Abstract
Renal xenobiotic transporters are important determinants of urinary secretion and reabsorption of chemicals. In addition to glomerular filtration, these processes are key to the overall renal clearance of a diverse array of drugs and toxins. Alterations in kidney transporter levels and function can influence the efficacy and toxicity of chemicals. Studies in experimental animals have revealed distinct patterns of renal transporter expression in response to sex hormones, pregnancy, and growth hormone. Likewise, a number of disease states including diabetes, obesity, and cholestasis alter the expression of kidney transporters. The goal of this review is to provide an overview of the major xenobiotic transporters expressed in the kidneys and an understanding of metabolic conditions and hormonal factors that regulate their expression and function.
Keywords: kidney, transporter, diabetes, pregnancy, obesity, gender, sex hormones, cholestasis
INTRODUCTION
While some chemicals enter and exit cells by passive diffusion, others require carriers or transport proteins. Transporters are membrane-spanning proteins that assist in the uptake and efflux of a large variety of compounds into and out of the cell, respectively. Transporters are widely distributed throughout the body and have an affinity for a range of substrates including endogenous compounds and xenobiotics. Because of their location in the plasma membrane, broad substrate specificities, and diverse patterns of tissue distribution, transporters are essential proteins that influence the disposition of endogenous and exogenous substrates.
Two superfamilies of transporters are recognized in humans and rodents: the solute carrier (SLC) and ATP-binding cassette (ABC) families (Table 1). The SLC family predominantly consists of uptake transporters, but there are some examples of SLC proteins with efflux and bidirectional transport activity. SLC proteins translocate substrates by either secondary or tertiary active transport. Secondary active transport couples the movement of a substrate to a co-substrate (such as an ion), whereas tertiary active transport requires coordinated transport by multiple membrane proteins including an ATPase pump. Transporters that are members of the SLC family include organic anion transporters (Oats/OATs), organic cation transporters (Octs/OCTs), organic anion transporting polypeptides (Oatps/OATPs), organic cation/carnitine transporters (Octns/OCTNs), glucose transporters (Gluts/GLUTS) and peptide transporters (Pepts/PEPTs), along with multidrug and toxin extrusion transporters (Mates/MATEs). While most of these transporters are responsible for the influx of chemicals, Oatp1a4 mediates the bidirectional transport of chemicals in exchange with gluthathione (1). Similarly, Mate transporters utilize an antiport mechanism to efflux cationic drugs using energy from proton exchange (reviewed in (2)). Lowercase letters denote rodent species and uppercase letters refer to human isoforms. The ABC family consists exclusively of efflux transporters that require energy liberated from ATP hydrolysis to actively translocate substrates against concentration gradients, a process called primary active transport. Members of the ABC family include multidrug resistance-associated proteins (Mrps/MRPs), multidrug resistance proteins (Mdrs/MDRs), and the breast cancer resistance protein (Bcrp/BCRP).
Table 1.
Transporter nomenclature.a
| SLC Transporters | ABC Transporters | ||
|---|---|---|---|
| Gene | Protein | Gene | Protein |
| SLC22A6 | OAT1 | ABCB1 | MDR1 (human), Mdr1a and 1b (rodent) |
| SLC22A7 | OAT2 | ABCB4 | MDR3 |
| SLC22A8 | OAT3 | ||
| SLC22A11 | OAT4 | ABCC1 | MRP1 |
| SLC22A10/19 | OAT5 | ABCC2 | MRP2 |
| SLC22A12 | URAT | ABCC3 | MRP3 |
| ABCC4 | MRP4 | ||
| SLCO1a1 | Oatp1a1 (rodent) | ABCC5 | MRP5 |
| SLCO1A2 | OATP1A2 (human) | ABCC6 | MRP6 |
| SLCO1a4 | Oatp1a4 (rodent) | ||
| SLCO1a5 | Oatp1a5 (rodent) | ABCG2 | BCRP |
| SLCO1a6 | Oatp1a6 (rodent) | ||
| SLCO2B1 | OATP2B1 | ||
| SLCO3A1 | OATP3A1 | ||
| SLCO4A1 | OATP4A1 | ||
| SLCO4C1 | OATP4C1 | ||
| SLCO5A1 | OATP5A1 | ||
| SLCO6A1 | OATP6A1 | ||
| SLC22A1 | OCT1 | ||
| SLC22A2 | OCT2 | ||
| SLC22A3 | OCT3 | ||
| SLC22A4 | OCTN1 | ||
| SLC22A5 | OCTN2 | ||
| SLC2A9 | GLUT9 | ||
| SLC15A1 | PEPT1 | ||
| SLC15A2 | PEPT2 | ||
| SLC47A1 | MATE1 | ||
| SLC47A2 | MATE2-K (human), Mate2 (rodent) | ||
The gene and protein names are listed for the solute carrier (SLC) and ATP-binding cassette (ABC) transporters. This is not intended to be a complete list, rather highlights transporters expressed in the kidneys. Transporters with different human and rodent isoforms are indicated.
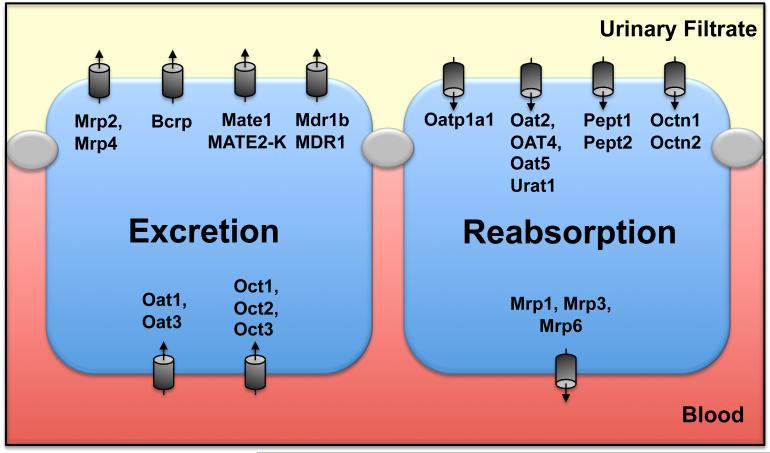
Transport systems are abundant within the kidneys and facilitate the movement of toxicants and drugs from the blood to the urine (tubular secretion) and from the urine to the blood (tubular reabsorption). To accommodate transcellular movement of substrates, SLC and ABC transporters within the epithelial lining of proximal tubules are distributed on the apical (or brush border) membrane facing the tubule lumen and the basolateral membrane facing the peritubular capillaries and interstitium (Figure 1).
Figure 1. Transporters in kidneys.
The tubule cell on the left illustrates the proposed and demonstrated localization of basolateral uptake and apical efflux transporters involved in tubular secretion or excretion. The tubule cell on the right illustrates the apical uptake and basolateral efflux transporters involved in tubular reabsorption. It has been proposed that Oatp1a4, 1a6, 2a1, 2b1, and 3a1 are localized to the luminal surface with Oatp4c1 on the basolateral membrane (136).
Work has been done to identify specific kidney transporters and their physiological roles in renal function. Additionally, multiple laboratories have elucidated the involvement of renal transporters in the disposition and urinary clearance of drugs and toxins. In recent years, research on transporters has begun to focus on regulatory mechanisms involved in their expression and function. Described below are key features of each transporter family, including localization within the kidneys and substrate profiles. Additional attention is placed upon a variety of regulatory mechanisms that alter SLC and ABC transporter expression and function, including gender and hormonal regulation as well as metabolic conditions such as diabetes, obesity, and cholestasis.
EXPRESSION AND REGULATION OF DRUG TRANSPORTERS
SLC Transporter Families
Organic Anion Transporters
Organic anions are removed from the systemic circulation by the kidneys through glomerular filtration and/or tubular secretion in part by the organic anion transporters (Oats/OATs). Within the SLC22 family, the OATs (humans) or Oats (rodents) are a subfamily with seven members that differ in species and tissue distribution as well as substrate specificity. Five of these members (Oat1-5/OAT1-5) along with Urat1/URAT1 are strongly expressed in the kidneys. These transporters are found on both the apical and basolateral membranes of proximal tubule cells (3-10). Of note, OAT4 is expressed in human kidneys (11) but not in mouse kidneys. As organic anion/dicarboxylate exchangers, Oat/OAT transporters facilitate the uptake of substrates including p-aminohippurate (PAH), cyclic nucleotides, uric acid, and prostaglandins as well as antibiotics (cephaloridine), antivirals (cidofovir, tenofovir, adefovir), toxins (ochratoxin A), and antineoplastics (6,12-15). The substrates of Oat transporters include not only anionic compounds but also some cationic chemicals such as cimetidine and 1-methyl-4-phenylpyridium (MPP) (16) and the zwitterion trigonelline (17).
Organic Anion Transporting Polypeptides
Similar to the Oat family of transporters, the organic anion transporting polypeptides (Oatp/OATP) facilitate the influx of a number of organic anionic compounds such as bile acids, drugs (rifampin and digoxin), toxins (ochratoxin A), xenobiotic conjugates, and thyroid hormones (reviewed in (18)). In the SLCO family, there are 11 human, 13 rat, and at least 11 mouse isoforms of the Oatp/OATP transporters (19). Numerous mouse Oatp mRNA isoforms have been identified, but only a few are expressed in the kidneys. Mouse renal Oatp transporters include Oatp1a1, 1a6, 2b1, 3a1, and 4c1 (20). Human isoforms of these transporters often differ from rodents in their tissue distribution. For instance, mouse Oatp2b1 mRNA is strongly expressed in kidneys (20) whereas human OATP2B1 is predominantly expressed in the liver (21). Therefore, when studying Oatp transporters in mice, it is crucial to take species differences into consideration when extrapolating to humans.
Organic Cation Transporters
Members of the SLC22 family of transporters that enable the uptake of cationic compounds belong to the organic cationic transporter (Oct/OCT) family, of which there are three members. Like other SLC families, Oct1-3/OCT1-3 overlap in tissue distribution and substrate specificity. Each member of the Oct/OCT family is abundantly expressed in the kidneys (22-25). Expressed on the basolateral membrane of proximal tubule cells (26,27), Oct/OCT transporters facilitate the electrogenic uptake of substrates including antivirals (lamivudine and zalcitabine) as well as the antidiabetic drug metformin, antihypertensives, and neurotransmitters and the prototypical cations tetraethylammonium (TEA) and MPP (28-31).
Organic Cation/Carnitine Transporters
The organic cation/carnitine transporters (Octn/OCTN) are also members of the SLC22 family and enable the uptake of cationic compounds as well as carnitine. Octn1/OCTN1 and Octn2/OCTN2 are highly localized to the apical membrane of the proximal tubules (32-34). Members of the Octn/OCTN family act as proton/organic cation antiporters or simple organic cation uniporters, which influx carnitine, TEA, pyrilamine, quinidine, and verapamil (35,36).
Multidrug and Toxin Extrusion Transporters
The SLC transporter class is mostly comprised of uptake carriers with the exception of the multidrug and toxin extrusion transporters (Mate/MATE) that belong to the SLC47 subfamily and participate in the efflux of organic cations from the cell. In rodents, Mate1 mRNA is predominantly expressed in the kidneys, liver, heart, as well as placenta (rats only) (37,38). In humans, MATE1 mRNA is found abundantly in the kidneys, liver, and skeletal muscle, with lesser amounts in the heart, and no expression in placenta (39). In contrast, rodent Mate2 is found only in the testes while human MATE2/MATE2-K is found in the kidneys (37,39). Both Mate1/MATE1 and MATE2 are expressed on the apical membrane of renal proximal tubule cells (32,39). In order to transport substrates such as TEA, metformin, MPP, cimetidine, cephalexin, acyclovir, and anticancer drugs (oxaliplatin, topotecan), Mate transporters exchange protons for organic cations using energy generated from antiport transport (38-41).
Peptide Transporters
Additional members of the SLC family that influence renal chemical transport include the peptide transporters (Pept/PEPT) that facilitate the uptake of di- and tripeptides (42,43). Members of the SLC15 subfamily, both Pept1/PEPT1 and Pept2/PEPT2 are ubiquitously expressed with high levels in the kidneys of rodents and humans. Pept1/PEPT1 has been identified along the apical surface of the S1 segment of proximal tubule cells whereas Pept2/PEPT2 is found on the S3 segment (44-46). The Pept/PEPT transporters utilize proton/peptide cotransport to transport substrates including valacyclovir, valganciclovir, and beta-lactam antibiotics (45,47-49).
Glucose Transporters
In addition to Urat1, the glucose transporter (GLUT) 9 (SLC2A9) is also involved in urate homeostasis in the liver and kidneys of mice (50) and humans (reviewed in (51)). In the kidneys, mouse Glut9 is expressed on the apical and basolateral membranes of distal tubules, and to a lesser extent proximal tubules (50). Overexpression of GLUT9 in oocytes from Xenopus frogs demonstrates that GLUT9 mediates the efflux of urate using voltage-driven facilitated transport (52). Mice lacking Glut9 expression exhibit hyperuricemia, hyperuricosuria, and mild renal insufficiency (50).
ABC Transporter Families
Multidrug Resistance Proteins
Members of the ABC class of transporters participate in the active efflux of chemicals from renal tubules. ABC transporters possess ATP-binding domains that bind and hydrolyze ATP to remove substrates from cells. The first family of ABC transporters identified was the ABCB1 family and included the multidrug resistance proteins (Mdr/MDR) or P-glycoprotein. There are several members of the Mdr/MDR transporters including human MDR1 and MDR3 and the rodent orthologs Mdr1a/Mdr1b and Mdr2, respectively. Of these transporters, only Mdr1b/MDR1 is highly expressed in the kidneys (53,54). Substrates of MDR1 include anticancer drugs (vinblastine, doxorubicin, paclitaxel), antihyperlipidemic drugs, digoxin, verapamil, and colchicine (55-61).
Breast Cancer Resistance Protein
An additional ABC transporter encoded by the ABCG2 gene was identified in a human breast cancer cell line resistant to the anticancer agent mitoxantrone and thus was named the breast cancer resistance protein (Bcrp/BCRP) (62). Soon after its initial discovery in breast cancer cells, the Bcrp/BCRP transporter was identified in other tumor types and found to be ubiquitously expressed in normal tissues, including the apical membrane of proximal tubules (63). BCRP transports numerous substrates such as anticancer drugs (mitoxantrone, doxorubicin, daunorubicin, topotecan, imatinib), miscellaneous pharmaceuticals (albendazole, prazosin, and nitrofurantoin), and sulfated conjugates of estrogens, genistein, and biochanin A (62-70).
Multidrug Resistance-Associated Proteins
The ABCC subfamily encodes the multidrug resistance-associated proteins (Mrp/MRP). Similar to their related ABC members, the Mrp/MRP family confers chemoresistance to multiple substrates. There are nine members in the MRP family, but only six (Mrp1-6) are expressed in mouse kidneys (71). Mrp3/MRP3 and Mrp6/MRP6 are basolaterally-located whereas Mrp2/MRP2 and Mrp4/MRP4 are apically-located within proximal tubules and some other kidney cell types (72-79). Mrp proteins transport substrates such as cephalosporin antibiotics, diuretics (furosemide), PAH, glutathione conjugates and free glutathione, glucuronide conjugates, chemotherapeutics (vincristine, cisplatin, methotrexate, and daunorubicin), and endogenous molecules (urate, leukotrienes, cyclic nucleotides, bilirubin glucuronides, and bile salts) (73,80-87) and are reviewed in (88,89).
Endocrine and Metabolic Regulation of Renal Transporter Expression and Function
Diabetes and Obesity
Since renal transporters play an important role in nutrient, toxin, and drug disposition, altered expression in disease states can change pharmacokinetics and susceptibility to adverse effects. Diabetes affects over 350 million individuals worldwide (90). Hyperglycemia resulting from diabetes has significant effects on the kidneys causing glomerular structural changes, increases in extracellular matrix of the mesangium, tubular atrophy, and expansion of basement membranes (91). Patients with diabetes produce higher amounts of urine, and in some cases, greater clearance of drugs. Research has begun to characterize the influence of diabetes on renal transporter expression. Initial studies exploring the effect of Type I (insulin-dependent or juvenile) diabetes (TID) on renal drug handling demonstrated altered renal transport of specific organic cations and anions. The clinical features of TID including hypoinsulinemia and hyperglycemia can be recapitulated in rodents using streptozotocin (STZ). STZ accumulates in the pancreas and causes selective destruction of beta islet cells (92). In renal cortical slices and proximal tubule cells isolated from male rats with STZ-induced TID, the cellular accumulation of the organic cation 14C-TEA was reduced in comparison to non-diabetic rats (93). Lower cellular levels of 14C-TEA in diabetic kidney slices and proximal tubule cells suggested down-regulation of organic cation transport. Administration of insulin to TID rats restored 14C-TEA accumulation in kidney slices and proximal tubule cells, suggesting preservation or up-regulation of organic cation transporters (93).
Additional mechanistic work by the same group focused on transporter expression in renal cortical slices from STZ-treated diabetic male rats. Protein expression of Oct1 and Oct2 was significantly decreased in kidneys of TID rats in comparison to non-diabetic controls and returned to normal levels following insulin treatment (Table 2) (94). Down-regulation of basolaterally-located Oct1 and Oct2 transporters during diabetes translates to reduced organic cation transport across proximal tubule epithelial cells (93,94). Subsequent work implicated glycation of Oct proteins as a mechanism for reduced expression and function in TID rats (95). In addition to reversal of Oct down-regulation by insulin, treatment of diabetic rats with the angiotensin converting enzyme inhibitor, ramipril, also preserved Oct1 and Oct2 protein expression and function possibly by altering angiotensin II signaling (96). Similarly, treatment of TID patients with a related drug, perindopril, preserves tubular organic ion clearance (97).
Table 2.
Transporter expression in kidneys from hyperglycemic and obese rodents.a
| Type I Diabetes Models | Type II Diabetes Models | |||||
|---|---|---|---|---|---|---|
| Model: | STZ | STZ | STZ | Db/Db | Ob/Ob | HFD/STZ |
| Species: | Rat | Rat | Mice | Mice | Mice | Rats |
| Tissue: | Glomeruli | Total | Total | Total | Total | Total |
| Gender: | Male | Male or Female | Female | Male & Female | Male & Female | Male |
| Uptake | ||||||
| Oatp1a1 | ND | ND | ND | ↓(M) | ↓ | ND |
| Oatp1a4 | ND | ND | ND | ND | ND | ND |
| Oatp1a6 | ND | ND | ↔ | ↓(F) | ND | ND |
| Oatp2b1 | ND | ND | ↑ | ND | ND | ND |
| Oatp4c1 | ND | ND | ↑ | ND | ND | ND |
| Oat1 | ND | ND | ↔ | ↓(M) | ND | ND |
| Oat2 | ND | ND | ↓ | ↓ | ↓ | ↑ |
| Oat3 | ND | ND | ↔ | ND | ND | ND |
| Oat5 | ND | ND | ↓ | ND | ND | ND |
| Urat1 | ND | ND | ↔ | ND | ↑ (M) | ND |
| Glut9 | ND | ND | ↑ b | ND | ↑ (M) | ND |
| Oct1 | ND | ↓ (M) | ↔ | ↔ | ND | ND |
| Oct2 | ND | ↓ (M) | ↔ | ↓ (M) | ND | ↓ |
| Oct3 | ND | ↓ (M) | ↔ | ND | ND | ND |
| Octn1 | ND | ND | ↔ | ND | ND | ND |
| Octn2 | ND | ND | ↔ | ND | ND | ND |
| Pept1 | ND | ↑ (F,M) | ↑ | ND | ND | ND |
| Pept2 | ND | ↔ (F) | ↔ | ND | ND | ND |
| Efflux | ||||||
| Mate1 | ND | ND | ↔ | ND | ND | ND |
| Mdr1a | ↔ | ↑ (F) | ND | ND | ND | ND |
| Mdr1b | ↑ | ↔ (F) | ↓ ↔ | ↓ (M) | ND | ND |
| Bcrp | ND | ND | ↔ | ND | ↑ (M) | ↔ |
| Mrp1 | ↑ | ND | ↑ ↔ | ND | ND | ↔ |
| Mrp2 | ↔ | ND | ↑ ↔ | ↔ | ND | ↑ |
| Mrp3 | ND | ND | ↓ | ↓ (F) ↑ (M) | ↓ (F) | ND |
| Mrp4 | ↔ | ND | ↑ | ↑ (M) | ↑ | ↑ |
| Mrp5 | ND | ND | ↑ | ND | ND | ↔ |
| Mrp6 | ND | ND | ↓ ↔ | ND | ND | ND |
The mRNA and/or protein expression of transporters in kidneys of mice or rats after streptozotocin (STZ) treatment alone or in combination with high fat diet (HFD). Studies are also included from transgenic obese (ob/ob) and diabetic (db/db) mice. Species, tissue (either isolated glomeruli or total tissue), and gender (M: male or F: female) are noted. ↑ denotes up-regulation, ↓ denotes down-regulation, and ↔ denotes no change. ND denotes transporter expression that was not determined. Type I diabetes references include (94-96,100-102,161,162). Type II diabetes references include (103-106).
The gender was not included in the Methods of this paper (162).
Expression and/or function of other transporters have been assessed in STZ-treated rodents (Table 2). Rats treated with STZ show increased expression of Mdr1b mRNA (but not protein) as well as Pept1 protein (98-101). Recently, Quezada et al. demonstrated up-regulation of Mrp1 expression and function in isolated glomeruli from STZ-treated rats (102). There were similar increases in Mdr1 levels in glomeruli although this did not translate to changes in function.
Type II diabetes (TIID), or noninsulin-dependent diabetes, is more prevalent than TID and is associated with impairment of insulin secretion as well as reduced insulin sensitivity of peripheral tissues. Because the incidence of TIID is increasing in Western countries, it is important to also investigate how this disorder influences renal transporter expression and/or function. Three rodent models have been used to recapitulate various clinical features of TIID such as increased food consumption and altered body composition, weight gain, hyperglycemia, and hypoinsulinemia. These models include ob/ob (obese) mice, db/db (diabetic) mice, and rats fed a high fat diet and administered a low dose of STZ (Table 2). Ob/ob and db/db mice have deficient leptin and leptin receptor signaling, respectively. In addition to excessive weight gain, ob/ob mice exhibit a 2.5-fold increase in circulating glucose levels (103). Analysis of kidney transporters in ob/ob mice revealed decreased mRNA and protein expression of Mrp3 in female mice, decreased Oatp1a1 and Oat2 mRNA expression, and increased Mrp4 mRNA and protein expression (103). In addition, the kidneys of ob/ob mice have increased expression of Bcrp, Urat1, and Glut9 protein and trends for elevated Bcrp and Glut9 mRNAs (104). This pattern of transporter regulation would favor enhanced secretion and reduced reabsorption of organic anions in TIID. Db/db mice are used as an alternate model of TIID because they also exhibit hyperglycemia, hyperlipidemia, and weight gain. Db/db mice have reduced expression of Oatp1a1 and Oatp1a6 mRNA as well as lower levels of Oat1, Oat2, and Oct2 mRNA (105). It should be noted that some changes in transporter expression were observed in only male or female db/db mice. For example, female db/db mice have reduced Mrp3 mRNA whereas male counterparts exhibit up-regulation of this transporter (105). The mechanism(s) for gender-divergent regulation of transporters in TIID models is currently unknown.
Dietary models are also used in combination with diabetogenic chemicals to study TIID. In rats fed a high fat diet and administered a single low dose of STZ, protein expression of renal organic anion transporters Oat2, Mrp2, and Mrp4 was elevated (106). In addition, TIID rats had reduced Oct2 protein levels similar to rats with TID. It is important to note that mice fed a high fat diet (no STZ) exhibit only mild hyperglycemia and relatively constant renal transporter expression, suggesting that disease severity may be critical in transcriptionally regulating ABC and SLC genes (107). When comparing the various rodent models of diabetes, there are some fairly consistent changes in renal transporter expression including down-regulation of Oct2 and up-regulation of Pept1 and Mrp4. There are currently no studies that have investigated the regulation of renal transporters in patients with TIID. Collectively, this work suggests enhanced organic anion secretion and impaired organic cation clearance in rodents with moderate to severely uncontrolled hyperglycemia.
Cholestasis
Cholestasis is a condition of slowed or blocked bile flow within the liver or bile ducts. Cholestasis can result from obstruction of the common bile duct (termed extrahepatic cholestasis) or from altered bile acid handling within the liver (called intrahepatic cholestasis). Extrahepatic cholestasis can result from stones or tumors and is recapitulated in rodents using surgical ligation of the common bile duct. Intrahepatic cholestasis can occur during pregnancy or from genetic disorders in bile acid transporters. Rodent models of intrahepatic cholestasis include feeding with toxic bile acids such as cholic acid or lithocholic acid or administration of chemicals such as alpha-naphthylisothiocyanate (ANIT) or the synthetic estrogen, ethinyl estradiol.
Most research in the field of cholestasis has focused on how defects in hepatobiliary transporters contribute to disease development as well as adaptive changes in transporter expression. Several previously identified bile acid transporters include the sodium taurocholate co-transporting polypeptide (Ntcp), Oatp1a1, and Oatp1b2 which are localized on the sinusoidal membrane and the bile salt export pump and Mrp2 located on the canalicular membrane (reviewed in (108)). Cooperative efforts between sinusoidal and canalicular transporters produce the directional flow of bile acids through hepatocytes and into the bile. When bile flow is impaired or decreased, bile constituents accumulate in the liver and spill over into the circulation. To model the cholestasis phenotype, rodents undergo ligation of the common bile duct (109). Livers from bile duct-ligated rodents have decreased mRNA expression of sinusoidal uptake transporters Oatp1a1 and Oatp1b2 and increases in efflux transporters Mrp1-5 over a 14 day period following surgery (109,110). Similarly, sinusoidal Oct1 protein and hepatic accumulation of TEA are reduced by bile duct ligation, reflecting a reduced uptake of cations into hepatocytes during cholestasis (111). Together, decreased expression of uptake transporters along with up-regulation of efflux transporters appears to be an adaptive mechanism to remove bile acids and other chemicals and protect the liver.
Retrograde transport of bile acids and bilirubin from hepatocytes to circulating blood during cholestasis has been shown to alter expression of transporters in other organs including the kidneys (Table 3). In kidneys from mice that have undergone bile duct ligation, the expression of Oatp1a1 mRNA was reduced and levels of Oatp1a4 and Mrp1-5 mRNA were increased (109). Additional work confirmed Mrp4 mRNA up-regulation as well as Bcrp protein down-regulation in the kidneys of mice during cholestasis (112). Because Mrp4 can transport bile acids, higher levels of this transporter should aid in reducing bile acid accumulation and enhancing urinary excretion (113). Extensive work has been done in bile duct-ligated rats revealing elevated urinary clearance levels of PAH, bile salts, and compounds such as bromosulfophthalein, glucuronidated acetaminophen, and cimetidine (114-119). In response to cholestasis in rats, the kidneys have elevated protein expression of Oct2, Oatp1a1, and Mrp2 (114,116-123). Treatment of rats with ANIT also results in cholestatic liver injury that is accompanied by up-regulation of renal Mrp2 protein and down-regulation of Oat1 and 3 proteins (124). Down-regulation of Oct2 mRNA and protein has also been observed in the kidneys of rats administered a cholestatic dose of ethinyl estradiol (125). Furthermore, treatment of mice with cholic acid increases the renal expression of Mrp2 and Mrp4 mRNAs which correlates with increased urinary excretion of bile acids (126,127). The regulatory mechanisms underlying transporter regulation in the kidneys of rodents after cholestasis are unknown. Cumulatively, the up-regulation of basolateral uptake carriers and apical efflux transporters may enhance urinary clearance of bile acids and other chemicals. Therefore, renal transporters appear to be regulated in a fashion that would compensate for a loss in biliary secretion by enhancing urinary elimination.
Table 3.
Transporter expression in kidneys from rodents with cholestasis.a
| Model: | BDL | BDL | ANIT |
|---|---|---|---|
| Species: | Rat | Mouse | Rat |
| Uptake | |||
| Oatplal | ↑ | ↓ | ND |
| Oatp1a4 | ND | ↑ | ND |
| Oatp1a5 | ND | ↑ ↓ | ND |
| Oatp1a6 | ND | ↔ | ND |
| Oatp4a1 | ND | ↑ | ND |
| Oat1 | ↓ ↑ | ND | ↓ |
| Oat3 | ↑ | ND | ↓ |
| Oct1 | ↔ ↓ ↑ | ND | ND |
| Oct2 | ↑ | ND | ND |
| Efflux | |||
| Mate1 | ↔ | ND | ND |
| Bcrp | ND | ↓ | ND |
| Mrp1 | ↑ | ↑ | ND |
| Mrp2 | ↑ | ↑ | ↑ |
| Mrp3 | ↔ | ↑ | ND |
| Mrp4 | ↓ | ↑ | ND |
| Mrp5 | ND | ↑ | ND |
The mRNA and/or protein expression of transporters in kidneys of male rats or mice after bile duct ligation (BDL) after varying time points. ↑ denotes up-regulation, ↓ denotes down-regulation, and ↔ denotes no change. ND denotes transporter expression that was not determined. Rat BDL references include (110,111,114-119,122,123). Mouse BDL references include (109,112). The ANIT reference is (124).
Hormonal Influence: Sex Hormones, Growth Hormones, and Pregnancy
It is becoming increasingly evident that hormonal signaling is important in regulating transporters in the kidneys. Renal transporter expression and function has been explored in studies evaluating gender differences, pregnancy, disruption of growth hormone secretion, surgical removal of sex organs (gonadectomy) or the pituitary gland (hypophysectomy), and exogenous administration of hormones. Mechanisms likely involved in hormonal regulation of transporters include the activation of steroid and hormone nuclear receptors. As mechanisms of gene control continue to become better understood, additional levels of transporter regulation by hormones may be revealed.
Sex hormones
As early as 1955, it was demonstrated that the organic anion, PAH, accumulated in rat kidney slices in response to testosterone (128). This work was further supported by pharmacokinetic studies demonstrating comparable clearance of PAH between male and testosterone-treated female rats, which was higher than untreated female rats (129). Likewise, castration of male rats reduced PAH clearance, which could be reversed by administering testosterone (129). Preliminary studies depicting gender-specific patterns of renal transport and potential involvement of sex hormones set the stage for future mechanistic work.
By the 2000s, researchers began to speculate that the expression and/or activity of transporter proteins might explain gender differences in the renal clearance of organic ions. Gender-specific expression patterns have been demonstrated in rodents for many SLC and ABC transporters (Table 4). Messenger RNA and protein expression analysis have revealed rodent Oat1 as a male-predominant transporter and Oat2 and Oat5 as female-predominant transporters (8,130-137). As expected, Oat1 protein expression and uptake of PAH was lower in basolateral membrane vesicles prepared from kidneys of female rats compared to males (138,139). Furthermore, castration of adult male rats decreased Oat1 protein expression in kidney cortex that was reversed by treatment with testosterone (8). These findings suggest that testosterone is a stimulator of Oat1 expression in the kidneys.
Table 4.
Sex differences in renal transporter expression between male and female rodents.a
| Transporter | Mice | Rats | References |
|---|---|---|---|
| Uptake | |||
| Oatplal | M > F | M > F | (153,163,164) |
| Oatp1a6 | M = F | M = F | (163,164) |
| Oatp2b1 | M = F | N.D. | (163) |
| Oatp3a1 | M > F | N.D. | (153,163) |
| Oatp4c1 | M > F | N.D. | (136,163) |
| Oatl | M > F | M > F | (8,130,131,133,141) |
| Oat2 | F ≥ M | F > M | (130-133,141) |
| Oat3 | F ≥ Mb | M ≥ F | (8,130,131,133,141) |
| Oat5 | F > M | F > M | (135,136) |
| Urat1 | M > F | N.D. | (136) |
| Oct1 | M = F | F > M | (22,25) |
| Oct2 | M > F | M > F | (22,25,141,144) |
| Oct3 | M = F | M = F | (22,25) |
| Octn1 | M = F | M = F | (22,25) |
| Octn2 | M = F | M = F | (22,25) |
| Pept1 | M = F | M = F | (165) |
| Pept2 | M = F | F > M | (165) |
| Efflux | |||
| Mate1 | M > F | N.D. | (37) |
| Mdr1b | F > M | N.D. | (53) |
| Bcrp | M = F | M > F | (166) |
| Mrp1 | M = F | N.D. | (71) |
| Mrp2 | M = F | N.D. | (71) |
| Mrp3 | F > M | N.D. | (71,146) |
| Mrp4 | F > M | N.D. | (71,146) |
| Mrp5 | M = F | N.D. | (71) |
| Mrp6 | M = F | N.D. | (71) |
The mRNA and/or protein expression of transporters in kidneys of adult male (M) and female (F) rats or mice. ND denotes transporter expression that was not determined.
Compared to male counterparts, elevated Oat3 mRNA was higher in female 129 J mice, but not female C57BL/6 mice.
Gonadectomy of rats and mice demonstrates that female-predominant Oat2 and Oat5 expression is dependent upon hormonal regulation. The mRNA and protein expression of both transporters are up-regulated in kidneys of castrated males (132,134-137). Moreover, the administration of estrogen to castrated males further increased Oat2 and Oat5 expression. Conversely, testosterone replacement decreases expression to match that of control or sham-operated males (132,134-137). Ovarectomized female rodents experience small reductions in Oat2 and Oat5 mRNA and protein. Treatment of gonadectomized females with testosterone further suppresses Oat5 expression while estrogen supplementation increases expression to that of control females (136). Collectively, data from rodents suggest that testosterone suppresses and estrogen weakly stimulates renal Oat2 and Oat5 expression.
Similar to organic anions, the renal uptake of the organic cation, TEA, occurs at a greater rate in male rats than females suggesting that testosterone may enhance basolateral organic cation uptake or that estrogens may inhibit this process (140).The organic cation transporter Oct2 is expressed at a higher level in male rodents and rabbits compared to females (22,25,141). It has been suggested that elevated Oct2 levels enhance renal clearance of the nephrotoxicant cisplatin in male rodents (142). Gonadectomy of male rats and mice decreases Oct2 mRNA as well as renal clearance of the organic cation TEA (22,143). With testosterone treatment, renal Oct2 mRNA and protein expression and TEA uptake increase to control male levels while estrogen supplementation has no effect (22,143,144). Thus, gender differences in Oct2 expression have been attributed to testosterone stimulation. Subsequent work investigating a mechanistic link between testosterone regulation and rodent Oct2 expression analyzed the rat Oct2 promoter region and revealed two potential androgen response elements that may be transactivated by testosterone binding to the androgen receptor (145).
Efflux transporters also exhibit gender divergent regulation in the kidneys of mice (Table 4). Mdr1b, Mrp3, and Mrp4 are female-predominant transporter genes in kidneys of C57Bl/6 mice (53,146). Despite this similar gender predominance, different sex hormones are responsible for each of these ABC transporters. Gonadectomy causes Mdr1b and Mrp3 expression to decrease in female mice and increase in male mice. Estrogen treatment increases Mrp3 levels in castrated mice of both sexes whereas testosterone treatment has the opposing response (146). Thus, hormonal regulation of renal Mrp3 is balanced by testosterone (suppression) and estrogen (stimulation) (146). Interestingly, both estradiol and testosterone reduce Mdr1b mRNA in kidneys of gonadectomized male mice (53). In contrast, gonadectomy of male mice increases Mrp4 expression relative to that of female controls (146). While estrogen addition had no effect, testosterone replacement caused Mrp4 expression to decrease to control male levels. Testosterone, but not estrogen, appears to have a suppressive effect on renal Mrp4 expression and explains female-predominant expression of this transporter (146).
When considering gender divergence in transporter expression, it is important to acknowledge that sex differences occurring in one species or strain may not occur in another. Rabbits exhibit similar levels of Oat1 between the sexes, which contrasts with previous studies in rodents that indicated male-predominant Oat1 expression (130,131,133,138,141). Gender expression patterns may also differ between strains. For example, Oat3 mRNA is higher in kidneys of female 129J mice, but not C57Bl/6 mice (131). Considering the potential differences that may exist between species and strains, it is critical to exercise caution when attempting to extrapolate gender differences in transporter expression between species.
Growth hormone
Pituitary growth hormone secretion differs between genders resulting in distinct patterns of gene expression. In male rats, growth hormone is secreted in high-amplitude pulses at regular intervals, with periods of little to no growth hormone in between (147,148). On the other hand, female rats secrete growth hormone more frequently as low-amplitude pulses resulting in continuously detectable levels of hormone (148,149). As early as 1991, it was evident that gender divergent patterns of growth hormone secretion could explain differential expression of hepatic cytochrome p450 enzymes between males and females (130,150,151). As cytochrome P450 enzymes and transporters are often coordinately regulated by similar mechanisms, more recent studies have begun to explore whether growth hormone may participate in these differences. Several methods are used to assess the influence of growth hormone secretion on transporter expression including 1) hypophysectomy, which is the surgical removal of the pituitary that obliterates not only production of growth hormone but also luteinizing and follicle-stimulating hormones and 2) mutant lit/lit mice that have a spontaneous mutation in the growth hormone-releasing hormone receptor that prevents release of growth hormone (152).
The influence of growth hormone on the endocrine regulation of rat renal Oat transporters (male-predominant Oat1 and female-predominant Oat2) has been assessed in hypophysectomized rats in the absence and presence of growth hormone supplementation (130). Messenger RNA expression of renal Oat1 in female rats was unchanged by hypophysectomy, suggesting little to no involvement of growth hormone in regulating this transporter. In contrast, hypophysectomy decreased renal Oat2 mRNA expression in female rats which could be reversed by restoration of growth hormone signaling (130). Based on gonadectomy and hypophysectomy studies, regulation of Oat1 appears to be more sensitive to sex hormone signaling while growth hormone secretion participates in Oat2 predominance in female rats along with sex hormones (130).
Contribution of growth hormone secretory patterns to female-predominant Mrp3 and Mrp4 expression in kidneys of mice was examined using hypophysectomized and lit/lit mice (146). In hypophysectomized female mice, Mrp3 mRNA expression was dramatically reduced. Treatment of these mice with growth hormone did not alter Mrp3 mRNA. Rather, the administration of 17β-estradiol increased Mrp3 expression significantly. These results suggest that female-predominant Mrp3 expression in the kidneys is dependent on sex hormones and not growth hormone (146). In contrast, in hypophysectomized and lit/lit mice, the absence of growth hormone increased Mrp4 mRNA expression in both genders. Testosterone replacement, as well as growth hormone addition (in a male-specific pattern) in hypophysectomized mice caused Mrp4 expression to decrease to levels observed in male control mice (146). Therefore, Mrp4 female-dominant expression is due to repression by testosterone and male-specific growth hormone secretion. Further analysis of the 5’ flanking region of the mouse Mrp3 promoter identified two estrogen response elements potentially responsive to estrogen regulation. Unlike Mrp3, the Mrp4 promoter region contains response elements that are responsive to androgens and growth-hormone secretory patterns suggesting this transporter is regulated by both hormones (146).
Regulation of Oatp transporters in the kidneys of mice is solely dependent upon sex hormones (153). Regulation of male-predominant renal Oatp1a1 and 3a1 transporters has been explored in gonadectomized, hypophysectomized, and lit/lit mice. In gonadectomized male mice, Oatp1a1 and Oatp3a1 mRNA expression was reduced. Treatment with testosterone caused Oatp1a1 and Oatp3a1 to dramatically increase while exogenous 17β-estradiol had no effect, suggesting testosterone acts as a stimulator of male-predominant Oatp1a1 and Oatp3a1 (153). Additional studies demonstrated that Oatp1a1 and 3a1 mRNA expression in male mice is independent of growth hormone signaling since hypophysectomy of male mice reduces renal Oatp1a1 and 3a1 mRNA, which is restored by testosterone but not growth hormone supplementation (153). Male lit/lit mice do have reduced Oatp1a1 mRNA, however growth hormone supplementation has no effect, further confirming a key role for androgens in regulating these two renal transporters. Taken together, individual transporter isoforms can be regulated by sex and/or growth hormones, which allows for different mechanisms to influence renal chemical secretion and reabsorption.
Pregnancy
Pregnancy causes both hormonal (progesterone, estradiol, prolactin, etc) and hemodynamic changes that influence maternal kidney function. Pregnancy-induced renal alterations include elevations in renal plasma flow and glomerular filtration rate resulting in an increased clearance of nutrients, toxins, and drugs (154-157). Studies exploring the influence of pregnancy on renal transport have largely focused on the accelerated excretion of drugs into urine. For instance, urinary elimination of the cardiac glycoside, digoxin, and the antidiabetic drug, glyburide, are elevated during human and rodent pregnancies, respectively (158,159). More recent work has begun to shed light on the role of transporters in pregnancy-induced renal clearance. In a pharmacokinetic study using Bcrp knockout mice and its known substrate glyburide, the absence of apical Bcrp in the kidneys had little to no effect on the elevated renal clearance of glyburide during pregnancy (158). This was despite a modest increase in renal Bcrp protein expression in pregnant dams (160). This suggested that the glomerular hyperfiltration that accompanies pregnancy may be more important for enhanced glyburide elimination than changes in active tubular secretion. More recent unpublished work from our laboratory demonstrates down-regulation of apical renal transporters (Mrp2, Mrp4, Mate1, Mdr1b) in pregnant mice during mid-gestation that would favor retention of chemicals by the kidney and may in fact be a compensatory mechanism to the hyperfiltration that accompanies pregnancy. Further research is needed in this field to understand how fluctuations in sex hormones during pregnancy might participate in the altered expression of renal transporters.
CONCLUSIONS AND FUTURE PERSPECTIVES
Renal transporters are tubular proteins involved in the secretion and reabsorption of nutrients, drugs, and toxins. Sex hormones, pregnancy, and growth hormone secretory patterns as well as hyperglycemia, obesity, and cholestasis have a variety of effects on renal transporter expression and function. As a result of metabolic and endocrine regulation of renal transport, drug efficacy and toxicity may be altered. Although significant strides in this field have been made, much work is needed to understand the molecular signaling mechanisms as well as relevance between species.
Acknowledgments
This work was supported by the National Institutes of Health Institute of Diabetes and Digestive and Kidney Diseases [Grant DK080774] and the National Institutes of Environmental Health Sciences [Grants ES020522, ES007148, and ES005022], components of the National Institutes of Health.
Non-Standard Abbreviations
- Abc
ATP-binding cassette
- Bcrp
breast cancer resistance protein
- BDL
bile duct ligation
- Glut
glucose transporter
- HFD
high fat diet
- Mate
multidrug and toxin extrusion transporter
- Mdr
multidrug resistance protein
- Mrp
multidrug resistance-associated protein
- MPP
1-methyl-4-phenylpyridium
- Oat
organic anion transporter
- Oatp
organic anion transporting polypeptide
- Oct
organic cation transporter
- Octn
organic cation/carnitine transporter
- PAH
p-aminohippurate
- Pept
peptide transporter
- Slc
solute carrier
- STZ
streptozotocin
- TEA
tetraethylammonium
- TID
type I diabetes
- TIID
type II diabetes
- Urat
urate transporter
REFERENCES
- 1.Li L, Meier PJ, Ballatori N. Oatp2 mediates bidirectional organic solute transport: a role for intracellular glutathione. Mol Pharmacol. 2000;58(2):335–40. doi: 10.1124/mol.58.2.335. [DOI] [PubMed] [Google Scholar]
- 2.Terada T, Inui K. Physiological and pharmacokinetic roles of H+/organic cation antiporters (MATE/SLC47A). Biochem Pharmacol. 2008;75(9):1689–96. doi: 10.1016/j.bcp.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Knorr BA, Lipkowitz MS, Potter BJ, Masur SK, Abramson RG. Isolation and immunolocalization of a rat renal cortical membrane urate transporter. J Biol Chem. 1994;269(9):6759–64. [PubMed] [Google Scholar]
- 4.Hosoyamada M, Sekine T, Kanai Y, Endou H. Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Physiol. 1999;276(1 Pt 2):F122–8. doi: 10.1152/ajprenal.1999.276.1.F122. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Nieto CE, You G, Bush KT, Barros EJ, Beier DR, Nigam SK. Molecular cloning and characterization of NKT, a gene product related to the organic cation transporter family that is almost exclusively expressed in the kidney. J Biol Chem. 1997;272(10):6471–8. doi: 10.1074/jbc.272.10.6471. [DOI] [PubMed] [Google Scholar]
- 6.Sekine T, Watanabe N, Hosoyamada M, Kanai Y, Endou H. Expression cloning and characterization of a novel multispecific organic anion transporter. J Biol Chem. 1997;272(30):18526–9. doi: 10.1074/jbc.272.30.18526. [DOI] [PubMed] [Google Scholar]
- 7.Tojo A, Sekine T, Nakajima N, Hosoyamada M, Kanai Y, Kimura K, Endou H. Immunohistochemical localization of multispecific renal organic anion transporter 1 in rat kidney. J Am Soc Nephrol. 1999;10(3):464–71. doi: 10.1681/ASN.V103464. [DOI] [PubMed] [Google Scholar]
- 8.Ljubojevic M, Herak-Kramberger CM, Hagos Y, Bahn A, Endou H, Burckhardt G, Sabolic I. Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am J Physiol Renal Physiol. 2004;287(1):F124–38. doi: 10.1152/ajprenal.00029.2004. [DOI] [PubMed] [Google Scholar]
- 9.Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H. Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J Am Soc Nephrol. 2002;13(4):848–57. doi: 10.1681/ASN.V134848. [DOI] [PubMed] [Google Scholar]
- 10.Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, Kanai Y, Sophasan S, Endou H. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubules. J Pharmacol Sci. 2004;94(3):297–304. doi: 10.1254/jphs.94.297. [DOI] [PubMed] [Google Scholar]
- 11.Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000;275(6):4507–12. doi: 10.1074/jbc.275.6.4507. [DOI] [PubMed] [Google Scholar]
- 12.Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56(3):570–80. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- 13.Takeda M, Tojo A, Sekine T, Hosoyamada M, Kanai Y, Endou H. Role of organic anion transporter 1 (OAT1) in cephaloridine (CER)-induced nephrotoxicity. Kidney Int. 1999;56(6):2128–36. doi: 10.1046/j.1523-1755.1999.00789.x. [DOI] [PubMed] [Google Scholar]
- 14.Uwai Y, Ida H, Tsuji Y, Katsura T, Inui K. Renal transport of adefovir, cidofovir, and tenofovir by SLC22A family members (hOAT1, hOAT3, and hOCT2). Pharm Res. 2007;24(4):811–5. doi: 10.1007/s11095-006-9196-x. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda M, Sekine T, Takeda M, Cha SH, Kanai Y, Kimura M, Endou H. Transport of ochratoxin A by renal multispecific organic anion transporter 1. J Pharmacol Exp Ther. 1999;289(3):1301–5. [PubMed] [Google Scholar]
- 16.Ahn SY, Eraly SA, Tsigelny I, Nigam SK. Interaction of organic cations with organic anion transporters. J Biol Chem. 2009;284(45):31422–30. doi: 10.1074/jbc.M109.024489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fork C, Bauer T, Golz S, Geerts A, Weiland J, Del Turco D, Schomig E, Grundemann D. OAT2 catalyses efflux of glutamate and uptake of orotic acid. Biochem J. 2011;436(2):305–12. doi: 10.1042/BJ20101904. [DOI] [PubMed] [Google Scholar]
- 18.Kim RB. Organic anion-transporting polypeptide (OATP) transporter family and drug disposition. Eur J Clin Invest. 2003;33(Suppl 2):1–5. doi: 10.1046/j.1365-2362.33.s2.5.x. [DOI] [PubMed] [Google Scholar]
- 19.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/ SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447(5):653–65. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- 20.Cheng X, Maher J, Chen C, Klaassen CD. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps). Drug Metab Dispos. 2005;33(7):1062–73. doi: 10.1124/dmd.105.003640. [DOI] [PubMed] [Google Scholar]
- 21.Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120(2):525–33. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- 22.Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos. 2006;34(3):477–82. doi: 10.1124/dmd.105.006932. [DOI] [PubMed] [Google Scholar]
- 23.Grundemann D, Gorboulev V, Gambaryan S, Veyhl M, Koepsell H. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994;372(6506):549–52. doi: 10.1038/372549a0. [DOI] [PubMed] [Google Scholar]
- 24.Schweifer N, Barlow DP. The Lx1 gene maps to mouse chromosome 17 and codes for a protein that is homologous to glucose and polyspecific transmembrane transporters. Mamm Genome. 1996;7(10):735–40. doi: 10.1007/s003359900223. [DOI] [PubMed] [Google Scholar]
- 25.Slitt AL, Cherrington NJ, Hartley DP, Leazer TM, Klaassen CD. Tissue distribution and renal developmental changes in rat organic cation transporter mRNA levels. Drug Metab Dispos. 2002;30(2):212–9. doi: 10.1124/dmd.30.2.212. [DOI] [PubMed] [Google Scholar]
- 26.Karbach U, Kricke J, Meyer-Wentrup F, Gorboulev V, Volk C, Loffing-Cueni D, Kaissling B, Bachmann S, Koepsell H. Localization of organic cation transporters OCT1 and OCT2 in rat kidney. Am J Physiol Renal Physiol. 2000;279(4):F679–87. doi: 10.1152/ajprenal.2000.279.4.F679. [DOI] [PubMed] [Google Scholar]
- 27.Sugawara-Yokoo M, Urakami Y, Koyama H, Fujikura K, Masuda S, Saito H, Naruse T, Inui K, Takata K. Differential localization of organic cation transporters rOCT1 and rOCT2 in the basolateral membrane of rat kidney proximal tubules. Histochem Cell Biol. 2000;114(3):175–80. doi: 10.1007/s004180000186. [DOI] [PubMed] [Google Scholar]
- 28.Urakami Y, Okuda M, Masuda S, Saito H, Inui KI. Functional characteristics and membrane localization of rat multispecific organic cation transporters, OCT1 and OCT2, mediating tubular secretion of cationic drugs. J Pharmacol Exp Ther. 1998;287(2):800–5. [PubMed] [Google Scholar]
- 29.Jung N, Lehmann C, Rubbert A, Knispel M, Hartmann P, van Lunzen J, Stellbrink HJ, Faetkenheuer G, Taubert D. Relevance of the organic cation transporters 1 and 2 for antiretroviral drug therapy in human immunodeficiency virus infection. Drug Metab Dispos. 2008;36(8):1616–23. doi: 10.1124/dmd.108.020826. [DOI] [PubMed] [Google Scholar]
- 30.Kimura N, Masuda S, Tanihara Y, Ueo H, Okuda M, Katsura T, Inui K. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet. 2005;20(5):379–86. doi: 10.2133/dmpk.20.379. [DOI] [PubMed] [Google Scholar]
- 31.Zolk O, Solbach TF, Konig J, Fromm MF. Functional characterization of the human organic cation transporter 2 variant p.270Ala>Ser. Drug Metab Dispos. 2009;37(6):1312–8. doi: 10.1124/dmd.108.023762. [DOI] [PubMed] [Google Scholar]
- 32.Masuda S, Terada T, Yonezawa A, Tanihara Y, Kishimoto K, Katsura T, Ogawa O, Inui K. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006;17(8):2127–35. doi: 10.1681/ASN.2006030205. [DOI] [PubMed] [Google Scholar]
- 33.Tamai I, China K, Sai Y, Kobayashi D, Nezu J, Kawahara E, Tsuji A. Na(+)-coupled transport of L-carnitine via high-affinity carnitine transporter OCTN2 and its subcellular localization in kidney. Biochim Biophys Acta. 2001;1512(2):273–84. doi: 10.1016/s0005-2736(01)00328-5. [DOI] [PubMed] [Google Scholar]
- 34.Gisler SM, Pribanic S, Bacic D, Forrer P, Gantenbein A, Sabourin LA, Tsuji A, Zhao ZS, Manser E, Biber J. PDZK1: I. a major scaffolder in brush borders of proximal tubular cells. Kidney Int. 2003;64(5):1733–45. doi: 10.1046/j.1523-1755.2003.00266.x. others. [DOI] [PubMed] [Google Scholar]
- 35.Tamai I, Yabuuchi H, Nezu J, Sai Y, Oku A, Shimane M, Tsuji A. Cloning and characterization of a novel human pH-dependent organic cation transporter, OCTN1. FEBS Lett. 1997;419(1):107–11. doi: 10.1016/s0014-5793(97)01441-5. [DOI] [PubMed] [Google Scholar]
- 36.Yabuuchi H, Tamai I, Nezu J, Sakamoto K, Oku A, Shimane M, Sai Y, Tsuji A. Novel membrane transporter OCTN1 mediates multispecific, bidirectional, and pH-dependent transport of organic cations. J Pharmacol Exp Ther. 1999;289(2):768–73. [PubMed] [Google Scholar]
- 37.Lickteig AJ, Cheng X, Augustine LM, Klaassen CD, Cherrington NJ. Tissue distribution, ontogeny and induction of the transporters Multidrug and toxin extrusion (MATE) 1 and MATE2 mRNA expression levels in mice. Life Sci. 2008;83(1-2):59–64. doi: 10.1016/j.lfs.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terada T, Masuda S, Asaka J, Tsuda M, Katsura T, Inui K. Molecular cloning, functional characterization and tissue distribution of rat H+/organic cation antiporter MATE1. Pharm Res. 2006;23(8):1696–701. doi: 10.1007/s11095-006-9016-3. [DOI] [PubMed] [Google Scholar]
- 39.Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci U S A. 2005;102(50):17923–8. doi: 10.1073/pnas.0506483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007;74(2):359–71. doi: 10.1016/j.bcp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 41.Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T, Inui K. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 2007;74(3):477–87. doi: 10.1016/j.bcp.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Steel A, Nussberger S, Romero MF, Boron WF, Boyd CA, Hediger MA. Stoichiometry and pH dependence of the rabbit proton-dependent oligopeptide transporter PepT1. J Physiol. 1997;498(Pt 3):563–9. doi: 10.1113/jphysiol.1997.sp021883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramamoorthy S, Liu W, Ma YY, Yang-Feng TL, Ganapathy V, Leibach FH. Proton/peptide cotransporter (PEPT 2) from human kidney: functional characterization and chromosomal localization. Biochim Biophys Acta. 1995;1240(1):1–4. doi: 10.1016/0005-2736(95)00178-7. [DOI] [PubMed] [Google Scholar]
- 44.Smith DE, Pavlova A, Berger UV, Hediger MA, Yang T, Huang YG, Schnermann JB. Tubular localization and tissue distribution of peptide transporters in rat kidney. Pharm Res. 1998;15(8):1244–9. doi: 10.1023/a:1011996009332. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi K, Nakamura N, Terada T, Okano T, Futami T, Saito H, Inui KI. Interaction of beta-lactam antibiotics with H+/peptide cotransporters in rat renal brush-border membranes. J Pharmacol Exp Ther. 1998;286(2):1037–42. [PubMed] [Google Scholar]
- 46.Shen H, Smith DE, Yang T, Huang YG, Schnermann JB, Brosius FC., 3rd Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am J Physiol. 1999;276(5 Pt 2):F658–65. doi: 10.1152/ajprenal.1999.276.5.F658. [DOI] [PubMed] [Google Scholar]
- 47.Ganapathy ME, Huang W, Wang H, Ganapathy V, Leibach FH. Valacyclovir: a substrate for the intestinal and renal peptide transporters PEPT1 and PEPT2. Biochem Biophys Res Commun. 1998;246(2):470–5. doi: 10.1006/bbrc.1998.8628. [DOI] [PubMed] [Google Scholar]
- 48.Sugawara M, Huang W, Fei YJ, Leibach FH, Ganapathy V, Ganapathy ME. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J Pharm Sci. 2000;89(6):781–9. doi: 10.1002/(SICI)1520-6017(200006)89:6<781::AID-JPS10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 49.Raeissi SD, Li J, Hidalgo IJ. The role of an alpha-amino group on H+ -dependent transepithelial transport of cephalosporins in Caco-2 cells. J Pharm Pharmacol. 1999;51(1):35–40. doi: 10.1211/0022357991772060. [DOI] [PubMed] [Google Scholar]
- 50.Preitner F, Bonny O, Laverriere A, Rotman S, Firsov D, Da Costa A, Metref S, Thorens B. Glut9 is a major regulator of urate homeostasis and its genetic inactivation induces hyperuricosuria and urate nephropathy. Proc Natl Acad Sci U S A. 2009;106(36):15501–6. doi: 10.1073/pnas.0904411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Doblado M, Moley KH. Facilitative glucose transporter 9, a unique hexose and urate transporter. Am J Physiol Endocrinol Metab. 2009;297(4):E831–5. doi: 10.1152/ajpendo.00296.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, Srivastava S, Kitamura K, Hisatome I, Endou H. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283(40):26834–8. doi: 10.1074/jbc.C800156200. others. [DOI] [PubMed] [Google Scholar]
- 53.Cui YJ, Cheng X, Weaver YM, Klaassen CD. Tissue distribution, gender-divergent expression, ontogeny, and chemical induction of multidrug resistance transporter genes (Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab Dispos. 2009;37(1):203–10. doi: 10.1124/dmd.108.023721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84(21):7735–8. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsuruoka S, Sugimoto KI, Fujimura A, Imai M, Asano Y, Muto S. P-glycoprotein-mediated drug secretion in mouse proximal tubule perfused in vitro. J Am Soc Nephrol. 2001;12(1):177–81. doi: 10.1681/ASN.V121177. [DOI] [PubMed] [Google Scholar]
- 56.Bosch I, Dunussi-Joannopoulos K, Wu RL, Furlong ST, Croop J. Phosphatidylcholine and phosphatidylethanolamine behave as substrates of the human MDR1 P-glycoprotein. Biochemistry. 1997;36(19):5685–94. doi: 10.1021/bi962728r. [DOI] [PubMed] [Google Scholar]
- 57.de Lannoy IA, Silverman M. The MDR1 gene product, P-glycoprotein, mediates the transport of the cardiac glycoside, digoxin. Biochem Biophys Res Commun. 1992;189(1):551–7. doi: 10.1016/0006-291x(92)91593-f. [DOI] [PubMed] [Google Scholar]
- 58.Sakaeda T, Takara K, Kakumoto M, Ohmoto N, Nakamura T, Iwaki K, Tanigawara Y, Okumura K. Simvastatin and lovastatin, but not pravastatin, interact with MDR1. J Pharm Pharmacol. 2002;54(3):419–23. doi: 10.1211/0022357021778493. [DOI] [PubMed] [Google Scholar]
- 59.Santoni-Rugiu E, Silverman JA. Functional characterization of the rat mdr1b encoded P-glycoprotein: not all inducing agents are substrates. Carcinogenesis. 1997;18(11):2255–63. doi: 10.1093/carcin/18.11.2255. [DOI] [PubMed] [Google Scholar]
- 60.Ueda K, Cardarelli C, Gottesman MM, Pastan I. Expression of a full-length cDNA for the human “MDR1” gene confers resistance to colchicine, doxorubicin, and vinblastine. Proc Natl Acad Sci U S A. 1987;84(9):3004–8. doi: 10.1073/pnas.84.9.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yusa K, Tsuruo T. Reversal mechanism of multidrug resistance by verapamil: direct binding of verapamil to P-glycoprotein on specific sites and transport of verapamil outward across the plasma membrane of K562/ADM cells. Cancer Res. 1989;49(18):5002–6. [PubMed] [Google Scholar]
- 62.Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95(26):15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ, Heemskerk S, Russel FG, Masereeuw R. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73(2):220–5. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]
- 64.Imai Y, Asada S, Tsukahara S, Ishikawa E, Tsuruo T, Sugimoto Y. Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol Pharmacol. 2003;64(3):610–8. doi: 10.1124/mol.64.3.610. [DOI] [PubMed] [Google Scholar]
- 65.Merino G, van Herwaarden AE, Wagenaar E, Jonker JW, Schinkel AH. Sex-dependent expression and activity of the ATP-binding cassette transporter breast cancer resistance protein (BCRP/ABCG2) in liver. Mol Pharmacol. 2005;67(5):1765–71. doi: 10.1124/mol.105.011080. [DOI] [PubMed] [Google Scholar]
- 66.Muenster U, Grieshop B, Ickenroth K, Gnoth MJ. Characterization of substrates and inhibitors for the in vitro assessment of Bcrp mediated drug-drug interactions. Pharm Res. 2008;25(10):2320–6. doi: 10.1007/s11095-008-9632-1. [DOI] [PubMed] [Google Scholar]
- 67.Zhou L, Schmidt K, Nelson FR, Zelesky V, Troutman MD, Feng B. The effect of breast cancer resistance protein and P-glycoprotein on the brain penetration of flavopiridol, imatinib mesylate (Gleevec), prazosin, and 2-methoxy-3-(4-(2-(5-methyl-2-phenyloxazol-4-yl)ethoxy)phenyl)propanoic acid (PF-407288) in mice. Drug Metab Dispos. 2009;37(5):946–55. doi: 10.1124/dmd.108.024489. [DOI] [PubMed] [Google Scholar]
- 68.Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol. 2005;67(5):1758–64. doi: 10.1124/mol.104.010439. [DOI] [PubMed] [Google Scholar]
- 69.Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res. 1999;59(17):4237–41. [PubMed] [Google Scholar]
- 70.An G, Morris ME. The sulfated conjugate of biochanin A is a substrate of breast cancer resistant protein (ABCG2). Biopharm Drug Dispos. 2011;32(8):446–57. doi: 10.1002/bdd.772. [DOI] [PubMed] [Google Scholar]
- 71.Maher JM, Slitt AL, Cherrington NJ, Cheng X, Klaassen CD. Tissue distribution and hepatic and renal ontogeny of the multidrug resistance-associated protein (Mrp) family in mice. Drug Metab Dispos. 2005;33(7):947–55. doi: 10.1124/dmd.105.003780. [DOI] [PubMed] [Google Scholar]
- 72.Cui Y, Konig J, Buchholz JK, Spring H, Leier I, Keppler D. Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, MRP2, permanently expressed in human and canine cells. Mol Pharmacol. 1999;55(5):929–37. [PubMed] [Google Scholar]
- 73.Leier I, Hummel-Eisenbeiss J, Cui Y, Keppler D. ATP-dependent para-aminohippurate transport by apical multidrug resistance protein MRP2. Kidney Int. 2000;57(4):1636–42. doi: 10.1046/j.1523-1755.2000.00007.x. [DOI] [PubMed] [Google Scholar]
- 74.Schaub TP, Kartenbeck J, Konig J, Vogel O, Witzgall R, Kriz W, Keppler D. Expression of the conjugate export pump encoded by the mrp2 gene in the apical membrane of kidney proximal tubules. J Am Soc Nephrol. 1997;8(8):1213–21. doi: 10.1681/ASN.V881213. [DOI] [PubMed] [Google Scholar]
- 75.Scheffer GL, Kool M, de Haas M, de Vree JM, Pijnenborg AC, Bosman DK, Elferink RP, van der Valk P, Borst P, Scheper RJ. Tissue distribution and induction of human multidrug resistant protein 3. Lab Invest. 2002;82(2):193–201. doi: 10.1038/labinvest.3780411. [DOI] [PubMed] [Google Scholar]
- 76.van Aubel RA, Smeets PH, Peters JG, Bindels RJ, Russel FG. The MRP4/ABCC4 gene encodes a novel apical organic anion transporter in human kidney proximal tubules: putative efflux pump for urinary cAMP and cGMP. J Am Soc Nephrol. 2002;13(3):595–603. doi: 10.1681/ASN.V133595. [DOI] [PubMed] [Google Scholar]
- 77.Gorgels TG, Hu X, Scheffer GL, van der Wal AC, Toonstra J, de Jong PT, van Kuppevelt TH, Levelt CN, de Wolf A, Loves WJ. Disruption of Abcc6 in the mouse: novel insight in the pathogenesis of pseudoxanthoma elasticum. Hum Mol Genet. 2005;14(13):1763–73. doi: 10.1093/hmg/ddi183. others. [DOI] [PubMed] [Google Scholar]
- 78.Beck K, Hayashi K, Nishiguchi B, Le Saux O, Hayashi M, Boyd CD. The distribution of Abcc6 in normal mouse tissues suggests multiple functions for this ABC transporter. J Histochem Cytochem. 2003;51(7):887–902. doi: 10.1177/002215540305100704. [DOI] [PubMed] [Google Scholar]
- 79.Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, Scheper RJ. MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest. 2002;82(4):515–8. doi: 10.1038/labinvest.3780444. [DOI] [PubMed] [Google Scholar]
- 80.Bakos E, Evers R, Sinko E, Varadi A, Borst P, Sarkadi B. Interactions of the human multidrug resistance proteins MRP1 and MRP2 with organic anions. Mol Pharmacol. 2000;57(4):760–8. doi: 10.1124/mol.57.4.760. [DOI] [PubMed] [Google Scholar]
- 81.Hooijberg JH, Broxterman HJ, Kool M, Assaraf YG, Peters GJ, Noordhuis P, Scheper RJ, Borst P, Pinedo HM, Jansen G. Antifolate resistance mediated by the multidrug resistance proteins MRP1 and MRP2. Cancer Res. 1999;59(11):2532–5. [PubMed] [Google Scholar]
- 82.Keppler D, Leier I, Jedlitschky G. Transport of glutathione conjugates and glucuronides by the multidrug resistance proteins MRP1 and MRP2. Biol Chem. 1997;378(8):787–91. [PubMed] [Google Scholar]
- 83.Renes J, de Vries EG, Nienhuis EF, Jansen PL, Muller M. ATP- and glutathione-dependent transport of chemotherapeutic drugs by the multidrug resistance protein MRP1. Br J Pharmacol. 1999;126(3):681–8. doi: 10.1038/sj.bjp.0702360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zaman GJ, Flens MJ, van Leusden MR, de Haas M, Mulder HS, Lankelma J, Pinedo HM, Scheper RJ, Baas F, Broxterman HJ. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A. 1994;91(19):8822–6. doi: 10.1073/pnas.91.19.8822. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kato Y, Takahara S, Kato S, Kubo Y, Sai Y, Tamai I, Yabuuchi H, Tsuji A. Involvement of multidrug resistance-associated protein 2 (Abcc2) in molecular weight-dependent biliary excretion of beta-lactam antibiotics. Drug Metab Dispos. 2008;36(6):1088–96. doi: 10.1124/dmd.107.019125. [DOI] [PubMed] [Google Scholar]
- 86.Lai L, Tan TM. Role of glutathione in the multidrug resistance protein 4 (MRP4/ABCC4)-mediated efflux of cAMP and resistance to purine analogues. Biochem J. 2002;361(Pt 3):497–503. doi: 10.1042/0264-6021:3610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirohashi T, Suzuki H, Takikawa H, Sugiyama Y. ATP-dependent transport of bile salts by rat multidrug resistance-associated protein 3 (Mrp3). J Biol Chem. 2000;275(4):2905–10. doi: 10.1074/jbc.275.4.2905. [DOI] [PubMed] [Google Scholar]
- 88.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62(1):1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 1996;56(5):988–94. [PubMed] [Google Scholar]
- 90.Whiting DR, Guariguata L, Weil C, Shaw J. IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011 doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 91.Vargas R, Repke JT, Ural SH. Type 1 diabetes mellitus and pregnancy. Rev Obstet Gynecol. 2010;3(3):92–100. [PMC free article] [PubMed] [Google Scholar]
- 92.Melmed RN, Benitez CJ, Holt SJ. Intermediate cells of the pancreas. 3. Selective autophagy and destruction of beta-granules in intermediate cells of the rat pancreas induced by alloxan and streptozotocin. J Cell Sci. 1973;13(1):297–315. doi: 10.1242/jcs.13.1.297. [DOI] [PubMed] [Google Scholar]
- 93.Grover B, Auberger C, Sarangarajan R, Cacini W. Functional impairment of renal organic cation transport in experimental diabetes. Pharmacol Toxicol. 2002;90(4):181–6. doi: 10.1034/j.1600-0773.2002.900402.x. [DOI] [PubMed] [Google Scholar]
- 94.Grover B, Buckley D, Buckley AR, Cacini W. Reduced expression of organic cation transporters rOCT1 and rOCT2 in experimental diabetes. J Pharmacol Exp Ther. 2004;308(3):949–56. doi: 10.1124/jpet.103.058388. [DOI] [PubMed] [Google Scholar]
- 95.Thomas MC, Tikellis C, Kantharidis P, Burns WC, Cooper ME, Forbes JM. The role of advanced glycation in reduced organic cation transport associated with experimental diabetes. J Pharmacol Exp Ther. 2004;311(2):456–66. doi: 10.1124/jpet.104.070672. [DOI] [PubMed] [Google Scholar]
- 96.Thomas MC, Tikellis C, Burns WC, Thallas V, Forbes JM, Cao Z, Osicka TM, Russo LM, Jerums G, Ghabrial H. Reduced tubular cation transport in diabetes: prevented by ACE inhibition. Kidney Int. 2003;63(6):2152–61. doi: 10.1046/j.1523-1755.2003.00006.x. others. [DOI] [PubMed] [Google Scholar]
- 97.Thomas MC, Jerums G, Tsalamandris C, Macisaac R, Panagiotopoulos S, Cooper ME. Increased tubular organic ion clearance following chronic ACE inhibition in patients with type 1 diabetes. Kidney Int. 2005;67(6):2494–9. doi: 10.1111/j.1523-1755.2005.00359.x. [DOI] [PubMed] [Google Scholar]
- 98.Zhang LL, Lu L, Jin S, Jing XY, Yao D, Hu N, Liu L, Duan R, Liu XD, Wang GJ. Tissue-specific alterations in expression and function of P-glycoprotein in streptozotocin-induced diabetic rats. Acta Pharmacol Sin. 2011;32(7):956–66. doi: 10.1038/aps.2011.33. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Anger GJ, Magomedova L, Piquette-Miller M. Impact of acute streptozotocin-induced diabetes on ABC transporter expression in rats. Chem Biodivers. 2009;6(11):1943–59. doi: 10.1002/cbdv.200900053. [DOI] [PubMed] [Google Scholar]
- 100.Tramonti G, Xie P, Wallner EI, Danesh FR, Kanwar YS. Expression and functional characteristics of tubular transporters: P-glycoprotein, PEPT1, and PEPT2 in renal mass reduction and diabetes. Am J Physiol Renal Physiol. 2006;291(5):F972–80. doi: 10.1152/ajprenal.00110.2006. [DOI] [PubMed] [Google Scholar]
- 101.Gangopadhyay A, Thamotharan M, Adibi SA. Regulation of oligopeptide transporter (Pept-1) in experimental diabetes. Am J Physiol Gastrointest Liver Physiol. 2002;283(1):G133–8. doi: 10.1152/ajpgi.00445.2001. [DOI] [PubMed] [Google Scholar]
- 102.Quezada C, Alarcon S, Carcamo JG, Yanez A, Casanello P, Sobrevia L, San Martin R. Increased expression of the multidrug resistance-associated protein 1 (MRP1) in kidney glomeruli of streptozotocin-induced diabetic rats. Biol Chem. 2011;392(6):529–37. doi: 10.1515/BC.2011.052. [DOI] [PubMed] [Google Scholar]
- 103.Cheng Q, Aleksunes LM, Manautou JE, Cherrington NJ, Scheffer GL, Yamasaki H, Slitt AL. Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol Pharm. 2008;5(1):77–91. doi: 10.1021/mp700114j. [DOI] [PubMed] [Google Scholar]
- 104.Doshi M, Takiue Y, Saito H, Hosoyamada M. The increased protein level of URAT1 was observed in obesity/metabolic syndrome model mice. Nucleosides Nucleotides Nucleic Acids. 2011;30(12):1290–4. doi: 10.1080/15257770.2011.603711. [DOI] [PubMed] [Google Scholar]
- 105.More V, X W, Thomas P, Aleksunes L, Slitt A. Severe diabetes and leptin resistance causes differential hepatic and renal transporter expression in mice. Comparative Hepatology. 2012;11(1):1. doi: 10.1186/1476-5926-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nowicki MT, Aleksunes LM, Sawant SP, Dnyanmote AV, Mehendale HM, Manautou JE. Renal and hepatic transporter expression in type 2 diabetic rats. Drug Metab Lett. 2008;2(1):11–7. doi: 10.2174/187231208783478425. [DOI] [PubMed] [Google Scholar]
- 107.More VR, Slitt AL. Alteration of hepatic but not renal transporter expression in diet-induced obese mice. Drug Metab Dispos. 2011;39(6):992–9. doi: 10.1124/dmd.110.037507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Trauner M, Boyer JL. Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev. 2003;83(2):633–71. doi: 10.1152/physrev.00027.2002. [DOI] [PubMed] [Google Scholar]
- 109.Slitt AL, Allen K, Morrone J, Aleksunes LM, Chen C, Maher JM, Manautou JE, Cherrington NJ, Klaassen CD. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta. 2007;1768(3):637–47. doi: 10.1016/j.bbamem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 110.Denk GU, Soroka CJ, Takeyama Y, Chen WS, Schuetz JD, Boyer JL. Multidrug resistance-associated protein 4 is up-regulated in liver but down-regulated in kidney in obstructive cholestasis in the rat. J Hepatol. 2004;40(4):585–91. doi: 10.1016/j.jhep.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 111.Denk GU, Soroka CJ, Mennone A, Koepsell H, Beuers U, Boyer JL. Down-regulation of the organic cation transporter 1 of rat liver in obstructive cholestasis. Hepatology. 2004;39(5):1382–9. doi: 10.1002/hep.20176. [DOI] [PubMed] [Google Scholar]
- 112.Mennone A, Soroka CJ, Harry KM, Boyer JL. Role of breast cancer resistance protein in the adaptive response to cholestasis. Drug Metab Dispos. 2010;38(10):1673–8. doi: 10.1124/dmd.110.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mennone A, Soroka CJ, Cai SY, Harry K, Adachi M, Hagey L, Schuetz JD, Boyer JL. Mrp4-/- mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43(5):1013–21. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- 114.Brandoni A, Quaglia NB, Torres AM. Compensation increase in organic anion excretion in rats with acute biliary obstruction: role of the renal organic anion transporter 1. Pharmacology. 2003;68(2):57–63. doi: 10.1159/000069529. [DOI] [PubMed] [Google Scholar]
- 115.Brandoni A, Torres AM. Characterization of the mechanisms involved in the increased renal elimination of bromosulfophthalein during cholestasis: involvement of Oatp1. J Histochem Cytochem. 2009;57(5):449–56. doi: 10.1369/jhc.2009.952986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brandoni A, Villar SR, Picena JC, Anzai N, Endou H, Torres AM. Expression of rat renal cortical OAT1 and OAT3 in response to acute biliary obstruction. Hepatology. 2006;43(5):1092–100. doi: 10.1002/hep.21142. [DOI] [PubMed] [Google Scholar]
- 117.Kurata T, Muraki Y, Mizutani H, Iwamoto T, Okuda M. Elevated systemic elimination of cimetidine in rats with acute biliary obstruction: the role of renal organic cation transporter OCT2. Drug Metab Pharmacokinet. 2010;25(4):328–34. doi: 10.2133/dmpk.dmpk-10-rg-004. [DOI] [PubMed] [Google Scholar]
- 118.Lee J, Azzaroli F, Wang L, Soroka CJ, Gigliozzi A, Setchell KD, Kramer W, Boyer JL. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology. 2001;121(6):1473–84. doi: 10.1053/gast.2001.29608. [DOI] [PubMed] [Google Scholar]
- 119.Villanueva SS, Ruiz ML, Ghanem CI, Luquita MG, Catania VA, Mottino AD. Hepatic synthesis and urinary elimination of acetaminophen glucuronide are exacerbated in bile duct-ligated rats. Drug Metab Dispos. 2008;36(3):475–80. doi: 10.1124/dmd.107.018127. [DOI] [PubMed] [Google Scholar]
- 120.Brandoni A, Anzai N, Kanai Y, Endou H, Torres AM. Renal elimination of p-aminohippurate (PAH) in response to three days of biliary obstruction in the rat. The role of OAT1 and OAT3. Biochim Biophys Acta. 2006;1762(7):673–82. doi: 10.1016/j.bbadis.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 121.Denk GU, Cai SY, Chen WS, Lin A, Soroka CJ, Boyer JL. A comparison of gene expression in mouse liver and kidney in obstructive cholestasis utilizing high-density oligonucleotide microarray technology. World J Gastroenterol. 2006;12(16):2536–48. doi: 10.3748/wjg.v12.i16.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pei QL, Kobayashi Y, Tanaka Y, Taguchi Y, Higuchi K, Kaito M, Ma N, Semba R, Kamisako T, Adachi Y. Increased expression of multidrug resistance-associated protein 1 (mrp1) in hepatocyte basolateral membrane and renal tubular epithelia after bile duct ligation in rats. Hepatol Res. 2002;22(1):58–64. doi: 10.1016/s1386-6346(01)00110-3. [DOI] [PubMed] [Google Scholar]
- 123.Tanaka Y, Kobayashi Y, Gabazza EC, Higuchi K, Kamisako T, Kuroda M, Takeuchi K, Iwasa M, Kaito M, Adachi Y. Increased renal expression of bilirubin glucuronide transporters in a rat model of obstructive jaundice. Am J Physiol Gastrointest Liver Physiol. 2002;282(4):G656–62. doi: 10.1152/ajpgi.00383.2001. [DOI] [PubMed] [Google Scholar]
- 124.Liu T, Guo X, Meng Q, Wang C, Liu Q, Sun H, Ma X, Kaku T, Liu K. Effect of JBP485 on obstructive jaundice is related to regulation of renal Oat1, Oat3 and Mrp2 expression in ANIT-treated rats. Peptides. 2012;36(1):78–85. doi: 10.1016/j.peptides.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 125.Jin HE, Hong SS, Choi MK, Maeng HJ, Kim DD, Chung SJ, Shim CK. Reduced antidiabetic effect of metformin and down-regulation of hepatic Oct1 in rats with ethynylestradiol-induced cholestasis. Pharm Res. 2009;26(3):549–59. doi: 10.1007/s11095-008-9770-5. [DOI] [PubMed] [Google Scholar]
- 126.Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU. Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G923–32. doi: 10.1152/ajpgi.00490.2005. others. [DOI] [PubMed] [Google Scholar]
- 127.Zollner G, Fickert P, Fuchsbichler A, Silbert D, Wagner M, Arbeiter S, Gonzalez FJ, Marschall HU, Zatloukal K, Denk H. Role of nuclear bile acid receptor, FXR, in adaptive ABC transporter regulation by cholic and ursodeoxycholic acid in mouse liver, kidney and intestine. J Hepatol. 2003;39(4):480–8. doi: 10.1016/s0168-8278(03)00228-9. others. [DOI] [PubMed] [Google Scholar]
- 128.Huang KC, McIntosh BJ. Effect of sex hormones on renal transport of p-aminohippuric acid. Am J Physiol. 1955;183(3):387–90. doi: 10.1152/ajplegacy.1955.183.3.387. [DOI] [PubMed] [Google Scholar]
- 129.Reyes JL, Melendez E, Alegria A, Jaramillo-Juarez F. Influence of sex differences on the renal secretion of organic anions. Endocrinology. 1998;139(4):1581–7. doi: 10.1210/endo.139.4.5930. [DOI] [PubMed] [Google Scholar]
- 130.Buist SC, Cherrington NJ, Klaassen CD. Endocrine regulation of rat organic anion transporters. Drug Metab Dispos. 2003;31(5):559–64. doi: 10.1124/dmd.31.5.559. [DOI] [PubMed] [Google Scholar]
- 131.Buist SC, Klaassen CD. Rat and mouse differences in gender-predominant expression of organic anion transporter (Oat1-3; Slc22a6-8) mRNA levels. Drug Metab Dispos. 2004;32(6):620–5. doi: 10.1124/dmd.32.6.620. [DOI] [PubMed] [Google Scholar]
- 132.Ljubojevic M, Balen D, Breljak D, Kusan M, Anzai N, Bahn A, Burckhardt G, Sabolic I. Renal expression of organic anion transporter OAT2 in rats and mice is regulated by sex hormones. Am J Physiol Renal Physiol. 2007;292(1):F361–72. doi: 10.1152/ajprenal.00207.2006. [DOI] [PubMed] [Google Scholar]
- 133.Buist SC, Cherrington NJ, Choudhuri S, Hartley DP, Klaassen CD. Gender-specific and developmental influences on the expression of rat organic anion transporters. J Pharmacol Exp Ther. 2002;301(1):145–51. doi: 10.1124/jpet.301.1.145. [DOI] [PubMed] [Google Scholar]
- 134.Kobayashi Y, Hirokawa N, Ohshiro N, Sekine T, Sasaki T, Tokuyama S, Endou H, Yamamoto T. Differential gene expression of organic anion transporters in male and female rats. Biochem Biophys Res Commun. 2002;290(1):482–7. doi: 10.1006/bbrc.2001.6180. [DOI] [PubMed] [Google Scholar]
- 135.Breljak D, Ljubojevic M, Balen D, Zlender V, Brzica H, Micek V, Kusan M, Anzai N, Sabolic I. Renal expression of organic anion transporter Oat5 in rats and mice exhibits the female-dominant sex differences. Histol Histopathol. 2010;25(11):1385–402. doi: 10.14670/HH-25.1385. [DOI] [PubMed] [Google Scholar]
- 136.Cheng X, Klaassen CD. Tissue distribution, ontogeny, and hormonal regulation of xenobiotic transporters in mouse kidneys. Drug Metab Dispos. 2009;37(11):2178–85. doi: 10.1124/dmd.109.027177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kudo N, Katakura M, Sato Y, Kawashima Y. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem Biol Interact. 2002;139(3):301–16. doi: 10.1016/s0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 138.Cerrutti JA, Brandoni A, Quaglia NB, Torres AM. Sex differences in p-aminohippuric acid transport in rat kidney: role of membrane fluidity and expression of OAT1. Mol Cell Biochem. 2002;233(1-2):175–9. doi: 10.1023/a:1015563021602. [DOI] [PubMed] [Google Scholar]
- 139.Cerrutti JA, Quaglia NB, Torres AM. Characterization of the mechanisms involved in the gender differences in p-aminohippurate renal elimination in rats. Can J Physiol Pharmacol. 2001;79(9):805–13. [PubMed] [Google Scholar]
- 140.Bowman HM, Hook JB. Sex differences in organic ion transport by rat kidney. Proc Soc Exp Biol Med. 1972;141(1):258–62. doi: 10.3181/00379727-141-36754. [DOI] [PubMed] [Google Scholar]
- 141.Groves CE, Suhre WB, Cherrington NJ, Wright SH. Sex differences in the mRNA, protein, and functional expression of organic anion transporter (Oat) 1, Oat3, and organic cation transporter (Oct) 2 in rabbit renal proximal tubules. J Pharmacol Exp Ther. 2006;316(2):743–52. doi: 10.1124/jpet.105.094979. [DOI] [PubMed] [Google Scholar]
- 142.Yonezawa A, Masuda S, Nishihara K, Yano I, Katsura T, Inui K. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 2005;70(12):1823–31. doi: 10.1016/j.bcp.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 143.Meetam P, Srimaroeng C, Soodvilai S, Chatsudthipong V. Regulatory role of testosterone in organic cation transport: in vivo and in vitro studies. Biol Pharm Bull. 2009;32(6):982–7. doi: 10.1248/bpb.32.982. [DOI] [PubMed] [Google Scholar]
- 144.Urakami Y, Okuda M, Saito H, Inui K. Hormonal regulation of organic cation transporter OCT2 expression in rat kidney. FEBS Lett. 2000;473(2):173–6. doi: 10.1016/s0014-5793(00)01525-8. [DOI] [PubMed] [Google Scholar]
- 145.Asaka J, Terada T, Okuda M, Katsura T, Inui K. Androgen receptor is responsible for rat organic cation transporter 2 gene regulation but not for rOCT1 and rOCT3. Pharm Res. 2006;23(4):697–704. doi: 10.1007/s11095-006-9665-2. [DOI] [PubMed] [Google Scholar]
- 146.Maher JM, Cheng X, Tanaka Y, Scheffer GL, Klaassen CD. Hormonal regulation of renal multidrug resistance-associated proteins 3 and 4 (Mrp3 and Mrp4) in mice. Biochem Pharmacol. 2006;71(10):1470–8. doi: 10.1016/j.bcp.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 147.Tannenbaum GS, Martin JB. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology. 1976;98(3):562–70. doi: 10.1210/endo-98-3-562. [DOI] [PubMed] [Google Scholar]
- 148.MacLeod JN, Pampori NA, Shapiro BH. Sex differences in the ultradian pattern of plasma growth hormone concentrations in mice. J Endocrinol. 1991;131(3):395–9. doi: 10.1677/joe.0.1310395. [DOI] [PubMed] [Google Scholar]
- 149.Saunders A, Terry LC, Audet J, Brazeau P, Martin JB. Dynamic studies of growth hormone and prolactin secretion in the female rat. Neuroendocrinology. 1976;21(3):193–203. doi: 10.1159/000122525. [DOI] [PubMed] [Google Scholar]
- 150.Waxman DJ, Pampori NA, Ram PA, Agrawal AK, Shapiro BH. Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc Natl Acad Sci U S A. 1991;88(15):6868–72. doi: 10.1073/pnas.88.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Waxman DJ, O'Connor C. Growth hormone regulation of sex-dependent liver gene expression. Mol Endocrinol. 2006;20(11):2613–29. doi: 10.1210/me.2006-0007. [DOI] [PubMed] [Google Scholar]
- 152.Beamer WH, Eicher EM. Stimulation of growth in the little mouse. J Endocrinol. 1976;71(1):37–45. doi: 10.1677/joe.0.0710037. [DOI] [PubMed] [Google Scholar]
- 153.Cheng X, Maher J, Lu H, Klaassen CD. Endocrine regulation of gender-divergent mouse organic anion-transporting polypeptide (Oatp) expression. Mol Pharmacol. 2006;70(4):1291–7. doi: 10.1124/mol.106.025122. [DOI] [PubMed] [Google Scholar]
- 154.Conrad KP. Mechanisms of renal vasodilation and hyperfiltration during pregnancy. J Soc Gynecol Investig. 2004;11(7):438–48. doi: 10.1016/j.jsgi.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 155.Cornelis T, Odutayo A, Keunen J, Hladunewich M. The kidney in normal pregnancy and preeclampsia. Semin Nephrol. 2011;31(1):4–14. doi: 10.1016/j.semnephrol.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 156.Maynard SE, Thadhani R. Pregnancy and the kidney. J Am Soc Nephrol. 2009;20(1):14–22. doi: 10.1681/ASN.2008050493. [DOI] [PubMed] [Google Scholar]
- 157.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54(6):2056–63. doi: 10.1046/j.1523-1755.1998.00217.x. others. [DOI] [PubMed] [Google Scholar]
- 158.Zhou L, Zhang Y, Hebert MF, Unadkat JD, Mao Q. Increased glyburide clearance in the pregnant mouse model. Drug Metab Dispos. 2010;38(9):1403–6. doi: 10.1124/dmd.110.033837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hebert MF, Easterling TR, Kirby B, Carr DB, Buchanan ML, Rutherford T, Thummel KE, Fishbein DP, Unadkat JD. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84(2):248–53. doi: 10.1038/clpt.2008.1. [DOI] [PubMed] [Google Scholar]
- 160.Wang H, Wu X, Hudkins K, Mikheev A, Zhang H, Gupta A, Unadkat JD, Mao Q. Expression of the breast cancer resistance protein (Bcrp1/Abcg2) in tissues from pregnant mice: effects of pregnancy and correlations with nuclear receptors. Am J Physiol Endocrinol Metab. 2006;291(6):E1295–304. doi: 10.1152/ajpendo.00193.2006. [DOI] [PubMed] [Google Scholar]
- 161.Yacovino LL, Aleksunes LM. Renal efflux transporter expression in pregnant mice with Type I diabetes. Toxicol Lett. 2012;211(3):304–311. doi: 10.1016/j.toxlet.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Keembiyehetty C, Augustin R, Carayannopoulos MO, Steer S, Manolescu A, Cheeseman CI, Moley KH. Mouse glucose transporter 9 splice variants are expressed in adult liver and kidney and are up-regulated in diabetes. Mol Endocrinol. 2006;20(3):686–97. doi: 10.1210/me.2005-0010. [DOI] [PubMed] [Google Scholar]
- 163.Cheng X, Maher J, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab Dispos. 2005;33(9):1276–82. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- 164.Li N, Hartley DP, Cherrington NJ, Klaassen CD. Tissue expression, ontogeny, and inducibility of rat organic anion transporting polypeptide 4. J Pharmacol Exp Ther. 2002;301(2):551–60. doi: 10.1124/jpet.301.2.551. [DOI] [PubMed] [Google Scholar]
- 165.Lu H, Klaassen C. Tissue distribution and thyroid hormone regulation of Pept1 and Pept2 mRNA in rodents. Peptides. 2006;27(4):850–7. doi: 10.1016/j.peptides.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 166.Tanaka Y, Slitt AL, Leazer TM, Maher JM, Klaassen CD. Tissue distribution and hormonal regulation of the breast cancer resistance protein (Bcrp/Abcg2) in rats and mice. Biochem Biophys Res Commun. 2005;326(1):181–7. doi: 10.1016/j.bbrc.2004.11.012. [DOI] [PubMed] [Google Scholar]