5.2 mL/min/kg, respectively). Even without consideration of plasma protein binding, this result indicated net tubular secretion of CMS into urine. During the first 24 h after dosing 61.1% ± 14.4% of the total dose of CMS was recovered in urine, with approximately half present as colistin. However, PK analysis revealed that only ~7% of the administered dose of CMS was converted to colistin systemically. Considering this low systemic conversion of CMS to colistin, extensive renal tubular reabsorption of colistin, and the conversion of CMS to colistin in aqueous media including urine, the high urinary recovery of colistin after administration of CMS was very likely due to conversion of CMS at 37°C within renal tubular cells, the bladder and/or at room temperature in the collection vessel. The terminal t1/2 of formed colistin was approximately twice that of the administered CMS (55.7 ± 19.3 min versus 23.6 ± 3.9 min), suggesting that the elimination of colistin is not rate limited by its formation from CMS, and was similar to the t1/2 of colistin administered directly. An independent rat PK study by Marchand et al. using a wide range of CMS doses (5 – 120 mg/kg intravenously) confirmed the observations of Li et al. in regard to the fundamental aspects of the overall disposition of CMS and formed colistin. In addition, linear PK of CMS and formed colistin was observed in rats treated with the wide range of intravenous doses of CMS.
6.2 Clinical pharmacokinetic studies
Over the last decade, our knowledge on the clinical PK of CMS and formed colistin and polymyxin B has increased substantially. As the old CMS/colistin PK data obtained with microbiological assays are invalid due to conversion of CMS to colistin during the assay, this section will only review those clinical PK studies that were conducted with HPLC or LC-MS/MS assays which are able to quantify CMS and formed colistin separately.
As CMS/colistin and polymyxin B are increasingly being used as salvage therapy in critically-ill patients with MDR Gram-negative infections, a comprehensive understanding of their PK is vital to optimize their dosage regimens in this patient population. This review will focus on the clinical studies after intravenous administration of CMS and polymyxin B in critically-ill patients. Please refer to our Lancet Infect Dis review regarding the very different and confusing product content labelling (i.e. International Units [IU] versus colistin base activity [CBA]) used in various parts of the world; here, we express doses in both conventions to facilitate interpretation for the reader.
Several studies investigated the PK of formed colistin after CMS dosing in critically-ill patients, but did not measure CMS. Imberti et al. studied the steady-state PK of formed colistin in 13 critically-ill patients with ventilator-associated pneumonia caused by Gram-negative bacteria. With 2 million IU (i.e. 65 CBA) intravenous CMS 8-hourly, the maximum plasma concentrations of formed colistin at steady state (Cmax,ss) were 2.21 ± 1.08 μg/ml at 1 h after the start of the infusion with an apparent terminal t1/2 of 5.9 ± 2.6 h. Bronchoalveolar lavage (BAL) was performed at 2 h after the start of the CMS infusion but colistin was undetectable in BAL (the detection limit of the assay in terms of epithelial lining fluid was ~10 μg/ml after considering the dilution during BAL collection). This PK study suggested that intravenous administration of CMS 2 million IU (i.e. 65 CBA) 8-hourly leads to suboptimal plasma concentrations of colistin. In another clinical PK study with 14 critically-ill patients administered 2.8 million IU (i.e. 84 mg CBA) 8-hourly or 12-hourly, Cmax,ss of formed colistin was 2.93 ± 1.24 μg/ml and the apparent total body clearance, apparent volume of distribution, and t1/2 of formed colistin were 13.6 ± 5.8 L/h, 139.9 ± 60.3 L and 7.4 ± 1.7 h, respectively. After intravenous administration of CMS (1.88 – 8.44 million IU per day, i.e. 56 – 253 mg CBA per day) in five critically-ill adult patients, concentrations of formed colistin in the cerebrospinal fluid (CSF) varied between 0.041 – 0.099 μg/ml and the CSF-to-serum ratios were 0.051 – 0.057 in the patients at the sampling times examined. This indicates that intravenous administration of CMS is not suitable for treatment of meningitis.
The disposition of both CMS and formed colistin was examined by Plachouras et al. in 18 critically-ill patients (age range 40 – 83 years; 6 females) with moderate to good renal function (creatinine clearance of 41 – 126 mL/min) receiving maintenance doses of 3 million IU (i.e. 90 CBA) of CMS 8-hourly intravenously (two patients with creatinine clearance < 50 mL/min received 2 million IU 8 hourly). More recently, the same group reported clinical PK data on a further 10 critically-ill patients (age range 32 – 88 years; 4 females) with creatinine clearance values ranging from 24 to 214 mL/min; CMS maintenance doses were 1 – 3 million IU (30 – 90 mg CBA) every 8 hours. Population PK analysis of the data from the combined 28 patients revealed that the estimated PK parameters for CMS were clearance 13.1 L/h and terminal half-life (t1/2) 2.2 h, while the terminal t1/2 of formed colistin was 18.5 h. As a consequence of these dispositional events relating to CMS and formed colistin, patients were exposed to sub-optimal plasma colistin concentrations for 2 – 3 days before reaching steady state. Even at an average plasma colistin Cmax,ss of 2.3 μg/ml and without considering plasma protein binding, a large fraction of the patients had plasma colistin concentrations below the MIC breakpoint of 2 μg/ml (Table 1). Based upon these considerations, the need for a loading dose and a change in the dosing strategy for CMS was suggested. Unfortunately, due to the relatively small patient numbers and the limited range of creatinine clearance values, it was not possible to identify patient covariates, including creatinine clearance, as a driver of disposition in both studies.
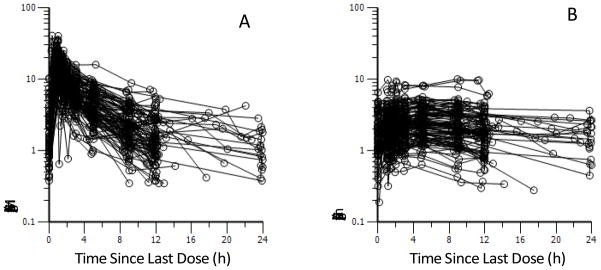
A recent population PK study developed the first scientifically based dosing suggestions for CMS to achieve a desired target steady-state plasma concentration of formed colistin in various categories of critically ill patients. Of the 105 critically-ill patients, 89 were not on renal replacement and had a large range of renal functions (creatinine clearance 3 – 169 mL/min/1.73m2), 12 were on intermittent hemodialysis and 4 on continuous renal replacement. The median daily dose across the 105 patients was 200 mg CBA (range 75 – 410 mg CBA; i.e. 2.5 – 13.7 million IU). Both CMS and formed colistin were efficiently cleared by hemodialysis and continuous renal replacement therapy (see below). The average steady-state plasma concentration (Css,avg) of formed colistin in all patients ranged from 0.48 to 9.38 μg/ml (median 2.36 μg/ml; Figure 3). Importantly, creatinine clearance was identified, for the first time, as an important covariate for the total clearance of CMS (mainly eliminated renally) and also the apparent clearance of formed colistin (predominantly non-renally cleared). With decreasing renal function, a larger fraction of the CMS dose was converted to colistin; this explains the observed decrease in the apparent clearance of formed colistin with declining creatinine clearance. On the basis of the population PK modelling, including the identification of creatinine clearance as an important covariate for clearance of CMS and colistin, the authors were able to propose maintenance dosage regimens for CMS to achieve a given Css,avg of formed colistin in patients with various degrees of renal function and, as discussed below, those receiving renal replacement therapy. An important finding of this study was that with the currently recommended dosage regimens, it is very difficult to achieve what are likely to be adequate plasma concentrations of formed colistin with CMS monotherapy, especially in patients with moderate to good renal function and if the MIC of the isolate is ≥ 1 μg/ml. It should also be noted that nephrotoxicity is a dose-limiting factor for intravenous CMS; even with the currently recommended dosage regimens, ~50% of patients developed various degrees of nephrotoxicity. Therefore, CMS/colistin might best be used as part of a highly active combination, in particular when treating an infection caused by an isolate with an MIC of ≥ 1 μg/ml in a patient with a creatinine clearance greater than ~70 mL/min/1.73m2.
Figure 3.
Steady-state plasma concentration versus time profiles of (A) CMS and (B) formed colistin in 105 critically-ill patients. Physician-selected CMS dosage intervals ranged from 8 to 24 h; therefore, the inter-dosing blood sampling interval spanned the same range. Reproduced from Garonzik et al. [2011] with permission.
In a PK study conducted in 12 young healthy volunteers (29.5 ± 5.5 years), a single dose of 1 million IU (i.e. 30 mg CBA) CMS was infused over 1 h. Similar to the findings in rats, CMS was predominantly excreted in urine (70% on average as both CMS and colistin, with the majority of the latter formed in the urinary tract).
There were only a very limited number of clinical PK studies on CMS and formed colistin in patients on renal replacement therapy. Li et al. described the steady-state PK in a critically-ill patient on continuous venovenous hemodiafiltration (CVVHDF) treated with intravenous CMS (150 mg CBA, 5 million IU) 48 hourly, a dosage regimen recommended by the Product Information. This dosage regimen was used, as there was a lack of scientifically based information to guide dose adjustment in patients on continuous renal replacement therapy. This CMS dose led to the plasma concentrations of formed colistin falling below the MIC of 1 μg/ml approximately 6 h after the dose; unfortunately the patient died. This study first revealed that both CMS and colistin are cleared by CVVHDF (clearances of CMS and colistin were 11.2 mL/min and 11.9 mL/min, respectively), and highlighted the urgent need to investigate the PK of CMS and formed colistin in this subset of critically-ill patients. In another case study, Marchand et al. examined the PK of CMS and formed colistin in two patients on intermittent hemodialysis. With intravenous administration of 1 million IU (i.e. 30 mg CBA) every 48 h or 2 million IU (i.e. 60 mg CBA) every 12 h, time-averaged dialysis clearances were ~140 mL/min for colistin and ~90 mL/min for CMS. In a recent study, both CMS and colistin were shown to undergo efficient extracorporeal clearance in a total of 16 patients; based upon the population PK analysis, the authors were able to suggest loading and maintenance doses of CMS for patients on intermittent hemodialysis or continuous renal replacement and supplemental doses for patients in the former category after a dialysis session.
Although significant progress has been made recently in understanding how to optimize clinical use of CMS, PK/PD data and dosing recommendations for parenteral CMS/colistin cannot be extrapolated to polymyxin B. First, polymyxin B is not administered as a prodrug. Secondly, the overall PK of CMS/colistin is very complex. CMS is predominantly cleared via the renal route, while colistin formed in vivo is inefficiently cleared by the kidneys. Lastly, in a subject with normal renal function only a very small fraction of the administered dose of CMS is converted slowly to colistin. Without administration of a loading dose of CMS, the slow accumulation of formed colistin in plasma may cause sub-optimal exposure over the first day or two of therapy, thereby leading to emergence of resistance. However, because polymyxin B is administered in its active form (i.e. not as an inactive prodrug), administration of a suitable intravenous loading dose of polymyxin B will more rapidly achieve higher exposure than can occur with generation of colistin, even when an intravenous loading dose of CMS is used.
As polymyxin B is used much less widely than CMS, there are fewer clinical PK studies on polymyxin B and they incorporate only a relatively small number of patients receiving intravenous administration (100 – 400 mg/day). Kwa et al. examined the disposition of polymyxin B1 (one major component of polymyxin B; Figure 1) in 9 non-critically-ill adult patients with normal renal function. The average volume of distribution and elimination t1/2 were 47.2 L and 13.6 h, respectively. In another clinical PK study, 8 critically-ill patients (APACHE II score: 20 – 27) received 1-h infusions of 0.5 to 1.5 mg/kg polymyxin B 12- or 48-hourly. Renal function of the 8 patients ranged widely (creatinine clearance from <10 to 246 mL/min). The Cmax, total body clearance and volume of distribution were 2.38 – 13.9 μg/ml, 0.27 – 0.81 mL/min/kg and 71 – 194 mL/kg, respectively. Only 0.04 – 0.86% of the dose was recovered unchanged in urine in 4 patients where urine collection was possible; this finding indicates that total body clearance of polymyxin B is relatively insensitive to renal function, which is similar to colistin observed in rats. In a case report, a 50-year-old Chinese patient with a creatinine clearance of 18 mL/min was administered an initial intravenous infusion of 50 mg polymyxin B over 60 min, followed by infusions of 75 mg over 90 min every 24 h. The elimination t1/2 of polymyxin B1 in this renally insufficient patient was 11.5 h, which was similar to those previously observed in patients with normal renal function. In a 9-month-old infant treated with intravenous polymyxin B for MDR K. pneumoniae bacteremia, the elimination t1/2 of polymyxin B1 and isoleucine polymyxin B1 were 3.1 and 4.7 h, respectively, which are much shorter than those observed in the limited number of adult patients studied. Overall, polymyxin B is predominantly non-renally eliminated and larger clinical studies, including in patients on renal replacement therapy, are needed for optimizing dosage regimens for different patient populations.
7. Combination therapy with polymyxins
As outlined in Sections 4 and 5 above, the emerging PK and PD data on CMS/colistin suggest that caution is required with monotherapy due to sub-optimal exposure and emergence of resistance. Therefore, combination therapy has been suggested as a possible means to overcome these limitations.
7.1 In vitro time-kill studies
Numerous in vitro studies have employed time-kill methods to examine CMS/colistin or polymyxin B combination therapy. However, given that CMS is an inactive prodrug that undergoes conversion to the antibacterial entity colistin in aqueous media, employing CMS in these in vitro systems is invalid and studies identified as utilizing CMS will not be considered here. While many antimicrobial agents have been combined with colistin or polymyxin B, rifampicin and the carbapenems feature most often. The most common organisms studied are P. aeruginosa, A. baumannii and K. pneumoniae. Unless otherwise stated below, synergy is defined as a ≥2 log10 CFU/mL lower bacterial count with the combination than with its most active component antibiotic at a specified time; additivity is a 1 to <2 log10 CFU/mL lower bacterial count with the combination.
7.1.1 Static time-kill studies
Against 42 unique clinical isolates of blaVIM-1-type metallo-β-lactamase producing K. pneumoniae, the combination of colistin (5 μg/ml) and imipenem (10 μg/ml) resulted in synergy against 12 of 24 colistin-susceptible isolates, with antagonism observed against 10 of 18 colistin-resistant isolates. Resistance to colistin (MICs 64 – 256 μg/ml) was observed in 7 of 12 isolates initially susceptible to colistin. Urban et al. examined antibiotic combinations using polymyxin B, doripenem, and rifampicin against MDR carbapenem-resistant isolates of P. aeruginosa, A. baumannii, K. pneumoniae and E. coli; all antibiotics were used at 0.25 × MIC. As monotherapy, none of the tested antibiotics was bactericidal (defined as a ≥3 log10 CFU/mL decrease in 24 h at 0.25 × MIC). Triple therapy with the combination of polymyxin B, doripenem and rifampicin was most effective, with bactericidal activity achieved against 5 of 5 P. aeruginosa isolates, 5 of 5 E. coli isolates, 4 of 5 K. pneumoniae isolates, and 3 of 5 A. baumannii isolates. Combinations utilizing only two antibiotics were less effective, with polymyxin B plus doripenem or rifampicin bactericidal against only 1 – 2 of 5 isolates for all bacterial species except E. coli; against E. coli, polymyxin B plus doripenem was bactericidal against 4 of 5 isolates. In another study, Jernigan et al. investigated the activity of ‘colistin’ (1 μg/ml) in combination with doripenem (8 μg/ml), gentamicin (2 μg/ml) or doxycycline (2 μg/ml) against 12 carbapenemase-producing isolates of K. pneumoniae; all isolates were resistant to doripenem and most were resistant to the other agents. The colistin/doripenem combination was most active against all isolates. With this combination, synergy was observed in 6 of 12 isolates at 24 h (log-kills ranged from 2.02 to 6.01 log10 CFU/mL). Of the remaining combinations, synergy was reported in only 3 of 12 and 1 of 12 isolates when colistin was combined with gentamicin and doxycycline, respectively. Unfortunately, none of the studies above considered emergence of polymyxin resistance during the static time-kill.
Bergen et al. examined colistin/imipenem combinations and were the first to employ PAPs to investigate the emergence of colistin resistance with colistin combination therapy. This systematic study conducted at two inocula (~106 and 108 CFU/mL) over 48 h utilized MDR and non-MDR P. aeruginosa (6 isolates in total) and included colistin-resistant and heteroresistant isolates. Based upon the clinical PK of colistin and imipenem, 9 colistin/imipenem combinations were studied using concentrations of 0.5, 4 and 16× MIC for susceptible isolates; for resistant isolates, concentrations of 1, 4 and 32 μg/ml for colistin and 1, 8, and 32 μg/ml for imipenem were employed. Substantial improvements in activity (including additivity or synergy) with combinations were observed across 48 h with all colistin concentrations at the low inoculum, and with colistin at 4 and 16 × MIC (or 4 and 32 μg/ml) at the high inoculum. Notably, against a colistin-resistant isolate (MIC 128 μg/ml) the 9 combinations were additive or synergistic at 24 h in 9 and 8 cases at the 106 and 108 CFU/mL inocula, respectively, and in 5 and 7 cases at 48 h.
7.1.2 Dynamic time-kill studies
While numerous studies have employed static time-kill methods to examine colistin or polymyxin B combination therapy, remarkably few have employed in vitro dynamic models. Gunderson et al. was the first to utilize a one-compartment PK/PD model to examine colistin combination therapy with either ceftazidime or ciprofloxacin over 24 h against two colistin-susceptible MDR isolates of P. aeruginosa. The combination of colistin plus ceftazidime was synergistic. However, only changes in bacterial counts between colistin monotherapy and combination therapy were employed in the determination of synergy and when data for ceftazidime monotherapy (which was performed for only one of the two isolates tested) is considered, synergy was not observed. More recently, Bergen et al. investigated bacterial killing and the emergence of colistin resistance with colistin and doripenem combinations against a colistin-heteroresistant reference strain ATCC 27853 and colistin-resistant MDR clinical isolate of P. aeruginosa. Four combinations utilizing clinically achievable concentrations of each drug (constant colistin concentrations of 0.5 or 2 μg/ml, mimicking the ‘flat’ plasma concentration - time profiles across a dosing interval at steady state (Figure 3); doripenem with peaks of 2.5 or 25 μg/ml 8-hourly, t1/2 1.5 h) were investigated at two inocula (~106 and ~108 CFU/mL). All combinations at the low inoculum, and combinations containing 2 μg/ml colistin at the high inoculum, substantially increased bacterial killing against both strains. Against the colistin-resistant MDR isolate, combinations containing doripenem at 25 μg/ml resulted in bacterial eradication at the low inoculum. Importantly, combination therapy against the colistin-heteroresistant strain at both inocula substantially reduced or delayed the emergence of colistin-resistant subpopulations. This important finding was in contrast to earlier work by the same group utilizing static time-kill studies for colistin/imipenem combination therapy against colistin-susceptible isolates (including heteroresistant isolates) in which little effect was observed on the proportion of colistin-resistant subpopulations; loss of imipenem due to degradation in the static experiments may have contributed to these different results. This observation highlights the importance of in vitro dynamic PK/PD models in assessing the activity and the emergence of resistance to antimicrobial therapy.
7.2 Animal studies
The number of animal studies examining colistin combination therapy is small and most, like clinical studies discussed below, suffer from a number of significant shortcomings that make the results difficult to interpret. These include ambiguity around whether the administered ‘colistin’ was colistin (sulfate) or CMS (sodium), and failure to provide a rationale for the doses of CMS/colistin administered or to recognize the importance of animal scaling. Crucially, PK data for CMS/colistin and the second antibiotic are absent from virtually all investigations, preventing comparisons with PK profiles achieved in patients.
Cirioni et al. examined ‘colistin’ (although it is unclear whether colistin or CMS was administered) in combination with imipenem or rifampicin against P. aeruginosa using rat and mouse sepsis models; all antibiotics were administered intravenously. Both combinations resulted in significantly enhanced bacterial killing across 72 h when compared with monotherapy, although only the colistin/imipenem combination had significantly lower mortality. In a mouse pneumonia model, all control mice receiving monotherapy (n = 14 to 16) with CMS (total daily dose of 20 mg/kg administered subcutaneously [SC] or 10 mg/kg administered intranasally; both divided into two doses administered every 12 h), imipenem (SC; total daily dose of 60 mg/kg divided into two doses administered every 12 h) or rifampicin (25 mg/kg orally every 24 h) died within 42 h of infection with P. aeruginosa PAO1. Combinations of CMS plus imipenem or rifampicin increased survival to 62.5% and 75% at 72 h, respectively; intranasal CMS was also superior to SC CMS when combined with imipenem. Similar trends were observed using a MDR clinical isolate of P. aeruginosa. In contrast, no differences were observed in survival or bacterial clearance from the lungs in mouse pneumonia models with rifampicin monotherapy (25 mg/kg intraperitoneally [IP] as a single daily dose or 100 mg/kg/day IP in four divided doses) and rifampicin/CMS (40 mg/kg/day IP in four divided doses or 60 mg/kg/day IM in three divided doses) combination therapy against MDR A. baumannii. Against E. coli in a septic shock rat model, mortality at 48 h was 93.3%, 33.3%, 33.3%, and 0% for animals following a single IP administration of ‘colistin’ monotherapy (1 mg/kg; unclear as to whether CMS sodium or colistin sulfate was administered), piperacillin monotherapy (60 mg/kg), and ‘colistin’ plus piperacillin combination, respectively (n = 15). In a recent mouse thigh and lung infection study, PK/PD of colistin, doripenem and rifampicin alone was considered in the design of this combination study ; the colistin/doripenem combination was superior (at least >1 log better kill) to the most active monotherapy in both lungs and thighs for 2 of 3 strains of both P. aeruginosa and K. pneumoniae. Against the A. baumannii strains in the thigh infection, the colistin/rifampicin combination was more active than monotherapy.
7.3 Clinical studies of CMS combination therapy
While a number of clinical studies have involved CMS combination therapy, major limitations with all published clinical studies exist due to practical and ethical considerations. Notably, these clinical investigations are primarily retrospective in nature and do not include PK information on CMS, formed colistin, or concomitant antibiotics. Further, the number of patients participating is usually small and there is heterogeneity in the definitions of outcomes (e.g. mortality or clinical cure), variability in the dosing regimens, differences in the susceptibility testing methods (disc or broth dilution), and often no clear rationale for the choice of the second antibiotic. Moreover, most studies did not stratify outcome by severity of illness, an important consideration as combination therapy is most likely to be given to the sickest patients who are more likely to die. Thus, although clinical studies to date have not shown any clear advantage for CMS combination therapy, the limitations associated with these studies significantly hinder interpretation of the data and, as such, the benefits to patients of CMS combination therapy remain unclear. As a consequence, clinical studies will only be briefly reviewed here.
Few studies directly compared the effectiveness of CMS monotherapy with combination therapy. In a recent study, Falagas et al. reported on 258 patients infected with MDR Gram-negative pathogens. Infection was cured in an equal proportion of patients (83.3%) who received CMS monotherapy or CMS combined with meropenem, whereas patients treated with CMS combined with piperacillin/tazobactam, ampicillin/sulbactam or other agents had significantly lower rates of infection cure (64.7%, 75.0% and 61.3%, respectively); average daily doses or dosage ranges for patients receiving mono- or combination therapy were not specified. When only patients infected with polymyxin-only-susceptible bacteria were included, 18 (90%) of 20 patients treated with CMS monotherapy were cured of the infection compared with 70 (83.3%) of 84 patients treated with CMS combined with meropenem and 17 (54.8%) of 31 patients treated with other antimicrobial agents. Multivariate analysis for this subset of patients showed that treatment with CMS monotherapy or CMS/meropenem combination therapy was an independent factor for cure of infection. In contrast, a much smaller review of studies involving a total of 18 patients with infections caused by KPC β-lactamase-producing K. pneumoniae treated with polymyxins (CMS or polymyxin B) alone or in combination reported that infections were successfully treated in 1 of 7 patients receiving polymyxin monotherapy and 8 of 11 patients receiving combination therapy (mainly with tigecycline or gentamicin). In patients receiving antimicrobial therapy for acute respiratory exacerbation of cystic fibrosis, no substantial differences were observed between patients receiving 2 million IU of CMS (i.e. 60 mg CBA) intravenously every 8 hours as monotherapy (n = 36) or CMS plus a second anti-pseudomonal antibiotic (n = 35).
8. Conclusion
In summary, over the last decade significant progress has been made in understanding the PK/PD of CMS/colistin and polymyxin B. As both polymyxins have been off patent for decades, it is very difficult to seek funding from pharmaceutical companies to re-develop ‘old’ polymyxins through contemporary drug development procedures. Fortunately, with significant funding support from government agencies (e.g. US National Institutes of Health (NIH; National Institute of Allergy and Infectious Diseases (NIAID)), Australian National Health and Medical Research Council (NHMRC) and European Commission), the knowledge derived over the last decade from investigations into the PK, PD, and PK/PD of polymyxins means we are now in a much better position to optimize the dosing regimens for maximizing efficacy while minimizing development of resistance. Importantly, systematic pre-clinical and clinical PK and PD investigations on polymyxin B are urgently needed and are being undertaken in several laboratories, including ours. The current poor Product Information for CMS and polymyxin B products needs to be updated by incorporating the recent modern PK and PD information, which will greatly help clinicians to optimize their clinical use. As new antibiotics will not become available for many years, CMS/colistin and polymyxin B will continue being a last-line therapeutic option against MDR Gram-negative ‘superbugs’. In the battle against rapidly emerging Gram-negative bacterial resistance we can no longer rely on discovery of new antibiotics. We must pursue rational approaches to the use of polymyxins and other existing antibiotics through the application of PK/PD principles.
Acknowledgments
JL and RLN are supported by Award Numbers R01AI098771, R01AI079330 and R01AI070896 from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. JL is an Australian National Health and Medical Research Council (NHMRC) Senior Research Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MD, Nickel GC, Bajaksouzian S, Lavender H, Murthy AR, Jacobs MR, Bonomo RA. Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother. 2009;53:3628–3634. doi: 10.1128/AAC.00284-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N, Tateda K, Kikuchi Y, Kimura S, Miyazaki C, Ishii Y, Tanabe Y, Gejyo F, Yamaguchi K. Efficacy of colistin combination therapy in a mouse model of pneumonia caused by multidrug-resistant Pseudomonas aeruginosa. J Antimicrob Chemother. 2009;63:534–542. doi: 10.1093/jac/dkn530. [DOI] [PubMed] [Google Scholar]
- Balaji V, Jeremiah SS, Baliga PR. Polymyxins: Antimicrobial susceptibility concerns and therapeutic options. Indian J Med Microbiol. 2011;29:230–242. doi: 10.4103/0255-0857.83905. [DOI] [PubMed] [Google Scholar]
- Barnett M, Bushby SR, Wilkinson S. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol Chemother. 1964;23:552–574. doi: 10.1111/j.1476-5381.1964.tb01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, Dhanji H, Chart H, Bou G, Livermore DM, Woodford N. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother. 2011;55:3370–3379. doi: 10.1128/AAC.00079-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:1953–1958. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother. 2008;61:636–642. doi: 10.1093/jac/dkm511. [DOI] [PubMed] [Google Scholar]
- Bergen PJ, Bulitta JB, Forrest A, Tsuji BT, Li J, Nation RL. Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob Agents Chemother. 2010;54:3783–3789. doi: 10.1128/AAC.00903-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen PJ, Forrest A, Bulitta JB, Tsuji BT, Sidjabat HE, Paterson DL, Li J, Nation RL. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob Agents Chemother. 2011a;55:5134–5142. doi: 10.1128/AAC.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen PJ, Tsuji BT, Bulitta JB, Forrest A, Jacob J, Sidjabat HE, Paterson DL, Nation RL, Li J. Synergistic killing of multidrug-resistant Pseudomonas aeruginosa at multiple inocula by colistin combined with doripenem in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2011b;55:5685–5695. doi: 10.1128/AAC.05298-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge EG, Martin AJ. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmac Chemother. 1967;29:125–135. doi: 10.1111/j.1476-5381.1967.tb01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulitta JB, Yang JC, Yohonn L, Ly NS, Brown SV, D’Hondt RE, Jusko WJ, Forrest A, Tsuji BT. Attenuation of colistin bactericidal activity by high inoculum of Pseudomonas aeruginosa characterized by a new mechanism-based population pharmacodynamic model. Antimicrob Agents Chemother. 2010;54:2051–2062. doi: 10.1128/AAC.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos MA, Vargas MA, Regueiro V, Llompart CM, Alberti S, Bengoechea JA. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect Immun. 2004;72:7107–7114. doi: 10.1128/IAI.72.12.7107-7114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirioni O, Ghiselli R, Orlando F, Silvestri C, Mocchegiani F, Rocchi M, Chiodi L, Abbruzzetti A, Saba V, Scalise G, Giacometti A. Efficacy of colistin/rifampin combination in experimental rat models of sepsis due to a multiresistant Pseudomonas aeruginosa strain. Crit Care Med. 2007a;35:1717–1723. doi: 10.1097/01.CCM.0000266685.25436.03. [DOI] [PubMed] [Google Scholar]
- Cirioni O, Ghiselli R, Silvestri C, Kamysz W, Orlando F, Mocchegiani F, Di Matteo F, Riva A, Lukasiak J, Scalise G, Saba V, Giacometti A. Efficacy of tachyplesin III, colistin, and imipenem against a multiresistant Pseudomonas aeruginosa strain. Antimicrob Agents Chemother. 2007b;51:2005–2010. doi: 10.1128/AAC.01576-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausell A, Garcia-Subirats M, Pujol M, Busquets MA, Rabanal F, Cajal Y. Gram-negative outer and inner membrane models: insertion of cyclic cationic lipopeptides. J Phys Chem B. 2007;111:551–563. doi: 10.1021/jp064757+. [DOI] [PubMed] [Google Scholar]
- Clements A, Tull D, Jenney AW, Farn JL, Kim SH, Bishop RE, McPhee JB, Hancock RE, Hartland EL, Pearse MJ, Wijburg OL, Jackson DC, McConville MJ, Strugnell RA. Secondary acylation of Klebsiella pneumoniae lipopolysaccharide contributes to sensitivity to antibacterial peptides. J Biol Chem. 2007;282:15569–15577. doi: 10.1074/jbc.M701454200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway SP, Pond MN, Watson A, Etherington C, Robey HL, Goldman MH. Intravenous colistin sulphomethate in acute respiratory exacerbations in adult patients with cystic fibrosis. Thorax. 1997;52:987–993. doi: 10.1136/thx.52.11.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornaglia G, Giamarellou H, Rossolini GM. Metallo-beta-lactamases: a last frontier for beta-lactams? Lancet Infect Dis. 2011;11:381–393. doi: 10.1016/S1473-3099(11)70056-1. [DOI] [PubMed] [Google Scholar]
- Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, Mimoz O. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin Pharmacol Ther. 2011;89:875–879. doi: 10.1038/clpt.2011.48. [DOI] [PubMed] [Google Scholar]
- Decolin D, Leroy P, Nicolas A, Archimbault P. Hyphenated liquid chromatographic method for the determination of colistin residues in bovine tissues. J Chromatogr Sci. 1997;35:557–564. doi: 10.1093/chromsci/35.12.557. [DOI] [PubMed] [Google Scholar]
- Dotsikas Y, Markopoulou CK, Koundourellis JE, Loukas YL. Validation of a novel LC-MS/MS method for the quantitation of colistin A and B in human plasma. J Sep Sci. 2011;34:37–45. doi: 10.1002/jssc.201000680. [DOI] [PubMed] [Google Scholar]
- Dudhani RV, Nation RL, Li J. Evaluating the stability of colistin and colistin methanesulphonate in human plasma under different conditions of storage. J Antimicrob Chemother. 2010a;65:1412–1415. doi: 10.1093/jac/dkq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudhani RV, Turnidge JD, Coulthard K, Milne RW, Rayner CR, Li J, Nation RL. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother. 2010b;54:1117–1124. doi: 10.1128/AAC.01114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudhani RV, Turnidge JD, Nation RL, Li J. fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J Antimicrob Chemother. 2010c;65:1984–1990. doi: 10.1093/jac/dkq226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elverdam I, Larsen P, Lund E. Isolation and characterization of three new polymyxins in polymyxins B and E by high-performance liquid chromatography. J Chromatogr. 1981;218:653–661. doi: 10.1016/s0021-9673(00)82091-9. [DOI] [PubMed] [Google Scholar]
- Evans ME, Feola DJ, Rapp RP. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann Pharmacother. 1999;33:960–967. doi: 10.1345/aph.18426. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Bliziotis IA, Kasiakou SK, Samonis G, Athanassopoulou P, Michalopoulos A. Outcome of infections due to pandrug-resistant (PDR) Gram-negative bacteria. BMC Infect Dis. 2005;5:24. doi: 10.1186/1471-2334-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Kasiakou SK, Kofteridis DP, Roditakis G, Samonis G. Effectiveness and nephrotoxicity of intravenous colistin for treatment of patients with infections due to polymyxin-only-susceptible (POS) gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 2006;25:596–599. doi: 10.1007/s10096-006-0191-2. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Rafailidis PI, Matthaiou DK, Virtzili S, Nikita D, Michalopoulos A. Pandrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii infections: characteristics and outcome in a series of 28 patients. Int J Antimicrob Agents. 2008;32:450–454. doi: 10.1016/j.ijantimicag.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Rafailidis PI, Ioannidou E, Alexiou VG, Matthaiou DK, Karageorgopoulos DE, Kapaskelis A, Nikita D, Michalopoulos A. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents. 2010;35:194–199. doi: 10.1016/j.ijantimicag.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Gooderham WJ, Bains M, McPhee JB, Wiegand I, Hancock RE. Adaptive resistance to the “last hope” antibiotics polymyxin B and colistin in Pseudomonas aeruginosa is mediated by the nnovel two-component regulatory system ParR-ParS. Antimicrob Agents Chemother. 2010;54:3372–3382. doi: 10.1128/AAC.00242-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galani I, Kontopidou F, Souli M, Rekatsina PD, Koratzanis E, Deliolanis J, Giamarellou H. Colistin susceptibility testing by Etest and disk diffusion methods. Int J Antimicrob Agents. 2008;31:434–439. doi: 10.1016/j.ijantimicag.2008.01.011. [DOI] [PubMed] [Google Scholar]
- Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: results from the SENTRY Antimicrobial Surveillance Program (2006–09) J Antimicrob Chemother. 2011;66:2070–2074. doi: 10.1093/jac/dkr239. [DOI] [PubMed] [Google Scholar]
- Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother. 2011a;55:3284–3294. doi: 10.1128/AAC.01733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garonzik SM, Forrest A, Thamlikitkul V, Li J, Paterson DL, Silveira FP, Nation RL. Predictors of changes in renal function in critically-ill patients on colistin methanesulfonate. A-1101 Slide Session 156; Program and abstract of the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept 17–20; Chicago, IL: American Society for Microbiology; 2011b. [Google Scholar]
- Giacometti A, Cirioni O, Ghiselli R, Orlando F, Mocchegiani F, D’Amato G, Silvestri C, Riva A, Del Prete MS, Saba V, Scalise G. Antiendotoxin activity of antimicrobial peptides and glycopeptides. J Chemother. 2003;15:129–133. doi: 10.1179/joc.2003.15.2.129. [DOI] [PubMed] [Google Scholar]
- Gobin P, Lemaitre F, Marchand S, Couet W, Olivier JC. Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrob Agents Chemother. 2010;54:1941–1948. doi: 10.1128/AAC.01367-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govaerts C, Adams E, Van Schepdael A, Hoogmartens J. Hyphenation of liquid chromatography to ion trap mass spectrometry to identify minor components in polypeptide antibiotics. Anal Bioanal Chem. 2003;377:909–921. doi: 10.1007/s00216-003-2173-x. [DOI] [PubMed] [Google Scholar]
- Gunderson BW, Ibrahim KH, Hovde LB, Fromm TL, Reed MD, Rotschafer JC. Synergistic activity of colistin and ceftazidime against multiantibiotic-resistant Pseudomonas aeruginosa in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2003;47:905–909. doi: 10.1128/AAC.47.3.905-909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock RE. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- Hancock RE, Chapple DS. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, Vishnepolsky M, Weintrob A, Wortmann G. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis. 2009;48:1724–1728. doi: 10.1086/599225. [DOI] [PubMed] [Google Scholar]
- Helander IM, Kato Y, Kilpeläinen I, Kostiainen R, Lindner B, Nummila K, Sugiyama T, Yokochi T. Characterization of lipopolysaccharides of polymyxin-resistant and polymyxin-sensitive Klebsiella pneumoniae O3. Eur J Biochem. 1996;237:272–278. doi: 10.1111/j.1432-1033.1996.0272n.x. [DOI] [PubMed] [Google Scholar]
- Helander IM, Kilpelainen I, Vaara M. Increased substitution of phosphate groups in lipopolysaccharides and lipid A of the polymyxin-resistant pmrA mutants of Salmonella typhimurium: a 31P-NMR study. Mol Microbiol. 1994;11:481–487. doi: 10.1111/j.1365-2958.1994.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumoniae carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother. 2010;65:1119–1125. doi: 10.1093/jac/dkq108. [DOI] [PubMed] [Google Scholar]
- Infectious Diseases Society of America. Bad bugs, no drugs. Alexandria, VA: Infectious Diseases Society of America; 2004. www.idsociety.org. [Google Scholar]
- Infectious Diseases Society of America. The 10 × ‘20 Initiative: Pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010;50:1081–1083. doi: 10.1086/652237. [DOI] [PubMed] [Google Scholar]
- Imberti R, Cusato M, Villani P, Carnevale L, Iotti GA, Langer M, Regazzi M. Steady-state pharmacokinetics and bronchoalveolar lavage concentration of colistin in critically ill patients after intravenous colistin methanesulfonate administration. Chest. 2010;138:1333–1339. doi: 10.1378/chest.10-0463. [DOI] [PubMed] [Google Scholar]
- Jansson B, Karvanen M, Cars O, Plachouras D, Friberg LE. Quantitative analysis of colistin A and colistin B in plasma and culture medium using a simple precipitation step followed by LC/MS/MS. J Pharm Biomed Anal. 2009;49:760–767. doi: 10.1016/j.jpba.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Jernigan MG, Press EG, Nguyen MH, Clancy CJ, Shields RK. The combination of doripenem and colistin is bactericidal and synergistic against colistin-resistant, carbapenemase-producing. Klebsiella pneumoniae Antimicrob Agents Chemother. 2012;56:3395–3398. doi: 10.1128/AAC.06364-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketthireddy S, Lee DG, Murakami Y, Stamstad T, Andes DR, Craig WA. In vivo pharmacodynamics of colistin against Pseudomonas aeruginosa in thighs of neutropenic mice (abstract A-1). Program and abstract of the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; Sept 17–20; Chicago, Illinois: American Society for Microbiology; 2007. p. 1. [Google Scholar]
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, Chaudhary U, Doumith M, Giske CG, Irfan S, Krishnan P, Kumar AV, Maharjan S, Mushtaq S, Noorie T, Paterson DL, Pearson A, Perry C, Pike R, Rao B, Ray U, Sarma JB, Sharma M, Sheridan E, Thirunarayan MA, Turton J, Upadhyay S, Warner M, Welfare W, Livermore DM, Woodford N. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa AL, Lim TP, Low JG, Hou J, Kurup A, Prince RA, Tam VH. Pharmacokinetics of polymyxin B1 in patients with multidrug-resistant Gram-negative bacterial infections. Diagn Microbiol infect Dis. 2008;60:163–167. doi: 10.1016/j.diagmicrobio.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kwa AL, Abdelraouf K, Low JG, Tam VH. Pharmacokinetics of polymyxin B in a patient with renal insufficiency: a case report. Clin Infect Dis. 2011;52:1280–1281. doi: 10.1093/cid/cir137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Ku C, Tsuji BT, Forrest F, Bulitta JB, Li J, Nation RL. Efficacy of colistin combination therapy against multidrug-resistant Gram-negative bacteria in mouse lung and thigh infection models. Program and abstract of the 22nd European Congress of Clinical Microbiology and Infectious Diseases; March 31 - April 3; London, UK: European Society of Clinical Microbiology and Infectious Diseases; 2012. p. 1. [Google Scholar]
- Li J, Turnidge J, Milne R, Nation RL, Coulthard K. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob Agents Chemother. 2001a;45:781–785. doi: 10.1128/AAC.45.3.781-785.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Johnson DW. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 2001b;761:167–175. doi: 10.1016/s0378-4347(01)00326-7. [DOI] [PubMed] [Google Scholar]
- Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K, Valentine J. Simple method for assaying colistin methanesulfonate in plasma and urine using high-performance liquid chromatography. Antimicrob Agents Chemother. 2002;46:3304–3307. doi: 10.1128/AAC.46.10.3304-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob Agents Chemother. 2003a;47:1766–1770. doi: 10.1128/AAC.47.5.1766-1770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Milne RW, Nation RL, Turnidge JD, Coulthard K. Stability of colistin and colistin methanesulfonate in aqueous media and plasma as determined by high-performance liquid chromatography. Antimicrob Agents Chemother. 2003b;47:1364–1370. doi: 10.1128/AAC.47.4.1364-1370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Coulthard K, Milne R, Nation RL, Conway S, Peckham D, Etherington C, Turnidge J. Steady-state pharmacokinetics of intravenous colistin methanesulphonate in patients with cystic fibrosis. J Antimicrob Chemother. 2003c;52:987–992. doi: 10.1093/jac/dkg468. [DOI] [PubMed] [Google Scholar]
- Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. Pharmacokinetics of colistin methanesulphonate and colistin in rats following an intravenous dose of colistin methanesulphonate. J Antimicrob Chemother. 2004;53:837–840. doi: 10.1093/jac/dkh167. [DOI] [PubMed] [Google Scholar]
- Li J, Nation RL, Milne RW, Turnidge JD, Coulthard K. Evaluation of colistin as an agent against multi-resistant Gram-negative bacteria. Int J Antimicrob Agents. 2005a;25:11–25. doi: 10.1016/j.ijantimicag.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Li J, Rayner CR, Nation RL, Deans R, Boots R, Widdecombe N, Douglas A, Lipman J. Pharmacokinetics of colistin methanesulfonate and colistin in a critically ill patient receiving continuous venovenous hemodiafiltration. Antimicrob Agents Chemother. 2005b;49:4814–4815. doi: 10.1128/AAC.49.11.4814-4815.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- Li Y, Lv Y, Wang S. Mohnarin report 2010: surveillance of antimicrobial resistance in non-fermenting Gram-negative bacteria. Chin J Nosocomiol. 2011;21:5133–5105. [Google Scholar]
- Linden PK, Kusne S, Coley K, Fontes P, Kramer DJ, Paterson D. Use of parenteral colistin for the treatment of serious infection due to antimicrobial-resistant Pseudomonas aeruginosa. Clin Infect Dis. 2003;37:e154–160. doi: 10.1086/379611. [DOI] [PubMed] [Google Scholar]
- Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22:582–610. doi: 10.1128/CMR.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet E, Tomas JM, Bengoechea JA. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology. 2008;154:3877–3886. doi: 10.1099/mic.0.2008/022301-0. [DOI] [PubMed] [Google Scholar]
- Lo-Ten-Foe JR, de Smet AM, Diederen BM, Kluytmans JA, van Keulen PH. Comparative Evaluation of the VITEK 2, Disk Diffusion, Etest, Broth Microdilution, and Agar Dilution Susceptibility Testing Methods for Colistin in Clinical Isolates, Including Heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob Agents Chemother. 2007;51:3726–3730. doi: 10.1128/AAC.01406-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Wang J, Gerber JP, Milne RW. Determination of colistin in human plasma, urine and other biological samples using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;862:205–212. doi: 10.1016/j.jchromb.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Ma Z, Wang J, Nation RL, Li J, Turnidge JD, Coulthard K, Milne RW. Renal disposition of colistin in the isolated perfused rat kidney. Antimicrob Agents Chemother. 2009;53:2857–2864. doi: 10.1128/AAC.00030-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalej SM, Meziou MR, Rhimi FM, Hammami A. Comparison of disc diffusion, Etest and agar dilution for susceptibility testing of colistin against Enterobacteriaceae. Lett Appl Microbiol. 2011;53:546–551. doi: 10.1111/j.1472-765X.2011.03145.x. [DOI] [PubMed] [Google Scholar]
- Marchand S, Frat JP, Petitpas F, Lemaitre F, Gobin P, Robert R, Mimoz O, Couet W. Removal of colistin during intermittent haemodialysis in two critically ill patients. J Antimicrob Chemother. 2010a;65:1836–1837. doi: 10.1093/jac/dkq185. [DOI] [PubMed] [Google Scholar]
- Marchand S, Lamarche I, Gobin P, Couet W. Dose-ranging pharmacokinetics of colistin methanesulphonate (CMS) and colistin in rats following single intravenous CMS doses. J Antimicrob Chemother. 2010b;65:1753–1758. doi: 10.1093/jac/dkq183. [DOI] [PubMed] [Google Scholar]
- Markantonis SL, Markou N, Fousteri M, Sakellaridis N, Karatzas S, Alamanos I, Dimopoulou E, Baltopoulos G. Penetration of colistin into cerebrospinal fluid. Antimicrob Agents Chemother. 2009;53:4907–4910. doi: 10.1128/AAC.00345-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou N, Markantonis SL, Dimitrakis E, Panidis D, Boutzouka E, Karatzas S, Rafailidis P, Apostolakos H, Baltopoulos G. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, gram-negative bacilli infections: A prospective, open-label, uncontrolled study. Clin Ther. 2008;30:143–151. doi: 10.1016/j.clinthera.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, Henry R, Crane B, St Michael F, Cox AD, Adler B, Nation RL, Li J, Boyce JD. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;54:4971–4977. doi: 10.1128/AAC.00834-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt JH, Harper M, Adler B, Nation RL, Li J, Boyce JD. Insertion sequence ISAba11 is involved in colistin resistance and loss of lipopolysaccharide in Acinetobacter baumannii. Antimicrob Agents Chemother. 2011;55:3022–3024. doi: 10.1128/AAC.01732-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi T, Murase Y, Mori M, Shiomi K, Omura S, Paranagama MP, Kita K. Polymyxin B identified as an inhibitor of alternative NADH dehydrogenase and malate: quinone oxidoreductase from the Gram-positive bacterium Mycobacterium smegmatis. J Biochem. 2009;146:491–499. doi: 10.1093/jb/mvp096. [DOI] [PubMed] [Google Scholar]
- Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. Application of a loading dose of colistin methanesulphonate (CMS) in critically ill patients: population pharmacokinetics, protein binding and prediction of bacterial kill. Antimicrob Agents Chemother. 2012 May 21; doi: 10.1128/AAC.06426-11. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero A, Ariza J, Corbella X, Domenech A, Cabellos C, Ayats J, Tubau F, Borraz C, Gudiol F. Antibiotic combinations for serious infections caused by carbapenem-resistant Acinetobacter baumannii in a mouse pneumonia model. J Antimicrob Chemother. 2004;54:1085–1091. doi: 10.1093/jac/dkh485. [DOI] [PubMed] [Google Scholar]
- Montero M, Horcajada JP, Sorli L, Alvarez-Lerma F, Grau S, Riu M, Sala M, Knobel H. Effectiveness and safety of colistin for the treatment of multidrug-resistant Pseudomonas aeruginosa infections. Infection. 2009;37:461–465. doi: 10.1007/s15010-009-8342-x. [DOI] [PubMed] [Google Scholar]
- Moskowitz SM, Ernst RK, Miller SI. PmrAB, a two-component regulatory system of Pseudomonas aeruginosa that modulates resistance to cationic antimicrobial peptides and addition of aminoarabinose to lipid A. J Bacteriol. 2004;186:575–579. doi: 10.1128/JB.186.2.575-579.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I, Cohen S, Rahmani R, Kabha K, Tamarkin D, Herzig Y, Rubinstein E. Antibacterial synergism of polymyxin B nonapeptide and hydrophobic antibiotics in experimental gram-negative infections in mice. Antimicrob Agents Chemother. 1994;38:374–377. doi: 10.1128/aac.38.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orwa JA, Van Gerven A, Roets E, Hoogmartens J. Development and validation of a liquid chromatography method for analysis of colistin sulphate. Chromatographia. 2000;51:433–436. [Google Scholar]
- Orwa JA, Govaerts C, Busson R, Roets E, Van Schepdael A, Hoogmartens J. Isolation and structural characterization of colistin components. J Antibiot. 2001;54:595–599. doi: 10.7164/antibiotics.54.595. [DOI] [PubMed] [Google Scholar]
- Owen RJ, Li J, Nation RL, Spelman D. In vitro pharmacodynamics of colistin against Acinetobacter baumannii clinical isolates. J Antimicrob Chemother. 2007;59:473–477. doi: 10.1093/jac/dkl512. [DOI] [PubMed] [Google Scholar]
- Ozbek B, Senturk A. Postantibiotic effects of tigecycline, colistin sulfate, and levofloxacin alone or tigecycline-colistin sulfate and tigecycline-levofloxacin combinations against Acinetobacter baumannii. Chemotherapy. 2010;56:466–471. doi: 10.1159/000321015. [DOI] [PubMed] [Google Scholar]
- Pachon-Ibanez ME, Docobo-Perez F, Lopez-Rojas R, Dominguez-Herrera J, Jimenez-Mejias ME, Garcia-Curiel A, Pichardo C, Jimenez L, Pachon J. Efficacy of rifampin and its combinations with imipenem, sulbactam, and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54:1165–1172. doi: 10.1128/AAC.00367-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Drugs for bad bugs: confronting the challenges of antibacterial discovery. Nat Rev Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- Perez JC, Groisman EA. Acid pH activation of the PmrA/PmrB two-component regulatory system of Salmonella enterica. Mol Microbiol. 2007;63:283–293. doi: 10.1111/j.1365-2958.2006.05512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo N, Ioannidou E, Falagas ME. Colistin monotherapy vs. combination therapy: evidence from microbiological, animal and clinical studies. Clin Microbiol Infect. 2008;14:816–827. doi: 10.1111/j.1469-0691.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- Pintado V, San Miguel LG, Grill F, Mejia B, Cobo J, Fortun J, Martin-Davila P, Moreno S. Intravenous colistin sulphomethate sodium for therapy of infections due to multidrug-resistant gram-negative bacteria. J Infect. 2008;56:185–190. doi: 10.1016/j.jinf.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Plachouras D, Giamarellos-Bourboulis EJ, Kentepozidis N, Baziaka F, Karagianni V, Giamarellou H. In vitro postantibiotic effect of colistin on multidrug-resistant Acinetobacter baumannii. Diagn Microbiol infect Dis. 2007;57:419–422. doi: 10.1016/j.diagmicrobio.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. Population pharmacokinetic analysis of colistin methanesulphonate and colistin after intravenous administration in critically ill patients with Gram-negative bacterial infections. Antimicrob Agents Chemother. 2009;53:3430–3436. doi: 10.1128/AAC.01361-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poudyal A, Howden BP, Bell JM, Gao W, Owen RJ, Turnidge JD, Nation RL, Li J. In vitro pharmacodynamics of colistin against multidrug-resistant Klebsiella pneumoniae. J Antimicrob Chemother. 2008;62:1311–1318. doi: 10.1093/jac/dkn425. [DOI] [PubMed] [Google Scholar]
- Quale J, Shah N, Kelly P, Babu E, Backer M, Rosas-Garcia G, Salamera J, George A, Bratu S, Landman D. Activity of polymyxin B and the novel polymyxin analogue CB-182,804 against contemporary Gram-negative pathogens in New York City. Microb Drug Resist. 2012;18:132–136. doi: 10.1089/mdr.2011.0163. [DOI] [PubMed] [Google Scholar]
- Queenan AM, Pillar CM, Deane J, Sahm DF, Lynch AS, Flamm RK, Peterson J, Davies TA. Multidrug resistance among Acinetobacter spp. in the USA and activity profile of key agents: results from CAPITAL Surveillance 2010. Diagn Microbiol infect Dis. 2012 May 12; doi: 10.1016/j.diagmicrobio.2012.04.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Rahal JJ. Novel antibiotic combinations against infections with almost completely resistant Pseudomonas aeruginosa and Acinetobacter species. Clin Infect Dis. 2006;43(Suppl 2):S95–99. doi: 10.1086/504486. [DOI] [PubMed] [Google Scholar]
- Rolain JM, Parola P, Cornaglia G. New Delhi metallo-beta-lactamase (NDM-1): towards a new pandemia? Clin Microbiol Infect. 2010;16:1699–1701. doi: 10.1111/j.1469-0691.2010.03385.x. [DOI] [PubMed] [Google Scholar]
- Salvatore CM, Abdelraouf K, Hsing DD, Tam VH. Pharmacokinetics of polymyxin B in an infant with multidrug-resistant Klebsiella pneumoniae bacteremia. Pediatr Infect Dis J. 2011;30:537–539. doi: 10.1097/INF.0b013e318207a7c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souli M, Rekatsina PD, Chryssouli Z, Galani I, Giamarellou H, Kanellakopoulou K. Does the activity of the combination of imipenem and colistin in vitro exceed the problem of resistance in metallo-beta-lactamase-producing Klebsiella pneumoniae isolates? Antimicrob Agents Chemother. 2009;53:2133–2135. doi: 10.1128/AAC.01271-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot GH, Bradley J, Edwards JE, Jr, Gilbert D, Scheld M, Bartlett JG. Bad bugs need drugs: an update on the development pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin Infect Dis. 2006;42:657–668. doi: 10.1086/499819. [DOI] [PubMed] [Google Scholar]
- Tam VH, Schilling AN, Vo G, Kabbara S, Kwa AL, Wiederhold NP, Lewis RE. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2005;49:3624–3630. doi: 10.1128/AAC.49.9.3624-3630.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CH, Li J, Nation RL. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2007;51:3413–3415. doi: 10.1128/AAC.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan TY, Ng LS. Comparison of three standardized disc susceptibility testing methods for colistin. J Antimicrob Chemother. 2006;58:864–867. doi: 10.1093/jac/dkl330. [DOI] [PubMed] [Google Scholar]
- Tascini C, Gemignani G, Palumbo F, Leonildi A, Tedeschi A, Lambelet P, Lucarini A, Piaggesi A, Menichetti F. Clinical and microbiological efficacy of colistin therapy alone or in combination as treatment for multidrug resistant Pseudomonas aeruginosa diabetic foot infections with or without osteomyelitis. J Chemother. 2006;18:648–651. doi: 10.1179/joc.2006.18.6.648. [DOI] [PubMed] [Google Scholar]
- Tsioutis C, Kritsotakis EI, Maraki S, Gikas A. Infections by pandrug-resistant gram-negative bacteria: clinical profile, therapeutic management, and outcome in a series of 21 patients. Eur J Clin Microbiol Infect Dis. 2010;29:301–305. doi: 10.1007/s10096-009-0857-7. [DOI] [PubMed] [Google Scholar]
- Urban C, Mariano N, Rahal JJ. In vitro double and triple bactericidal activities of doripenem, polymyxin B, and rifampin against multidrug-resistant Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Escherichia coli. Antimicrob Agents Chemother. 2010;54:2732–2734. doi: 10.1128/AAC.01768-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden IM, Levin AS, De Pedri EH, Fung L, Rossi F, Duboc G, Barone AA, Costa SF. Comparison of disc diffusion, Etest and broth microdilution for testing susceptibility of carbapenem-resistant P. aeruginosa to polymyxins. Ann Clin Microbiol Antimicrob. 2007;6:8. doi: 10.1186/1476-0711-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velkov T, Thompson PE, Nation RL, Li J. Structure-activity relationships of polymyxin antibiotics. J Med Chem. 2010;53:1898–1916. doi: 10.1021/jm900999h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace SJ, Li J, Rayner CR, Coulthard K, Nation RL. Stability of colistin methanesulfonate in pharmaceutical products and solutions for administration to patients. Antimicrob Agents Chemother. 2008;52:3047–3051. doi: 10.1128/AAC.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu H, Ciofu O, Song Z, Hoiby N. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2011;55:4469–4474. doi: 10.1128/AAC.00126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu H, Ciofu O, Song Z, Hoiby N. In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrob Agents Chemother. 2012;56:2683–2690. doi: 10.1128/AAC.06486-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zak O, O’Reilly T. Animal models in the evaluation of antimicrobial agents. Antimicrob Agents Chemother. 1991;35:1527–1531. doi: 10.1128/aac.35.8.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavascki AP, Goldani LZ, Cao GY, Superti SV, Lutz L, Barth AL, Ramos F, Boniatti MM, Nation RL, Li J. Pharmacokinetics of intravenous polymyxin B in critically-ill patients. Clin Infect Dis. 2008;47:1298–1304. doi: 10.1086/592577. [DOI] [PubMed] [Google Scholar]