Abstract
Background and Aims
Fatigue is the most frequent and often debilitating symptom of chronic hepatitis C. It is unclear whether successful therapy of hepatitis C leads to its clinical improvement. In the Virahep-C study, patients with hepatitis C virus (HCV) genotype 1 infection were treated with peginterferon alfa-2a and ribavirin for up to 48 weeks while undergoing assessment of viral kinetics and clinical symptoms.
Methods
Fatigue measurements were conducted, before, during and after therapy, as `presence' (yes/no) and `severity' (visual analogue scale: 0 to 100mm). The clinical, histologic and virologic features that correlated with the presence and degree of fatigue were assessed focusing upon changes associated with sustained virological response (SVR).
Results
At baseline, 52% (n= 401) participants reported having fatigue, which was more common in women than men (59% vs. 48%, p=0.02) and slightly more severe (30 vs. 22mm, p=0.056). Fatigue was frequent and worse in cirrhotics versus those with lesser fibrosis (66% vs. 49%; 34 vs. 24mm). Fatigue did not correlate with other parameters. The proportion of patients and median fatigue scores increased on treatment (52% to 78%; 25 to 40mm, p<0.0001) with higher fatigue noted amongst those who ultimately achieved SVR (p<0.0001). On achieving SVR, there was a significant decrease in both frequency and severity of fatigue compared to their baseline (53% to 33%; 27 to 13mm, both p<0.0001).
Conclusion
Fatigue is common in patients with chronic hepatitis C but associated poorly with biochemical parameters. Sustained response is accompanied by substantial improvement of fatigue.
Keywords: liver, anti-viral therapy, tiredness, cirrhosis, peginterferon, ribavirin, side-effects
Introduction
Fatigue is the most frequent symptom of liver disease and has a major effect on quality of life and daily activity in patients chronically infected with hepatitis C virus (HCV) [1–6]. However, the degree of fatigue varies considerably among patients and often correlates poorly with the severity of disease. In many instances, it is unclear whether fatigue is caused by hepatitis C and, if so, whether it is due to the chronic viral infection or to the degree of liver inflammation, injury or dysfunction [2, 3, 7]. A better understanding of the nature of fatigue in liver disease is hampered by (1) the fact that it is a subjective experience and difficult to measure and quantify; (2) is often multifactorial; and (3) its quality and severity are similar among the different etiologies.
Many clinical trials in liver disease have tried to capture the effects of medical treatments on fatigue using self-report instruments, with variable success [2, 3, 8–12]. Estimating the presence and severity of fatigue is particularly important in studies of interferon-based therapies for hepatitis C [7]. Even with recent advances in treatment for this disease, regimens still include peginterferon, administered for 24 to 48 weeks, based upon viral genotype and viral response [13–16]. During interferon therapy, fatigue is the most common side effect and can lead to early termination of therapy and treatment failure [7, 17, 18].
Perhaps just as important as capturing the degree of fatigue that occurs during therapy, is whether eradication of HCV ultimately leads to improvement in symptoms. While several studies have evaluated and noted improvements in quality of life (QoL) with successful therapy [18–20], the typical QoL construct can be diffuse, encompassing mental, physical, social, occupational and role functioning, without a detailed analysis of changes in specific symptoms. While the rationale for interferon treatment is usually to prevent long-term complications of hepatitis C, another reason is to improve clinical symptoms and quality of life.
The data from the Viral Resistance to Antiviral Therapy of Hepatitis C (Virahep-C) study offered a unique opportunity to evaluate the effect of therapy on fatigue in hepatitis C patients. Virahep-C enrolled 401 African-Americans (AAs) and Caucasian Americans (CAs), with HCV genotype 1 who were treated with peginterferon and ribavirin for up to 48 weeks while undergoing careful and extensive longitudinal evaluation of clinical, biochemical, virologic, immunologic factors and clinical symptoms [21–24], including fatigue. Fatigue severity was measured using a visual analogue scale (VAS), which is easy to administer, understand and not restricted by literacy levels [25, 26]. Notably, VASs have been used in multiple clinical contexts to study fatigue, including liver diseases [27–31], and are highly correlated with other validated fatigue measurements [25, 28, 31, 32]. The aim of this study was to assess whether fatigue was associated with disease severity and whether fatigue improved with successful treatment.
Methods
The Virahep-C study design
Virahep-C was a prospective clinical study conducted at 8 U.S. medical centers between 2002 and 2005 in which 196 AAs and 205 CAs patients with chronic hepatitis C, genotype 1, were treated with the standard antiviral regimen of peginterferon alfa-2a (Pegasus, Roche Pharmaceuticals; Nutley, NJ) and ribavirin (Copegus, Roche) while undergoing extensive studies of HCV viral kinetics, immune function, genetics and interferon signaling pathways [21–24, 33]. The major aims of Virahep-C were to define response rates to conventional therapy among AA versus CA patients and investigate factors associated with nonresponse in the two racial groups. Details of Virahep-C are described elsewhere [21]. All patients provided fully informed written consent and the study design and all details were approved by local Institutional Review Boards.
Patients were evaluated and had blood tests taken twice before therapy (Screening Visits 1 and 2), at treatment weeks 1, 2, 4, 8, 12, 16, 20, 24, 32, 36, 40, 44 and 48, and 4, 12 and 24 weeks after treatment discontinuation. Patients completed symptom questionnaires and were asked about side effects at Screen 2 and at all the treatment and the follow-up weeks. Blood samples were taken for routine liver tests (including serum alanine and aspartate aminotransferase levels: ALT, AST), blood counts and HCV RNA concentrations, which were measured centrally using an assay with a lower limit of sensitivity of 500 IU/mL (COBAS Amplicor Hepatitis C Virus Monitor Test, version 2.0 assay: Roche Molecular Diagnostics, Alameda CA).
Patients who remained HCV RNA positive after 24 weeks of therapy stopped treatment and were considered non-responders (NR) whereas those who were HCV RNA negative at week 24 (responders) continued treatment for a total of 48 weeks. Patients who remained HCV RNA negative for at least 24 weeks after treatment were considered to have a sustained virological response (SVR), while those who had rebound of HCV RNA positivity were considered to have had a relapse (if it occurred after treatment) or breakthrough (if while on treatment). For the current analyses, the major focus was on the responder versus the non-responder populations and especially those who achieved SVR.
Assessment of `presence' of fatigue (`fatigue presence')
The presence or absence of fatigue was assessed directly by study personnel (usually a research nurse coordinator) asking, “Do you have fatigue?” Patients were asked to respond “Yes” or “No.”
Assessment of `severity' of fatigue (`fatigue severity')
A VAS for fatigue was administered at each clinic visit (Supplement figure 3). The scales were displayed on a computer screen and patients were asked to touch a point along a 10 cm line between “None” and “Worst ever”, the position of which best reflected how much fatigue they had experienced during the previous week. The results were sent directly to the Data Coordinating Center, and study personnel were masked to patient responses. The degree of fatigue was scored from 0 to 100 mm by measuring from the start of the line to the patient mark.
Depression was also measured at each clinic visit using a VAS (Depression VAS). Analyses of depression during Virahep-C have been published [33].
Statistical Methods
The prevalence of fatigue at baseline by categorical patient characteristics was summarized using frequency and percent and the associated p-values were obtained from univariate logistic regression. Fatigue severity was summarized using median and quartiles. Due to its non-normality, fatigue severity between two groups was compared using Wilcoxon rank-sum test and across multiple groups using Kruskal-Wallis test. Univariate and multivariate logistic regression was used in assessing the association between incidence of fatigue (binary) and patient characteristics. Wilcoxon rank sum test was used in assessing the association between fatigue presence (non-normal) and patient characteristics; signed rank test was used to assess significant changes in fatigue presence between any two time points. All analyses were conducted using SAS 9.3 (SAS Institute Inc., Carey, NC).
Results
Baseline Results
At the time of screening, 207 of 401 (52%) study participants reported the presence of fatigue (Table 1). Fatigue was more common among women (59%) than men (48%) (p= 0.02). There were no statistically significant differences in the presence of fatigue by age, race, body mass index (BMI), ALT or AST level, viral subtype (1a vs. 1b), HCV RNA concentration, or any other baseline variable (Table 1). Liver histology taken within one year before enrollment was available from 399 patients and the presence of fatigue did not vary by inflammatory scores, but was more common in patients with cirrhosis (Ishak fibrosis score 5–6: 66%) than those with minimal (Ishak 0–2: 49%) or moderate fibrosis (Ishak 3–4: 53%) although not statistically significant (p=0.25). Multivariable logistic regression analysis at screening showed that no variables, except gender, to have a significant association with the presence of fatigue.
Table 1.
Presence of fatigue at the time of enrollment by selected clinical, virological and histologic features.
| n | Fatigue (n, %) | p value | |
|---|---|---|---|
| Total | 401 | 207 (52%) | |
| Gender: | |||
| Male | 261 | 124 (48%) | 0.02* |
| Female | 140 | 83 (59%) | |
| Race: | |||
| White | 205 | 113 (55%) | 0.15 |
| Black | 196 | 94 (48%) | |
| Age: | |||
| <40 years | 49 | 25 (51%) | |
| >40 years | 352 | 182 (51%) | 0.93 |
| BMI: | |||
| <25 | 95 | 46 (48%) | |
| 25–30 | 149 | 78 (52%) | 0.64 |
| >30 | 150 | 77 (51%) | 0.86 |
| ALT: | |||
| <40U/L | 68 | 36 (53%) | |
| 40–100U/L | 220 | 116 (53%) | 0.77 |
| >100U/L | 111 | 55 (50%) | 0.58 |
| AST: | |||
| <30U/L | 58 | 30 (52%) | |
| 30–100U/L | 283 | 147 (52%) | 0.97 |
| >100U/L | 58 | 30 (52%) | 0.98 |
| HCV genotype: | |||
| 1a | 210 | 115 (55%) | |
| 1b | 148 | 73 (49%) | 0.98 |
| HCV RNA: | |||
| >400,000 | 31 | 18 (58%) | |
| <400,000 | 368 | 188 (51%) | 0.46 |
| HAI Score: | |||
| 1–5 | 34 | 17 (50%) | |
| 5–10 | 163 | 86 (53%) | 0.71 |
| 11–18 | 202 | 103 (51%) | 0.95 |
| Fibrosis: | |||
| 0–2 | 253 | 125 (49%) | |
| 3–4 | 117 | 62 (53%) | 0.49 |
| 5–6 | 29 | 19 (66%) | 0.14 |
p<0.05
The severity of fatigue was measured at baseline by the fatigue VAS (n=390), and the median fatigue VAS score was 25 mm. Of 390, 205 patients (53%) reported having fatigue, of which 181 (88%) patients marked a score of greater than 10 mm on the fatigue severity VAS. Fatigue severity was slightly higher for women than men (median 30 vs. 22 mm: p=0.056) (Table 2). Baseline fatigue severity was similar between AA and CA patients, and did not vary consistently by BMI, HCV RNA levels or histological inflammatory scores. Again noted was fatigue severity higher among patients with cirrhosis than in those with minimal or moderate fibrosis (median 34 vs. 24 and 25 mm), although not statistically significant (p= 0.62). Interestingly, fatigue severity was worse among patients with normal ALT levels (< 40 U/L: 37 mm) than in those with mild (40–100 U/L: 25 mm) or moderate-to-high ALT elevations (>100 U/L: 21 mm) (p= 0.02). In contrast, fatigue scores did not vary by AST levels. In multivariable analysis at screening, controlling for sex, race, baseline viral level and Ishak score, younger age and lower ALT was associated with more severe fatigue (p<0.05 for both).
Table 2.
Median and 75% interquartile range of fatigue VAS scores and their distribution by baseline clinical, virologic and histologic features
| n | Fatigue VAS Median (Q1,Q3) | p value | None to Mild fatigue (VAS 0–10) | Moderate fatigue (VAS 10–40) | Severe fatigue (VAS 40–100) | |
|---|---|---|---|---|---|---|
| Total | 390 | 25 (6, 55) | 33% | 29% | 38% | |
| Gender: | ||||||
| Male | 261 | 22 (5, 49) | 0.06 | 35% | 30% | 35% |
| Female | 140 | 30 (9, 68) | 29% | 26% | 45% | |
| Race: | ||||||
| White | 205 | 24 (9, 55) | 0.48 | 26% | 37% | 37% |
| Black | 196 | 27 (5, 56) | 39% | 20% | 40% | |
| Age: | ||||||
| <40 | 49 | 39 (15,70) | 0.12 | 22% | 29% | 49% |
| >40 | 352 | 24 (5, 53) | 34% | 29% | 37% | |
| BMI: | ||||||
| <25 | 95 | 23 (5, 55) | 0.85 | 35% | 28% | 37% |
| 25–30 | 149 | 24 (5,51) | 32% | 30% | 39% | |
| >30 | 150 | 27 (6, 60) | 33% | 27% | 40% | |
| ALT: | ||||||
| <40 U/L | 68 | 37 (6, 63) | 0.02* | 31% | 22% | 47% |
| 40–100 U/L | 220 | 25 (6, 55) | 31% | 27% | 41% | |
| >100 U/L | 111 | 21 (5,44) | 36% | 36% | 28% | |
| AST: | ||||||
| <30 U/L | 58 | 28 (3, 60) | 0.89 | 40% | 17% | 43% |
| 30–100 U/L | 283 | 25 (6, 55) | 31% | 29% | 39% | |
| >100 U/L | 58 | 21 (6, 46) | 31% | 38% | 31% | |
| HCV genotype: | ||||||
| 1a | 210 | 29 (6, 55) | 0.4 | 31% | 27% | 42% |
| 1b | 148 | 24 (5, 59) | 33% | 30% | 36% | |
| HCV RNA: | ||||||
| >400,000 | 368 | 33 (5, 47) | 0.58 | 33% | 29% | 38% |
| <400,000 | 31 | 25 (6, 56) | 32% | 23% | 45% | |
| HAI Score: | ||||||
| 1–5 | 34 | 24 (3, 67) | 0.99 | 44% | 21% | 35% |
| 5–10 | 163 | 26 (8,55) | 31% | 31% | 39% | |
| 11–18 | 202 | 25 (6, 55) | 32% | 29% | 40% | |
| Fibrosis: | ||||||
| 0–2 | 253 | 24 (4, 54) | 0.62 | 36% | 26% | 38% |
| 3–4 | 117 | 25 (8, 63) | 29% | 32% | 39% | |
| 5–6 | 29 | 34 (21,55) | 14% | 38% | 48% |
p<0.05
To better classify fatigue severity, fatigue VAS scores (n=390) were grouped into three categories: no or minimal fatigue (0 to 10 mm: n =120), mild-to-moderate fatigue (>10 to 40 mm: n=115) and severe fatigue (>40 to 100 mm: n= 155). The clinical, demographic, biochemical and histologic features of patients with these three degrees of fatigue are tabulated in Table 2. Those with cirrhosis had significantly higher proportion with severe fatigue compared to those with lesser degree of fibrosis (48% vs. 38%: p-value=0.005).
On Treatment Results
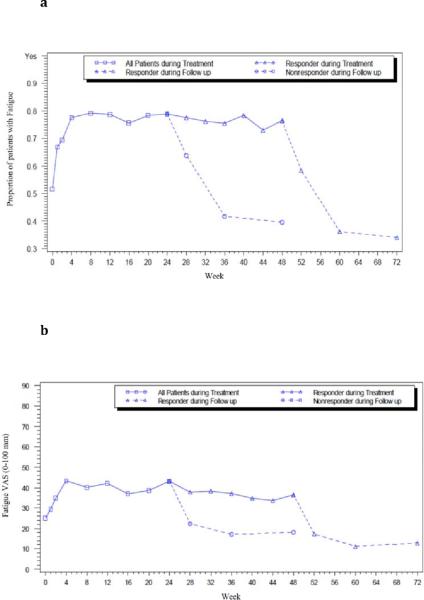
During treatment, the proportion of patients who admitted to having fatigue increased from 52% at baseline to 78% at week 4 (p<0.0001), and remained at this level for the duration of treatment (range: 67% to 79%) (Figure 1a). The median fatigue severity score increased during the first 4 weeks of therapy (from 25 to 40 mm, p<0.0001) and remained at this higher level for the duration of treatment (range: 29 to 43 mm).
Figure 1. `Presence of' and `severity' of fatigue at baseline, on treatment and follow-up of all patients.
(a) Proportion (`yes/no') of patients with fatigue and, (b) the Severity of fatigue (VAS), at baseline, on treatment and on follow-up. Each point marks the week of patient evaluation and fatigue measurement: at screening visit 2, treatment weeks 1, 2, 4, 8, 12, 16, 20, 24, 32, 36, 40, 44 and 48 (until week 24 for non-responders), and on follow-up at 4, 12 and 24 weeks after treatment discontinuation. ◻ All patients on treatment, ▵ Responder, ◯ Non-responders (NR). Solid line, on treatment; and dashed line: off-treatment follow-up weeks.
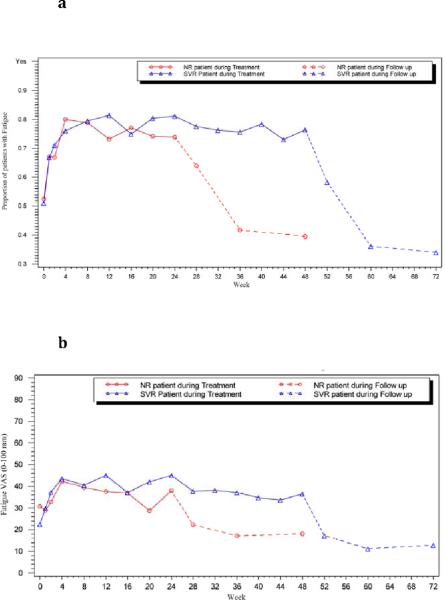
Patterns of changes in fatigue severity on treatment were assessed in subgroups of patients during treatment; comparing women vs. men (Supplemental Figure 1), AA vs. CA participants (Supplemental Figure 2). Fatigue severity was greater amongst women than men (p<0.001: Supplemental Figure 1) and amongst CA compared to AA patients during therapy (p<0.001: Supplemental Figure 2). In addition, both the frequency and severity of fatigue was greater among participants who ultimately achieved SVR vs. NRs (p<0.001: Figures 2a and 2b, respectively).
Figure 2. `Presence of' and `severity' of fatigue at baseline, on treatment and follow-up amongst those achieving SVR versus NR.
(a) Proportion of patients with fatigue and (b) severity of fatigue amongst those who achieved sustained virologic response (SVR) versus NR. ▵ SVR (blue line), ◯ NR patients (red line). Solid line: on treatment, and dashed line: off-treatment, follow-up weeks.
After Treatment Results
Once therapy terminated, the proportion of patients who admitted to feeling fatigued decreased. By 12 weeks after discontinuation, proportions of patients with fatigue were lower than that at baseline (36% in responders, 42% non-responders vs 52% at baseline, Figure 1a). The median fatigue VAS scores were also lower (11 mm in responders, 17 mm in non-responders vs 25 mm at baseline: Figure 1b).
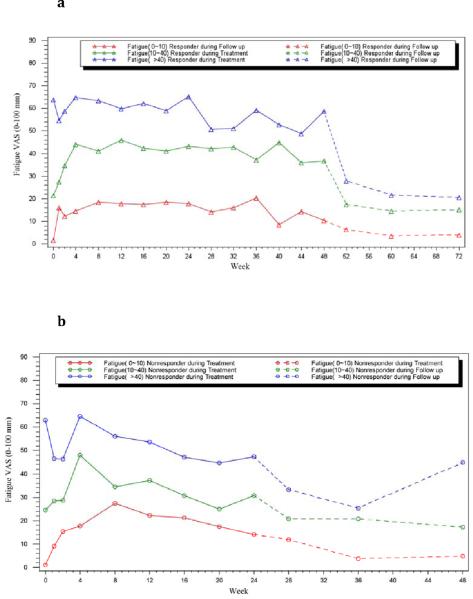
The improvement in fatigue was greater among patients who achieved an SVR than in those who never became HCV RNA negative (non-responders). Overall, the proportion of SVR patients who admitted to having fatigue decreased from 53% at baseline to 33% 24 weeks after treatment (p<0.0001; n=161), and the median VAS fatigue score decreased from 27 mm to 13 mm (p<0.0001; n=158). These changes were especially profound in patients who at baseline had severe levels of fatigue (fatigue VAS score >40 mm), in whom the median fatigue VAS score decreased from 64 mm at baseline to 21 mm at follow-up week 24 (p<0.0001, n= 66; Figure 3a). Among non-responders, the presence and severity of fatigue decreased but not significantly between baseline and 24 weeks after treatment regardless of the initial score (p>0.05, Figure 3b). Furthermore, there was no significant change in fatigue presence or severity among patients who had virologic relapse (n= 60) or breakthrough (n= 21) (data not shown).
Figure 3. Severity of fatigue categorized by baseline fatigue status in responders and NR.
Severity of fatigue by baseline fatigue status in (a) Responders (b) NR patients. Red line: none-to-mild fatigue: VAS ≤10; Green line: moderate fatigue: VAS >10 to ≤40 and; Blue line: severe fatigue: VAS >40. (a) ▵ Responder, (b) ◯ Non-responders. Solid line: on treatment, and dashed line: off-treatment follow-up weeks.
As expected, fatigue score was associated with depression, (Spearman correlation coefficients, rs=0.53 at baseline; 0.66 at treatment week 24; and 0.73 at follow-up week 24; all p < 0.0001). Controlling for the presence of depression did not alter the significance of the changes in fatigue severity after successful completion of therapy compared to baseline (p<0.0001).
Discussion
Fatigue is perhaps the most common symptom among patients with chronic hepatitis C and is a troublesome side effect of its therapy[1, 2, 4, 7, 9, 34, 35]. In this study, half of patients enrolled in a study of antiviral therapy of HCV admitted to having some degree of fatigue, of whom two-thirds rated it as moderate or severe. The current literature suggests that the presence and severity of fatigue correlates poorly with disease activity although it may be somewhat more common and severe in patients with cirrhosis [1, 9, 19, 36]. In the current study, the differences in frequency and severity of fatigue in patients with cirrhosis compared to those with lesser degrees of fibrosis were not statistically significant; however, the data were limited by numbers of patients with more advanced disease (n=29: 7% of the cohort) but notable was that more cirrhotics had worse fatigue than those with minimal fibrosis.
As expected, fatigue became more troublesome during interferon therapy [9, 14, 15, 18]. Fatigue worsened for the first 4 weeks of therapy, then plateaued, and did not completely resolve or return to baseline until 12 weeks after stopping therapy. The cause of fatigue induced from interferon therapy is likely multifactorial, but may include the systemic effects of cytokines, secondary effects of treatment-related side effects such as anemia [37–40], as well as the psychosocial stress of having to maintain occupational and family responsibilities while undergoing medical treatment. Thus, although attributing the cause of fatigue to a specific set of genes or proteins is an attractive and parsimonious notion, an interlinked pathway involving multiple genetic, biochemical and environmental processes is a more realistic probability [41], and an area for future research.
Importantly, the presence and severity of fatigue ultimately declined in patients with sustained clearance of HCV. The results remained consistent even after controlling for depression, a common cofounder of fatigue. These findings indicate that therapy of HCV can result in significant and sustained improvement in clinical symptoms, and that the measurement of fatigue using VASs is successful in capturing these changes. Improvements in fatigue were most convincing in patients with moderate to severe levels of fatigue at baseline. Thus, patients with relatively non-significant biochemical or histologic disease, but who have troublesome symptoms such as fatigue, should be considered for antiviral therapy.
The likely cause for the improvement of fatigue with eradication of HCV is unclear. It is also unclear if certain aspects of fatigue (i.e., physical, mental or cognitive) fare better, as the VAS is a quantitative measure rather than a qualitative one. While patients' awareness of virological response could have a beneficial psychological effect on perceptions of fatigue, the fatigue assessments were obtained before the results of virological testing were known, and improvements in fatigue were achieved well before knowledge of SVR was given to patients.
A few limitations of this study should be noted. The cohort tested was a relatively biased sample of patients with HCV infection, as these subjects all had genotype 1 and all were sufficiently motivated to undergo a rigorous, prolonged medical therapy with notable adverse side effects. Another caveat to consider is that the improvements in fatigue scores were seen predominantly among patients who had moderate or severe levels of fatigue before treatment, and there was little or no improvement in patients who reported minimal fatigue initially. Such findings suggest that there is little room for improvement in fatigue among those with lower levels at baseline, or that the VAS is not sensitive enough to detect minor improvements.
In conclusion, use of a simple fatigue VAS demonstrated that at least half of patients with chronic hepatitis C who participated in a clinical trial had complaints of fatigue at baseline, however, fatigue significantly improved in those who achieved viral eradication. Further analyses of the quality of fatigue in chronic liver disease, as well as the biologic and psychosocial pathways associated with this subjective symptom are needed to improve the management of chronic liver disease and assessment of the benefits of antiviral therapy, whether curative or ameliorative in nature.
Supplementary Material
Supplemental Figure 1 Severity of fatigue in male versus female patients, at baseline, on treatment and follow-up
Severity of fatigue of male (blue line) versus female (red line) patients. ◻ All patients on treatment; ▵ Responder, ◯ Non-responders. Solid line: on treatment; and dashed line: offtreatment follow-up weeks.
Supplemental-Figure 2 Severity of fatigue in African American versus Caucasian patients
Severity of fatigue of African American (AA, blue line) versus Caucasian (CA, red line) patients. ◻ All patients on treatment; ▵ Responder, ◯ Non-responders. Solid line: on treatment; and dashed line: off-treatment follow-up weeks.
Supplemental-Figure 3 Sample of Virahep-C study questionnaire to measure fatigue VAS
Sample of the questionnaire presented to the patients for recording fatigue severity on a visual analogue scale (VAS, question 3) on the Virahep-C study at different time-points.
Acknowledgments
We thank all individuals who contributed to the Virahep-C study: from the Beth Israel Deaconess Medical Center, Boston, MA: Nezam Afdhal, MD (principal investigator), Tiffany Geahigan, PA-C, MS (research coordinator); from the New York-Presbyterian Medical Center, New York, NY: Robert S. Brown, Jr., MD, MPH (principal investigator), Lorna Dove, MD, MPH (co-investigator), Shana Stovel, MPH (study coordinator), Maria Martin (study coordinator); from the University of California, San Francisco, San Francisco, CA: Norah Terrault, MD, MPH (principal investigator), Stephanie Straley, PA-C, Eliana Agudelo, PA-C, Melissa Hinds, BA (clinical research coordinator), Jake Heberlein (clinical research coordinator); from Rush University, Chicago, IL: Thelma E. Wiley, MD (principal investigator), Monique Williams, RN (study coordinator); from the University of Maryland, Baltimore, MD: Charles D. Howell, MD (principal investigator), Kelly Gibson (project coordinator), Karen Callison, RN (study coordinator), Jane Lewis, RN (study coordinator); from the University of Miami, Miami, FL: Lennox J. Jeffers, MD (principal investigator), Shvawn McPherson Baker, PharmD (co-investigator), Maria DeMedina, MSPH (project manager), Carol Hermitt, MD (project coordinator); from the University of Michigan, Ann Arbor, MI: Hari S. Conjeevaram, MD, MS (principal investigator), Robert J. Fontana, MD (co-investigator), Donna Harsh, MS (study coordinator); from the University of North Carolina at Chapel Hill, Chapel Hill, NC: Michael W. Fried, MD (principal investigator (K24 DK066144)), Scott R. Smith, PhD (co-investigator), Dickens Theodore, MD, MPH(co-investigator), Steven Zacks, MD, MPH, FRCPC (co-investigator), Roshan Shrestha, MD (co-investigator), Karen Dougherty, NP (co-investigator), Paris Davis (study coordinator), Shirley Brown (study coordinator); from St Louis University, St Louis, MO: John E. Tavis, PhD (principal investigator), Adrian Di Bisceglie, MD (co-investigator), Ermei Yao, PhD (co-investigator), Maureen Donlin, PhD (co-investigator), Nathan Cannon, BS (graduate student), Ping Wang, BS (lab technician); from Cedars- Sinai Medical Center, Los Angeles, CA: Huiying Yang, MD, PhD (principal investigator), George Tang, PhD (project scientist), Dai Wang, PhD (project scientist); from the University of Colorado Health Sciences Center, Denver, CO: Hugo R. Rosen, MD (principal investigator), James R. Burton, MD (co-investigator), Jared Klarquist (lab technician); from Veteran's Administration, Portland, OR: Scott Weston (lab technician); from Indiana University, Bloomington, IN: Milton W. Taylor, PhD (principal investigator), Corneliu Sanda, MD (post-doctoral associate), Takuma Tsukahara, MS (statistician), Mary Ferris (lab assistant); from the Data Coordinating Center, Graduate School of Public Health at the University of Pittsburgh, Pittsburgh, PA: Steven H. Belle, PhD (principal investigator), Geoffrey Block, MD (co-investigator), Jennifer Cline, BS (data manager), KyungAh Im, MS (statistician), Stephanie Kelley, MS (data manager), Laurie Koozer, BA (project coordinator), Sharon Lawlor, MBA (data coordinator), Stephen B. Thomas, PhD (co-investigator), Yuling Wei, MS (project coordinator), Leland J. Yee, PhD (consultant), Song Zhang, MS, MD (statistician); from the National Institute of Diabetes and Digestive and Kidney Diseases: Patricia Robuck, PhD, MPH (project scientist), James Everhart, MD, MPH (scientific adviser), Edward Doo, MD (scientific adviser), T. Jake Liang, MD (scientific adviser), Leonard B. Seeff, MD (scientific adviser); from the National Cancer Institute: David E. Kleiner, MD, PhD (central pathologist).
Source of Funding: The Virahep-C Study was funded as a cooperative agreement by NIDDK. Peginterferon, ribavirin, HCV RNA detection assays and funds for ancillary studies were provided by Roche Pharmaceutics through a Cooperative Research and Development Agreement (CRADA) between Roche and NIDDK. [See acknowledgements] NCT Trial Number: NCT00038974. Further support for this study was from the Intramural Program of NIDDK and a K23 award (Evon: K23-DK089004)
Abbreviations Used
- HCV
Hepatitis C virus
- AA
African American
- CA
Caucasian American
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- SVR
sustained virological response
- NR
non-response
- VAS
visual analogue scale
- HRQoL
health related quality of life.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: D.M.E. had grant funding from Roche/Genentech and served as advisor for Vertex in the last 12 months. Other authors have no conflicts of interest to report.
References
- [1].Foster GR, Goldin RD, Thomas HC. Chronic hepatitis C virus infection causes a significant reduction in quality of life in the absence of cirrhosis. Hepatology. 1998;27:209–212. doi: 10.1002/hep.510270132. [DOI] [PubMed] [Google Scholar]
- [2].Poynard T, Cacoub P, Ratziu V, Myers RP, Dezailles MH, Mercadier A, et al. Fatigue in patients with chronic hepatitis C. J Viral Hepat. 2002;9:295–303. doi: 10.1046/j.1365-2893.2002.00364.x. [DOI] [PubMed] [Google Scholar]
- [3].Glacken M, Coates V, Kernohan G, Hegarty J. The experience of fatigue for people living with hepatitis C. J Clin Nurs. 2003;12:244–252. doi: 10.1046/j.1365-2702.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- [4].Kallman J, O'Neil MM, Larive B, Boparai N, Calabrese L, Younossi ZM. Fatigue and health-related quality of life (HRQL) in chronic hepatitis C virus infection. Dig Dis Sci. 2007;52:2531–2539. doi: 10.1007/s10620-006-9708-x. [DOI] [PubMed] [Google Scholar]
- [5].Karaivazoglou K, Iconomou G, Triantos C, Hyphantis T, Thomopoulos K, Lagadinou M, et al. Fatigue and depressive symptoms associated with chronic viral hepatitis patients. health-related quality of life (HRQOL) Ann Hepatol. 2010;9:419–427. [PubMed] [Google Scholar]
- [6].Tillmann HL, Wiese M, Braun Y, Wiegand J, Tenckhoff S, Mossner J, et al. Quality of life in patients with various liver diseases: patients with HCV show greater mental impairment, while patients with PBC have greater physical impairment. J Viral Hepat. 2011;18:252–261. doi: 10.1111/j.1365-2893.2010.01292.x. [DOI] [PubMed] [Google Scholar]
- [7].Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237–244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- [8].Goh J, Coughlan B, Quinn J, O'Keane JC, Crowe J. Fatigue does not correlate with the degree of hepatitis or the presence of autoimmune disorders in chronic hepatitis C infection. Eur J Gastroenterol Hepatol. 1999;11:833–838. doi: 10.1097/00042737-199908000-00004. [DOI] [PubMed] [Google Scholar]
- [9].Hassoun Z, Willems B, Deslauriers J, Nguyen BN, Huet PM. Assessment of fatigue in patients with chronic hepatitis C using the Fatigue Impact Scale. Dig Dis Sci. 2002;47:2674–2681. doi: 10.1023/a:1021040702370. [DOI] [PubMed] [Google Scholar]
- [10].Kleinman L, Zodet MW, Hakim Z, Aledort J, Barker C, Chan K, et al. Psychometric evaluation of the fatigue severity scale for use in chronic hepatitis C. Qual Life Res. 2000;9:499–508. doi: 10.1023/a:1008960710415. [DOI] [PubMed] [Google Scholar]
- [11].McDonald J, Jayasuriya J, Bindley P, Gonsalvez C, Gluseska S. Fatigue and psychological disorders in chronic hepatitis C. J Gastroenterol Hepatol. 2002;17:171–176. doi: 10.1046/j.1440-1746.2002.02669.x. [DOI] [PubMed] [Google Scholar]
- [12].Wessely S, Pariante C. Fatigue, depression and chronic hepatitis C infection. Psychol Med. 2002;32:1–10. doi: 10.1017/s0033291701004615. [DOI] [PubMed] [Google Scholar]
- [13].Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405–2416. doi: 10.1056/NEJMoa1012912. [DOI] [PubMed] [Google Scholar]
- [15].Poordad F, McCone J, Jr., Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- [17].Van Vlierberghe H, Adler M, Bastens B, Colle I, Delwaide J, Henrion J, et al. Effectiveness and tolerability of pegylated interferon alfa-2b in combination with ribavirin for treatment of chronic hepatitis C: the PegIntrust study. Acta Gastroenterol Belg. 2010;73:5–11. [PubMed] [Google Scholar]
- [18].Hassanein T, Cooksley G, Sulkowski M, Smith C, Marinos G, Lai MY, et al. The impact of peginterferon alfa-2a plus ribavirin combination therapy on health-related quality of life in chronic hepatitis C. J Hepatol. 2004;40:675–681. doi: 10.1016/j.jhep.2003.12.014. [DOI] [PubMed] [Google Scholar]
- [19].Bonkovsky HL, Snow KK, Malet PF, Back-Madruga C, Fontana RJ, Sterling RK, et al. Health-related quality of life in patients with chronic hepatitis C and advanced fibrosis. J Hepatol. 2007;46:420–431. doi: 10.1016/j.jhep.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McHutchison JG, Ware JE, Jr., Bayliss MS, Pianko S, Albrecht JK, Cort S, et al. The effects of interferon alpha-2b in combination with ribavirin on health related quality of life and work productivity. J Hepatol. 2001;34:140–147. doi: 10.1016/s0168-8278(00)00026-x. [DOI] [PubMed] [Google Scholar]
- [21].Conjeevaram HS, Fried MW, Jeffers LJ, Terrault NA, Wiley-Lucas TE, Afdhal N, et al. Peginterferon and ribavirin treatment in African American and Caucasian American patients with hepatitis C genotype 1. Gastroenterology. 2006;131:470–477. doi: 10.1053/j.gastro.2006.06.008. [DOI] [PubMed] [Google Scholar]
- [22].Hoofnagle JH, Wahed AS, Brown RS, Jr., Howell CD, Belle SH. Early changes in hepatitis C virus (HCV) levels in response to peginterferon and ribavirin treatment in patients with chronic HCV genotype 1 infection. J Infect Dis. 2009;199:1112–1120. doi: 10.1086/597384. [DOI] [PubMed] [Google Scholar]
- [23].Howell CD, Dowling TC, Paul M, Wahed AS, Terrault NA, Taylor M, et al. Peginterferon pharmacokinetics in African American and Caucasian American patients with hepatitis C virus genotype 1 infection. Clin Gastroenterol Hepatol. 2008;6:575–583. doi: 10.1016/j.cgh.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Terrault NA, Im K, Boylan R, Bacchetti P, Kleiner DE, Fontana RJ, et al. Fibrosis progression in African Americans and Caucasian Americans with chronic hepatitis C. Clin Gastroenterol Hepatol. 2008;6:1403–1411. doi: 10.1016/j.cgh.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Research in nursing & health. 1990;13:227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- [26].Gift AG. Visual analogue scales: measurement of subjective phenomena. Nurs Res. 1989;38:286–288. [PubMed] [Google Scholar]
- [27].Hamer C. The impact of combination therapy with peginterferon alpha-2a and ribavirin on the energy intake and body weight of adult hepatitis C patients. J Hum Nutr Diet. 2008;21:486–493. doi: 10.1111/j.1365-277X.2008.00882.x. [DOI] [PubMed] [Google Scholar]
- [28].Kos D, Nagels G, D'Hooghe MB, Duportail M, Kerckhofs E. A rapid screening tool for fatigue impact in multiple sclerosis. BMC Neurol. 2006;6:27. doi: 10.1186/1471-2377-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Norheim KB, Harboe E, Goransson LG, Omdal R. Interleukin-1 inhibition and fatigue in primary Sjogren's syndrome--a double blind, randomised clinical trial. PLoS One. 2012;7:e30123. doi: 10.1371/journal.pone.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Spath M, Welzel D, Farber L. Treatment of chronic fatigue syndrome with 5-HT3 receptor antagonists--preliminary results. Scand J Rheumatol. 2000;113(Suppl):72–77. [PubMed] [Google Scholar]
- [31].ter Borg PC, van Os E, van den Broek WW, Hansen BE, van Buuren HR. Fluvoxamine for fatigue in primary biliary cirrhosis and primary sclerosing cholangitis: a randomised controlled trial [ISRCTN88246634] BMC Gastroenterol. 2004;4:13. doi: 10.1186/1471-230X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kleinman L, Zodet MW, Hakim Z, Aledort J, Barker C, Chan K, et al. Psychometric evaluation of the fatigue severity scale for use in chronic hepatitis C. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2000;9:499–508. doi: 10.1023/a:1008960710415. [DOI] [PubMed] [Google Scholar]
- [33].Evon DM, Ramcharran D, Belle SH, Terrault NA, Fontana RJ, Fried MW. Prospective analysis of depression during peginterferon and ribavirin therapy of chronic hepatitis C: results of the Virahep-C study. Am J Gastroenterol. 2009;104:2949–2958. doi: 10.1038/ajg.2009.528. [DOI] [PubMed] [Google Scholar]
- [34].Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr., et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- [35].Lang CA, Conrad S, Garrett L, Battistutta D, Cooksley WG, Dunne MP, et al. Symptom prevalence and clustering of symptoms in people living with chronic hepatitis C infection. J Pain Symptom Manage. 2006;31:335–344. doi: 10.1016/j.jpainsymman.2005.08.016. [DOI] [PubMed] [Google Scholar]
- [36].Bajaj JS, Thacker LR, Wade JB, Sanyal AJ, Heuman DM, Sterling RK, et al. PROMIS computerised adaptive tests are dynamic instruments to measure health-related quality of life in patients with cirrhosis. Alimentary pharmacology & therapeutics. 2011;34:1123–1132. doi: 10.1111/j.1365-2036.2011.04842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Antonelli A, Ferri C, Ferrari SM, Marchi S, De Bortoli N, Sansonno D, et al. N-terminal pro-brain natriuretic peptide and tumor necrosis factor-alpha both are increased in patients with Hepatitis C. J Interferon Cytokine Res. 2010;30:359–363. doi: 10.1089/jir.2009.0059. [DOI] [PubMed] [Google Scholar]
- [38].Felger JC, Cole SW, Pace TW, Hu F, Woolwine BJ, Doho GH, et al. Molecular signatures of peripheral blood mononuclear cells during chronic interferon-alpha treatment: relationship with depression and fatigue. Psychol Med. 2011:1–13. doi: 10.1017/S0033291711002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gershon AS, Margulies M, Gorczynski RM, Heathcote EJ. Serum cytokine values and fatigue in chronic hepatitis C infection. J Viral Hepat. 2000;7:397–402. doi: 10.1046/j.1365-2893.2000.00249.x. [DOI] [PubMed] [Google Scholar]
- [40].Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alphainduced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Broderick G, Fuite J, Kreitz A, Vernon SD, Klimas N, Fletcher MA. A formal analysis of cytokine networks in chronic fatigue syndrome. Brain Behav Immun. 2010;24:1209–1217. doi: 10.1016/j.bbi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 Severity of fatigue in male versus female patients, at baseline, on treatment and follow-up
Severity of fatigue of male (blue line) versus female (red line) patients. ◻ All patients on treatment; ▵ Responder, ◯ Non-responders. Solid line: on treatment; and dashed line: offtreatment follow-up weeks.
Supplemental-Figure 2 Severity of fatigue in African American versus Caucasian patients
Severity of fatigue of African American (AA, blue line) versus Caucasian (CA, red line) patients. ◻ All patients on treatment; ▵ Responder, ◯ Non-responders. Solid line: on treatment; and dashed line: off-treatment follow-up weeks.
Supplemental-Figure 3 Sample of Virahep-C study questionnaire to measure fatigue VAS
Sample of the questionnaire presented to the patients for recording fatigue severity on a visual analogue scale (VAS, question 3) on the Virahep-C study at different time-points.