Abstract
We report a fourteen year old adolescent girl with growth deficiency, microcephaly, intellectual disability, distinctive dysmorphic features (bulbous nose with wide nasal base, hypotelorism, deeply set eyes, protruding cupped ears and thick lips), cataract, pigmentary retinopathy, hypoplastic thorax, kyphoscoliosis and unusual skeletal changes but without chromosomal imbalances detected by array-CGH who probably represents a novel phenotype.
Keywords: Short stature, intellectual disability, retinitis pigmentosa, cataract, narrow thorax, microcephaly, hypotonia, laxity, kyphosis, scoliosis, dislocation of hip, slender bones
INTRODUCTION
Identification of a multiple congenital anomaly-intellectual disability (MCA/ID) syndrome requires documentation of two or more cases, preferably from different families. Because of the rarity of some of these conditions, it may often be difficult to collect several cases by a single observer. We report on a child with MCA/ID syndrome presumably a new phenotype. These features are distinctive enough to suggest a specific dysmorphic syndrome.
CLINICAL REPORT
A 14-year-old girl was referred for evaluation of facial anomalies, intellectual disability and hip dislocations. She is the only child of a nonconsanguineous healthy couple of average stature. Her gestation was unremarkable except for pregnancy-induced hypertension near term and she was delivered by cesarean. Her birth weight was 3.5 kg. Her parents noted neonatal feeding difficulties. She was admitted for 22 days for suspected intracranial infection and septicemia. Though exact details are not available, she had undergone a lumbar puncture and treatment with intravenous antibiotics and dexamethasone. Her parents noticed severe developmental delay. She achieved head control at one year, sitting with support at 1.5 years, standing at 2 years, walking at 2.5 years. She spoke her first word at about 2 years and at age 14 she had a vocabulary of about 20 words and could not form sentences. At the time of presentation, she was dry by day but had nocturnal enuresis. Her parents noticed unusual facial features by age 6 months and reported floppiness in infancy and early childhood. She never had seizures and her hearing is normal.
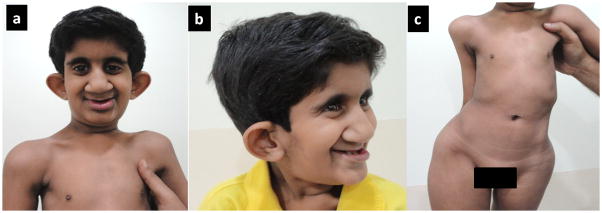
Her height was 118 cm, weight 17.4 kg (both between 5 and 6 SD below the mean) and head circumference 46 cm (between 6 and 7 SD below the mean for age 14 years). Her bone age was 10.3 years. Her arm span was 113 cm, shortened upper segment to lower segment (ratio 54:64, normal for her age) and decreased chest circumference (46 cm, 4SD below the mean). She had microcephaly, long and narrow face, hypotelorism, deeply set eyes, bulbous nose with wide nasal base, prominent nasolabial folds, thin vermilion of upper lip with absent Cupid’s bow thick lower lip with everted vermilion, open anterior fontanel, low posterior hairline and dental crowding with caries (Fig. 1). Her ears were protruding, cupped and low set, without antihelices (Figs. 1a and 1b). She had bilateral cataract and pigmentary retinal changes. She had a hypoplastic thorax, narrow abdomen and thoracolumbar kyphoscoliosis (Fig. 1c). Hands were normal and she had short third and fourth metatarsals (Figs. 2a and 2b). There was generalized ligamentous laxity with hypotonia. Her liver was palpable up to 1 cm below the right costal margin. She was pre-pubertal at age 14 years.
Figure 1.
Hypotelorism, bulbous nose, thin vermilion of upper lip, thick lower lip with everted vermilion, prominent nasolabial folds, cupped protruding ears (a), deeply set eyes (b) and hypoplastic thorax (c) are seen in the subject at 14 years of age.
Figure 2.
Hands are normal (a) and feet show short 3rd and 4th metatarsals (b).
Radiographic evaluation revealed thoracolumbar scoliosis with anterior central concavity of vertebral bodies (Figs. 3a and 3b). She had bilateral dislocation of hips with valgus slip of the capital femoral epiphysis, irregular cortices of the femur and varied bone density (Fig. 4). Spine and iliac bones were sclerotic. Her long bones were slender. Radiography of both hands showed short 4th and 5th metacarpals and hypoplastic distal phalanges (Fig. 5a). There were short 3rd and 4th metatarsals (Fig. 5b). Radiographs of knees were unremarkable (Figs. 5c and 5d)
Figure 3.

Radiographs of spine show increased density of axial skeleton with thoracolumbar scoliosis (a), slender long bones and ribs, and anterior central concavity of vertebra bodies (b).
Figure 4.
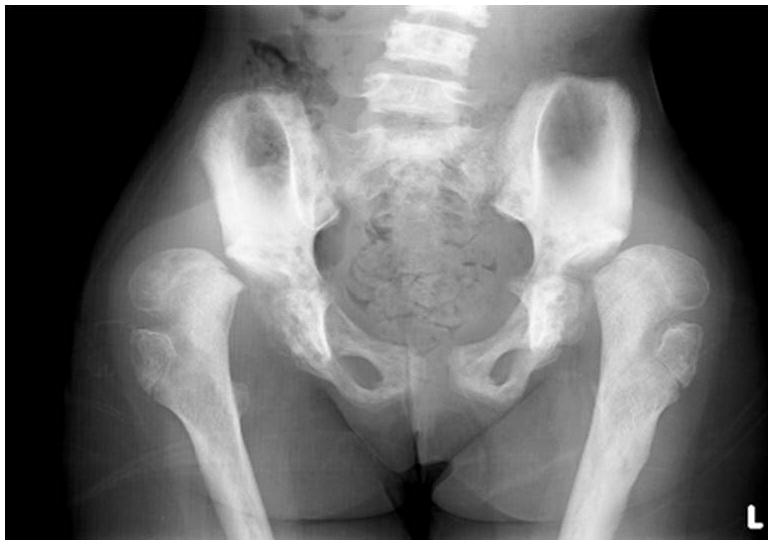
Radiograph of pelvis shows bilateral hip dislocations with valgus slip of capital femoral epiphysis and irregular cortex with increased bone density of iliac bones.
Figure 5.
Radiographs of both hands (a) and feet (b) show short 4th and 5th metacarpals, and 3rd, 4th and 5th metatarsals. Knee radiographs (c and d) are normal.
Her complete blood count, liver functions, renal functions, calcium, phosphorous and alkaline phosphatase levels were unremarkable. Ultrasonography of abdomen revealed mild hepatomegaly and minimal post-void residual urine. Echocardiography revealed a normal cardiac shape and function. Radionuclide bone scan was unremarkable. Magnetic resonance imaging of her brain was normal. The G-banded karyotype showed 46,XX at a band resolution of 450. Bleomycin induced cultures did not show increased chromosomal breakage. Growth hormone and parathyroid hormone levels were normal adjusted for her age and gender.
Molecular karyotyping
Microarray analysis was performed using the SurePrint G3 Human CGH 180k Microarray platform (overall mean probe spacing 13 Kb, Agilent Technologies, Santa Clara, USA). Experimental procedures were performed according to the manufacturer’s instructions. 1 μg of test DNA and 1 μg of reference DNA from a pool of 10 healthy donors with a female karyotype were hybridized. The array was scanned with the G2565CA Microarray Scanner System (Agilent Technologies, Santa Clara, USA) at a resolution of 2 μm/pixel. Signal intensities from the generated images were measured and evaluated with the Feature Extraction v10.7.3.1 and the Agilent Genomic Workbench Standard Edition 6.5 software packages (Agilent Technologies). Regions, which were determined as significantly deviated by the software and which included more than 3 single oligonucleotides (overall resolution 0.04 Mb), were regarded as aberrant. The differentiation between pathologic aberrations and benign copy number polymorphisms (CNPs) was performed by using the database integrated into the software and by using public databases.
DISCUSSION
We report on an adolescent girl with severe postnatal growth retardation, microcephaly, short stature, intellectual disability, speech delay, hypotonia, joint laxity, distinctive facial features (bulbous nose, ocular hypotelorism, protruding ears and thick lower lips with everted vermilion), cataracts, pigmentary retinopathy, bilateral hip dislocations with valgus slip of femoral heads, slender long bones, kyphoscoliosis, abnormal pelvic bones and hypoplastic thorax. Extensive evaluation including a microarray analysis did not reveal any obvious etiology for her features. We could not ascribe this constellation of dysmorphic features, growth and developmental retardation and skeletal features to any previously described syndrome despite a thorough search of published literature and the London Medical Database.
We do not have her anthropometry at birth except for a normal birth weight. Hence it is difficult to comment on the onset of growth retardation. Her clinical features point to a definitive multiple congenital anomaly-mental retardation syndrome rather than any acquired etiology.
We found Gurrieri syndrome (OMIM 601187) to be the closest syndrome sharing growth and intellectual disability, dental anomalies, deep set eyes, brachydactyly, kyphosis, joint laxity, hypoplastic iliac alae, thick-everted lower lips and delayed speech [Gurrieri et al., 1992a & 1992b; Oricco et al., 1999]. However absence of seizures, osteoporosis, advanced bone age, prognathism, sparse eyebrows, presence of microcephaly, cataracts, pigmentary retinopathy, hip dislocations, valgus slip of femoral heads and hypoplastic thorax differentiate the phenotype of our subject from this possible autosomal recessive condition. As no gene is yet identified for this condition, this condition was excluded on clinical basis only.
The other three conditions we considered were: geroderma osteodysplasticum (OMIM 231070) with premature ageing of skin [Hennies et al., 2008; Skidmore et al., 2011], microcephalic osteodysplastic primordial dwarfisms (OMIM 210710, 210720, 210730) [Abdel-Salam et al., 2012; Hall et al., 2004], filamin A related disorders (OMIM 300017) [Robertson SP. 2007] and osteomesopyknosis (OMIM 166450) [Porschek et al., 1985]. However, normal skin, unique facial features, cataracts, pigmentary retinopathy and hypoplastic thorax differentiate these conditions. Since this is an isolated case with normal microarray results, it is difficult to postulate on the etiology of this condition.
Acknowledgments
The authors gratefully acknowledge the cooperation of the child and her parents for this study. The authors thank Dr Benjamin Joseph, Professor, Pediatric Orthopedics Service, Department of Orthopedics, Kasturba Medical College, Manipal for his valuable inputs and comments in preparation of this manuscript, Dr Reiner Siebert, Professor, Institute of Human Genetics, Kiel, for continuous support. JMG is supported by NIH/NICHD program project grant (HD22657), and the Medical Genetics NIH/NIGMS training program grant (5-T32-GM08243). KMG is a recipient of Hargobind Foundation Medical Fellowship. The authors declare no conflict of interest.
Footnotes
The authors declare no conflict of interest.
References
- Abdel-Salam GM, Abdel-Hamid MS, Issa M, Magdy A, El-Kotoury A, Amr K. Expanding the phenotypic and mutational spectrum in microcephalic osteodysplastic primordial dwarfism type I. Am J Med Genet Part A. 2012;158:1455–1461. doi: 10.1002/ajmg.a.35356. [DOI] [PubMed] [Google Scholar]
- Gurrieri F, Sammito V, Ricci B, Lossa M, Bellussi A, Neri G. Possible new type of oral-facial-digital syndrome with retinal abnormalities: OFDS type (VIII) Am J Med Genet. 1992a;42:789–792. doi: 10.1002/ajmg.1320420608. [DOI] [PubMed] [Google Scholar]
- Gurrieri F, Sammito V, Bellussi A, Neri G. New autosomal recessive syndrome of mental retardation, epilepsy, short stature, and skeletal dysplasia. Am J Med Genet. 1992b;44:315–320. doi: 10.1002/ajmg.1320440310. [DOI] [PubMed] [Google Scholar]
- Hall JG, Flora C, Scott CI, Jr, Pauli RM, Tanaka KI. Majewski osteodysplastic primordial dwarfism type II (MOPD II): natural history and clinical findings. Am J Med Genet A. 2004;130:55–72. doi: 10.1002/ajmg.a.30203. [DOI] [PubMed] [Google Scholar]
- Hennies HC, Kornak U, Zhang H, Egerer J, Zhang X, Seifert W, Kühnisch J, Budde B, Nätebus M, Brancati F, Wilcox WR, Müller D, Kaplan PB, Rajab A, Zampino G, Fodale V, Dallapiccola B, Newman W, Metcalfe K, Clayton-Smith J, Tassabehji M, Steinmann B, Barr FA, Nürnberg P, Wieacker P, Mundlos S. Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab-6 interacting golgin. Nat Genet. 2008;40:1410–1412. doi: 10.1038/ng.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proschek R, Labelle H, Bard C, Marton D. Osteomesopycnosis: case report. J Bone Joint Surg Am. 1985;67:652–653. [PubMed] [Google Scholar]
- Robertson SP. Otopalatodigital syndrome spectrum disorders: Otopalatodigital syndrome types 1 and 2, frontometaphyseal dysplasia and Melnick-Needles syndrome. Eur J Hum Genet. 2007;15:3–9. doi: 10.1038/sj.ejhg.5201654. [DOI] [PubMed] [Google Scholar]
- Skidmore DL, Chitayat D, Morgan T, Hinek A, Fischer B, Dimopoulou A, Somers G, Halliday W, Blaser S, Diambomba Y, Lemire EG, Kornak U, Robertson SP. Further expansion of the phenotypic spectrum associated with mutations in ALDH18A1, encoding Δ1-pyrroline-5-carboxylate synthase (P5CS) Am J Med Genet A. 2011;155:1848–1856. doi: 10.1002/ajmg.a.34057. [DOI] [PubMed] [Google Scholar]