Abstract
We hypothesize that the beneficial effects estradiol on cognitive performance may be mediated through GPR30, a putative membrane target of estrogens. Recently we showed that administration of a selective GPR30 agonist (G-1) to ovariectomized rats enhanced acquisition of a delayed matching-to-position (DMP) T-maze task and increased potassium-stimulated acetylcholine release in the hippocampus, similar to estradiol (E2) (Hammond et al., 2009). The present study tested whether treating with a selective GPR30 antagonist (G-15) would impair spatial learning in gonadally intact rats and in ovariectomized (OVX) rats treated with E2. As predicted, G-15 dose-dependently impaired DMP acquisition both in gonadally intact rats and in OVX rats treated with E2. G-15 specifically reduced the rate of acquisition, and this effect was associated with an increased predisposition to adopt a persistent turn. In contrast, G-15 alone at the highest dose had no significant effect on DMP acquisition in OVX controls. The effects were task dependent, as similar effects of G-15 were not observed in gonadally intact rats tested on an operant discrimination/reversal learning task motivated by the same food reward. This suggests that the effects on DMP acquisition were not due to effects on motivation for food. Effects of G-15 on DMP acquisition were similar to previously published work showing significant impairment produced by selective cholinergic denervation of the hippocampus. These data suggest that GPR30 can play an important role in mediating the effects of estradiol on spatial learning, possibly by mediating estradiol effects on basal forebrain cholinergic function.
Keywords: estradiol, G-15, T-maze, working memory, reversal learning
Introduction
Animal studies show that estrogens can have beneficial effects on the brain, improving cognitive performance and preventing or slowing age-related cognitive decline (Daniel, 2006; Dohanich, 2002; Frick, 2009; Gibbs and Gabor, 2003; Gibbs, 2006). These effects are selective and vary depending on the type of learning and memory tested (Davis et al., 2005; Fader et al., 1999; Galea et al., 2001; Korol and Kolo, 2002). Our lab has repeatedly shown that 17β-estradiol (E2) enhances spatial learning on a delayed matching-to-position (DMP) T-maze task in young and middle-aged rats (Gibbs et al., 2004; Gibbs, 2007; Gibbs et al., 2009).
The mechanisms that underlie the effects of E2 on the DMP task remain unclear. A number of studies implicate effects on basal forebrain cholinergic function (reviewed in Gibbs, 2010). For example, E2 increases the expression of choline acetyltransferase (ChAT) mRNA and protein (Bohacek et al., 2008; Gibbs, 1996; McMillan et al., 1996) in the medial septum and nucleus basalis magnocellularis, and increases both ChAT activity (Gibbs, 2000b; Luine, 1985) and high affinity choline uptake (O’Malley et al., 1987; Singh et al., 1995) in the hippocampus and cortex. E2 also increases potassium-stimulated acetylcholine (ACh) release in the hippocampus (Gabor et al., 2003; Gibbs et al., 1997), which correlates with effects on place learning (Marriott and Korol, 2003). Effects of E2 on acquisition of T-maze and radial arm maze tasks are blocked by either cholinergic denervation of the hippocampus (Gibbs, 2007), or by the inhibition of M2 muscarinic ACh receptors (Daniel and Dohanich, 2001). These findings demonstrate that E2 enhances basal forebrain cholinergic function, and that the cholinergic projections play a critical role in mediating effects of E2 on cognitive performance.
The mechanisms by which E2 affects the cholinergic neurons are unclear. Estradiol can exert its effects via specific receptors. Estrogen receptors (ER) α and β are nuclear receptors that belong to a large superfamily of nuclear receptors that regulate gene transcription and which can activate specific second messenger signaling pathways such as MAPK, CamKII, and CREB (Kuiper et al., 1996; Manavathi and Kumar, 2006; McEwen, 2002; Toran-Allerand, 2004). ERα has been implicated in mediating estrogen effects on object recognition and spatial learning (Frye et al., 2007; Hammond et al., 2009). ERβ also has been implicated in mediating effects on spatial learning, as well as on anxiety-like behaviors and depression (Rhodes and Frye, 2006; Walf et al., 2004; Walf et al., 2008).
GPR30 is a novel G-protein coupled estrogen receptor (Filardo et al., 2000; Filardo et al., 2002; Revankar, 2005). It is structurally unrelated to ERα or ERβ, yet binds E2 with high affinity (Thomas et al., 2005) and promotes rapid estrogen signaling in a variety of cell types (Albanito et al., 2007; Filardo and Thomas, 2005; Thomas et al., 2005; Vivacqua et al., 2006). GPR30 has been detected in many brain regions, such as the hippocampus, hypothalamus, septum, frontal cortex, and regions containing basal forebrain cholinergic neurons (Brailoiu et al., 2007; Hammond et al., 2010). Recently we reported that the vast majority of cholinergic neurons in the medial septum, diagonal band of Broca, nucleus basalis magnocellularis, and striatum contain GPR30-like immunoreactivity (Hammond et al., 2010). Neurons in the septum and nucleus basalis magnocellularis are the primary source of cholinergic inputs to the hippocampus and cortex (Mesulam, 1996; Woolf, 1991) and play an important role in learning and memory processes (Baxter and Chiba, 1999; Everitt and Robbins, 1997; Gibbs, 1994; Wenk, 1997). These neurons also are critical for estrogen-mediated enhancement of a spatial learning task (Gibbs, 2002).
Recently we showed that G-1, a selective GPR30 agonist, enhances potassium-stimulated acetylcholine release in the hippocampus and increases the rate of acquisition on a delayed matching-to-position (DMP) spatial learning task similar to E2. This suggests that GPR30 may mediate the effects of E2 on basal forebrain cholinergic function with corresponding effects on cognitive performance. The purpose of the present study was to test this hypothesis further by evaluating the effects of G-15, a selective GPR30 antagonist, on cognitive performance in gonadally intact rats and in OVX rats with and without E2 replacement. G-15 at concentrations below 1 mM has been shown to be a highly selective antagonist of GPR30 in vitro (Dennis et al., 2009). G-15 has a similar binding affinity to GPR30 as G-1, displays minimal binding to ERs α and β (Ki > 10 μM), has been shown to block estradiol-mediated intracellular calcium mobilization and PI3K activation in SKBR3 cells, and decreases estrogen-mediated epithelial cell proliferation (Dennis et al., 2009). G-15 also has been shown to block the protective effects of estradiol against glutamate-induced neurotoxicity in certain hippocampal cell lines (Gingerich et al., 2010). We predicted that systemically administered G-15 would impair performance in gonadally intact rats and would block the effects of E2 on performance in OVX rats while having no effect in OVX controls. This would support the hypothesis that GPR30 plays a role in mediating effects of E2 on spatial learning.
Materials and Methods
Animals
A total of 75 3–4 month old female Sprague-Dawley rats (300–350 g) were purchased from Hilltop Laboratories. Thirty-seven rats were ovariectomized (OVX) by the supplier prior to delivery. Rats were individually housed on a 12-hour day/night cycle (7 am to 7 pm) with food and water freely available. Behavioral tests were conducted in the early afternoon during the lights-on phase. All procedures were carried out in accordance with PHS policies on the use of animals in research, and with the approval of the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Treatments
Two weeks prior to treatment and testing, rats were handled daily for 5 minutes, food restricted, and maintained at 85% of normal body weight during acquisition and testing on both tasks. Handling consisted of picking up the rat and placing it on the handler’s arm or holding it against the body for five minutes. The time interval between OVX and drug treatment was at least 3 weeks. Rats were administered G-15 (a gift from Eric Prossnitz, University of New Mexico, also purchased from Calbiochem, Inc.; La Jolla, CA) or vehicle by Alzet model 2006 mini-osmotic pumps implanted s.c. in the dorsal neck region. The pumps delivered a volume of 0.15 μl per hour over 42 days. G-15 was administered at a rate of 5, 10, or 40 μg/day in a vehicle of 33.7% DMSO + 20% hydroxypropyl-β-cyclodextrin (HPβCD). Doses were selected based on our previous work showing that 5 μg/day of the selective GPR30 agonist G-1 enhanced learning on the DMP task similar to estradiol (Hammond et al., 2009). Rats receiving E2 received silastic capsules (6 mm length, 1.98 mm I.D., 3.18 mm O.D.) packed with 3 mm of powdered 17β-E2 (Sigma-Aldrich, Inc.) implanted s.c. in the dorsal neck region. The E2 capsules produce levels of E2 in the physiologic range for up to 2 months post-implantation. These levels have been shown to enhance DMP acquisition in young adult OVX female rats (Gibbs, 1999; Gibbs, 2002; Gibbs et al., 2004). One week following implantation of pumps and/or capsules rats began T-maze training.
DMP T-maze Testing
The DMP task is a spatial learning and memory T-maze task. The T-maze consists of an approach alley (4 in. wide × 14 in. long) and two goal arms (4 in. wide × 12 in. long). The walls of the maze are 5 in. high, and the doors are constructed of clear plexiglass, thus allowing animals to view the surrounding room. Sliding doors are positioned 8 in. down the approach alley and at the entrance to each goal arm.
Behavior training was performed as previously described (Gibbs, 1999). Rats were first adapted to the maze by placing them in the maze with food (formula 5TUM 45 mg pellets from Test Diets, Inc.) for 5 days. Starting on day 6 through 9 rats were trained to run to the ends of the goal arms by using a series of forced choices and rewarding with four pellets. Right and left arms were alternated to avoid introducing a side bias. Next, rats began DMP testing, which was performed as 8 trial pairs per day. The first trial of each pair consisted of a forced choice in which one arm was blocked, forcing the rat to enter the unblocked arm to receive the food reward. The rat was then returned to the approach alley for the second trial in which both goal arms were open. A choice was defined as the rat placing both front legs and part of both rear legs into a goal arm. Returning to the same arm as the forced choice trial resulted in a food reward, while entering the incorrect arm resulted in no food reward and confinement for 10 seconds. Forced choices were randomized and balanced to avoid introducing a side bias. Rats were run in squads of 4 to 6. After each trial pair, the rat was returned to its home cage for 5–10 min while other rats were tested. Rats received 8 trial pairs per day until they reached the criterion of at least 15/16 correct choices over two days.
After reaching criterion, rats received a probe trial during which the T-maze was rotated 180° (relative to extramaze cues) between the forced and open trial. This was done to assess whether rats were using a place strategy (relying on extramaze cues) or a response strategy (independent of extramaze cues) to perform the task. Rats relying on extramaze cues are expected to turn in the opposite direction (i.e. enter the opposite physical arm now located in the same position of the room as the goal arm during the “forced” trial), while rats relying on internal or kinetic cues would be expected to turn in the same direction (i.e., enter the same physical arm as during the “forced” trial) even though the arm occupies a different position in the room. After the probe trial, animals received 8 trial pairs per day for 4 days with increased intertrial delays (10, 30, 60, 90 seconds on each of the 4 consecutive days) to assess delay-dependent effects on performance.
Operant Discrimination/Reversal Learning (OD/RL) Task
Following DMP T-maze testing, a subset of rats were trained on the OD/RL task. This task was chosen since it uses the same food reward, requires the ability to discriminate between stimuli, and tests the ability to alter behavior in response to change in reinforcement contingency. Four treatment groups were tested to evaluate the effects of G-15 treatment in gonadally intact rats as well as the effects of ovariectomy and E2 replacement. Training was performed in operant chambers (Med. Associates, Inc., Georgia, VT) connected to a computer running Med-PC software. Each operant chamber contained a dim red house light, a ventilation fan, a 6 W stimulus panel light, a speaker calibrated to present a 1500 Hz tone, a pellet dispenser, and a recessed food cup located immediately below the panel light. Entry into the food cup was monitored by a photosensor. Rats were adapted to the chamber by receiving one 60 min session during which they received a total of 16 food pellets delivered at intervals ranging from 2 to 6 min. Testing began the following day. Each rat received one training session per day for a total of 14 days. During the session, rats received 30 presentations of a tone stimulus and 30 presentations of a light stimulus. Each treatment group was randomly divided into 2 subgroups and trained to respond to only one of the stimuli (either tone or light), which will be referred to as the reinforced stimuli. If the rat entered the food cup within 10 sec of the presentation of the reinforced stimuli, the stimulus was discontinued and the rat received a food reward (one 45 mg pellet). If the rat entered the food cup during the non-reinforced stimulus, the stimulus was discontinued, the house light was turned off for 60 sec. and no food was delivered. This is referred to as a time out. The stimuli presentations were randomly distributed throughout the session and occurred at one of 30 randomly selected intertrial intervals ranging from 12 to 70 sec. After 6 days of testing, the stimulus contingency was reversed such that rats were now rewarded for responding to the initially non-reinforced stimulus. Training continued for an additional 8 days.
Estradiol Assay
Following training, rats were given an overdose of ketamine (40 mg/kg) and xylazine (28 mg/kg) injected i.p. and euthanized by decapitation. Trunk blood was collected for the determination of serum estradiol levels, which were determined using a sensitive LC-MS/MS assay recently developed by the Small Molecules Biomolecular Core Facility in our department. Samples were spiked with internal standard (2,4,16,16,17-d3-17β-estradiol) and then extracted with n-butyl chloride. After centrifugation and evaporation, the residue was derivatized in 0.1 mL buffered dansyl chloride solution (pH 10.5). E2 was eluted from a Waters Acquity UPLC BEH C18, 1.7 um, 2.1 × 150 mm reversed-phase column, with an acetonitrile:water (0.1% formic acid) gradient. Detection and quantitation were achieved in the positive mode with a Thermo Fisher TSQ Quantum Ultra mass spectrometer interfaced via an electrospray ionization (ESI) probe with the Waters UPLC Acquity solvent delivery system. Transitions used for analysis were 506 → 171 for E2, and 511 → 171 for the deuterated internal standard. Area under the peak was quantified and used to determine absolute levels of E2/mL of sample by comparison with a series of standards. The limit of detectability for this assay is 2.5 pg/mL. Intra-assay statistics show errors below 8.1% and relative standard deviations below 10.4%. Inter-assay statistics show errors below 5.0% with relative standard deviations below 7.4%.
Data Analysis
DMP Task
Rate of acquisition was defined as the number of days to reach criterion (DTC). DTC was analyzed for each treatment group by one-way ANOVA. Learning curves were constructed by plotting the mean performance (percent correct) for each group across Blocks of training. Each Block represented average performance across three consecutive days of training. Upon reaching criterion, a value of 93.8% correct, reflecting the criterion of 15/16 correct choices over 2 days, was used in calculating group performance on subsequent days. The learning curves were compared using a two factor (Treatment x Block) ANOVA with repeated measures on Block. Performance on the probe trial was analyzed by contingency table and Chi-square. Performance during the increased intertrial delays was analyzed by ANOVA with repeated measures on Delay. Post-hoc comparisons were made using a Tukey test. Significance was set at p ≤ 0.05. All statistical analysis was performed using JMP software for Macintosh.
OD/RL Task
Learning curves were constructed by plotting the number of correct and incorrect choices for each treatment group across Days of training. The learning curves were compared using a two factor (Treatment x Day) ANOVA with repeated measures on Day. Significance was set at p ≤ 0.05.
Effects of G-15 on estrous cycles and E2 levels
Partway through the study we realized that E2 levels in many of the gonadally intact rats were relatively low. This was likely due to the fact that rats were euthanized randomly at different points along the estrous cycle. Nevertheless, we thought it best to test any potential effect of G-15 on the cycle and on levels of E2. A separate cohort of gonadally intact rats were treated with G-15 (40 μg/day) or vehicle for a period of four weeks. Daily vaginal smears were collected beginning several days following the initiation of treatment. Smears were stained with cresyl violet and analyzed to determine cycle stage. After four weeks of treatment, rats were euthanized and plasma was collected and analyzed to determine serum levels of E2 as described above.
Results
Serum estradiol levels
Mean levels of E2 in the gonadally intact rats were 4.6 ± 0.65 pg/ml, 1.9 ± 0.14 pg/ml, and 2.32 ± 0.34 pg/ml in the vehicle-, G-15 (5 μg/day)- and G-15(10 μg/day)-treated rats. Levels in many rats were below detectability and were assigned values of 0 for the purpose of calculating averages. However, these low values are not unexpected given that rats were euthanized randomly with respect to the estrous cycle. Group differences were not statistically significant. E2 levels for all OVX rats were undetectable. Levels in the OVX+E2 group were 50.7 ± 1.52 pg/ml, with a range of 32.32 – 75.28 pg/ml. Levels in the OVX+E2+G-15 group were 49.56 ± 1.47 pg/ml, with a range of 34.52 – 77.31 pg/ml. These levels are consistent with the reported peak levels obtained on the afternoon of proestrus (McGinnis et al., 1981). In the separate cohort of rats whose cycles were monitored, mean levels of E2 were 5.05 ± 4.40 pg/ml and 9.60 ± 5.17 pg/ml for vehicle- and G-15-treated rats. These differences were not statistically significant. Again, levels were undetectable in some rats, consistent with the fact that some rats were sacrificed during diestrus or metestrus. Analysis of the vaginal smears indicated that all rats in both the vehicle and the G-15-treated groups experienced estrous cycles of 4–5 days in length.
DMP T-maze Acquisition
Effect of G-15 on DTC in Gonadally Intact Rats
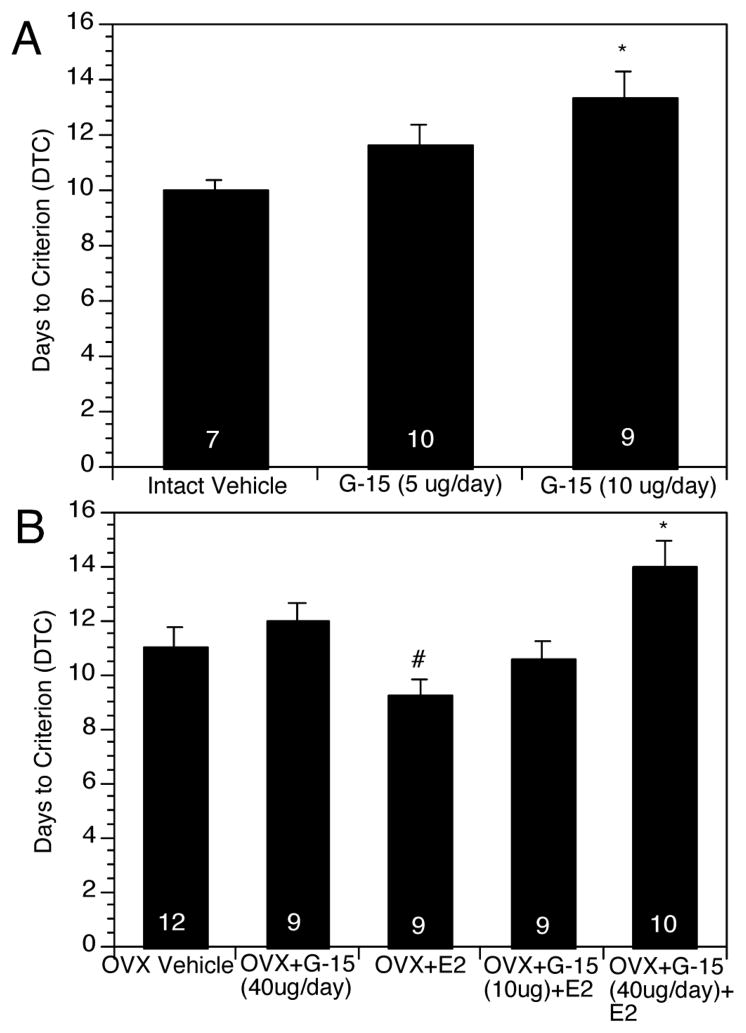
G-15 dose-dependently impaired performance in gonadally intact rats (Figure 1). Gonadally intact rats treated with 10 μg/day of G-15 required significantly more days on average to reach criterion (13.3 ±0.9 days for rats treated with 10 μg/day G-15) than vehicle treated intact controls (10.0 ± 0.3 days) and performed similarly to OVX controls. ANOVA revealed a significant effect of Treatment (F(2,23)=7.66, p < 0.01). Post-hoc analysis revealed that in intact rats treated with G-15 at a dose of 10 μg/day differed significantly from gonadally intact controls.
Figure 1.
Effects of G-15 on DTC in gonadally intact rats and in OVX rats. 1A shows G-15 dose-dependently increased DTC in gonadally intact rats, similar to the effects of OVX. 1B shows E2 treatment significantly decreased DTC in OVX rats and this was dose-dependently reversed by G-15. G-15 alone had no significant effect on DTC in OVX rats. Bars indicate mean number of days to react criterion ± s.e.m. *p < 0.05 relative to gonadally intact controls or OVX+E2-treated rats. # p < 0.05 relative to OVX vehicle controls.
Effect of G-15 on DTC in Ovx Rats
G-15 dose-dependently impaired performance in OVX+E2-treated rats (Figure 1). OVX rats treated with E2 + 40 μg/day G-15 required significantly more days to reach criterion (14.0 ± 0.9) than OVX rats treated with E2 alone (9.2 ± 0.6). ANOVA revealed a significant effect of Treatment (F(4,44)=5.64, p < 0.01). Among OVX rats, those treated with E2 learned significantly faster than OVX controls, and OVX+E2+G-15 (40 μg/day) rats took significantly longer than OVX+E2 rats (p<0.05 in each case). Rats treated with G-15 alone did not differ significantly from OVX controls but differed significantly from OVX+E2-treated rats.
DMP Learning Curves
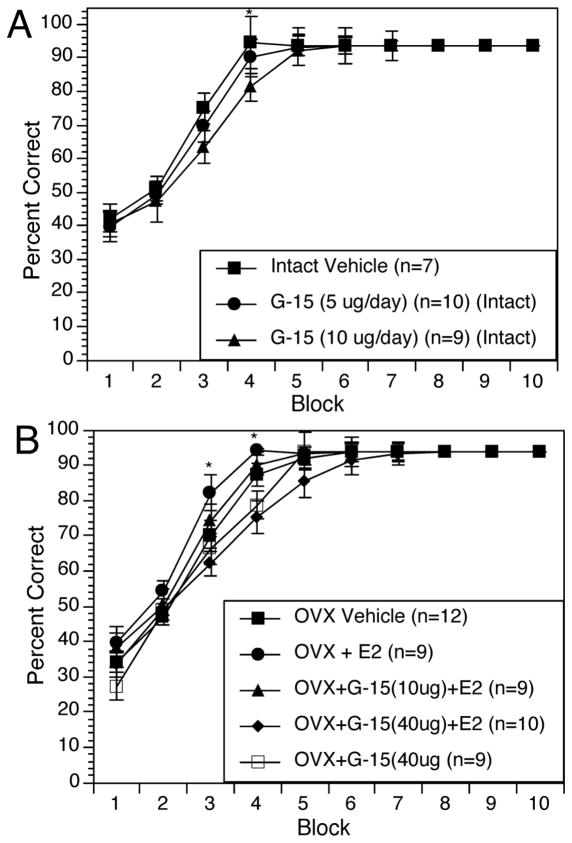
Learning curves show that all groups performed similarly below chance at the start of training and then improved at different rates. Among the gonadally intact rats, controls acquired the task at a significantly faster rate than those that recevied G-15 at 10 μg/day (Figure 2A). In analyzing Blocks 3–5, ANOVA revealed a significant effect of Treatment (F(2,23)=3.43, p < 0.05) and a significant effect of Block (F(2,22)= 18.5, p < 0.01). Post-hoc analysis revealed significant differences between the controls and the G-15 (10 μg/day) group (p < 0.01) on Block 4.
Figure 2.
Learning curves showing acquisition of the DMP task over time. Values represent the mean percent correct ± s.e.m. within a 3-day block of training for each treatment group. Panel 2A shows learning curves for all gonadally intact rats. *p < 0.05 for G-15 (10 μg/day) relative to intact vehicles. Panel 2B shows learning curves for all OVX rats. *p < 0.05 for OVX+G-15 (40 μg/day) and OVX+E2+G-15(40 μg/day) relative to OVX+E2-treated rats.
Among OVX rats, those treated with E2 acquired the task at a significantly faster rate than all other OVX groups (Figure 2B). OVX controls did not differ significantly from any of the G-15-treated groups. In analyzing Blocks 3–5 ANOVA revealed a significant effect of Treatment (F(4,44)= 4.83, p < 0.01) a significant effect of Block (F(2,43)= 44.2, p < 0.01), and a significant Treatment x Block interaction (F(8,86)=2.1, p < 0.05). Post-hoc analysis revealed the OVX+G-15 (40 μg/day) and OVX+E2+G-15 (40 μg/day) groups were significantly different from the OVX+E2 group on Blocks 3 and 4.
Effects on Persistent Turn
Previous studies show that some rats adopt a persistent turn early on during DMP training and that this can affect DTC (Gibbs, 2007; Gibbs and Johnson, 2007). To quantify this, we examined whether rats adopted a persistent turn, defined as entering the same arm at least 15/16 times during the choice trial over a two day period. Results show that ovariectomy significantly increased the percentage of rats that adopted a persistent turn (28.6% of gonadally intact controls vs. 83.3% of OVX controls). Treatments also affected the percentage of rats that adopted a persistent turn. Among gonadally intact rats, G-15 dose-dependently increased the percentage of rats that adopted a persistent turn (X2=6.9, p < 0.05) as well as the average numbers of days that rats engaged in a persistent turn (Table 1). Among OVX rats, E2 significantly decreased the percentage of rats that adopted a persistent turn and decreased the average number of days that rats engaged in a persistent turn. However, G-15 at the highest dose blocked this effect. G-15 alone had no effect on persistent turn on OVX rats (X2=23.1, p < 0.01, Table 1).
Table 1.
Effects of Treatments on Adoption of a Persistent Turn During DMP Training
| Group | Percentage that Adopted a Persistent Turn | Average Length of Persistent Turn (Days ± s.e.m.) |
|---|---|---|
| Intact Vehicle Controls | 28.6 | 0.6 ± 0.4† |
| Intact+G15 (5ug/day) | 50.0 | 2.0 ± 0.8 |
| Intact+G15 (10ug/day) | 88.9* | 3.6 ± 0.7* |
| OVX Vehicle Controls | 83.3* | 4.1 ± 0.6* |
| OVX+G15 (40ug/day) | 88.9* | 3.4 ± 0.6 |
| OVX+E2 | 33.3† | 2.0 ± 0.3† |
| OVX+E2+G15 (10ug/day) | 11.1† | 2.0 ± 0.2† |
| OVX+E2+G15 (40ug/day) | 90.0* | 5.4 ± 1.2* |
p<0.05 relative to Intact Vehicle controls;
p<0.05 relative to OVX Vehicle Controls
Effects of Probe Trial
After reaching criterion, rotating the maze 180° between the forced and open choices caused a significant decrease in the percentage of rats within each group that selected the arm located in the same physical location in the room. This percentage dropped from 93.8% (criterion level) to 42% for intact groups and to 49% for all OVX groups. Analysis showed no significant effects of Treatment on performance during the probe trial (X2=0.95, p > 0.6 for intact groups; X2=2.1, p > 0.7 for OVX groups). These findings suggest that a significant number of rats in each group rely on extramaze cues (i.e., a place strategy) to solve the task and that treatments did not differentially affect the degree to which rats relied upon place vs. response strategy.
Effects on Short-Term Spatial Memory
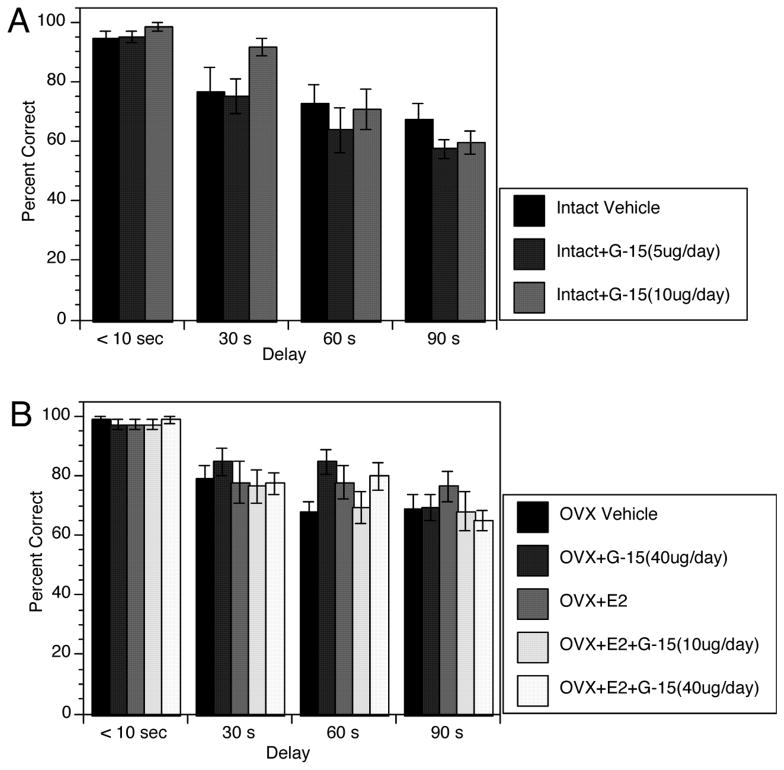
During post-criterion testing, increasing the intertrial delay impaired performance for all treatment groups (Figure 3). Treatments did not differentially alter this effect. For gonadally intact rats, ANOVA revealed a significant effect of Delay (F(3,21)=5.7, p < 0.01), no significant effect of Treatment (F(2,23)=0.11, p > 0.3) and no significant interaction between Treatment and Delay (F(6,42)=0.71, p > 0.3). For OVX groups, ANOVA revealed a significant effect of Delay (F(3,42)=13.4, p < 0.01), no significant effect of Treatment (F(4,44)=0.46, p > 0.8), and no significant interaction between Treatment and Delay (F(12,111)=0.77, p > 0.5).
Figure 3.
Bar graphs showing effects of increasing the delay between the forced and open trials to 30, 60, and 90 seconds on performance. Bars represent the percentage of correct choices ± s.e.m. for each group. Panel A shows results for gonadally intact rats. Panel B shows results for OVX rats.
OD/RL Testing
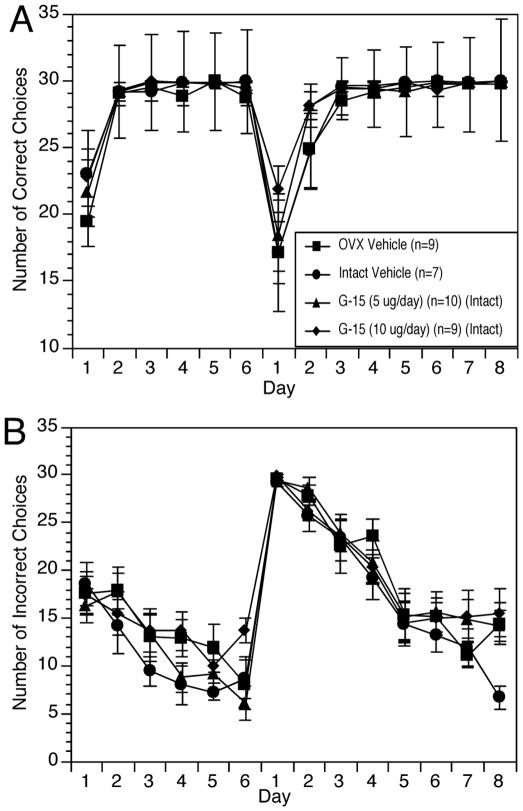
In contrast to effects on DMP acquisition, neither OVX nor E2 treatment had any significant effect on OD/RL performance. Likewise, G-15 treatment had no effect on OD/RL acquisition or reversal learning in gonadally intact rats (Figure 4). ANOVA revealed no significant effect of Treatment on number of correct choices (F(3,29)= 0.95, p > 0.4) (Figure 4A) or incorrect choices (F(3,29)= 0.51, p > 0.7) (Figure 4B) during the acquisition or reversal learning phases.
Figure 4.
Learning curves showing number of correct choices (A) and number of incorrect choices (B) made by rats during successive choice discrimination. Rats were trained to respond to either light or sound for the first six days and then trained to respond to the opposite stimulus for the next eight days. The arrow indicates the day on which the stimulus contingency was reversed.
Discussion
Effects of E2 and G-15 on DMP acquisition
Our findings corroborate previous work showing that ovariectomy slows the rate of DMP acquisition in young adult rats, and that E2 can restore the rate of DMP acquisition to that of gonadally intact controls (Gibbs, 1999; Gibbs, 2007). The present study showed that G-15 dose-dependently impaired acquisition in gonadally intact rats and blocked the effect of E2 on DMP acquisition in OVX rats. Rats treated with the higher doses of G-15 performed comparable to OVX controls. Notably, the dose required to block the effect of E2 in OVX rats was higher than that needed to affect performance in gonadally intact controls, possibly because OVX+E2-treated rats had sustained elevated levels of E2 in the high physiological range.
G-15 alone had no apparent effect on DMP acquisition in OVX rats, suggesting that results were not due to a non-specific effect of G-15 on performance. Recent work from Smejkalova et al. suggests that E2 synthesized in the brain may be important for neural plasticity in the hippocampus (Smejkalova and Woolley, 2010). The finding that G15 fails to influence learning or working memory in OVX rats also suggests that local release of E2 and subsequent signaling through non-nuclear means does not play a substantial role in the observed effects of E2 treatment on this task. Moreover, there were no treatment effects on delay-dependent performance once rats had reached criterion. This is consistent with previously published work from our lab showing that E2 enhances the rate of acquisition but not performance during increasing intertrial delays (Gibbs et al., 2004; Gibbs, 2007). These finding are consistent with the hypothesis that the effects of E2 on DMP acquisition are task-specific and may be mediated, at least in part, via GPR30.
Previous work from our lab showed that, like E2, DPN (ERβ agonist), PPT (ERα agonist), and G-1 (GPR30 agonist) were able enhance acquisition of this task in OVX rats (Hammond et al., 2009), suggesting that E2 effects on this task may be mediated by multiple estrogen receptors. In this study, G-15 impaired performance to that of ovariectomy, suggesting that GPR30 is necessary for estradiol to enhance the rate of acquisition on this task.
Effects of G-15 were not due to effects on estrous cycles or ovarian function
E2 levels in the gonadally intact rats were low. This can be explained by the fact that rats were killed randomly with respect to the estrous cycle. Nevertheless, we considered the possibility that G-15 might affect performance by interfering with ovarian function. In a separate cohort of rats, we observed no effect of sustained G-15 delivery on estrous cycles or on serum levels of E2. This is consistent with the fact that in mice, knock-out of the GPR30 gene does not impair normal reproduction (Otto et al., 2009). This suggests that the effects of G-15 on DMP acquisition in gonadally intact rats were not due to an effect on ovarian function or on circulating levels of E2.
Effects on learning strategy and persistent turn
Rats use various strategies to solve spatial tasks such as place strategies (use of extramaze visual cues) and response strategies (use body positioning and kinetic cues) (Dudchenko, 2001; Korol et al., 2004). Learning strategy can vary across the estrous cycle (Korol et al., 2004), and studies suggest that this is due to changes in circulating levels of estradiol. Specifically Ovx has been shown to favor use of a response strategy whereas acute and chronic E2 treatment improves the ability of rats to use a place strategy (Daniel and Lee, 2004; Davis et al., 2005; Korol and Kolo, 2002). In the current study, once rats had reached criterion, rotating the maze 180° between the forced and open choices resulted in a significant decrease in the percentage of rats within each group that selected the arm located in the same physical location in the room. There are two possible interpretations of the data: (1) a proportion of rats in each group rely on extramaze cues and the remainder do not, or (2) all rats rely on extramaze cues to some degree such that rotation of the maze causes all of the rats to become confused, resulting in a random choice. Both possibilities are consistent with the observation that within each treatment group, the percentage of rats that selected the arm located in the same physical location in the room did not differ significantly from chance during the probe trial. We cannot distinguish between these two possibilities; however, what we can say is that by the time rats reach criterion, enough rats within each group rely on extramaze cues to result in a significant effect on this measure during the probe trial. Prior work shows that this change, the increase in reliance on extramaze cues, occurs during the course of training and is impaired by cholinergic lesions (Fitz et al., 2008). Since there was no main effect of treatment, we conclude that treatments did not differentially affect the degree to which rats relied upon extramaze cues to solve the task. The findings are consistent with prior observations showing little effect of ovariectomy and estradiol treatment on performance during the probe trial despite seeing reproducible effects on acquisition (Gibbs, 2002; Gibbs, 2007).
In the present study, gonadally intact rats treated with G-15, and OVX+E2-treated rats that received G-15, were more likely to adopt a persistent turn during DMP acquisition than rats that did not receive G-15. In fact, in cycling rats 8 out of 9 rats receiving 10 μg/day of G-15 adopted a persistent turn, in comparison with only 2 out of 7 vehicle treated controls. G-15 also increased the average number of days that rats engaged in a persistent turn. The same trend was observed in the OVX+E2 rats treated with the highest dose of G-15. This suggests that G-15 impairs performance on this task by increasing the predisposition to adopt and maintain a persistent turn. The effect is consistent with a predisposition to utilize a response strategy during early stages of acquisition and agrees nicely with the studies cited above describing effects of Ovx and E2 on learning strategy. On the DMP task, adoption of a persistent turn limits performance to 50%; however, with additional training, the rats ultimately were able to abandon the persistent turn and adopt a more effective strategy thus enabling them to reach criterion. This accounts for the increased number of days required to reach criterion. Notably, these same effects are produced by basal forebrain cholinergic lesions (Gibbs and Johnson, 2007), suggesting that both G-15 and cholinergic lesions affect response pattern, strategy selection, and the ability to alter strategy (i.e., cognitive flexibility) in a similar way.
Role of Basal Forebrain Cholinergic Neurons
As mentioned above, cholinergic neurons in the medial septum and nucleus basalis magnocellularis are the primary source of cholinergic inputs to the hippocampus and cortex and play an important role in learning, memory, and attentional processes (Baxter and Chiba, 1999; Everitt and Robbins, 1997; Gritti et al., 1997; Mesulam, 1996). Studies show that selectively lesioning these cells produces an increase in the predisposition to adopt a persistent turn during DMP acquisition (Gibbs, 2002; Gibbs, 2007). A similar effect is observed in aged rats and can be reversed, in part, by treating with selective cholinesterase inhibitors (Bohacek et al., 2008; Gibbs et al., 2009) (Gibbs et al., 2011a; Gibbs et al., 2011b). This suggests that cholinergic inputs to the hippocampus and cortex have a strong effect on perseveration and the adoption of a persistent turn during DMP training. Prior studies show that E2 has a number of effects on basal forebrain cholinergic neurons including increasing choline acetyltransferase mRNA and protein (Gibbs et al., 1997; Gibbs, 2000b; Singh et al., 1994), increasing high affinity choline uptake in the hippocampus and frontal cortex (O’Malley et al., 1987; Tinkler et al., 2004), and increasing potassium-stimulated acetylcholine release (Gabor et al., 2003; Gibbs, 1997). In addition, we have shown that septal cholinergic neurons are essential for E2-mediated enhancement of DMP acquisition (Gibbs, 2002). Recently we showed that cholinergic neurons in the medial septum and nucleus basalis magnocellularis also contain GPR30, and that G-1, a selective GPR30 agonist, enhances potassium-stimulated acetylcholine release in the hippocampus similar to E2 (Hammond et al., 2010). Collectively, these data suggest that E2 can enhance basal forebrain cholinergic function via GPR30, and support the hypothesis that the effects of G-15 on DMP acquisition are due, at least in part, to a blockade of E2 effects on basal forebrain cholinergic neurons. Further studies will focus on testing this hypothesis.
Effects of estradiol and GPR30 antagonism on OD/RL learning
In contrast to effects on the DMP task, no effects of ovariectomy or E2 replacement were observed on the OD/RL task. Likewise, G-15 had no effect on OD/RL learning in gonadally intact rats. The OD/RL task is a non-spatial operant learning and memory task. Learning requires that rats be able to distinguish visual and auditory cues and to associate individual cues with a food reward. The fact that neither OVX, E2 nor G-15 had any affect on this task suggests that treatments did not significantly impact these processes. In addition, as both the DMP task and the OD/RL task utilize the same food reward, this suggests that effects on the DMP task are not due to an effect on the motivation for food. Many studies have shown that effects of E2 on cognitive performance are task selective. For example, E2 has been shown to improve performance on spatial learning (Daniel et al., 1997; Gibbs, 1999), object placement and recognition (Fernandez et al., 2008; Gresack and Frick, 2006), and fear condition tasks (Jasnow et al., 2006), but not on specific reference or working memory tasks (Fader et al., 1999; Galea et al., 2001), nor on a configural association operant conditioning task (Gibbs and Gabor, 2003; Gibbs et al., 2009). Thus, our results agree with previous work showing that the effects of E2 on learning and memory tasks are task-specific, and suggest that the effects of G-15 are due to interference with the effects of E2, rather than to non-specific effects on learning and memory processes.
Conclusions
In summary, our data show that G-15 impairs DMP acquisition in gonadally intact rats and blocks the effects of E2 on DMP acquisition in OVX rats. Treatment with G-15 alone had no significant effect. The effects of G-15 are similar to the effects of removing cholinergic inputs to the hippocampus and frontal cortex. These findings support the hypothesis that G-15 impairs DMP acquisition by blocking the effects of E2 on basal forebrain cholinergic neurons that provide cholinergic inputs to the hippocampus and cortex. Studies are underway to elucidate the cellular and neurobiological processes that underlie these effects.
Highlights.
G-15 dose-dependently impairs acquisition of a T-maze task in intact rats and in ovariectomized rats treated with estradiol
G-15 reduces the rate of acquisition by increasing the predisposition to adopt a persistent turn
No G-15 effects were observed in intact rats tested on an operant discrimination/reversal learning task
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Albanito L, Madeo A, Lappano R, Vivacqua A, Rago V, Carpino A, Oprea TI, Prossnitz ER, Musti AM, Ando S, Maggiolini M. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007;67:1859–66. doi: 10.1158/0008-5472.CAN-06-2909. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Chiba AA. Cognitive functions of the basal forebrain. Curr Opin Neurobiol. 1999;9:178–83. doi: 10.1016/s0959-4388(99)80024-5. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. J Neuroendocrinol. 2008;20:1023–7. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–21. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–25. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Dohanich GP. Acetylcholine mediates the estrogen-induced increase in NMDA receptor binding in CA1 of the hippocampus and the associated improvement in working memory. J Neurosci. 2001;21:6949–6956. doi: 10.1523/JNEUROSCI.21-17-06949.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Lee CD. Estrogen replacement in ovariectomized rats affects strategy selection in the Morris water maze. Neurobiol Learn Mem. 2004;82:142–9. doi: 10.1016/j.nlm.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–95. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–7. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009;5:421–7. doi: 10.1038/nchembio.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohanich GP. Gonadal Steroids, Learning, and Memory. In: Pfaff AA, Etgen DW, Fahrbach AM, Rubin SE, Hormones RI, editors. Brain, and Behavior. Vol. 2. Academic Press; San Diego: 2002. pp. 265–327. [Google Scholar]
- Dudchenko PA. How do animals actually solve the T maze? Behav Neurosci. 2001;115:850–60. [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Johnson PEM, Dohanich GP. Estrogen improves working but not reference memory and prevents amnestic effects of scopolamine on a radial-arm maze. Pharmacology Biochemistry and Behavior. 1999;62:711–717. doi: 10.1016/s0091-3057(98)00219-6. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal extracellular signal-regulated kinase activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–7. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–60. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. GPR30: a seven-transmembrane-spanning estrogen receptor that triggers EGF release. Trends Endocrinol Metab. 2005;16:362–7. doi: 10.1016/j.tem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Fitz NF, Gibbs RB, Johnson DA. Selective lesion of septal cholinergic neurons in rats impairs acquisition of a delayed matching to position T-maze task by delaying the shift from a response to a place strategy. Brain Res Bull. 2008;77:356–60. doi: 10.1016/j.brainresbull.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Estrogens and age-related memory decline in rodents: what have we learned and where do we go from here? Horm Behav. 2009;55:2–23. doi: 10.1016/j.yhbeh.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–16. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabor R, Nagle R, Johnson DA, Gibbs RB. Estrogen enhances potassium-stimulated acetylcholine release in the rat hippocampus. Brain Res. 2003;962:244–7. doi: 10.1016/s0006-8993(02)04053-2. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behav Brain Res. 2001;126:115–26. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen and nerve growth factor-related systems in brain. Effects on basal forebrain cholinergic neurons and implications for learning and memory processes and aging. Ann N Y Acad Sci. 1994;743:165–96. doi: 10.1111/j.1749-6632.1994.tb55792.x. discussion 197–9. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: effects of estrogen and progesterone. J Neurosci. 1996;16:1049–55. doi: 10.1523/JNEUROSCI.16-03-01049.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Effects of estrogen on basal forebrain cholinergic neurons vary as a function of dose and duration of treatment. Brain Res. 1997;757:10–6. doi: 10.1016/s0006-8993(96)01432-1. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Hashash A, Johnson DA. Effects of estrogen on potassium-stimulated acetylcholine release in the hippocampus and overlying cortex of adult rats. Brain Res. 1997;749:143–6. doi: 10.1016/s0006-8993(96)01375-3. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen replacement enhances acquisition of a spatial memory task and reduces deficits associated with hippocampal muscarinic receptor inhibition. Horm Behav. 1999;36:222–33. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000a;21:107–16. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Effects of gonadal hormone replacement on measures of basal forebrain cholinergic function. Neuroscience. 2000b;101:931–938. doi: 10.1016/s0306-4522(00)00433-4. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Basal forebrain cholinergic neurons are necessary for estrogen to enhance acquisition of a delayed matching-to-position T-maze task. Horm Behav. 2002;42:245–57. doi: 10.1006/hbeh.2002.1825. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R. Estrogen and cognition: applying preclinical findings to clinical perspectives. J Neurosci Res. 2003;74:637–43. doi: 10.1002/jnr.10811. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Gabor R, Cox T, Johnson DA. Effects of raloxifene and estradiol on hippocampal acetylcholine release and spatial learning in the rat. Psychoneuroendocrinology. 2004;29:741–8. doi: 10.1016/S0306-4530(03)00118-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Preclinical data relating to estrogen’s effects on cognitive performance. In: Rasgon NL, editor. The Effects of Estrogen on Brain Function. 2006. [Google Scholar]
- Gibbs RB. Estradiol enhances DMP acquisition via a mechanism not mediated by turning strategy but which requires intact basal forebrain cholinergic projections. Horm Behav. 2007;52:352–9. doi: 10.1016/j.yhbeh.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Johnson DA. Cholinergic lesions produce task-selective effects on delayed matching to position and configural association learning related to response pattern and strategy. Neurobiol Learn Mem. 2007;88:19–32. doi: 10.1016/j.nlm.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Mauk R, Nelson D, Johnson DA. Donepezil treatment restores the ability of estradiol to enhance cognitive performance in aged rats: evidence for the cholinergic basis of the critical period hypothesis. Horm Behav. 2009;56:73–83. doi: 10.1016/j.yhbeh.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen Therapy and Cognition: A Review of the Cholinergic Hypothesis. Endocrine Reviews. 2010;31 doi: 10.1210/er.2009-0036. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Hammond R, Nelson D. Galanthamine plus estradiol treatment enhances cognitive performance in aged ovariectomized rats. Horm Behav. 2011a;60:607–16. doi: 10.1016/j.yhbeh.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs RB, Chipman AM, Nelson D. Donepezil plus estradiol treatment enhances learning and delay-dependent memory performance by young ovariectomized rats with partial loss of septal cholinergic neurons. Horm Behav. 2011b;59:503–11. doi: 10.1016/j.yhbeh.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingerich S, Kim GL, Chalmers JA, Koletar MM, Wang X, Wang Y, Belsham DD. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience. 2010;170:54–66. doi: 10.1016/j.neuroscience.2010.06.076. [DOI] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Post-training estrogen enhances spatial and object memory consolidation in female mice. Pharmacol Biochem Behav. 2006;84:112–9. doi: 10.1016/j.pbb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Gritti I, Mainville L, Mancia M, Jones BE. GABAergic and other noncholinergic basal forebrain neurons, together with cholinergic neurons, project to the mesocortex and isocortex in the rat. J Comp Neurol. 1997;383:163–77. [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–14. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–20. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Korol DL, Malin EL, Borden KA, Busby RA, Couper-Leo J. Shifts in preferred learning strategy across the estrous cycle in female rats. Horm Behav. 2004;45:330–8. doi: 10.1016/j.yhbeh.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–30. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN. Estradiol increases choline acetyltransferase activity in specific basal forebrain nuclei and projection areas of female rats. Exp Neurol. 1985;89:484–90. doi: 10.1016/0014-4886(85)90108-6. [DOI] [PubMed] [Google Scholar]
- Manavathi B, Kumar R. Steering estrogen signals from the plasma membrane to the nucleus: two sides of the coin. J Cell Physiol. 2006;207:594–604. doi: 10.1002/jcp.20551. [DOI] [PubMed] [Google Scholar]
- Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiol Learn Mem. 2003;80:315–22. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–84. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- McGinnis MY, Krey LC, MacLusky NJ, McEwen BS. Steroid receptor levels in intact and ovariectomized estrogen-treated rats: an examination of quantitative, temporal and endocrine factors influencing the efficacy of an estradiol stimulus. Neuroendocrinology. 1981;33:158–65. doi: 10.1159/000123222. [DOI] [PubMed] [Google Scholar]
- McMillan PJ, Singer CA, Dorsa DM. The effects of ovariectomy and estrogen replacement on trkA and choline acetyltransferase mRNA expression in the basal forebrain of the adult female Sprague-Dawley rat. J Neurosci. 1996;16:1860–5. doi: 10.1523/JNEUROSCI.16-05-01860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. The systems-level organization of cholinergic innervation in the human cerebral cortex and its alterations in Alzheimer’s disease. Prog Brain Res. 1996;109:285–97. doi: 10.1016/s0079-6123(08)62112-3. [DOI] [PubMed] [Google Scholar]
- O’Malley CA, Hautamaki RD, Kelley M, Meyer EM. Effects of ovariectomy and estradiol benzoate on high affinity choline uptake, ACh synthesis, and release from rat cerebral cortical synaptosomes. Brain Res. 1987;403:389–92. doi: 10.1016/0006-8993(87)90082-5. [DOI] [PubMed] [Google Scholar]
- Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier K-H. GPR30 Does Not Mediate Estrogenic Responses in Reproductive Organs in Mice. Biology of Reproduction. 2009;80:34–41. doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Climino Daniel F, Sklar Laryy A, Arterburn Jeffrey B, Prossnitz Eric R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rhodes ME, Frye CA. ERbeta-selective SERMs produce mnemonic-enhancing effects in the inhibitory avoidance and water maze tasks. Neurobiol Learn Mem. 2006;85:183–91. doi: 10.1016/j.nlm.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Res. 1994;644:305–12. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Simpkins JW. The effect of ovariectomy and estradiol replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague-Dawley rats. Endocrinology. 1995;136:2320–4. doi: 10.1210/endo.136.5.7720680. [DOI] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–48. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–32. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Tinkler GP, Tobin JR, Voytko ML. Effects of two years of estrogen loss or replacement on nucleus basalis cholinergic neurons and cholinergic fibers to the dorsolateral prefrontal and inferior parietal cortex of monkeys. J Comp Neurol. 2004;469:507–21. doi: 10.1002/cne.11028. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–74. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Vivacqua A, Bonofiglio D, Recchia AG, Musti AM, Picard D, Ando S, Maggiolini M. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006;20:631–46. doi: 10.1210/me.2005-0280. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Antidepressant effects of ERbeta-selective estrogen receptor modulators in the forced swim test. Pharmacol Biochem Behav. 2004;78:523–9. doi: 10.1016/j.pbb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122:974–81. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]