Abstract
Event-related potentials (ERPs) may be particularly useful for examining emotional processing across development. Though a number of ERP components are sensitive to emotional content in adults, previous studies have yet to systematically examine the components sensitive to emotion in children. The current study used temporal-spatial principal components analysis (PCA) to identify ERP components in response to complex emotional images in nine-year-old children. Three components were modulated by emotional content and were similar to those previously observed in adults, including: the early posterior negativity, the P300, and a sustained relative positivity similar to the late positive potential (LPP). Compared to those previously observed in adults, the components sensitive to emotion in children were maximal over more occipital regions and the LPP component appeared to be less protracted in time, perhaps indicative of less elaborative processing of emotional stimuli.
Keywords: event-related potentials, emotion, children, early posterior negativity, P300, late positive potential
The last few decades have been marked by growing interest in neural activity involved in emotional processing (LeDoux, 2000). Psychophysiological measures in particular have the potential to identify aspects of emotion that cannot be assessed through observational or subjective measures –and even to identify emotional processes occurring in the absence of emotional experience (Larsen, Berntson, Poehlmann, Ito, & Cacioppo, 2008; Santerre & Allen, 2007). In addition, psychophysiological measures of emotion are particularly useful for developmental studies, given the challenges of obtaining subjective reports from young children. Because they are non-invasive, temporally-sensitive, and reliably measured throughout development, electroencephalogram (EEG) and event-related potential (ERP) measures are ideal for understanding neural processing across typical and atypical development (Banaschewski & Brandeis, 2007). However, little previous work has systematically examined ERP components sensitive to emotion in childhood.
Emotional stimuli, including complex images from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008), words, and faces are commonly used to examine emotional reactivity. Studies with adults indicate that compared to neutral stimuli, emotional stimuli automatically capture attention (Armony & Dolan, 2002; Öhman, Flykt, & Esteves, 2001), are more likely to be recalled (Hamann, Ely, Grafton, & Kilts, 1999; Phelps, LaBar, & Spencer, 1997), and are viewed for longer durations (Lang, Greenwald, Bradley, & Hamm, 1993). In addition, biased attention towards, and processing of, emotional compared to neutral images has been observed across development (Lindstrom et al., 2009; Waters, Lipp, & Spence, 2004).
There is emerging evidence that the allocation of attention to emotion can change rapidly over the course of stimulus presentation and that individual differences may interact with changes over time to determine the dynamic allocation of attention (Hajcak, Weinberg, MacNamara, & Foti, 2011). Because of their excellent temporal sensitivity, ERP measures are particularly useful in examining the temporal dynamics of reactivity to emotional stimuli. Among adults, a number of ERP components are sensitive to emotional content, though different components appear to index distinct processes (Hajcak, et al., 2011; MacNamara, Foti, & Hajcak, 2009; Olofsson, Nordin, Sequeira, & Polich, 2008; Weinberg & Hajcak, 2011). A negativity peaking at 136 ms after stimulus onset over parietal sites (N1) is among the earliest ERP components to be modulated by emotional content of IAPS images (Foti, Hajcak, & Dien, 2009). Following the N1, the early posterior negativity (EPN) appears maximal within 200–300 ms after stimulus onset over occipital sites and is enhanced for emotional images (Foti, et al., 2009; Schupp, Flaisch, Stockburger, & Junghöfer, 2006; Weinberg & Hajcak, 2011). The P300 is maximal over parietal sites approximately 300–400 ms after stimulus onset and is modulated by stimulus salience and relevance (e.g., Pritchard, 1981), as well as emotional content (Keil et al., 2002b).
Lastly, the late positive potential (LPP) is a sustained positivity to emotional pictures over centroparietal sites beginning around 300 ms after stimulus onset and continuing beyond stimulus offset (Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Hajcak & Olvet, 2008). Unlike earlier components, the LPP is thought to reflect more ongoing, elaborative processing of motivationally-salient stimuli (Hajcak et al., 2011; Olofsson et al., 2008; Weinberg, Ferri, & Hajcak, in press). Although there is likely to be some overlap between the P300 and LPP in response to emotional images in adults, evidence from PCA suggests that the later portion of the LPP appears to be a distinct component (Foti, et al., 2009). In addition, an enhanced LPP, rather than earlier components, is uniquely associated with behavioral interference by emotional images, suggesting that the later LPP may index sustained attention (Weinberg & Hajcak, 2011), while earlier components may represent more automatic processing of emotional images (Hajcak, et al., 2011).
Evidence from fMRI studies suggests that there may be important developmental changes in neural processing of emotional information, including transitions from greater processing in subcortical regions to enhanced processing in the prefrontal cortex (PFC; Monk, 2008). Many studies examining developmental changes in neural processing of emotional information have focused on transitions between adolescence and adulthood (e.g., Burnett, Bird, Moll, Frith, & Blakemore, 2008; Lau et al., 2011; Nelson et al., 2003), but there is also evidence of important changes from middle childhood to adolescence (e.g., Yurgelun-Todd & Killgore, 2006). Nonetheless, the development of ERPs sensitive to emotional stimuli in children remains unclear. Several studies of emotional face processing suggest that early components may be observable in young children, though these effects are not always apparent from middle childhood to adolescence. For example, Batty and Taylor (2006) found that emotional faces modulated the early P1 component only for very young children (4–5 year olds), with no emotion effects observable for this early component through later childhood or adolescence. Other studies using emotional faces have found emotional modulation of components peaking between 100 and 400 ms after stimulus onset in very young children (Curtis & Cicchetti, 2011; Dawson, Webb, Carver, Panagiotides, & McPartland, 2004; Nelson & Nugent, 1990), but a source localization study of face processing in 10–16-year-olds failed to find emotional modulation of early ERP components (Wong, Fung, McAlonan, & Chua, 2009). Though few studies have examined ERPs to more complex IAPS images in children, one previous study of five- to eight-year-old children found that unpleasant and pleasant IAPS images did not significantly modulate the EPN (Hajcak & Dennis, 2009). Taken together, these studies suggest that early processing of emotional stimuli may continue to develop from childhood into adolescence and may not be consistently observable across childhood.
With regard to later ERP components, there is evidence that the P300 in children and adolescents is enhanced for emotional compared to neutral facial expressions (Anokhin, Golosheykin, & Heath, 2010), and fearful relative to neutral IAPS images (Leutgeb, Schäfer, Köchel, Scharmüller, & Schienle, 2010). The LPP also appears to be enhanced in response to emotional IAPS images and emotional faces in children as young as five years old, with some important distinctions from the LPP observed in adults (Hajcak & Dennis, 2009; Kujawa, Hajcak, Torpey, Kim, & Klein, 2012). In early childhood, the LPP appears to be less protracted in time and maximal over more occipital sites than observed in adulthood (Kujawa, Hajcak et al., 2012). In addition, there is some evidence that between middle childhood and early adolescence, the scalp distribution of the LPP shifts from more occipital sites to primarily parietal regions (Kujawa, Klein, & Hajcak, 2012). Activation over more occipital sites, as opposed to parietal regions, may indicate that children rely more on visual processing regions, rather than activation of frontoparietal networks observed in adults (Moratti, Saugar, & Strange, 2011).
Previous ERP studies of emotional processing in children have scored components in particular time windows and electrode sites; however, more systematic methods are needed to identify the temporal and spatial dimensions of components sensitive to emotion in childhood. Across developmental studies, scoring and labeling of ERP components are often inconsistent, which makes it difficult to build a cohesive understanding of ERP measures of emotional processing in childhood. In addition, as EEG data obtained from children are likely to include more noise and artifacts than EEG data from adults, it may be particularly difficult to separate systematic effects from unsystematic variance in child data. Temporospatial PCA is a factor-analytic statistical approach that can be used to capture variance across electrode sites and time points to separate latent components. Several studies have used PCA to examine the components sensitive to emotion in adults (e.g., Foti, et al., 2009; Weinberg & Hajcak, 2011) and it has been used previously in children to identify error-related processing (Arbel & Donchin, 2011); however, this approach has yet to be applied to understand ERPs sensitive to emotion in children. Compared to traditional ERP scoring methods (i.e., scoring an ERP at a particular set of electrodes in a particular time window), PCA may be a more reliable method for separating sources of variability into components and differentiating latent components from unsystematic sources of noise.
The current study used a temporospatial PCA approach to identify neural responses to unpleasant, pleasant, and neutral IAPS images in children. Given the exploratory nature of the study, only nine-year-old children were included to control for variability in development.
Methods
Participants
Participants in the current study are part of a larger prospective sample recruited through a commercial mailing list when the children were between three and four years old. Children with no significant medical conditions or developmental disabilities and living with at least one English-speaking biological parent were eligible to participate. A subset of 45 nine-year-old children (40% female) with a mean age of 9.28 years (SD = 0.20) participated in the current study. With regard to racial/ethnic distributions, 93.3% of participants were Caucasian, 2.2% were African-American, and 4.4% were Hispanic/Latino.
Emotional-Interrupt Task
In order to simultaneously measure ERPs and behavioral measures of attention and to monitor task participation, a modified version of the emotional-interrupt paradigm (Mitchell, Richell, Leonard, & Blair, 2006; Weinberg & Hajcak, 2011) was used. The task was administered using Presentation software (Neurobehavioral Systems, Inc.). A total of 60 developmentally appropriate pictures were selected from the IAPS (Lang et al., 2008). Of these, 20 depicted pleasant scenes (e.g., children playing, cute animals, babies), 20 depicted neutral scenes (e.g., people in neutral situations, neutral outdoor scenes, household objects), and 20 depicted unpleasant scenes (e.g., sad or angry people, weapons, aggressive animals)1. Normative valence and arousal ratings of IAPS images were obtained from adults by Lang et al. (2008), and average normative valence and arousal ratings for the images in this paradigm are presented in Table 1. Lang et al. (2008) also collected valence and arousal ratings from 7- to 9-year-old children on a subset of IAPS images. Average normative valence and arousal ratings by children are also presented in Table 1; however, normative child ratings were only available for a small portion of the images included in the current paradigm (5 pleasant, 5 neutral and 2 unpleasant). Each image was randomly presented once in each of two blocks for a total of 120 trials. Each trial began with an 800 ms fixation (+), then an image was presented for 1000 ms followed by a target (< or >) presented for 150 ms and the same picture presented for an additional 400 ms. The target was an arrow pointed to the left or to the right, and participants were required to press either the left or right button on a mouse to indicate the direction of the arrow. The intertrial interval varied randomly between 1500 and 2000 ms.
Table 1.
Mean Normative Valence and Arousal Ratings for Included IAPS Images (from Lang et al., 2008)
| Pleasant (n = 20) | Neutral (n = 20) | Unpleasant(n = 20) | |
|---|---|---|---|
| Mean(SD) Valence (Adults) | 7.51(.51) | 5.27(.35) | 3.09(.76) |
| Mean(SD) Arousal (Adults) | 5.03(.77) | 2.99(.68) | 6.12(.58) |
| Pleasant (n = 6) | Neutral (n = 6) | Unpleasant (n = 2) | |
|
| |||
| Mean(SD) Valence (Children) | 8.55(.13) | 5.91(.20) | 4.01(.14) |
| Mean(SD) Arousal (Children) | 5.81(.40) | 3.02(.19) | 7.41(.42) |
Procedure
After obtaining written informed consent from parents and verbal assent from children, EEG sensors were connected. Children were instructed that pictures would appear on the screen followed by an arrow to which they would respond. They were instructed to press the left mouse button if the arrow was pointed to the left and the right mouse button if pointed to the right. The experimenter pointed out the direction to ensure left-right discrimination. The child then completed 10 practice trials while the experimenter monitored performance. Throughout the task, children were monitored by a video camera.
Electrophysiological data collection and reduction
The continuous EEG was recorded using a 34-channel Biosemi system based on the 10/20 system (32 channel cap with the addition of Iz and FCz). Two electrodes were placed on the left and right mastoids, and the electrooculogram (EOG) generated from eye blinks and movements was recorded from four facial electrodes: two approximately one cm above and below the participant’s left eye, one approximately one cm to the left of the left eye and one approximately one cm to the right of the right eye. The ground electrode during acquisition was formed by the Common Mode Sense active electrode and the Driven Right Leg passive electrode. The data were digitized at 24-bit resolution with a LSB value of 31.25nV and a sampling rate of 1024 Hz, using a low-pass fifth order sinc filter with −3dB cutoff points at 208 Hz. Off-line analysis was performed using Brain Vision Analyzer software (Brain Products). All data were converted to a mastoid reference and band-pass filtered with cutoffs of 0.1 and 30 Hz. The EEG was segmented for each trial, beginning 200 ms before each picture onset and continuing for 1000 ms after onset. The EEG was corrected for eye blinks using the method developed by Gratton, Coles and Donchin (1983). Artifact rejection was completed using semiautomated procedures and the following criteria: a voltage step of more than 50 μV between sample points, a voltage difference of 300 μV within a trial. In addition, data were identified as artifacts if a voltage difference of less than .50 μV within 100 ms intervals was present. Visual inspection was then used to reject trials in which additional artifacts were observed.
ERPs were averaged across condition (unpleasant, pleasant, and neutral) for each participant and were exported for PCA. Only correct trials with responses between 150–2000 ms after target onset were included in the averages. For each ERP average, the average activity in the 200 ms window prior to picture onset served as the baseline. Behavioral analyses were calculated with each participant’s mean reaction time (RT) for correct trials and overall accuracy for each emotion type. Responses with RTs quicker than 150 ms or slower than 2000 ms after target onset were counted as errors. These cut-offs are consistent with those previously used for adults (Mitchell et al., 2006; Weinberg & Hajcak, 2011), with a slightly longer ceiling cut-off to allow children more time to respond.
PCA
Three ERP averages for each subject were entered into the data matrix for the PCA (i.e., pleasant, neutral, and unpleasant). PCA extracts linear combinations of data that distinguish patterns of electrocortical activity across all time points and recording sites. Using the Matlab ERP PCA Toolkit (Version 2; Dien, 2010a), a temporal PCA was performed first in order to capture variance across time and to maximize the initial separation of ERP components (Dien & Frishkoff, 2005). Simulation studies suggest that promax rotations, which do not force orthogonality amongst the components, are most effective for temporal analyses (Dien, 2010b; Dien, Khoe, & Mangun, 2007); therefore promax rotation was used to rotate to simple structure in the temporal domain. The temporal PCA used all time points as variables and considered all 45 subjects, three conditions, and 34 recording sites as observations, thereby yielding linear combinations of time points (referred to as temporal factors) and reducing the 1229 temporal dimensions of the original data set (1024 samples per second multiplied by a total trial-plus-baseline length of 1200 ms).
Following the first rotation, a parallel test (Horn, 1965) was conducted on the resulting Scree plot (Cattell, 1966), in which the Scree of the actual dataset is compared to a Scree plot derived from a fully random dataset. The number of factors retained is thus based on the largest number of factors that account for a greater proportion of variance than the fully random dataset (see Dien, 2010a for more information). Based on this criterion, seven temporal factors were extracted for rotation, and the covariance matrix and Kaiser normalization were used for this PCA (Dien, Beal, & Berg, 2005). Each temporal factor may be considered to be a virtual epoch and can be described both by its factor loading (which describes the time course of that factor) and factor scores (which give that factor’s value for each combination of subject, picture type, and recording site). Spatial information is preserved by temporal PCA; scalp topography can be reconstructed for any time point, subject, and condition by multiplying the corresponding electrode scores by the factor loading and standard deviation (Dien, 1998).
Following the temporal PCA, a spatial PCA was then performed in order to reduce the spatial dimensions of the datasets. Simulation studies suggest that Infomax rotations are most effective for spatial analyses (Dien, 2010b; Dien, et al., 2007); therefore Infomax was used to rotate to independence in the spatial domain. In the spatial PCA, recording sites were used as variables and all subjects, conditions, and temporal factor scores were used as observations; thus a spatial PCA was conducted on each temporal factor retained in the previous step. Based on the results of the parallel test (Horn, 1965), three spatial factors were extracted from each temporal factor for Infomax rotation, yielding a total of 21 temporospatial factor combinations. To directly assess timing and spatial voltage distributions, we then translated the factors back into voltages.
Eight factor combinations accounted for more than 1% of the variance each; the total variance accounted for by the eight factor combinations was 80%. Each factor combination was statistically evaluated using SPSS (Version 18.0) General Linear Model software. In order to evaluate the effect of emotion (i.e., pleasant, neutral, and unpleasant), eight repeated-measures ANOVAs were conducted. Significant main effects of emotion were followed up with three pairwise comparisons to evaluate which emotion conditions differed from one another. Greenhouse-Geisser correction was applied to p values associated with multiple-df, repeated measures comparisons when necessitated by violation of the assumption of sphericity; p-values were adjusted with the Bonferroni correction for multiple comparisons.
Results
PCA
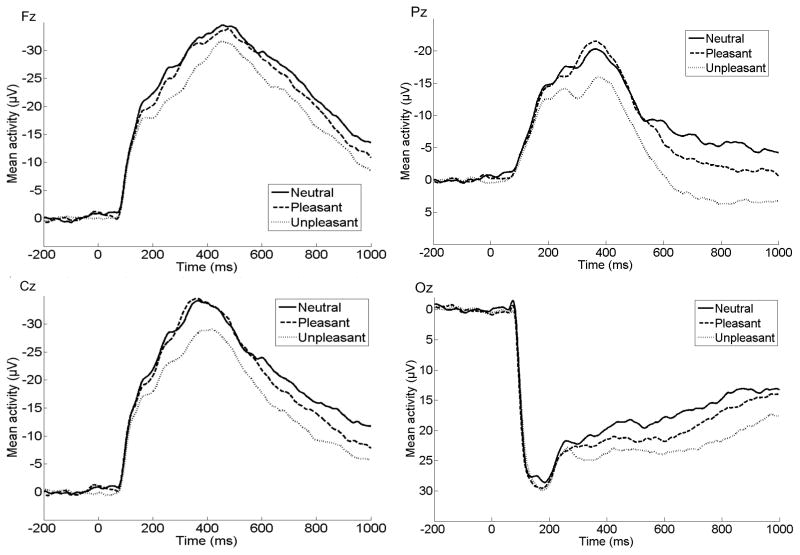
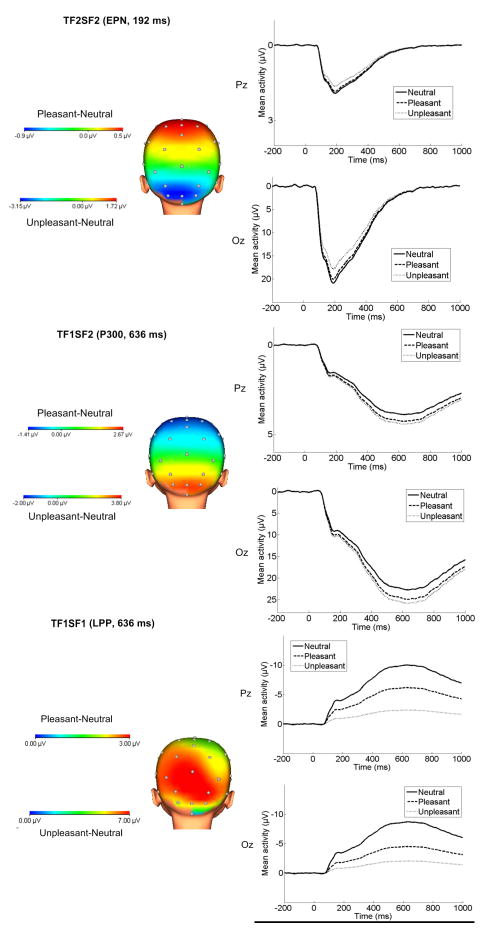
The results of all eight repeated-measures ANOVAs examining the effect of emotion on each ERP component are presented in Table 2. Of the eight factor combinations accounting for more than 1% of variance, four factor combinations were significantly sensitive to picture type using Bonferroni-corrected p values (p < .006). Three of the factor combinations accounted for a large proportion of variance combined (61%). An additional factor combination (TF1/SF3) will not be discussed because it accounted for a relatively small proportion of variance (3.1%) and had a focal, lateralized distribution that did not appear consistent with ERPs observed previously in children or adults. The original ERP waveforms at Fz, Cz, Pz, and Oz prior to PCA are presented in Figure 1, and spatial topographies and waveforms for each of the three factor combinations are presented in Figure 2. For presentation purposes, we have also overlaid the three PCA components for each image type on the original ERP waveforms to demonstrate how the original data can be reconstructed from the three components retained for further analysis. These are displayed in Figure 3.
Table 2.
Description and Analysis of Variance Results for Each Temporospatial Factor Accounting for More than 1% of the Variance
| Temporospatial factor combination | Temporal peak loading (ms) | Spatial distribution of emotional enhancement | Main effect of picture type F (2, 88) | Partial η2 | ε | Unpleasant vs. Neutral t (44) | Pleasant vs. Neutral t(44) | Unpleasant vs. Pleasant t (44) |
|---|---|---|---|---|---|---|---|---|
| TF2SF2/(EPN) | 192 | Occipital | 9.97** | .19 | .95 | −4.22† | −1.11 | −3.53† |
| TF1SF2/(P300) | 636 | Occipital | 6.87** | .14 | .98 | 3.84† | 2.58† | 1.00 |
| TF1SF1/(slow wave) | 636 | Occipital/Parietal | 16.31** | .27 | 1.00 | 5.68† | 2.71† | 3.02† |
| TF1/SF3 | 636 | Left Temporal | 5.64** | .11 | .89 | 3.28† | −1.35 | 1.92 |
| TF2/SF1 | 192 | Occipital/Parietal | 1.30 | .03 | .93 | - | - | - |
| TF2/SF3 | 192 | Posterior | 3.51* | .07 | .98 | - | - | - |
| TF3/SF1 | 529 | Parietal | .296 | .01 | .97 | - | - | - |
| TF4/SF1 | 450 | Parietal | 2.88 | .06 | .96 | - | - | - |
Note: ε indicates Greenhouse-Geisser epsilon value;
indicates p < .05;
indicates p<.006: note that this significance level was used for repeated-measures ANOVAs to control for multiple analyses (.05/8),
indicates p<.017: note that this significance level was used for post-hoc t-tests (.05/3) to control for multiple comparisons
Figure 1.
ERPs (negative up) for neutral, pleasant and unpleasant stimuli at Fz, Pz, Cz, and Oz prior to PCA.
Figure 2.
Scalp distributions depicting the pleasant-neutral and unpleasant-neutral difference and ERPs (negative up) for each of the three components identified through temporospatial PCA.
Figure 3.
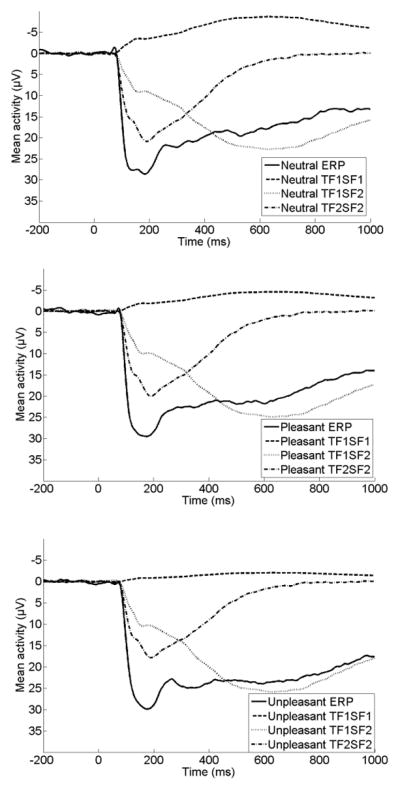
Original ERPs overlaid with the three components identified through temporospatial PCA at Oz for neutral (top), pleasant (middle), and unpleasant (bottom) images.
The first factor combination consisted of a relatively early positivity (Temporal Factor 2/Spatial Factor 2; TF2/SF2), sensitive to emotional images. This factor combination peaks at 192 ms at occipital recording sites (see Figure 2), consistent with previous work on the EPN (Foti, et al., 2009; Keil et al., 2002a; Weinberg & Hajcak, 2010). The three possible pairwise comparisons across picture type were performed, using a significance cutoff of p<.017 for three contrasts (.05/3). This factor combination showed a significantly reduced positivity (i.e., a relative negativity) for unpleasant compared to neutral pictures, though the contrast between pleasant and neutral images did not reach significance. Pleasant and unpleasant pictures also significantly differed from one another, such that unpleasant images were associated with a relative negativity compared to both neutral and pleasant images (Table 2).
Following this was a later relative positivity (Temporal Factor 1/Spatial Factor 2; TF1/SF2), observable over occipital sites. This factor peaks at 636 ms, and appears consistent with previous work on the P300/early portion of the LPP, though peaking later and maximal over more occipital sites than is typically observed in adults (Foti, et al., 2009; Polich, 2007; Weinberg & Hajcak, 2011). The three pairwise comparisons across picture type were again performed, using a significance cutoff of p<.017 for three contrasts (.05/3). This factor combination showed an enhanced positivity for both pleasant and unpleasant compared to neutral pictures, though pleasant and unpleasant images did not differ significantly from one another (Table 2).
Finally, a slower relative positivity was apparent (Temporal Factor 1/Spatial Factor 1; TF1/SF1) peaking at 636 ms, at occipital and parietal sites. The three pairwise comparisons across picture type were again performed, using a significance cutoff of p<.017 for three contrasts (.05/3). This factor combination showed a significantly reduced negativity (i.e., a relative positivity) for both pleasant and unpleasant compared to neutral pictures, as well as a significant difference between pleasant and unpleasant, such that unpleasant images were associated with the greatest relative positivity (Table 2). Although this component peaks around 600 ms, differentiation between emotional stimuli is observable around 200 ms and appears to continue throughout the stimulus presentation time (see Figure 2).
Behavioral Results
Repeated-measures ANOVAs were conducted to examine the effect of emotional stimuli on accuracy and reaction time (Table 3). The effect of image type on accuracy was significant, and paired comparisons indicated that both unpleasant and pleasant trials were associated with poorer accuracy compared to neutral trials. No significant differences in accuracy were observed for pleasant compared to unpleasant trials. The effect of image type on reaction time (RT) was also significant, and paired comparisons indicated that pleasant trials were associated with slower RTs compared to neutral trials. When using a Bonferroni-corrected significance cut-off of p<.017, the difference between unpleasant and neutral RT approached significance. Again, no significant differences in RT were observed when comparing pleasant and unpleasant RT. These results indicate that the presentation of emotional images was associated with more errors and lower accuracy compared to the presentation of neutral images.
Table 3.
Means and Analysis of Variance Results for Behavioral Measures
| Neutral M(SD) | Pleasant M(SD) | Unpleasant M(SD) | Main effect of picture type F (2, 88) | Partial η2 | ε | Unpleasant vs. Neutral t (44) | Pleasant vs. Neutral t(44) | Unpleasant vs. Pleasant t (44) | |
|---|---|---|---|---|---|---|---|---|---|
| Accuracy | 86.89 (8.78) | 81.94 (10.04) | 81.33 (9.84) | 11.60** | .21 | .98 | −4.35† | −3.70† | −.52 |
| Reaction Time | 562.98 (124.76) | 579.50 (146.96) | 580.66 (139.52) | 3.99* | .08 | .96 | 2.30* | 2.55† | .17 |
Note: ε indicates Greenhouse-Geisser epsilon value;
indicates p<.05,
indicates p<.001,
indicates p<.017; note that this significance level was used only for post-hoc t-tests (.05/3) to control for multiple comparisons
Associations between ERP and Behavioral Measures
Bivariate correlations were computed to examine associations between the temporospatial factor combinations and accuracy and RT within each emotional category (Table 4). An enhanced P300 (TF1/SF2) on unpleasant trials was associated with poorer accuracy on unpleasant trials. Though the association between the LPP (TF1/SF1) on unpleasant trials and unpleasant accuracy approached significance (p = .07), the direction indicates that an enhanced LPP is associated with higher accuracy. No other significant associations were found between behavioral and ERP measures.
Table 4.
Bivariate Associations between Behavioral and ERP Measures
| Neutral TF2SF2/EPN | Neutral TF1SF2/P300 | Neutral TF1SF1/LPP | |
|---|---|---|---|
| Neutral Accuracy | .003 | .02 | .08 |
| Neutral RT | .00 | .15 | −.07 |
|
| |||
| Pleasant TF2SF2/EPN | Pleasant TF1SF2/P300 | Pleasant TF1SF1/LPP | |
|
| |||
| Pleasant Accuracy | −.08 | −.12 | −.20 |
| Pleasant RT | −.06 | .20 | .20 |
|
| |||
| Unpleasant TF2SF2/EPN | Unpleasant TF1SF2/P300 | Unpleasant TF1SF1/LPP | |
|
| |||
| Unpleasant Accuracy | −.17 | −.30* | .28 |
| Unpleasant RT | −.03 | .05 | .05 |
p < .05
Discussion
A temporospatial PCA identified three components sensitive to emotion in nine-year-old children. The first component (TF2/SF2) is a relative negativity for unpleasant compared to neutral images maximal around 200 ms after stimulus onset over occipital sites, and is consistent with the EPN observed in previous studies of adults using both a passive viewing (Foti, et al., 2009) and emotional-interrupt paradigm (Weinberg & Hajcak, 2011). The second component (TF1/SF2) is a relative positivity for both unpleasant and pleasant compared to neutral images peaking around 600 ms after stimulus onset and also maximal over occipital sites. This component appears consistent with the P300 previously observed in adult studies, though in adults it tends to peak earlier (around 350 ms after stimulus onset) and is maximal over more parietal sites (Foti, et al., 2009; Weinberg & Hajcak, 2011). The third component (TF1/SF1) is a sustained relative positivity for both unpleasant and pleasant compared to neutral images peaking around 600 ms after stimulus onset and maximal over occipital and parietal sites. This component appears consistent with the LPP identified in adult studies, though the LPP has been shown to peak later in adults, perhaps because in adult populations it is linked to more elaborative processing of emotional content (Foti, et al., 2009; Hajcak, et al., 2011; Olofsson et al., 2008; Weinberg & Hajcak, 2011; Wiens, Sand, Olofsson, 2011).
The three components observed to be sensitive to emotional stimuli in children are in many ways comparable to those previously observed in adults, with a few important distinctions. First, consistent with previous studies (e.g., Batty & Taylor, 2006), very early components, such as the N1, were not sensitive to emotional content in middle childhood, suggesting that there may be delays in the rapid, automatic detection of emotional stimuli. However, the EPN did significantly differentiate emotional stimuli around 200 ms after stimulus onset, indicating that children are able to effectively differentiate emotional stimuli relatively quickly, though at this stage only unpleasant stimuli were differentiated from neutral images.
Although studies in adults using passive viewing and emotional-interrupt paradigms identify centroparietal activation in response to emotional images (Foti, et al., 2009; Weinberg & Hajcak, 2011), both the EPN and P300 in the current study were maximal at occipital sites, with the LPP also marked by enhanced activity over occipital regions. This is consistent with previous studies that find enhanced LPPs over occipital sites in children (Hajcak & Dennis, 2009; Kujawa, Hajcak et al., 2012; Kujawa, Klein et al., 2012) and may indicate that emotional reactivity in children is driven primarily by activation in visual processing areas with less engagement of frontoparietal attention networks linked to the LPP in adults (Moratti et al., 2011). This may also be consistent with neuroimaging evidence that PFC involvement in emotional processing increases across development (Monk, 2008; Yurgelun-Todd & Killgore, 2006). However, given the limited spatial resolution of ERP measures, additional research using combined fMRI and ERP techniques is needed to better understand the structures contributing to the LPP in children.
Although adult studies identify the LPP as a relatively late component associated with ongoing elaboration of emotional stimuli (Foti, et al., 2009; Weinberg & Hajcak, 2011), the slow wave component in the current study, which appeared to be the most consistent with the LPP, peaked earlier. Previous studies suggest the LPP in children may end earlier and be associated with less consistent effects in later time windows (Hajcak & Dennis, 2009; Kujawa, Hajcak et al., 2012). The LPP component identified in the current study may represent a developmental shift in emotional processing, such that processing in middle childhood is marked by sustained attention towards emotional stimuli but later, elaborative processing has yet to develop. In addition, though Weinberg & Hajcak (2011) found that enhanced LPPs were associated with slower reaction times using the same task in adults, few associations were found between behavioral and ERP measures in the current study. This could be attributed to the relatively early peak of the LPP or may indicate that behavioral measures are less consistently related to ERP measures in children.
It is important to note that differences between the stimuli in this study and those typically used with adults may contribute to the lack of a later component in the current study. Recent research suggests that within broad emotional categories, there is variability in response to specific types of stimuli (Briggs & Martin, 2009; Schupp et al., 2004; Weinberg & Hajcak, 2010; Weinberg, Hilgard, Bartholow, & Hajcak, in press). Because of the developmentally-appropriate nature of the stimuli selected for the current study, images that have been shown to be highly motivationally salient for adults (i.e., erotica and mutilation) were not included, and a number of pleasant images portrayed children playing or engaging in recreational activities—a category of pleasant images which tends to elicit a markedly smaller LPP in adults (Briggs & Martin, 2009; Schupp et al., 2004; Weinberg & Hajcak, 2010). Thus, we cannot rule out the possibility that the lack of later ERP components or lack of significant relationships between ERP and behavioral measures could be attributed to the types of stimuli included. Additional research is needed to identify classes of stimuli that most effectively elicit LPPs among children and to directly compare temporal-spatial factors across development using the same set of stimuli.
Lastly, it is important to mention differences in results for pleasant compared to unpleasant images. In the current study, only unpleasant images significantly differentiated the EPN from neutral images. Relatedly, while the P300 in the current study did not differentiate pleasant and unpleasant stimuli, the LPP was significantly larger for unpleasant relative to pleasant images. In adult studies, unpleasant images are sometimes associated with increased electrocortical reactivity relative to pleasant images; however, others have failed to find a negativity bias (e.g., Franken, Muris, Nijs, & van Strien, 2008; Schupp et al., 2006), and there is evidence that pleasant and unpleasant images which are well-matched on motivational relevance (e.g., erotica and mutilation images) elicit comparable electrocortical activity (Weinberg & Hajcak, 2010). As the type of pleasant images included in the present study typically elicit smaller LPPs among adults, it is possible that the nature of the images used account for these differences between pleasant and unpleasant images. The lack of a significant difference between pleasant and unpleasant images for the P300 could be attributed to limited power to identify differences or could represent functional differences between the P300 and the LPP. It is possible that attention is automatically captured by pleasant and unpleasant stimuli (as measured by the P300) at the same level, but the more salient unpleasant images are associated with enhanced sustained processing (as measured by the LPP) relative to pleasant images. This possibility is consistent with interpretations of the LPP as reflecting sustained processing (e.g., Hajcak et al., 2011) that appears specific to the motivational content of the stimuli (Bradley, Hamby, Löw, & Lang, 2007; Wiens et al., 2011).
There are a number of limitations to the current study. First, analyses focused on a subset of participants from a larger, longitudinal study. Additional work is needed to evaluate whether these results are consistent across other samples and age ranges. In addition, the lack of valence and arousal ratings by children for most of the stimuli used prevents us from examining the effects of valence and arousal on the structure of ERP components. Future work is needed to evaluate whether the structure changes depending on the salience of the images presented.
Taken together, the current findings suggest that emotional processing in children may be primarily driven by more automatic capture of visual attention rather than ongoing, elaborative processing. While the components modulated by emotion in children are somewhat consistent with those previously identified in adults, a number of differences emerge, indicating developmental differences in the time course and spatial distribution of emotional processing. Though very early components are not observable, there is evidence that children rapidly direct attention towards emotional, particularly unpleasant, stimuli. However, the relatively early P300 component peaks much later in children, and the slow wave component consistent with the LPP peaks earlier. Compared to those observed in adults, the components sensitive to emotion in children appear to be maximal over more occipital regions. Future research is needed to evaluate the possibility that emotional processing in childhood involves less functional connectivity between visual processing areas and frontoparietal networks. In addition, though the current study provides insight into emotional processing in middle childhood, it did not examine changes across childhood and into adolescence. Thus, future research should focus on identifying the ways in which the components sensitive to emotional information change across development.
Acknowledgments
This work was supported by National Institute of Mental Health Grants RO1 MH069942 to Daniel N. Klein and F31 MH09530701 to Autumn Kujawa.
Footnotes
IAPS pictures used were: pleasant (1463, 1710, 1750, 1811, 2070, 2091, 2092, 2224, 2340, 2345, 2347, 7325, 8031, 8200, 8461, 8496, 8497, 8370, 7400, 7330); neutral (5395, 7026, 7130, 7190, 7175, 2514, 7038, 2580, 5390, 7090, 5500, 5731, 5740, 7100, 5900, 7000, 7002, 7009, 7010, 7039); unpleasant (1050, 1052, 6571, 1205, 1200, 1300, 1304, 1930, 2458, 9600, 2691, 2703, 2800, 2811, 2900, 3022, 6190, 6213, 6231, 6510)
References
- Anokhin AP, Golosheykin S, Heath AC. Heritability of individual differences in cortical processing of facial affect. Behavior Genetics. 2010;40(2):178–185. doi: 10.1007/s10519-010-9337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbel Y, Donchin E. When a child errs: The ERN and the Pe complex in children. Psychophysiology. 2011;48(1):55–63. doi: 10.1111/j.1469-8986.2010.01042.x. [DOI] [PubMed] [Google Scholar]
- Armony JL, Dolan RJ. Modulation of spatial attention by fear-conditioned stimuli: An event-related fMRI study. Neuropsychologia. 2002;40(7):807–826. doi: 10.1016/s0028-3932(01)00178-6. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D. Annotation: What electrical brain activity tells us about brain function that other techniques cannot tell us – a child psychiatric perspective. Journal of Child Psychology and Psychiatry. 2007;48(5):415–435. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Batty M, Taylor MJ. The development of emotional face processing during childhood. Developmental Science. 2006;9(2):207–220. doi: 10.1111/j.1467-7687.2006.00480.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Löw A, Lang PJ. Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology. 2007;44(3):364–373. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Briggs KE, Martin FH. Affective picture processing and motivational relevance: Arousal and valence effects on ERPs in an oddball task. International Journal of Psychophysiology. 2009;72(3):299–306. doi: 10.1016/j.ijpsycho.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Burnett S, Bird G, Moll J, Frith C, Blakemore S-J. Development during Adolescence of the Neural Processing of Social Emotion. Journal of Cognitive Neuroscience. 2008;21(9):1736–1750. doi: 10.1162/jocn.2009.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB. The scree test for the number of factors. Multivariate Behavioral Research. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
- Curtis WJ, Cicchetti D. Affective facial expression processing in young children who have experienced maltreatment during the first year of life: An event-related potential study. Development And Psychopathology. 2011;23(02):373–395. doi: 10.1017/S0954579411000125. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52(2):95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, McPartland J. Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Developmental Science. 2004;7(3):340–359. doi: 10.1111/j.1467-7687.2004.00352.x. [DOI] [PubMed] [Google Scholar]
- Dien J. Addressing misallocation of variance in principal components analysis of event-related potentials. Brain Topography. 1998;11(1):43–55. doi: 10.1023/a:1022218503558. [DOI] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods. 2010a;187(1):138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J. Evaluating two-step PCA of ERP data with Geomin, Infomax, Oblimin, Promax, and Varimax rotations. Psychophysiology. 2010b;47(1):170–183. doi: 10.1111/j.1469-8986.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Dien J, Beal DJ, Berg P. Optimizing principal components analysis of event-related potentials: Matrix type, factor loading weighting, extraction, and rotations. Clinical Neurophysiology. 2005;116(8):1808–1825. doi: 10.1016/j.clinph.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Dien J, Frishkoff GA. Principal components analysis of event-related potential datasets. In: Handy T, editor. Event-related potentials: A methods handbook. Cambridge, MA: MIT Press; 2005. [Google Scholar]
- Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs. infomax rotations. Human Brain Mapping. 2007;28(8):742–763. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G, Dien J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology. 2009;46(3):521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- Franken I, Muris P, Nijs I, van Strien J. Processing of pleasant information can be as fast and strong as unpleasant information: Implications for the negativity bias. Netherlands Journal of Psychology. 2008;64(4):168–176. doi: 10.1007/bf03076419. [DOI] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography & Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Dennis TA. Brain potentials during affective picture processing in children. Biological Psychology. 2009;80(3):333–338. doi: 10.1016/j.biopsycho.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Olvet DM. The persistence of attention to emotion: Brain potentials during and after picture presentation. Emotion. 2008;8(2):250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Weinberg A, MacNamara A, Foti D. ERPs and the study of emotion. In: Luck SJ, Kappenman E, editors. Handbook of event-related potential components. New York: Oxford University Press; 2011. [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2(3):289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30(2):179–185. doi: 10.1007/bf02289447. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture-processing. Psychophysiology. 2002b;39(5):641–649. doi: 10.1017/s0048577202394162. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Hajcak G, Torpey D, Kim J, Klein DN. Electrocortical reactivity to emotional faces in young children and associations with maternal and paternal depression. Journal of Child Psychology and Psychiatry. 2012;53(2):207–215. doi: 10.1111/j.1469-7610.2011.02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Klein DN, Hajcak G. Electrocortical reactivity to emotional images and faces in middle childhood to early adolescence. Developmental Cognitive Neuroscience. 2012 doi: 10.1016/j.dcn.2012.03.005.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective ratings of pictures and instructional manual. Technical Report A-8. University of Florida; Gainesville, FL: 2008. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30(3):261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Larsen JT, Berntson GG, Poehlmann KM, Ito TA, Cacioppo JT. The psychophysiology of emotion. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. 3. New York, NY US: Guilford Press; 2008. pp. 180–195. [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Pine DS. Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences. 2011;108(11):4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leutgeb V, Schäfer A, Köchel A, Scharmüller W, Schienle A. Psychophysiology of spider phobia in 8- to 12-year-old girls. Biological Psychology. 2010;85(3):424–431. doi: 10.1016/j.biopsycho.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Lindstrom KM, Guyer AE, Mogg K, Bradley BP, Fox NA, Ernst M, Bar-Haim Y. Normative data on development of neural and behavioral mechanisms underlying attention orienting toward social-emotional stimuli: An exploratory study. Brain Research. 2009;1292:61–70. doi: 10.1016/j.brainres.2009.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNamara A, Foti D, Hajcak G. Tell me about it: Neural activity elicited by emotional pictures and preceding descriptions. Emotion. 2009;9(4):531–543. doi: 10.1037/a0016251. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Richell RA, Leonard A, Blair RJR. Emotion at the expense of cognition: Psychopathic individuals outperform controls on an operant response task. Journal of Abnormal Psychology. 2006;115(3):559–566. doi: 10.1037/0021-843x.115.3.559. [DOI] [PubMed] [Google Scholar]
- Monk CS. The development of emotion-related neural circuitry in health and psychopathology. Development and Psychopathology. 2008;20(04):1231–1250. doi: 10.1017/S095457940800059X. [DOI] [PubMed] [Google Scholar]
- Moratti S, Saugar C, Strange BA. Prefrontal-occipitoparietal coupling underlies late latency human neuronal responses to emotion. Journal of Neuroscience. 2011;31(47):17278–17286. doi: 10.1523/JNEUROSCI.2917-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Nugent KM. Recognition memory and resource allocation as revealed by children’s event-related potential responses to happy and angry faces. Developmental Psychology. 1990;26(2):171–179. doi: 10.1037/0012-1649.26.2.171. [DOI] [Google Scholar]
- Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, Ernst M. Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2003;44(7):1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77(3):247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, LaBar KS, Spencer DD. Memory for emotional words following unilateral temporal lobectomy. Brain and Cognition. 1997;35(1):85–109. doi: 10.1006/brcg.1997.0929. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard WS. Psychophysiology of P300. Psychological Bulletin. 1981;89(3):506–540. doi: 10.1037/0033-2909.89.3.506. [DOI] [PubMed] [Google Scholar]
- Santerre C, Allen JJB. Methods for Studying the Psychophysiology of Emotion. In: Rottenberg J, Johnson SL, editors. Emotion and psychopathology: Bridging affective and clinical science. Washington, DC US: American Psychological Association; 2007. pp. 53–79. [Google Scholar]
- Schupp HT, Cuthbert B, Bradley M, Hillman C, Hamm A, Lang P. Brain processes in emotional perception: Motivated attention. Cognition & Emotion. 2004;18(5):593–611. doi: 10.1080/02699930341000239. [DOI] [Google Scholar]
- Schupp HT, Flaisch T, Stockburger J, Junghöfer M. Emotion and attention: Event-related brain potential studies. Progress in Brain Research. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Stockburger J, Codispoti M, Junghöfer M, Weike AI, Hamm AO. Stimulus novelty and emotion perception: the near absence of habituation in the visual cortex. NeuroReport. 2006;17(4):365–369. doi: 10.1097/01.wnr.0000203355.88061.c6. 310.1097/1001.wnr.0000203355.0000288061.c0000203356. [DOI] [PubMed] [Google Scholar]
- Waters AM, Lipp OV, Spence SH. Attentional bias toward fear-related stimuli:: An investigation with nonselected children and adults and children with anxiety disorders. Journal of Experimental Child Psychology. 2004;89(4):320–337. doi: 10.1016/j.jecp.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Ferri J, Hajcak G. Bottom-up and top-down contributions to emotion: Reflections from ERP research. In: Robinson M, Watkins E, Harmon-Jones E, editors. Handbook of Cognition and Emotion. New York: Guilford Publications; in press. [Google Scholar]
- Weinberg A, Hajcak G. Beyond good and evil: The time-course of neural activity elicited by specific picture content. Emotion. 2010;10(6):767–782. doi: 10.1037/a0020242. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G. The late positive potential predicts subsequent interference with target processing. Journal of Cognitive Neuroscience. 2011;23(10):2994–3007. doi: 10.1162/jocn.2011.21630. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Hilgard J, Bartholow B, Hajcak G. Emotional targets: Evaluative categorization as a function of context and content. International Journal of Psychophysiology. doi: 10.1016/j.ijpsycho.2012.01.023. in press. [DOI] [PubMed] [Google Scholar]
- Wiens S, Sand A, et al. Nonemotional features suppress early and enhance late emotional electrocortical responses to negative pictures. Biological Psychology. 2011;86(1):83–89. doi: 10.1016/j.biopsycho.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Wong TKW, Fung PCW, McAlonan GM, Chua SE. Spatiotemporal dipole source localization of face processing ERPs in adolescents: A preliminary study. Behavioral and Brain Functions. 2009;5 doi: 10.1186/1744-9081-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WDS. Fear-related activity in the prefrontal cortex increases with age during adolescence: A preliminary fMRI study. Neuroscience Letters. 2006;406(3):194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]