Abstract
Saliva plays a major role in maintaining oral health. Patients afflicted with a decrease in saliva secretion (symptomatically, xerostomia) exhibit difficulty in chewing and swallowing foods, tooth decay, periodontal disease, and microbial infections. Despite recent improvements in treating xerostomia (e.g., saliva stimulants, saliva substitutes, and gene therapy), there is a need of more scientific advancements that can be clinically applied toward restoration of compromised salivary gland function. Here we provide a summary of the current salivary cell models that have been used to advance restorative treatments via development of an artificial salivary gland. These models represent initial steps toward clinical and translational research, to facilitate creation of clinically safe salivary glands. Further studies in salivary cell lines and primary cells are necessary to improve survival rates, cell differentiation, and secretory function. Additionally, the characterization of salivary progenitor and stem cell markers are necessary. Although these models are not fully characterized, their improvement may lead to the construction of an artificial salivary gland that is in high demand for improving the quality of life of many patients suffering from salivary secretory dysfunction.
Keywords: cell line, progenitor cells, primary culture, salivary gland dysfunction
Introduction
Hyposalivation is a significant clinical concern, as decreased saliva production leads to dental caries, periodontitis, microbial infections, and difficulties with basic oral functions (e.g., speaking, mastication, and swallowing), all of which significantly reduce the quality of life for afflicted patients (Thomson et al, 2006; Lawrence et al, 2008). Major causes of hyposalivation include (i) Sjögren’s Syndrome (SS), an autoimmune disease affecting approximately 1% of the population (Pillemer et al, 2001); (ii) γ-irradiation therapy administered to approximately 5% of the patients with head and neck cancer diagnosed each year in the United States (Jensen et al, 2010); (iii) side effects of medications used by a large portion of the population; and (iv) ectodermal dysplasias, a group of developmental disorders mainly affecting ectodermal tissues and organs (Pinheiro and Freire-Maia, 1994; Nordgarden et al, 2003; Clauss et al, 2008). Current treatments for hyposalivation are limited to (i) patient education, diet, and lifestyle modifications; (ii) prevention of dental and oral mucosal diseases; (iii) management of symptoms; (iv) sialogogues or salivary gland stimulants (e.g., the muscarinic receptor agonists pilocarpine and cevimeline) that induce saliva secretion from residual acinar cells (Vissink et al, 2010); and (v) use of artificial saliva (Silvestre et al, 2009). However, given that these therapies target surface-level symptoms and provide only temporary relief, development of an alternative treatment to provide a more permanent effect is essential. An in vivo gene therapy strategy involving viral vector-mediated transfer of the aquaporin-1 cDNA to irradiation-damaged salivary glands has been successfully tested in two pre-clinical models (irradiated rats and miniature pigs), as well as demonstrated its safety in a large toxicology and biodistribution study (Baum et al, 2009). This study represents an important advancement to treat secretory dysfunction; however, improvement of this therapeutic approach is required as incorporation of vital constituents of normal saliva (i.e., α-amylase and mucin) will be necessary in the future.
The development of a viable artificial salivary gland is a novel option in bringing relief to many patients afflicted with hyposalivation. Ideally, autologous primary cells should be used clinically (Hoekstra and Chamuleau, 2002). Hypothetically, a patient’s healthy tissue could be extracted prior to radiation therapy. During treatment, the cells could be grown on a scaffold and implanted back into the patient (Redman, 2008). In cases of severely damaged or absent salivary glands, however, this is not an option. These individuals would need to rely on an artificial salivary gland grown from donor cells. However, cells in primary cultures display slow growth, de-differentiation, and a finite lifespan (Redman et al, 1988; Yeh et al, 1991; Quissell et al, 1994a, b). Consequently, growing attention has been given to the use of salivary cell lines in developing an artificial gland (Demeter et al, 2009; Aure et al, 2010; Maria et al, 2011a, b). Currently, no cell line fully recapitulates the morphological and functional features of the native salivary acinar cells (Warner et al, 2008). Furthermore, cell lines are potentially tumorogenic. However, a study showed that the human submandibular gland (HSG) cell line could be transfected with a herpes simplex virus thymidine kinase (HSV-tk) suicide gene to provide additional safety for use in an artificial salivary gland. Unfortunately, the cell survival rate using this technology was low and needs to be optimized (Aframian et al, 2001). Therefore, we believe that salivary gland cell lines are good models for understanding physiology, behavior, pathological processes, genetic manipulations, and proof of concepts (See Table 1). However, with the current technology, available salivary cell lines are not ready to be used for implantation in vivo. In this review, we evaluate the potential advantages and limitations of commonly used salivary cell models.
Table 1.
Cell models utilized in the development of an artificial salivary gland. This table summarizes the current cell models used in the design of cell constructs that may provide insight for engineering an artificial salivary gland
| Cell line | Source | Immortalization action | Ability to acihieve polarity | Amylase expression | Ability to secrete fluid | References |
|---|---|---|---|---|---|---|
| HSY | Huma parotid adenocarcinoma | Tumor derived | + | + | + | Yanagawa et al (1986); Hayashi et al (1987) |
| HSG | Irradiated human submandibular gland intercalated duct cells | Tumor derived | + | + | + | Shirasuna et al (1981) |
| SMIE | Rat submandibular gland | 12S E1A adenovirus gene product | + | − | + | He et al (1990, 1998)) |
| RSMT-A5 | Rat submandibular gland | 3-Methylcholanthrene | − | − | − | Brown et al (1973); He et al (1989) |
| SMG-C6 | Rat submandibular gland | Transformed with a replication-deficient Simian Virus (pSV40) construct | + | a | a | Quissell et al (1997) |
| SMG-C10 | Rat submandibular gland | Transformed with a replication-deficient Simian Virus (pSV40) construct | + | a | a | Quissell et al (1997) |
| Par-C10 | Rat parotid gland | Transformed with a replication-deficient Simian Virus (pSV40) construct | + | − | + | Quissell et al (1997, 1998); Baker et al (2010) |
| Par-C5 | Rat parotid gland | Transformed with a replication-deficient Simian Virus (pSV40) construct | + | − | − | Quissell et al (1998) |
| Primary cells progenitor cells | Human submandibular and parotid glands | None | + | + | + | Pradhan et al (2010); Feng et al (2009) |
Not yet determined.
Tumor-derived cell lines
HSY
HSY is a neoplastic epithelial cell line that was established from athymic mice tumors after transplantation with surgical specimens of a human parotid gland adenocarcinoma (Yanagawa et al, 1986; Hayashi et al, 1987). Structurally, HSY cells are cuboidal in shape and show papillary infoldings by cytoplasmic processes and microvilli on their free border (Nagamine et al, 1990; Hayashi et al, 1987). These cells are able to establish intercellular connections such as desmosomes and tight junctions (TJ) (Yanagawa et al, 1986). The cytoplasmic organelles are situated to one side of the cell and display low levels of secretory granules (Nagamine et al, 1990). These morphological characteristics indicate HSY cells resemble intercalated duct cells as opposed to acinar cells.
There are several features that make HSY an attractive model for engineering an artificial salivary gland as follows: (i) they exhibit TJs to maintain polarized monolayer organization (Yanagawa et al, 1986), which is critical for engineering a gland capable of fluid secretion (Aframian et al, 2002); (ii) they express amylase (Imai et al, 2004), indicating that they are able to maintain the phenotypical and functional characteristics necessary to engineer functional salivary gland tissue, which should secrete consistent levels of α-amylase in vivo (Joraku et al, 2005); (iii) they respond to muscarinic and β-adrenergic autonomic agonists to increase intracellular free calcium concentration ([Ca2+]i) and intracellular cyclic AMP ([cAMP]i), respectively (Patton et al, 1991), features that are essential for saliva secretion in vivo (Turner and Sugiya, 2002); and (iv) they can be easily transfected allowing for protein and genetic manipulation to modulate HSY cell growth and differentiation (Zhang et al, 2001). Previous studies demonstrated that transfection of the keratinocyte growth factor receptor gene to HSY cells induced differentiation and apoptosis, while suppressed tumor cell growth in vitro and tumor development in vivo (Zhang et al, 2001). It has been proposed that salivary intercalated ducts function as the reservoir for progenitor cells in the salivary gland (Nanduri et al, 2011). As HSY cells have similar morphological features to that of intercalated duct cells, it would be interesting to study whether non-transfected HSY cells may behave like progenitor stem cells when transplanted in vivo. To the best of our knowledge, no groups have used HSY cells for bioengineering studies. However, these cells are strong candidates for differentiation studies toward their use in an artificial salivary gland.
HSG
HSG is a neoplastic intercalated duct cell line that was originated from an irradiated human submandibular gland (Sato et al, 1984). Histologically, HSG are cuboidal and conical in shape and have easily visible desmosomes with sporadic TJ complexes when grown on plastic (Shirasuna et al, 1981). Ultrastructurally, HSG have intercellular connections constituted of papillary infoldings, indicating secretory ability. Furthermore, they exhibit rough endoplasmic reticulum and Golgi complexes, indicating their ability to undergo exocytosis (Shirasuna et al, 1981).
HSG is a well-established in vitro model for salivary gland secretion, morphology, and regeneration (Kim et al, 2009; Wang et al, 2009). Several features indicate HSG as a potential source for developing an artificial salivary gland as follows: (i) they differentiate into acinar structures and express amylase when cultured on Matrigel (Royce et al, 1993; Hoffman et al, 1996; Vag et al, 2007); (ii) they have an innate capacity to increase [Ca2+]i concentration in response to muscarinic and purinergic agonists (Nagy et al, 2007); (iii) they can be modulated by regulators of apotosis, providing researchers with a safety mechanism when working with neoplastic cells in vivo (Fukuda et al, 2007); and (iv) they are regulated by growth factor receptors (i.e., epidermal growth factor receptor (EGFR)) that may be used to promote repair or regeneration of salivary tissue (Ratchford et al, 2010).
Our group has found that treatment with Lipoxin A4 [a lipid mediator derived from arachidonic acid that is involved in the resolution of inflammation (Serhan et al, 1984)] inhibits immune cell binding to salivary epithelium, indicating that Lipoxin A4 serves as a “stop signal” for immune cell–mediated tissue damage (Chinthamani et al, 2011). These studies implicate HSG as an attractive model for co-culture studies (using different cell types) as they are able to bind to human T lymphocytes.
There are many drawbacks regarding the use of HSG when grown on plastic as follows: (i) they are unable to form TJs, making them incapable of attaining and maintaining the polarized monolayer organization required for fluid secretion and (ii) they do not express aquaporins (AQP, essential water channel proteins) AQP1 and AQP5, making them unfit for water transport studies (Delporte and Steinfeld, 2006). However, a recent study indicated that HSG grown on permeable supports coated with Matrigel are able to express TJ proteins (claudin-1, -2, -3, -4, occludin, JAM-A, and ZO-1) and AQP (AQP5) (Maria et al, 2011a, b). Nevertheless, future studies will be necessary to understand the barrier properties of this model, such as characterization of TJ morphology and in-depth analysis of monolayer permeability.
SMIE and RSMT-A5
SMIE is an immortalized epithelial cell line derived from rat submandibular glands (He et al, 1990). This cell line was developed in 1990 and was originally named rat submandibular gland (RSMG); later, the cell line was re-named to SMIE because of the adenovirus (12S E1A gene product) used to immortalize the cells (He et al, 1998). SMIE cells were originally established to study polarized functions in salivary epithelium given their ability to form TJs when plated on collagen-coated permeable supports (He et al, 1990, 1998). Structurally, SMIE cells closely resemble salivary glandular epithelium with immature lumens; consequently, they appear relatively undifferentiated (He et al, 1998). SMIE cells express TJs (i.e., occludin and ZO-1) and adherens junctions proteins (i.e., E-cadherin), which are necessary for polarized functions in salivary epithelium (Michikawa et al, 2008). However, SMIE cells only express low levels of claudin-3 and lack other claudin family members (Michikawa et al, 2008). Claudin-3, -4, and -7 have been shown to play a role in salivary epithelium barrier function (Peppi and Ghabriel, 2004; Kawedia et al, 2007). SMIE cells display a leaky epithelium phenotype as a result of low protein levels of claudin-3 (He et al, 1998; Michikawa et al, 2008). This phenotype is exemplified with low transepithelial resistance (TER) values and high permeability to water-soluble, membrane-impermeant probes such as mannitol and dextran (He et al, 1998). However, SMIE cells are able to differentiate between various sized particles (4 kDa and 70 kDa dextran), allowing them to display selective barrier function and osmotically directed fluid transport (Michikawa et al, 2008). SMIE cells cultured together with IGF-1 increases TER (from approximately 3 to 40 Ωcm2) and permeability to 4 kDa fluorescein isothiocyanate dextran (FITC-dextran); these studies indicate that IGF-1 regulates paracellular barrier function in this cell line (Mitsui et al, 2010). SMIE cells have also been shown to secrete luciferase, a naturally non-secreted protein, when transfected with a pGL3-EGFSP construct (Aframian et al, 2007). These studies indicate that SMIE cells can be modulated to induce protein secretion. In summary, SMIE cells provide an intriguing model for investigating polarized functions of the salivary epithelium.
Another cell line of submandibular gland origin is the RSMT-A5 (A5), which was transformed into a cell line by treatment with 3-methylcholanthrene (Brown, 1973). This cell line displays a ductal epithelium phenotype and express a high density of α1-andrenergic receptors which show a metabolic behavior similar to smooth muscle cells (He et al, 1989). These results indicate that A5 cells could be used for receptor characterization and signaling studies. Previous studies indicate that A5 cells are difficult to transfect and therefore not suitable for protein secretion studies (Aframian et al, 2007). Together, these studies suggest that A5 cells could be used to study cell signaling in ductal epithelium.
Immortalized cell lines
SMG-C6 and SMG-C10
The SMG-C cell lines were isolated following transfection of a replication-defective simian virus (SV40) genome into rat submandibular acinar cells (Quissell et al, 1997). Only two clones, termed SMG-C6 and SMG-C10, were found to be both well differentiated and of epithelial origin (Quissell et al, 1997). Structurally, these cell lines are able to polarize because of their ability to form TJs and desmosomes (Quissell et al, 1997). Additionally, secretory features (i.e., domes, granules, and canaliculi) are observed in these cell lines (Quissell et al, 1997). Functionally, SMG-C6 respond to muscarinic and purinergic agonists (but not to α1 agonists) by increasing [Ca2+]i (Liu et al, 2000). Furthermore, both SMG-C6 and SMG-C10 respond to β-adrenergic agonists by increasing [cAMP]i (Liu et al, 2000). Of the two cell lines, SMG-C6 seems to be more cytodifferentiated than SMG-C10 owing to a greater quantity of secretory cellular structures and a more stable [Ca2+]i release (Quissell et al, 1997). SMG-C6 cells are of acinar origin given the lack of cytokeratin 19 expression (which is expressed only in cells of ductal origin) (Castro et al, 2000). Both SMG-C6 and SMG-C10 lines are able to develop a high TER when grown on collagen-coated polycarbonate filters (Castro et al, 2000). We have not found studies documenting SMG-C6 and SMG-C10 growth on three-dimensional (3D) extracellular matrices. It would be interesting to determine whether they are able to fully differentiate under these conditions.
Both SMG-C6 and SMG-C10 serve as excellent models to study Na+ channels and expression of the Epithelial Na+ Channel protein (ENaC) given their ability to modulate Na+ transport in response to growth in culture medium lacking glucocorticoids or mineralocorticoids (Vasquez et al, 2009). Studies on the SMG-C10 cell line indicated that the cation channel transient potential vanilloid receptor 4 (TRPV4) was functionally connected to AQP5 volume (Aure et al, 2010). These studies indicate SMG-C10 as potential candidates for salivary cell volume regulation, which is an important feature to develop an artificial salivary gland.
A critical issue when using salivary cell lines is the fact that they are tumorogenic; therefore, it is important to control apoptosis in these cells lines. Previous studies using SMG-C6 demonstrated that apoptosis could be modulated (through a Fas-mediated pathway) (Aiba-Masago et al, 2001). Consequently, these cells could be used in vivo without the risk of uncontrolled cellular growth.
Par-C10 and Par-C5
Following development of the SMG-C6 and SMG-C10 cell lines, another study was performed to isolate cells from a rat parotid gland (Quissell et al, 1998). Similarly to the SMG-C cell lines, parotid salivary cells were transfected with an origin-defective SV40 plasmid (Quissell et al, 1998). Both morphology and receptor-mediated calcium responses were used as a screening technique to monitor cell differentiation (Quissell et al, 1998). Both Par-C5 and Par-C10 cell lines form secretory granules, TJs, intermediate junctions, desmosomes, and microvilli (Quissell et al, 1998). When grown on plastic, Par-C10 form monolayers of cuboidal cells with thick extracellular matrices at their base while Par-C5 form layers of plump cells containing numerous intercellular lumen-like invaginations on their medial surfaces (Quissell et al, 1998).
Both Par-C5 and Par-C10 exhibit a significant elevation of [Ca2+]i in response to cholinergic, muscarinic, and α1-adrenergic agonists (Liu et al, 2001). These cell lines increase [cAMP]i in response to α1-adrenergic agonists similar to native tissues (Quissell et al, 1998). Additionally, they increase [Ca2+]i in response to M3 muscarinic agonists (Bockman et al, 2001). No functional amylase expression has been observed in Par-C10 cells when grown on plastic or on growth-factor-reduced (GFR) Matrigel, although an interesting study on the Par-C three to nine clones reported a 16-fold increase in amylase content following incubation with rat serum (Zhu et al, 1998). However, further studies are needed to improve amylase production in these cell lines.
The Par-C10 cell line has been widely studied given its ability to develop the highest TER as compared to Par-C5, SMG-C6, and SMG-C10. This high TER makes them ideal candidates for studies in Ussing chambers (Turner et al, 1998). In fact, transepithelial anion secretion in Par-C10 cells has been well characterized and is regulated by basolateral α1-adrenergic and muscarinic cholinergic receptors and apical P2Y2 receptors (Turner et al, 1998). Furthermore, Par-C10 cells express Na+/H+ exchangers, Na+-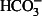 cotransporters, and anion exchange proteins on their basolateral surfaces (Demeter et al, 2009). These proteins, which regulate transepithelial transport, are sensitive to changes in both [Ca2+]i and [cAMP]i concentrations (Demeter et al, 2009). These studies demonstrate Par-C10 as an excellent model to characterize secretion, which is essential for the construction of an artificial salivary gland.
cotransporters, and anion exchange proteins on their basolateral surfaces (Demeter et al, 2009). These proteins, which regulate transepithelial transport, are sensitive to changes in both [Ca2+]i and [cAMP]i concentrations (Demeter et al, 2009). These studies demonstrate Par-C10 as an excellent model to characterize secretion, which is essential for the construction of an artificial salivary gland.
Our group has found that a 48-h treatment with the pro-inflammatory cytokines TNFα and IFNγ decrease TER in Par-C10 cells (Baker et al, 2008). This decrease is associated with increased permeability to normally impermeant molecules, disruption of TJ morphology, and a selective reduction of claudin-1 protein expression (Baker et al, 2008). TNFα and IFNγ are upregulated not only at the plasma level but also in salivary glands from patients with SS (Fox et al, 1994; Boumba et al, 1995). Understanding the mechanisms involved in cytokine-mediated disruption of TJs is important as it may lead to the development of a potential treatment for SS and to elucidate how TJs are modulated during saliva secretion. We recently demonstrated that RvD1 (a potent lipid mediator derived from docosahexaenoic acid (DHA), which promotes resolution of inflammation) blocked inflammatory responses caused by TNFα and enhanced epithelial integrity in Par-C10 monolayers (Odusanwo et al, 2012). These results suggest that RvD1 represents a novel therapeutic approach to block inflammatory responses in salivary glands. Additionally, they implicate RvD1 as an elicitor of acinar formation in salivary glands, which may lead to the improvement of culture conditions in salivary epithelial cells.
Par-C10 single cells form 3D acinar-like spheres when grown on GFR Matrigel. Under these conditions, Par-C10 acinar-like spheres are able to express TJs, AQP3, ion transporters, and M3 muscarinic receptors (Baker et al, 2010). Par-C10 cell spheres increased AQP5 expression when placed in a hypertonic medium (Baker et al, 2010). Furthermore, Par-C10 acinar-like spheres developed changes in potential difference in response to muscarinic agonist stimulation. These features make Par-C10 acinar-like spheres an intriguing model to characterize cell volume regulation and ion secretion in salivary epithelium.
Primary cells
Currently, the use of primary cells represents the best option for creation of an artificial salivary gland because they more closely resemble native tissue (as compared to cell lines). Furthermore, they can be manipulated in vitro and then transplanted to the organism in which they were derived. However, there are critical problems associated with the ability of primary cells to achieve acinar formation in vitro. For instance, primary cells have the tendency to de-differentiate when grown on plastic (Szlavik et al, 2008). Additionally, they are apoptotic when dissociated into single cell suspensions (Walsh et al, 1998). Therefore, new approaches are necessary to maintain high viability of primary salivary cells in culture by modulation of apoptosis.
Previous studies indicated that single human parotid cells are able to differentiate into acinar and ductal structures when grown in a 3D environment (Joraku et al, 2007). Human parotid acinar structures expressed α-amylase, AQP5, and TJ proteins when grown on GFR Matrigel (Joraku et al, 2007). Another study indicated that these cells grown on hyaluronic acid hydrogels are able to assemble into acinar lobules, form TJs, develop central lumens, and express α-amylase (Pradhan et al, 2010). More recently, a group utilized a panel of cell surface markers (that are commonly used to isolate mesenchymal stem cells), for the localization of selective subtypes of salivary acinar cells (i.e., mucous and serous) (Maria et al, 2012). Collectively, these studies suggest that in the future we will be able to create polarized salivary structures capable of secretory function. Furthermore, we will be able to purify different salivary cell types to meet the needs of a given patient.
Prior work using Rhesus parotid gland cells grown on plastic demonstrated their ability to form apical TJs and basolateral expression of Na+/K+ pumps, indicating polarization. Also, these cells have the ability to be transfected with adenoviral vectors to express AQP1 (Tran et al, 2006), indicating the potential of this model for secretion studies. Another potential model for secretion studies involves the use of human SMG. These cells are able to develop TJs, microvilli, secretory granules, and a relatively high TER when grown on permeable supports (Tran et al, 2005). These results indicate that salivary cells are able to polarize, differentiate, and have the potential to be regulated by secretory agonists.
Few studies have shown that salivary cells can be transplanted into living organisms. For instance, human parotid cells grown on polyglycolic acid polymers were implanted subcutaneously into athymic mice (Joraku et al, 2005). Later, the polymer scaffolds were retrieved, and differentiated acinar structures were observed (Joraku et al, 2005). Another study transplanted labeled rat SMG into atrophic salivary glands (Sugito et al, 2004). After several weeks, the labeled SMG cells were detectable over a broad area of the atrophic gland and localized around the acini and ductal region (Sugito et al, 2004). The studies above demonstrate that salivary tissue could be transplanted and maintain differentiation. However, further studies are needed to show whether transplanted salivary cells are able to function in response to neurotransmitters.
Progenitor cells
The use of stem and progenitor cells to create an artificial salivary gland is an exciting novel technique to improve current therapies. These cells have the potential to differentiate and replace damaged tissue, leading to the development of human stem cell therapy to restore the function of damaged salivary glands (Feng et al, 2009). However, there are no reliable methods to isolate sufficient numbers of human salivary gland stem cells for use in therapy (Lombaert et al, 2006).
Neonatal progenitor cells provide an attractive model for constructing an artificial salivary gland as they have been found to be involved in the regeneration of acinar, ductal, and myoepithelial salivary cells in vitro (Kishi et al, 2006). Isolating progenitor cells prior to radiation therapy, expanding the cells in vitro, and then transplanting the cells into the patient is an ideal therapeutic approach for treating patients whose salivary glands may potentially be damaged by irradiation (Lombaert et al, 2008). A transcription factor Ascl3 (which is essential for the determination of cell fate, development, and differentiation of numerous tissues) and a structural protein K5+ (a cytokeratin that forms cytoskeleton intermediate filaments) have been established as markers of progenitor cells (Bullard et al, 2008; Knox et al, 2010). Transplanted Ascl3-expressing cells were able to induce differentiation and tissue repair in salivary glands, indicating that these cells are potent inducers of salivary gland regeneration (Knox et al, 2010). Furthermore, Ascl3 was found to be expressed in ductal cells and demilune caps of submandibular glands. These studies indicate that they contribute to the maintenance of mature salivary tissue (Bullard et al, 2008). Conversely, K5 has been established as a marker for progenitor cells in tracheal and lung epithelial cells (Rock et al, 2009). Additionally, K5+ cells express Sox2, a transcription factor involved in the self-renewal of stem cells. However, K5+ cells make up a very small percentage (5–9%) of Sox2 cells (Lombaert et al, 2011). These studies suggest that K5 is not a well-established marker for salivary progenitor cells. Other studies demonstrated that cells located in the striated ducts of the salivary glands express other stem cell markers (i.e., CD24, CD49f, CD133, and c-Kit+) (Nanduri et al, 2011). Particularly, c-Kit was definitively established as a stem cell marker and therefore gained the highest priority, but flow cytometric analysis of cells obtained from submandibular glands indicated that only 0.058% of salivary cells expressed c-Kit (Nanduri et al, 2011). These results suggest that c-Kit is not an ideal marker for stem cell isolation in salivary glands, as it would not help to yield workable levels of stem cells. We believe that there are many types of progenitor cells in salivary glands that have not yet been characterized. Future studies are needed to identify more reliable progenitor cell populations and to investigate how they behave during tissue repair. These studies will be important for consolidation of the current methods to generate new functional salivary cells in culture.
α6β1 integrins have been found to be a marker for salivary progenitor cells in rats. Interestingly, this marker was only expressed after duct ligation (Okumura et al, 2003). These α6β1 integrins-expressing cells were used to establish an immortalized cell line of rat salivary progenitor cells (Yaniv et al, 2010). This cell line is able to differentiate into both acinar-like and ductal-like structures and has the ability to be modulated when grown on Matrigel-based 3D scaffolds; however, cells grown under these conditions display uncontrolled growth. Further studies are needed to determine whether the acinar-like and ductal-like structures generated from this cell line are capable of responding to salivary secretory agonists. Additionally, the uncontrolled cell growth has to be modulated before these structures can be used for transplantation in vivo.
The current techniques for isolating salivary stem cells continue to be the major limiting factor for their use in the creation of an artificial salivary gland. Further research is needed to develop a better marker for salivary stem cells. We are not aware of any studies that have been able to proliferate undifferentiated salivary stem cells in vitro. Once an isolating technique is developed, the next step will be growing and expanding the stem cells in vitro.
Conclusions and perspectives
The development of an artificial salivary gland is sought after by many patients, but the ideal salivary cell (or perhaps a combination of cells) needs to be acquired before construction of a gland may be started. However, there are many factors regarding the cells that need to be considered. Cell lines exhibit unlimited growth and therefore present a problem when transplanted in vivo. The insertion of a suicide gene that responds to a certain agonist may be one possible way to modulate these tumorigenic cells for use in vivo. The ideal salivary cell would be isolated from autologous tissue in the form of primary or stem cells. Autologous salivary cells would be accepted by the host with no risk of rejection. However, these cells have not been grown efficiently in vitro. Future studies are needed to improve cell differentiation and secretory function. Additionally, it is important to understand whether these cells are able to survive in co-culture with other cell types that are relevant for salivary secretory function (i.e., myoepithelial cells). Hopefully, future research using these cell models will bring tangible benefits to those who suffer from hyposalivation.
Acknowledgments
This work was supported by the NIH-NIDCR grant R21-DE19721-01A1 and RO1-DE021697-01A1. The authors would like to acknowledge Mr. Andrew McCall (Department of Oral Biology) for assistance in reviewing the manuscript.
Author contributions
K. Manzella had the original idea and performed literature review. J. Nelson analyzed the data and wrote the manuscript draft. O. Baker contributed to data interpretation, manuscript writing, advised on manuscript structure, and finalized the manuscript.
Conflict of interest
All authors of the present study disclose any actual or potential conflict of interest including any financial, personal, or other relationships with other people or organizations within that could inappropriately influence (bias) their work.
References
- Aframian DJ, Zheng C, Goldsmith CM. Using HSV-thymidine kinase for safety in an allogeneic salivary graft cell line. Tissue Eng. 2001;7:405–413. doi: 10.1089/10763270152436463. [DOI] [PubMed] [Google Scholar]
- Aframian DJ, Tran SD, Cukierman E, Yamada KM, Baum BJ. Absence of tight junction formation in an allogeneic graft cell line used for developing an engineered artificial salivary gland. Tissue Eng. 2002;8:871–878. doi: 10.1089/10763270260424231. [DOI] [PubMed] [Google Scholar]
- Aframian DJ, Amit D, David R, et al. Reengineering salivary gland cells to enhance protein secretion for use in developing artificial salivary gland device. Tissue Eng. 2007;13:995–1001. doi: 10.1089/ten.2006.0300. [DOI] [PubMed] [Google Scholar]
- Aiba-Masago S, Masago R, Vela-Roch N, Talal N, Dang H. Fas-mediated apoptosis in a rat acinar cell line is dependent on caspase-1 activity. Cell Signal. 2001;13:617–624. doi: 10.1016/s0898-6568(01)00183-8. [DOI] [PubMed] [Google Scholar]
- Aure MH, Roed A, Galtung HK. Intracellular Ca2+ responses and cell volume regulation upon cholinergic and purinergic stimulation in an immortalized salivary cell line. Eur J Oral Sci. 2010;118:237–244. doi: 10.1111/j.1600-0722.2010.00738.x. [DOI] [PubMed] [Google Scholar]
- Baker OJ, Camden JM, Redman RS, et al. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am J Physiol Cell Physiol. 2008;295:C1191–C1201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker OJ, Schulz DJ, Camden JM, et al. Rat parotid gland cell differentiation in three-dimensional culture. Tissue Eng Part C Methods. 2010;16:1135–1144. doi: 10.1089/ten.tec.2009.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum BJ, Zheng C, Cotrim AP, et al. Aquaporin-1 gene transfer to correct radiation-induced salivary hypofunction. Handb Exp Pharmacol. 2009;190:403–418. doi: 10.1007/978-3-540-79885-9_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman CS, Bradley ME, Dang HK, Zeng W, Scofield MA, Dowd FJ. Molecular and pharmacological characterization of muscarinic receptor subtypes in a rat parotid gland cell line: comparison with native parotid gland. J Pharmacol Exp Ther. 2001;297:718–726. [PubMed] [Google Scholar]
- Boumba D, Skopouli FN, Moutsopoulos HM, et al. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjogren’s syndrome. Br J Rheumatol. 1995;34:326–333. doi: 10.1093/rheumatology/34.4.326. [DOI] [PubMed] [Google Scholar]
- Brown AM. In vitro transformation of submandibular gland epithelial cells and fibroblasts of adult rats by methylcholanthrene. Cancer Res. 1973;33:2779–2789. [PubMed] [Google Scholar]
- Bullard T, Koek L, Roztocil E, Kingsley PD, Mirels L, Ovitt CE. Ascl3 expression marks a progenitor population of both acinar and ductal cells in mouse salivary glands. Dev Biol. 2008;320:72–78. doi: 10.1016/j.ydbio.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro R, Barlow-Walden L, Woodson T, Kerecman JD, Zhang GH, Martinez JR. Ion transport in an immortalized rat submandibular cell line SMG-C6. Proc Soc Exp Biol Med. 2000;225:39–48. doi: 10.1177/153537020022500105. [DOI] [PubMed] [Google Scholar]
- Chinthamani S, Odusanwo O, Mondal N, Nelson J, Neelamegham S, Baker OJ. Lipoxin A4 inhibits immune cell binding to salivary epithelium and vascular endothelium. Am J Physiol Cell Physiol. 2011;302:968–978. doi: 10.1152/ajpcell.00259.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss F, Maniere MC, Obry F, et al. Dento-craniofacial phenotypes and underlying molecular mechanisms in hypohidrotic ectodermal dysplasia (HED): a review. J Dent Res. 2008;87:1089–1099. doi: 10.1177/154405910808701205. [DOI] [PubMed] [Google Scholar]
- Delporte C, Steinfeld S. Distribution and roles of aquaporins in salivary glands. Biochim Biophys Acta. 2006;1758:1061–1070. doi: 10.1016/j.bbamem.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Demeter I, Szucs A, Hegyesi O, et al. Vectorial bicarbonate transport by Par-C10 salivary cells. J Physiol Pharmacol. 2009;60(Suppl. 7):197–204. [PubMed] [Google Scholar]
- Feng J, van der Zwaag M, Stokman MA, van Os R, Coppes RP. Isolation and characterization of human salivary gland cells for stem cell transplantation to reduce radiation-induced hyposalivation. Radiother Oncol. 2009;92:466–471. doi: 10.1016/j.radonc.2009.06.023. [DOI] [PubMed] [Google Scholar]
- Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren’s syndrome. J Immunol. 1994;152:5532–5539. [PubMed] [Google Scholar]
- Fukuda M, Tanaka S, Suzuki S, Kusama K, Kaneko T, Sakashita H. Cimetidine induces apoptosis of human salivary gland tumor cells. Oncol Rep. 2007;17:673–678. [PubMed] [Google Scholar]
- Hayashi Y, Yanagawa T, Yoshida H, et al. Expression of vasoactive intestinal polypeptide and amylase in a human parotid gland adenocarcinoma cell line in culture. J Natl Cancer Inst. 1987;79:1025–1037. [PubMed] [Google Scholar]
- He XJ, Wu XZ, Brown AM, Wellner RB, Baum BJ. Characteristics of alpha 1-adrenergic receptors in a rat salivary cell line, RSMT-A5. Gen Pharmacol. 1989;20:175–181. doi: 10.1016/0306-3623(89)90011-6. [DOI] [PubMed] [Google Scholar]
- He XJ, Frank DP, Tabak LA. Establishment and characterization of 12S adenoviral E1A immortalized rat submandibular gland epithelial cells. Biochem Biophys Res Commun. 1990;170:336–343. doi: 10.1016/0006-291x(90)91279-2. [DOI] [PubMed] [Google Scholar]
- He X, Kuijpers GA, Goping G, et al. A polarized salivary cell monolayer useful for studying transepithelial fluid movement in vitro. Pflugers Arch. 1998;435:375–381. doi: 10.1007/s004240050526. [DOI] [PubMed] [Google Scholar]
- Hoekstra R, Chamuleau RA. Recent developments on human cell lines for the bioartificial liver. Int J Artif Organs. 2002;25:182–191. doi: 10.1177/039139880202500304. [DOI] [PubMed] [Google Scholar]
- Hoffman MP, Kibbey MC, Letterio JJ, Kleinman HK. Role of laminin-1 and TGF-beta 3 in acinar differentiation of a human submandibular gland cell line (HSG) J Cell Sci. 1996;109(Pt 8):2013–2021. doi: 10.1242/jcs.109.8.2013. [DOI] [PubMed] [Google Scholar]
- Imai A, Nashida T, Shimomura H. Roles of Munc18-3 in amylase release from rat parotid acinar cells. Arch Biochem Biophys. 2004;422:175–182. doi: 10.1016/j.abb.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Jensen SB, Pedersen AM, Vissink A, et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 2010;18:1061–1079. doi: 10.1007/s00520-010-0837-6. [DOI] [PubMed] [Google Scholar]
- Joraku A, Sullivan CA, Yoo JJ, Atala A. Tissue engineering of functional salivary gland tissue. Laryngoscope. 2005;115:244–248. doi: 10.1097/01.mlg.0000154726.77915.cc. [DOI] [PubMed] [Google Scholar]
- Joraku A, Sullivan CA, Yoo J, Atala A. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation. 2007;75:318–324. doi: 10.1111/j.1432-0436.2006.00138.x. [DOI] [PubMed] [Google Scholar]
- Kawedia JD, Nieman ML, Boivin GP, et al. Interaction between transcellular and paracellular water transport pathways through Aquaporin 5 and the tight junction complex. Proc Natl Acad Sci USA. 2007;104:3621–3626. doi: 10.1073/pnas.0608384104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park SH, Moon YW, et al. Histamine H1 receptor induces cytosolic calcium increase and aquaporin translocation in human salivary gland cells. J Pharmacol Exp Ther. 2009;330:403–412. doi: 10.1124/jpet.109.153023. [DOI] [PubMed] [Google Scholar]
- Kishi T, Takao T, Fujita K, Taniguchi H. Clonal proliferation of multipotent stem/progenitor cells in the neonatal and adult salivary glands. Biochem Biophys Res Commun. 2006;340:544–552. doi: 10.1016/j.bbrc.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Knox SM, Lombaert IM, Reed X, Vitale-Cross L, Gutkind JS, Hoffman MP. Parasympathetic innervation maintains epithelial progenitor cells during salivary organogenesis. Science. 2010;329:1645–1647. doi: 10.1126/science.1192046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence HP, Thomson WM, Broadbent JM, Poulton R. Oral health-related quality of life in a birth cohort of 32-year olds. Community Dent Oral Epidemiol. 2008;36:305–316. doi: 10.1111/j.1600-0528.2007.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XB, Sun X, Mork AC, Dodds MW, Martinez JR, Zhang GH. Characterization of the calcium signaling system in the submandibular cell line SMG-C6. Proc Soc Exp Biol Med. 2000;225:211–220. doi: 10.1177/153537020022500308. [DOI] [PubMed] [Google Scholar]
- Liu X, Mork AC, Sun X, Castro R, Martinez JR, Zhang GH. Regulation of Ca(2+) signals in a parotid cell line Par-C5. Arch Oral Biol. 2001;46:1141–1149. doi: 10.1016/s0003-9969(01)00074-7. [DOI] [PubMed] [Google Scholar]
- Lombaert IM, Wierenga PK, Kok T, Kampinga HH, deHaan G, Coppes RP. Mobilization of bone marrow stem cells by granulocyte colony-stimulating factor ameliorates radiation-induced damage to salivary glands. Clin Cancer Res. 2006;12:1804–1812. doi: 10.1158/1078-0432.CCR-05-2381. [DOI] [PubMed] [Google Scholar]
- Lombaert IM, Brunsting JF, Wierenga PK, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE. 2008;3:e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert IM, Knox SM, Hoffman MP. Salivary gland progenitor cell biology provides a rationale for therapeutic salivary gland regeneration. Oral Dis. 2011;17:445–449. doi: 10.1111/j.1601-0825.2010.01783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria OM, Maria O, Liu Y, Komarova SV, Tran SD. Matrigel improves functional properties of human submandibular salivary gland cell line. Int J Biochem Cell Biol. 2011a;43:622–631. doi: 10.1016/j.biocel.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Maria OM, Zeitouni A, Gologan O, Tran SD. Matrigel improves functional properties of primary human salivary gland cells. Tissue Eng Part A. 2011b;17:1229–1238. doi: 10.1089/ten.TEA.2010.0297. [DOI] [PubMed] [Google Scholar]
- Maria OM, Maria AM, Cai Y, Tran SD. Cell surface markers CD44 and CD166 localized specific populations of salivary acinar cells. Oral Dis. 2012;18:162–168. doi: 10.1111/j.1601-0825.2011.01858.x. [DOI] [PubMed] [Google Scholar]
- Michikawa H, Fujita-Yoshigaki J, Sugiya H. Enhancement of barrier function by overexpression of claudin-4 in tight junctions of submandibular gland cells. Cell Tissue Res. 2008;334:255–264. doi: 10.1007/s00441-008-0689-2. [DOI] [PubMed] [Google Scholar]
- Mitsui R, Fujita-Yoshigaki J, Narita T. Maintenance of paracellular barrier function by insulin-like growth factor-I in submandibular gland cells. Arch Oral Biol. 2010;55:963–969. doi: 10.1016/j.archoralbio.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Nagamine S, Yanagawa T, Bando T, Yura Y, Yoshida H, Sato M. Induction of cells with phenotypic features of neuronal cells by treatment with dibutyryl cyclic adenosine 3′,5′-monophosphate in a human parotid gland adenocarcinoma cell line in culture. Cancer Res. 1990;50:6396–6404. [PubMed] [Google Scholar]
- Nagy K, Szlavik V, Racz G, Ovari G, Vag J, Varga G. Human submandibular gland (HSG) cell line as a model for studying salivary gland Ca2+ signalling mechanisms. Acta Physiol Hung. 2007;94:301–313. doi: 10.1556/APhysiol.94.2007.4.2. [DOI] [PubMed] [Google Scholar]
- Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van OsRP, Coppes RP. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol. 2011;99:367–372. doi: 10.1016/j.radonc.2011.05.085. [DOI] [PubMed] [Google Scholar]
- Nordgarden H, Storhaug K, Lyngstadaas SP, Jensen JL. Salivary gland function in persons with ectodermal dysplasias. Eur J Oral Sci. 2003;111:371–376. doi: 10.1034/j.1600-0722.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- Odusanwo O, Chinthamani S, McCall A, Duffey ME, Baker OJ. Resolvin D1 prevents TNF-alpha-mediated disruption of salivary epithelial formation. Am J Physiol Cell Physiol. 2012;302:1331–1345. doi: 10.1152/ajpcell.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumura K, Nakamura K, Hisatomi Y, et al. Salivary gland progenitor cells induced by duct ligation differentiate into hepatic and pancreatic lineages. Hepatology. 2003;38:104–113. doi: 10.1053/jhep.2003.50259. [DOI] [PubMed] [Google Scholar]
- Patton LL, Pollack S, Wellner RB. Responsiveness of a human parotid epithelial cell line (HSY) to autonomic stimulation: muscarinic control of K+ transport. In Vitro Cell Dev Biol. 1991;27A:779–785. doi: 10.1007/BF02631243. [DOI] [PubMed] [Google Scholar]
- Peppi M, Ghabriel MN. Tissue-specific expression of the tight junction proteins claudins and occludin in the rat salivary glands. J Anat. 2004;205:257–266. doi: 10.1111/j.0021-8782.2004.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillemer SR, Matteson EL, Jacobsson LT, et al. Incidence of physician-diagnosed primary Sjogren syndrome in residents of Olmsted County, Minnesota. Mayo Clin Proc. 2001;76:593–599. doi: 10.4065/76.6.593. [DOI] [PubMed] [Google Scholar]
- Pinheiro M, Freire-Maia N. Ectodermal dysplasias: a clinical classification and a causal review. Am J Med Genet. 1994;53:153–162. doi: 10.1002/ajmg.1320530207. [DOI] [PubMed] [Google Scholar]
- Pradhan S, Liu C, Zhang C, Jia X, Farach-Carson MC, Witt RL. Lumen formation in three-dimensional cultures of salivary acinar cells. Otolaryngol Head Neck Surg. 2010;142:191–195. doi: 10.1016/j.otohns.2009.10.039. [DOI] [PubMed] [Google Scholar]
- Quissell DO, Flaitz CM, Redman RS, Barzen KA. Primary culture of human labial salivary gland acini. In Vitro Cell Dev Biol Anim. 1994a;30A:736–740. doi: 10.1007/BF02631293. [DOI] [PubMed] [Google Scholar]
- Quissell DO, Redman RS, Barzen KA, McNutt RL. Effects of oxygen, insulin, and glucagon concentrations on rat submandibular acini in serum-free primary culture. In Vitro Cell Dev Biol Anim. 1994b;30A:833–842. doi: 10.1007/BF02639393. [DOI] [PubMed] [Google Scholar]
- Quissell DO, Barzen KA, Gruenert DC, Redman RS, Camden JM, Turner JT. Development and characterization of SV40 immortalized rat submandibular acinar cell lines. In Vitro Cell Dev Biol Anim. 1997;33:164–173. doi: 10.1007/s11626-997-0137-8. [DOI] [PubMed] [Google Scholar]
- Quissell DO, Barzen KA, Redman RS, Camden JM, Turner JT. Development and characterization of SV40 immortalized rat parotid acinar cell lines. In Vitro Cell Dev Biol Anim. 1998;34:58–67. doi: 10.1007/s11626-998-0054-5. [DOI] [PubMed] [Google Scholar]
- Ratchford AM, Baker OJ, Camden JM, et al. P2Y2 nucleotide receptors mediate metalloprotease-dependent phosphorylation of epidermal growth factor receptor and ErbB3 in human salivary gland cells. J Biol Chem. 2010;285:7545–7555. doi: 10.1074/jbc.M109.078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman RS. On approaches to the functional restoration of salivary glands damaged by radiation therapy for head and neck cancer, with a review of related aspects of salivary gland morphology and development. Biotech Histochem. 2008;83:103–130. doi: 10.1080/10520290802374683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman RS, Quissell DO, Barzen KA. Effects of dexamethasone, epidermal growth factor, and retinoic acid on rat submandibular acinar-intercalated duct complexes in primary culture. In Vitro Cell Dev Biol. 1988;24:734–742. doi: 10.1007/BF02623642. [DOI] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royce LS, Kibbey MC, Mertz P, Kleinman HK, Baum BJ. Human neoplastic submandibular intercalated duct cells express an acinar phenotype when cultured on a basement membrane matrix. Differentiation. 1993;52:247–255. doi: 10.1111/j.1432-0436.1993.tb00637.x. [DOI] [PubMed] [Google Scholar]
- Sato M, Hayashi Y, Yoshida H, Yanagawa T, Yura Y, Nitta T. Search for specific markers of neoplastic epithelial duct and myoepithelial cell lines established from human salivary gland and characterization of their growth in vitro. Cancer. 1984;54:2959–2967. doi: 10.1002/1097-0142(19841215)54:12<2959::aid-cncr2820541225>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Hamberg M, Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci USA. 1984;81:5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasuna K, Sato M, Miyazaki T. A neoplastic epithelial duct cell line established from an irradiated human salivary gland. Cancer. 1981;48:745–752. doi: 10.1002/1097-0142(19810801)48:3<745::aid-cncr2820480314>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Silvestre FJ, Minguez MP, Sune-Negre JM. Clinical evaluation of a new artificial saliva in spray form for patients with dry mouth. Med Oral Patol Oral Cir Bucal. 2009;14:E8–E11. [PubMed] [Google Scholar]
- Sugito T, Kagami H, Hata K, Nishiguchi H, Ueda M. Transplantation of cultured salivary gland cells into an atrophic salivary gland. Cell Transplant. 2004;13:691–699. doi: 10.3727/000000004783983567. [DOI] [PubMed] [Google Scholar]
- Szlavik V, Szabo B, Vicsek T, et al. Differentiation of primary human submandibular gland cells cultured on basement membrane extract. Tissue Eng Part A. 2008;14:1915–1926. doi: 10.1089/ten.tea.2007.0208. [DOI] [PubMed] [Google Scholar]
- Thomson WM, Lawrence HP, Broadbent JM, Poulton R. The impact of xerostomia on oral-health-related quality of life among younger adults. Health Qual Life Outcomes. 2006;4:86. doi: 10.1186/1477-7525-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran SD, Wang J, Bandyopadhyay BC, et al. Primary culture of polarized human salivary epithelial cells for use in developing an artificial salivary gland. Tissue Eng. 2005;11:172–181. doi: 10.1089/ten.2005.11.172. [DOI] [PubMed] [Google Scholar]
- Tran SD, Sugito T, Dipasquale G, et al. Re-engineering primary epithelial cells from rhesus monkey parotid glands for use in developing an artificial salivary gland. Tissue Eng. 2006;12:2939–2948. doi: 10.1089/ten.2006.12.2939. [DOI] [PubMed] [Google Scholar]
- Turner RJ, Sugiya H. Understanding salivary fluid and protein secretion. Oral Dis. 2002;8:3–11. doi: 10.1034/j.1601-0825.2002.10815.x. [DOI] [PubMed] [Google Scholar]
- Turner JT, Redman RS, Camden JM, Landon LA, Quissell DO. A rat parotid gland cell line, Par-C10, exhibits neurotransmitter-regulated transepithelial anion secretion. Am J Physiol. 1998;275:C367–C374. doi: 10.1152/ajpcell.1998.275.2.C367. [DOI] [PubMed] [Google Scholar]
- Vag J, Byrne EM, Hughes DH, et al. Morphological and functional differentiation of HSG cells: role of extracellular matrix and trpc 1. J Cell Physiol. 2007;212:416–423. doi: 10.1002/jcp.21035. [DOI] [PubMed] [Google Scholar]
- Vasquez MM, Mustafa SB, Choudary A, Seidner SR, Castro R. Regulation of epithelial Na+ channel (ENaC) in the salivary cell line SMG-C6. Exp Biol Med (Maywood) 2009;234:522–531. doi: 10.3181/0806-RM-209. [DOI] [PubMed] [Google Scholar]
- Vissink A, Mitchell JB, Baum BJ. Clinical management of salivary gland hypofunction and xerostomia in head-and-neck cancer patients: successes and barriers. Int J Radiat Oncol Biol Phys. 2010;78:983–991. doi: 10.1016/j.ijrobp.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh GM, Dewson G, Wardlaw AJ, Levi-Schaffer F, Moqbel R. A comparative study of different methods for the assessment of apoptosis and necrosis in human eosinophils. J Immunol Methods. 1998;217:153–163. doi: 10.1016/s0022-1759(98)00103-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Shnyra A, Africa C, Warholic C, McArthur C. Activation of the extrinsic apoptotic pathway by TNF-alpha in human salivary gland (HSG) cells in vitro, suggests a role for the TNF receptor (TNF-R) and intercellular adhesion molecule-1 (ICAM-1) in Sjogren’s syndrome-associated autoimmune sialadenitis. Arch Oral Biol. 2009;54:986–996. doi: 10.1016/j.archoralbio.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Warner JD, Peters CG, Saunders R, et al. Visualizing form and function in organotypic slices of the adult mouse parotid gland. Am J Physiol Gastrointest Liver Physiol. 2008;295:G629–G640. doi: 10.1152/ajpgi.90217.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa T, Hayashi Y, Nagamine S, Yoshida H, Yura Y, Sato M. Generation of cells with phenotypes of both intercalated duct-type and myoepithelial cells in human parotid gland adenocarcinoma clonal cells grown in athymic nude mice. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51:187–195. doi: 10.1007/BF02899028. [DOI] [PubMed] [Google Scholar]
- Yaniv A, Neumann Y, David R. Establishment of immortal multipotent rat salivary progenitor cell line toward salivary gland regeneration. Tissue Eng Part C Methods. 2010;17:69–78. doi: 10.1089/ten.TEC.2010.0228. [DOI] [PubMed] [Google Scholar]
- Yeh C, Mertz PM, Oliver C, Baum BJ, Kousvelari EE. Cellular characteristics of long-term cultured rat parotid acinar cells. In Vitro Cell Dev Biol. 1991;27A:707–712. doi: 10.1007/BF02633215. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Toratani S. Growth inhibition by keratinocyte growth factor receptor of human salivary adenocarcinoma cells through induction of differentiation and apoptosis. Proc Natl Acad Sci USA. 2001;98:11336–11340. doi: 10.1073/pnas.191377098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Aletta JM, Wen J, Zhang X, Higgins D, Rubin RP. Rat serum induces a differentiated phenotype in a rat parotid acinar cell line. Am J Physiol. 1998;275:G259–G268. doi: 10.1152/ajpgi.1998.275.2.G259. [DOI] [PubMed] [Google Scholar]


