Abstract
The endogenous cannabinoid anandamide (AEA) exerts the majority of its effects at CB1 and CB2 receptors and is degraded by fatty acid amide hydrolase (FAAH). FAAH KO mice and animals treated with FAAH inhibitors are impaired in their ability to hydrolyze AEA and other non-cannabinoid lipid signaling molecules, such as oleoylethanolamide (OEA) and palmitoylethanolamide (PEA). AEA and these other substrates activate non- cannabinoid receptor systems, including TRPV1 and PPAR-α receptors. In this mini review, we describe the functional consequences of FAAH inhibition on nicotine reward and dependence as well as the underlying endocannabinoid and non-cannabinoid receptor systems mediating these effects. Interestingly, FAAH inhibition seems to mediate nicotine dependence differently in mice and rats. Indeed, pharmacological and genetic FAAH disruption in mice enhances nicotine reward and withdrawal. However, in rats, pharmacological blockade of FAAH significantly inhibits nicotine reward and has no effect in nicotine withdrawal. Studies suggest that non-cannabinoid mechanisms may play a role in these species differences.
Keywords: cannabinoid, endocannabinoid, FAAH, nicotine, reward, withdrawal, URB597
Introduction
Tobacco use is one of the most widely abused drugs and the leading cause of preventable death worldwide. Nicotine, the main psychoactive component in tobacco, plays a major role in the initiation and maintenance of tobacco addiction. This drug induces its effects by acting on neuronal nicotinic acetylcholine receptors (nAChR), which are pentameric ligand gated ion channels. Multiple subtypes consisting of α (α2- α10) and β (β2- β4) subunits exist in the periphery and central nervous system (CNS). These subunits form either heteromeric or homomeric ligand-gated ion-channels of which α4β2* or α7 are the major nAChRs subtypes. In the CNS, nicotinic receptors are mainly distributed on presynaptic neurons where they modulate the release of many neurotransmitters. Nicotine stimulates the mesolimbic dopamine system (Di Chiara and Imperato, 1988), and can induce drug-seeking behavior in animals and humans, as seen with other addictive drugs of abuse (Stolerman and Shoaib, 1991).
Nicotine exerts its rewarding and reinforcing effects by inducing increased rates of dopaminergic neuron firing in the ventral tegmental area (VTA) (Grenhoff et al., 1986), which leads to increases in dopamine release in the nucleus accumbens (NAc) (Pontieri et al., 1996). In contrast, nicotine withdrawal has been shown to decrease dopamine neuronal activity in the VTA (Liu and Jin, 2004) and decrease dopamine output in the NAc (Hildebrand et al., 1998; Rada et al., 2001).
Based on research over the past decade, a variety of nicotine therapies have become available to patients. These therapies include nicotine replacement therapies such as gums and patches, the antidepressant bupropion (Zyban®), and the partial α4β2* nicotinic agonist varenicline (Chantix®) (Cummings and Mahoney, 2006; Jorenby et al., 2006). Unfortunately, the efficacy of these treatments remains quite modest with only 20% of patients remaining abstinent after one year (Prado et al., 2011). Consequently, there remains an essential need for more effective pharmacotherapy than existing treatments.
Nicotine activation of nAChRs causes a cascade of events by releasing several neurotransmitters that trigger various neuronal systems such as GABA and glutamate, which may regulate nicotine addiction (Castane et al., 2005; Wonnacott et al., 1989, 2005). Increased understanding of these neurobiological systems involved in nicotine intake and withdrawal will lead to the development of new targets and therapies. One neurobiological system implicated in the addictive properties of nicotine is the endocannabinoid (EC) system. This system consists of two receptors (CB1 and CB2), which are members of the superfamily of G protein coupled, and exert their actions predominantly through Gi/o proteins (Howlett et al., 2002, 2005), and several endogenous lipid-based signaling molecules (endocannabinoids) that bind to these receptors. CB1 receptors are distributed throughout the peripheral nervous system and CNS and CB2 receptors are mainly associated with immune cells in both the periphery and CNS. In particular, CB2 receptors were found to be present in microglia and brainstem neurons in the CNS (Cabral and Marciano-Cabral, 2005; Van Sickle et al., 2005; Xi et al., 2011). The two best characterized endogenous ligands, anandamide (AEA) and 2-arachindonoylglycerol (2-AG), are formed on-demand from membrane phospholipid precursors and then rapidly eliminated by enzymatic degradation (Clapper et al., 2009). The primary enzyme responsible for AEA degradation is fatty acid amid hydrolase (FAAH). The enzymatic degradation of 2-AG is primarily due to the activity of monoacylglycerol lipase (MAGL). This mini review will focus mainly on the role of FAAH blockade in nicotine intake and withdrawal. AEA is derived from multiple enzymatic pathways (Simon and Cravatt, 2006; Liu et. al., 2008). After release, AEA is rapidly taken up intracellularly and broken down into arachidonic acid and ethanolamine by FAAH (Devane et al., 1992; Cravatt et al., 1996; Rodriguez de Fonseca et al., 2005). AEA is a partial agonist at both CB1 and CB2 and also binds to TRPV1 receptors (Ahern, 2003). FAAH also degrades several other fatty acid amides with known physiological functions, such as oleoylethanolamide (OEA) and palmitoylethanolamide (PEA). These fatty acid amides, unlike AEA, are devoid of action at cannabinoid receptors (Fu et al., 2003), but activate peroxisome proliferator-activated receptor (PPAR-α) nuclear receptor. The PPAR receptor system consists of three subtypes (α, β, and ), which play important roles in lipid metabolism, insulin sensitivity, glucose homeostasis and inflammation (Berger and Moller, 2002). Thus, drugs that selectively inhibit FAAH will not only increase endogenous levels of AEA but also increase endogenous levels of OEA, PEA, and other lipid signaling molecules (Fegley et al., 2005).
Genetically engineered FAAH KO mice are severely impaired in their ability to degrade AEA and exhibit 10- to 15-fold increases in brain AEA levels. (Cravatt et al, 2001). The first selective FAAH inhibitor developed, URB597, produces a 3 to 5 fold increase in brain AEA levels, with enzyme inhibition persisting for approximately 12 hours (Fegley et al., 2005; Rodriguez de Fonseca et al., 2005; Piomelli et al., 2006; Zhang et al., 2007). Other FAAH inhibitors have been developed that are more selective and longer lasting than URB597, such as PF-3845 and PF-04457845, which elevate AEA levels 10–15 fold, and continues to inhibit FAAH for approximately 36 hours (Ahn et al., 2009, 2011). Genetic or pharmacological blockade of FAAH does not produce the typical cannabinoid tetrad effects that consist of hypothermia, catalepsy, antinociception, and locomotor inhibition (Ahn et al., 2008). Although exogenous administration of AEA to wild type mice does not produce cannabimimetic effects, administration to FAAH- impaired mice produces CB1 receptor-mediated tetrad behavioral effects (Cravatt et al., 2001; Fegley et al., 2005).
II. Evidence that the EC system is involved in nicotine dependence
Converging evidence indicates that the EC and nicotine systems interact with each other. Indeed, nAChRs and CB1 receptors overlap in many brain regions mediating the addictive properties of various drugs of abuse, such as the hippocampus, amygdala, and the mesolimbic dopamine system (Herkenham et al., 1990; Tsou et al., 1998; Matsuda et al., 2000; Picciotto et al., 2000). Furthermore, combination of nicotine and delta-9-tetrahydocannabinol (THC), the primary active constituent of marijuana, significantly enhanced nicotine reward, as measured in the conditioned-place preference (CPP) test (Valjent et al.,2002). Furthermore, WIN 55,212-2, a synthetic full CB1 receptor agonist, increased motivational for nicotine and nicotine seeking in a progressive-ratio (PR) i.v. nicotine self-administration paradigm (Gamaleddin et al., 2012). Alternatively, rimonabant, a CB1 receptor antagonist, decreases nicotine i.v. self-administration and CPP in rodents, a phenotype present in CB1 KO mice (LeFoll and Goldberg, 2004; Castane et al., 2005; Cohen et. al., 2005; Merritt et. al., 2008). Consistent with these findings, rimonabant blocked nicotine-induced dopamine release in the NAc (Cohen et al., 2002).
The EC system also plays a modulatory role in nicotine withdrawal models. THC significantly decreased the intensity of somatic withdrawal signs. (Balerio et al., 2004). However, precipitated somatic withdrawal signs in nicotine-dependent mice were not affected by moderate doses of rimonabant or deletion of the CB1 KO receptor (Castane et al., 2002, 2005; Balerio et al., 2004; Merritt et. al., 2008).
Overall, these results suggest the potential usefulness of a CB1 receptor antagonist as an anti-smoking agent. Indeed, initial clinical trials reported that rimonabant, which was originally marketed as Acomplia® for anti-obesity treatment, may increase quit rate of smokers (Cahill and Ussher, 2011). However, due to its psychiatric side effects, rimonabant was taken off the European market and was never approved in the United States. Thus, the strategy of directly inhibiting CB1 receptors as a treatment for nicotine dependence appears unlikely to gain approval.
On the other hand, the results of several studies suggest increased endocannabinoid tone and/or EC levels may play a role in nicotine intake. Interestingly, an initial study reported that endogenous AEA levels were increased in the limbic forebrain and brainstem, but were decreased in the hippocampus, striatum, and cerebral cortex of rats treated with nicotine for seven days. However, CB1 receptor mRNA and CB1 receptor binding were not altered by chronic nicotine exposure in the same animals (Gonzalez et al., 2002). While these results suggest that AEA levels are altered by chronic nicotine exposure, an inherent limitation of measuring total tissue AEA levels is that it does not provide insight into the amount of this EC signaling at the receptor. Using a different approach such as in vivo microdialysis in an awake animal would quantify the amount of EC release in the interstitial space in relevant brain areas while being tested in nicotine behavioral paradigms (Buczynski and Parsons, 2010).
Recent progress in the EC field has demonstrated that elevating endogenous cannabinoids by inhibiting their hydrolytic enzymes might have therapeutic utility, without the undesirable effects of direct CB1 agonists, such as THC (Solinas et al., 2007; Ahn et al., 2008, 2009; Justinova et al., 2008).
III. Role of FAAH blockade in Nicotine reward
The results of studies employing cannabinoid receptor agonists and antagonists suggest that CB1 receptor activation increases nicotine’s rewarding effects, while decreasing CB1 function reduces the reinforcing effects of nicotine. Based on these observations, increasing AEA levels would be expected to enhance nicotine’s reinforcing effects. Consistent with this prediction, our group found that genetic deletion or pharmacological inhibition of FAAH enhanced nicotine CPP (Merritt et al., 2008). Specifically, a subthreshold dose of nicotine (0.1 mg/kg), for producing CPP in wild-type mice, significantly increased CPP in FAAH KO mice that were mediated by CB1 receptors. Higher doses of nicotine produced a similar magnitude of nicotine CPP in FAAH KO and wild type mice. Complementary pharmacological blockade of FAAH produced a similar pattern of results. Specifically, URB597 enhanced the rewarding properties of low dose nicotine (0.1 mg/kg) in the CPP test. This enhancement of low dose nicotine in FAAH-compromised mice is in agreement with previous studies reporting that co-administration of subthreshold doses of nicotine and THC enhanced nicotine CPP (Valigent et al., 2002). These results are also in line with recent human studies that reported that a natural single nucleotide polymorphism (SNP) (rs324420) in the human FAAH gene, which shows reduced enzyme activity, is strongly associated with both street drug use and problem drug/alcohol use (Chiang et al., 2004).
In marked contrast to the study of Merritt et al., (2008), other studies reported that URB597 prevented the development and reinstatement of nicotine-induced CPP and blocked nicotine i.v. self-administration and reinstatement in rats (Scherma et al., 2008). URB597 also blocked nicotine-induced dopamine release in the shell of the nucleus accumbens (ShNAc) and suppressed nicotine-induced excitation of dopamine cells in the VTA of rats (Melis et al., 2008). However, URB597 failed to reduce break point of nicotine self-administration in a progressive-ratio schedule (Forget et al., 2009). Of importance, the anti-nicotine reward effect of URB597 was mimicked by other FAAH substrates, OEA and PEA, but not by methanadamide (a stable analog of AEA). Both OEA and PEA are agonists that activate PPAR-α receptors. The effects of URB597 on dopamine cells required both CB1 receptors and PPAR-α receptors (Luchicchi et al., 2010). More recently, PPAR-α agonists WY14643 and methyl oleoylethanolamide (methOEA; a long-lasting form of OEA) were reported to decrease nicotine i.v. self-administration and nicotine-induced reinstatement in rats and monkeys (Mascia et al., 2011). These agonists also decreased nicotine-induced excitation of dopamine neurons in the VTA and nicotine-induced elevations of dopamine levels in the ShNAc of rats. (Mascia et al.,2011). Importantly, PPAR-α receptor antagonist blocked the effects of PPAR-α agonists on nicotine’s actions.
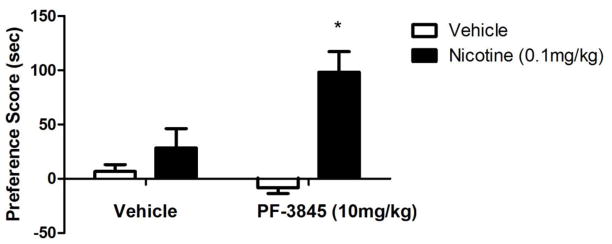
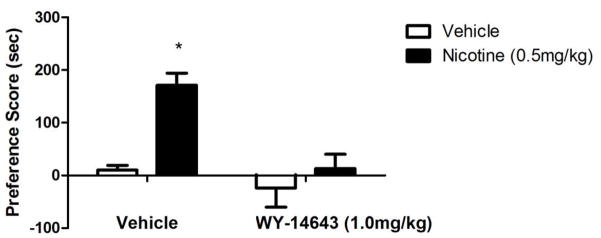
Similar to the results of URB597, VDM11 and AM404, inhibitors of the putative AEA transporter, was able to modulate nicotine's rewarding and reinforcing effects in rats. Indeed, VDM11 attenuated nicotine reinstatement induced by nicotine-associated cues and nicotine priming (Gamaleddin et al., 2011). In addition, AM404 blocked the development of nicotine CPP as well as the reinstatement of nicotine CPP. It also attenuated nicotine-induced release of dopamine in the ShNAc (Scherma et al., 2011). Several issues need to be considered in order to reconcile between the studies finding that FAAH inhibition or deletion augments the rewarding effects of nicotine in CPP (Merritt et al., 2008) and the reduction of nicotine reward by URB597 (Scherma et al.,, 2008). The first consideration is potential species differences to the effects of URB597. Merritt et al.,, (2008) employed mice, while Scherma et al., (2008) and Melis et al., (2008) used rats. However, the report of Fegley et al., (2004) does not suggest significant differences in URB597-induced increase in AEA brain levels between mice and rats. Furthermore, our preliminary studies show that an increase in nicotine reward in the CPP is observed with other FAAH inhibitors. Indeed, we assessed the effects of PF-3845, a highly selective FAAH inhibitor and structurally different from URB597 (Ahn et al., 2009), on nicotine reward in mice. Similar to URB597, PF-3845 (10mg/kg i.p) significantly enhanced a low dose of nicotine in the CPP test (Figure 1). Secondly, assessment of the full dose-response relationship of FAAH inhibitors in both rat and mouse nicotine paradigms might help reconcile the results. The effects of URB597 on nicotine reward in the mouse CPP test were indeed lost at doses higher than 3 mg/kg (Merritt et al., 2008). Third, several observations indicate that different neurochemical systems mediate the disparate effects of URB597 on nicotine reward between rats and mice. In rats, PPAR-α receptors appear to play a major role. In examining the study of Fegley et al., (2004), URB597 (after 2 hr pretreatment of 0.3 mg/kg) induced an approximately 2-fold increase in OEA and PEA brain levels in mice compared to 4 to 5-fold in rats. PPAR-α receptor antagonists block URB597-induced reduction in nicotine‘s effects on dopaminergic neurons (Luchicchi et al., 2010). Also, PPAR-α receptor agonists have a similar action to URB597 on nicotine’s effects. In support of this possibility, our preliminary data show that PPAR-α receptor agonist, WY14643, blocked nicotine’s rewarding effects in the CPP test in mice (Figure 2). Furthermore, it is also conceivable that AEA is modulating nicotinic behaviors by acting on non-CB targets such as TRPV1 and nicotine receptors. Indeed, studies have indicated that AEA can directly inhibit the function of expressed (Spivak et al., 2006) and native (Butt et al., 2008) α4β2* nAChRs in a CB1 receptor independent manner. Thus, the role of EC modulation of nicotine reward and reinforcing in the animals is complicated and influenced by variety of factors such as dose of nicotine, degree of inhibition of FAAH, species differences, and activation of non-cannabinoid receptor systems such as the PPAR-α receptor signaling. Assessment of the potency of PPAR-α receptor agonists in both rat and mouse nicotine paradigms might help reconcile the results. In addition, evaluation of the effects of PPAR-α receptor antagonist on URB597 in the two species will be very helpful in that regard.
Figure 1.
PF-3845 enhances nicotine CPP in mice. Pretreatment with the FAAH enzyme inhibitor PF-3845 (10mg/kg i.p.) or vehicle, given 2 h before an inactive dose of nicotine (0.1mg/kg s.c.) significantly enhances nicotine preference. *p<0.05 vs all treatment groups. Data are expressed as means ± SEM of 6–8mice. See Merritt et al. (2008) for nicotine CPP methods.
Figure 2.
WY14643 blocks nicotine CPP in mice. Pretreatment with the PPAR-α agonists WY14643 (1mg/kg i.p.) or vehicle, given 15 min before an active dose of nicotine (0.5mg/kg s.c.) significantly blocks nicotine preference. *p<0.05 vs all treatment groups. Data are expressed as means ± SEM of 6–8mice. See Merritt et al. (2008) for nicotine CPP methods.
IV. Nicotine withdrawal
Cannabinoids have also been implicated in alleviating the somatic and motivational effects of nicotine withdrawal. THC decreased somatic withdrawal signs and blocked the aversive motivational effects of nicotine (Balerio et al., 2004, 2006). In contrast, moderate doses of rimonabant or deletion of CB1 failed to alter precipitated withdrawal somatic signs in nicotine-dependent mice (Castane et al., 2002, 2005; Balerio et al., 2004; Merritt et. al., 2008). Overall, these studies suggest that CB1 receptor activation could have therapeutic utility in reducing nicotine withdrawal effects. However, FAAH KO mice showed significantly worsened nicotine somatic withdrawal signs and nicotine withdrawal-induced place aversion (as measured in the CPA test) compared to wild-type mice (Merritt et al., 2008). While a high dose of URB597 (10mg/kg) significantly enhanced spontaneous nicotine somatic withdrawal signs lower doses of this FAAH inhibitor were without effect. The effect of FAAH inhibition on nicotine withdrawal was recently reported in rats implanted with transdermal nicotine patches. Following the removal of chronically implanted transdermal nicotine patches, spontaneous nicotine somatic and anxiety-like induced withdrawal signs were measured at 16 and 36 hours, respectively (Cippitelli et al., 2011). While URB597 did not alter nicotine somatic withdrawal signs at 16 hours, it blocked nicotine withdrawal-induced anxiety-like behaviors as assessed in the elevated plus-maze and the shock-probe defensive burying tests at 34 hours (Cippitelli et al, 2011). The authors reported that AEA levels were significantly increased in the amygdala and hypothalamus at 34 hours. However, AEA levels in the hippocampus were significantly decreased at both 16 and 34 hours after patch removal. Importantly, there were no changes in the nucleus accumbens and caudate-putamen (Cippitelli et al., 2011). These studies indicate that FAAH has differential role in the regulation of nicotine somatic and affective signs in rats. Furthermore, FAAH inhibition seems to differentially impact affective signs of nicotine withdrawal in mice and rats. Although the effects of URB597 on nicotine affective signs of withdrawal may be species-dependent and/or may affect different neural pathways involved in these signs, the assessment of CB1 and PPAR-α receptors mediation of URB-597 was not reported in the mouse and rat studies.
Conclusion
Many studies show that manipulating targets of the EC system can modulate important aspects of nicotine dependence. CB1 receptor activation enhances the rewarding effects of nicotine, while CB1 inhibition attenuates nicotine reward in both rats and mice. However, while FAAH inhibition results in increased brain AEA levels and therefore increased CB1 receptor tone, distinct effects of FAAH inhibition on nicotine reward have been reported in mice and rats. Studies suggest that non-CB mechanisms may play a role in these species differences. A major PPAR-α receptor component appears to mediate the anti-nicotine reward effects of FAAH inhibitors in rats. A few studies suggest that CB1 agonists decrease nicotine somatic withdrawal signs however; CB1 blockade does not seem to have an effect in mice. Furthermore, no differences were reported on the effect of FAAH inhibition in nicotine somatic withdrawal signs in mice and rats. However, differences in affective signs of withdrawal, such as anxiety and aversion, after FAAH blockade seem to be emerging. More generally, the effects of FAAH inhibitors on nicotinic behavioral responses in animals may be influenced by procedural differences, species differences, level of nicotine exposure, and degree of FAAH inhibition. These factors seem to play an important role in understanding FAAH’s physiological function in nicotine reward and withdrawal. Pharmacological inhibition of FAAH was shown to block nicotine self-administration and prevent nicotine-induced reinstatement in rats, suggesting that FAAH is a promising molecular target for tobacco dependence. However, the actions of URB597 in nicotine-induced reward in mice are contrary to this notion. Furthermore, many human studies associated polymorphisms in the FAAH genes with drug-related behaviors, which seem to be in line with the mouse data. Care should be taken when generalizing the results from animal studies to the potential use of FAAH inhibitors as antismoking agents in humans.
Acknowledgments
The authors greatly appreciate the technical assistance of Tie Han.
This research was supported by the National Institutes Institute on Drug Abuse DA- 05274, DA009789, and DA017259.
Footnotes
Conflict of Interest statement
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003 Aug 15;278(33):30429–34. doi: 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, et al. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009;16:411–420. doi: 10.1016/j.chembiol.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Smith SE, Liimatta MB, Beidler D, Sadagopan N, Dudley DT, Young T, Wren P, Zhang Y, Swaney S, Van Becelaere K, Blankman JL, Nomura DK, Bhattachar SN, Stiff C, Nomanbhoy TK, Weerapana E, Johnson DS, Cravatt BF. Mechanistic and pharmacological characterization of PF-04457845: a highly potent and selective fatty acid amide hydrolase inhibitor that reduces inflammatory and noninflammatory pain. J Pharmacol Exp Ther. 2011;338:114–24. doi: 10.1124/jpet.111.180257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JP, Cravatt BF. Mechanism of carbamate inactivation of FAAH: implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chem Biol. 2005 Nov;12(11):1179–87. doi: 10.1016/j.chembiol.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Berrendero F, Murtra P, Maldonado R. Delta9- tetrahydrocannabinol decreases somatic and motivational manifestations of nicotine withdrawal in mice. Eur J Neurosci. 2004;20:2737–2748. doi: 10.1111/j.1460-9568.2004.03714.x. [DOI] [PubMed] [Google Scholar]
- Balerio GN, Aso E, Maldonado R. Role of the cannabinoid system in the effects induced by nicotine on anxiety-like behaviour in mice. Psychopharmacology. 2006;184(3–4):504–513. doi: 10.1007/s00213-005-0251-9. [DOI] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annual Review of Medicine. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Buczynski MW, Parsons LH. Quantification of brain endocannabinoid levels: methods, interpretations and pitfalls. British Journal of Pharmacology. 2010;160 (3):423–442. doi: 10.1111/j.1476-5381.2010.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt C, Alptekin A, Shippenberg T, Oz M. Endogenous cannabinoids anandamide inhibits nicotinic acetylcholine receptor function in mouse thalamic synaptosomes. J Neurochem. 2008;105:1235–1243. doi: 10.1111/j.1471-4159.2008.05225.x. [DOI] [PubMed] [Google Scholar]
- Cabral GA, Marciano Cabral F. Cannabinoid receptors in microglia of the central nervous system: immune functional relevance. Journal of leukocyte biology. 2005;78(6):1192–1197. doi: 10.1189/jlb.0405216. [DOI] [PubMed] [Google Scholar]
- Cahill K, Ussher MH. Cannabinoid type 1 receptor antagonists for smoking cessation. Cochrane database of systematic reviews. 2011;(3):CD005353–CD005353. doi: 10.1002/14651858.CD005353.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castané A, Valjent E, Ledent C, Parmentier M, Maldonado R, Valverde O. Lack of CB1 cannabinoid receptors modifies nicotine behavioural responses, but not nicotine abstinence. Neuropharmacology. 2002;43:857–867. doi: 10.1016/s0028-3908(02)00118-1. [DOI] [PubMed] [Google Scholar]
- Castané A, Berrendero F, Maldonado R. The role of the cannabinoid system in nicotine addiction. Pharmacol Biochem Behav. 2005;81:381–386. doi: 10.1016/j.pbb.2005.01.025. [DOI] [PubMed] [Google Scholar]
- Chiang KP, Gerber AL, Sipe JC, Cravatt BF. Reduced cellular expression and activity of the P129T mutant of human fatty acid amide hydrolase: evidence for a link between defects in the endocannabinoid system and problem drug use. Human molecular genetics. 2004;13(18):2113–2119. doi: 10.1093/hmg/ddh216. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Astarita G, Duranti A, Caprioli G, Ubaldi M, Stopponi S, Kallupi M, Sagratini G, Rodrìguez de Fonseca F, Piomelli D, Ciccocioppo R. Endocannabinoid regulation of acute and protracted nicotine withdrawal: effect of FAAH inhibition. PLoS One. 2011;6(11):e28142. doi: 10.1371/journal.pone.0028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Vacondio F, King AR, Duranti A, Tontini A, Silva C, Sanchini S, Tarzia G, Mor M, Piomelli D. A second generation of carbamate-based fatty acid amide hydrolase inhibitors with improved activity in vivo. ChemMedChem. 2009 Sep;4(9):1505–13. doi: 10.1002/cmdc.200900210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, Steinberg R, Soubrié P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 2002 Sep;13(5–6):451–63. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant(SR141716) Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fattyacid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KM, Mahoney M. Current and emerging treatment approaches for tobacco dependence. Curr Oncol Rep. 2006;8:475–483. doi: 10.1007/s11912-006-0077-6. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USa. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Characterization of the fatty acid amide hyrolase inhibittor cyclohexyl carbamic acid 3'-carbamoyl-biphenyl-3-yl ester (URB597): Effects on anandamide and oleoylethanolamide deactivation. J Pharmacol Exp Ther. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Forget B, Coen KM, Le Foll B. Inhibition of fatty acid amide hydrolase reduces reinstatement of nicotine seeking but not break point for nicotine self-administration--comparison with CB(1) receptor blockade. Psychopharmacology (Berl) 2009;205:613–24. doi: 10.1007/s00213-009-1569-5. [DOI] [PubMed] [Google Scholar]
- Fowler CJ. The cannabinoid system and its pharmacological manipulation--a review, with emphasis upon the uptake and hydrolysis of anandamide. Fundam Clin Pharmacol. 2006;20:549–562. doi: 10.1111/j.1472-8206.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, LoVerme J, Serrano A, Rodriguez de Fonseca F, Rosengarth A, Luecke H, Di Ciacomo B, Tarzia G, Piomelli D. Oleoylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-α. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Guranda M, Goldberg SR, Le Foll B. The selective anandamide transport inhibitor VDM11 attenuates reinstatement of nicotine seeking behaviour, but does not affect nicotine intake. Br J Pharmacol. 2011;164:1652–60. doi: 10.1111/j.1476-5381.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A, Goldberg SR, Le Foll B. Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol. 2012;17:47–61. doi: 10.1111/j.1369-1600.2011.00314.x. [DOI] [PubMed] [Google Scholar]
- Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzlez S, Cascio MG, Fernndez-Ruiz J, Fezza F, Di Marzo V, Ramos JA. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain research. 2002;954(1):73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of midbrain dopamine neurons. Acta Physiol Scand. 1986 Nov;128(3):351–8. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand BE, Nomikos GG, Hertel P, Schilström B, Svensson TH. Reduced dopamine output in the nucleus accumbens but not in the medial prefrontal cortex in rats displaying a mecamylamine-precipitated nicotine withdrawal syndrome. Brain Res. 1998 Jan 1;779(1–2):214–25. doi: 10.1016/s0006-8993(97)01135-9. [DOI] [PubMed] [Google Scholar]
- Howlett AC. The cannabinoid receptors. Prostaglandins & other lipid mediators. 2002;68–69:619–631. doi: 10.1016/s0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Cannabinoid receptor signaling. Handbook of experimental pharmacology. 2005;(168):53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Hays JT, Rigotti NA, Azoulay NA, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation. J Am Med Assoc. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, et al. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Rimonabant, a CB1 antagonist, blocks nicotine conditioned place preferences. Neuroreport. 2004;15:2139–2143. doi: 10.1097/00001756-200409150-00028. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Leung D, Shelton CC, Saghatelian A, Hardouin C, Boger DL, Cravatt BF. Reversible inhibitors of fatty acid amide hydrolase that promote analgesia: evidence for an unprecedented combination of potency and selectivity. J Pharmacol Exp Ther. 2004a;311:441–448. doi: 10.1124/jpet.104.069401. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004b;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Jin WQ. Decrease of ventral tegmental area dopamine neuronal activity in nicotine withdrawal rats. Neuroreport. 2004 Jun 28;15(9):1479–81. doi: 10.1097/01.wnr.0000126218.25235.b6. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquest S, Palmiter RD, Krystal G, Rai R, Mahadevan A, Razdan RK, Kunos G. Multiple pathways for anandamide. Proc Natl Acad Sci USA. 2008;103:13345–13350. [Google Scholar]
- Luchicchi A, Lecca S, Carta S, Pillolla G, Muntoni AL, Yasar S, Goldberg SR, Pistis M. Effects of fatty acid amide hydrolase inhibition on neuronal responses to nicotine, cocaine and morphine in the nucleus accumbens shell and ventral tegmental area: involvement of PPAR-alpha nuclear receptors. Addict Biol. 2010;15(3):277–88. doi: 10.1111/j.1369-1600.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, Pistis M. Endogenous fatty acid ethanolamides suppress nicotine-induced activation of mesolimbic dopamine neurons through nuclear receptors. J Neurosci. 2008 Dec 17;28(51):13985–94. doi: 10.1523/JNEUROSCI.3221-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt LL, Martin BR, Walters C, Lichtman AH, Damaj MI. The endogenous cannabinoid system modulates nicotine reward and dependence. J Pharmacol Exp Ther. 2008;326:483–492. doi: 10.1124/jpet.108.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008 Jan;54(1):141–50. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, King SL, Zachariou V. Nicotinic receptors in the brain. Links between molecular biology and behavior. Neuropsychopharmacology. 2000;22(5):451–465. doi: 10.1016/S0893-133X(99)00146-3. [DOI] [PubMed] [Google Scholar]
- Piomelli D. The molecular logic of endocannabinoid signalling. Nature reviews Neuroscience. 2003;4(11):873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Tarzia G, Duranti A, Tontini A, Mor M, Compton TR, Dasse O, Monaghan EP, Parrott JA, Putman D. Pharmacological profile of the selective FAAH inhibitor KDS-4103; (URB597) CNS Drug Rev. 2006;12:21–38. doi: 10.1111/j.1527-3458.2006.00021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996 Jul 18;382(6588):255–7. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Prado GF, Lombardi EM, Bussacos MA, Arrabal Fernandes FL, Terra-Filho M, Santos UdeP. A real-life study of the effectiveness of different pharmacological approaches to the treatment of smoking cessation: re-discussing the predictors of success. Clinics. 2011;66(1):65–71. doi: 10.1590/S1807-59322011000100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Jensen K, Hoebel BG. Effects of nicotine and mecamylamine-induced withdrawal on extracellular dopamine and acetylcholine in the rat nucleus accumbens. Psychopharmacology (Berl) 2001 Aug;157(1):105–10. doi: 10.1007/s002130100781. [DOI] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Del Arco I, Bermudez-Silva FJ, Bilbao A, Cippitelli A, Navarro M. The endocannabinoid system: physiology and pharmacology. Alcohol. 2005;40:2–14. doi: 10.1093/alcalc/agh110. [DOI] [PubMed] [Google Scholar]
- Scherma M, Justinová Z, Zanettini C, Panlilio LV, Mascia P, Fadda P, Fratta W, Makriyannis A, Vadivel SK, Gamaleddin I, Le Foll B, Goldberg SR. The anandamide transport inhibitor AM404 reduces the rewarding effects of nicotine and nicotine-induced dopamine elevations in the nucleus accumbens shell in rats. Br J Pharmacol. 2011 May 9; doi: 10.1111/j.1476-5381.2011.01467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston J, Thomas EA, Selley DE, Sim Selley LJ, Liu Q, Lichtman AH, Cravatt BF. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nature neuroscience. 2010;13(9):1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi S, Hasanein P. Behavioral effects of fatty acid amide hydrolase inhibition on morphine withdrawal symptoms. Brain Res Bull. 2011 Aug 10;86(1–2):118–22. doi: 10.1016/j.brainresbull.2011.06.019. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006 Sep 8;281(36):26465–72. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- Spivak CE, Lupica CR, Oz M. The endocannabinoid anandamide inhibits the function of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2006;72:1024–1032. doi: 10.1124/mol.107.036939. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Shoaib M. The neurobiology of tobacco addiction. Trends Pharmacol Sci. 1991;12:467–473. doi: 10.1016/0165-6147(91)90638-9. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Saudo-Pea MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, Vadivel SK, Makriyannis A, Goldberg SR. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. The journal of pharmacology and experimental therapeutics. 2007;321(1):370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Sun Yan, Bennett Andy. Cannabinoids: a new group of agonists of PPARs. PPAR Research. 2007;2007:23513–23513. doi: 10.1155/2007/23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson MJ, Caboche J, Maldonado R. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135(2):564–78. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310(5746):329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Irons J, Rapier C, Thorne B, Lunt GG. Presynaptic modulation of transmitter release by nicotinic receptors. Prog Brain Res. 1989a;79:157–63. doi: 10.1016/s0079-6123(08)62475-9. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behavior. Curr Opin Pharmacol. 2005b;5(1):53–9. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Peng X, Li X, Song R, Zhang H, Liu Q, Yang H, Bi G, Li J, Gardner EL. Brain cannabinoid CB2 receptors modulate cocaine. Nature neuroscience. 2011;14(9):1160–1166. doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Saraf A, Kolasa T, Bhatia P, Zheng GZ, Patel M, Lannoye GS, Richardson P, Stewart A, Rogers JC, Brioni JD, Surowy CS. Fatty acid amide hydrolase inhibitors display broad selectivity and inhibit multiple carboxylesterases as off-targets. Neuropharmacology. 2007 Mar;52(4):1095–105. doi: 10.1016/j.neuropharm.2006.11.009. [DOI] [PubMed] [Google Scholar]