Abstract
The aim of this study was to determine the effect of spinal manipulation therapy (SMT) force magnitude and force duration on the spinal stiffness of a feline preparation. A mechanical device performed simulated SMTs at the L6 spinous process in 22 anesthetised felines. Subjects were divided into four groups. Two groups (no preload, preload) received SMT having maximal displacements of 1.0mm, 2.0mm and 3.0mm of total displacement (displacement control). In two other groups (preload, no preload), SMTs were applied with maximal loads of 25%, 55% and 85% body weight (force control). Each of the SMTs were applied in order of increasing displacement or force amplitudes, at increasing durations ranging from 25 to 250 ms. Spinal stiffness was quantified by applying an indentation load to external surface of the back. Linear mixed effects models were fit for post-SMT stiffness variables. When SMT was applied under displacement control with and without a preceding preload, a significant interactive effect occurred between force magnitude and force duration (p≤0.05) for some of the stiffness variables. The findings from this experiment demonstrate that spinal stiffness in a feline model was affected by the interaction of the force amplitude and force duration parameters but the exact nature of this interaction remains unclear. This study provides guidance for further investigation given other SMT parameters not tested here may facilitate the ability of SMT to alter spinal stiffness.
Keywords: Spinal Manipulative Therapy, Biomechanics
INTRODUCTION
Spinal manipulative therapy (SMT) is one of many common approaches that clinicians use to treat low back pain (LBP). Although a common intervention, evidence supporting the efficacy of SMT is mixed (Assendelft et al. 2003; Bronfort et al. 2004). While various hypotheses exist to explain these mixed results, one possibility is variation in SMT application parameters between studies (Cleland et al. 2007; Cleland et al. 2009). As these parameters (e.g. force magnitude and force duration) are believed to modulate the neurophysiological and biomechanical mechanisms underlying the clinical effect of SMT (Herzog et al. 1993; Triano 2001; Pickar 2002; Colloca et al. 2006), differences in SMT application parameters between investigations may produce these mixed outcomes.
To better understand how SMT parameters may influence investigative outcomes, Pickar and Kang (2006) used a feline preparation to study neurophysiological responses to SMT. Specifically, afferent activity from muscle spindles in lumbar paraspinal muscles was measured in response to SMT delivered by a mechanical device (Pickar, and Kang 2006). From this preparation, higher discharge frequencies were observed from individual spindle afferents when the SMT duration was less than 100 ms. This observation suggests that SMT parameters can influence the effect of SMT on the neurological system.
While the above study highlights one relation between a SMT application parameter and a neurophysiological outcome, less is known about the influence of SMT parameters on spinal mechanics. In fact, SMT has only recently been shown to selectively alter spinal mechanics in those who benefit from SMT. Specifically, Fritz et al. demonstrated that following SMT application, spinal stiffness decreased significantly in those identified as “responders”, but does not change in those identified as “non-responders” (Fritz et al. 2011). These results suggest that the effect of SMT is greater in some presentations of back pain, but not all presentations of back pain. While these results help to further define the relation between SMT and spinal mechanics, SMT application parameters were not measured in this study. As such, the influence of these parameters on modulating SMT outcomes was not investigated.
While future clinical studies may choose to measure these parameters during SMT application, defining the relation between these parameters and clinical outcomes is a difficult task. Given the multitude of potential application parameters (e.g. force magnitude, duration, application site, application angle, etc.), investigators often limit the parameters they study to decrease experimental complexity. In many cases, the chosen parameters are force magnitude and force duration as these parameters can be readily quantified with a range of technologies including pressure mats (Kawchuk, and Herzog 1993), instrumented tables (Triano, and Schultz 1997) and manikins (Harvey et al. 2011). In addition, SMT force magnitude and force duration have significant clinical importance as their values are used to distinguish between types of manual therapy (e.g. SMT has a short force duration compared to mobilization) (Kawchuk et al. 2010). Presently, there is considerable evidence that suggests force magnitude and duration vary within and between clinicians (Herzog et al. 1993) but can become less variable with experience (Triano et al. 2011).
Given the above, the objective of this study was to use a reliable apparatus capable of controlling multiple SMT application parameters to determine the relation between SMT force magnitude, SMT force duration and a measure of spinal mechanics known to change in SMT responders (spinal stiffness).
METHODS
Overview
A mechanical device, similar to that used in previous investigations (Pickar 1999), was used to apply simulated high velocity SMT to the L6 spinous process in 22 anesthetized felines. Ethical approval for this study was obtained prior to commencement of the study from the Animal Care and Use Committee of the University of Alberta. Subjects were divided into 4 different SMT groups. In each group, 24 unique combinations of SMT duration and amplitude were employed in a non-randomized manner. Before and after each SMT event, spinal stiffness was quantified by applying a low velocity indentation load via the same mechanical device used to perform SMT.
Preparation
Experiments were performed on 22 felines in accordance with the guidelines of the Animal Care Committee of the University of Alberta. Each animal was given a pre-anesthesia subcutaneous injection of hydromorphone (0.05mg/kg), glycopyrolate (0.01mg/kg) and acepromazine (0.1mg/kg). Anesthesia was then induced with isofluorane via inhalation (up to 4.5% during induction, and maintained at 1.5–2% to effect). An endotracheal tube was then inserted to allow for controlled mechanical ventilation (ADS 2000, Engler, Florida, USA).
In preparation for SMT application and stiffness testing, the lumbar area of each animal was shaved and the L4 vertebra identified through palpation. A stereotaxic system (David Kopf instruments, Tujunga, California) was used to support the spine through the iliac crests and the L4 spinous process (Pickar 1999).
Indentation/Vertebral Loading
Spinal manipulative therapy and stiffness measurements were performed in a posteroanterior (PA) direction under displacement control using a variable rate force/displacement (VRFD) device placed on the skin overlaying the spinous process of the L6 vertebra. The high reliability of spinal stiffness measurements obtained with this device has been reported previously (Vaillant et al. 2010). The subject’s respiration was held at full exhalation through controlled ventilation for the duration of SMT applications and measurements of stiffness (<10s).
Contact Load
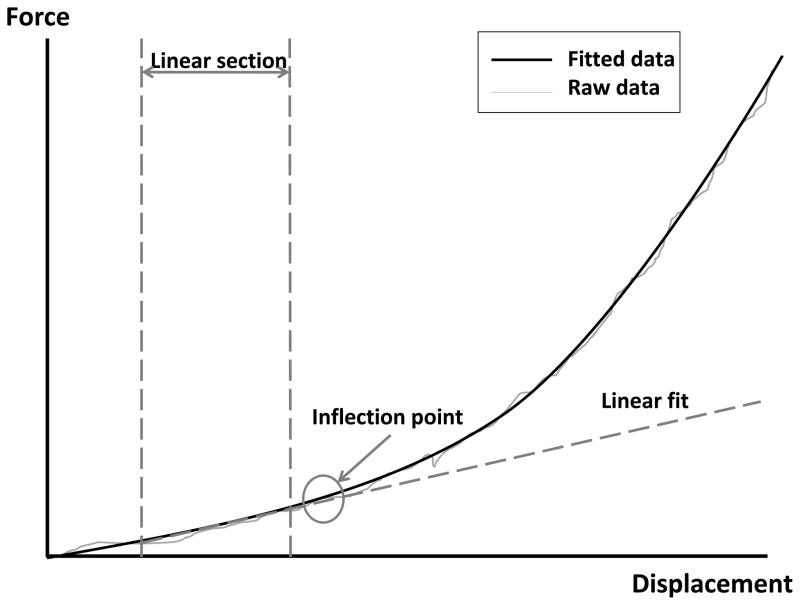
To ensure adequate physical contact between the VRFD and the spinous process for each SMT application and stiffness measurement, an initial contact load was given. Contact load was determined on a per subject basis by applying a 4mm indentation at a rate of 1.33mm/s. the applied force and resulting displacement (FD) data were collected from this procedure and plotted (see below: stiffness testing). To identify the contact load, a linear regression line was fit to the linear portion of the FD curve. The point where the FD curve diverged from the linear fit was considered to be the contact load (Figure 1). Prior work validating this choice was performed with a high resolution optical recording system (Motion Pro Digital Image System, Redlake MASD Inc, San Diego, CA) to track vertebral movement vs. soft tissue compression then synchronized to the FD curve. This process confirmed that loading beyond the contact load (as identified above) produced no further soft tissue compression without immediate vertebral displacement.
Figure 1.
A force vs. displacement curve showing the locations used to determine the contact load. To obtain this data, a 4mm indentation was performed at a rate of 1.33mm/s. Force and displacement data were obtained simultaneously then plotted. A linear regression line was fit to the linear portion of the FD curve prior to the curve inflection point. The point where this linear fit diverged away from the FD curve was identified as the contact load.
Stiffness testing
At the start of each experiment, five pre-conditioning stiffness measures were performed at 5 minute intervals to obtain a baseline stiffness value. In these pre-conditioning trials, and in each subsequent stiffness measure, the subject-specific contact load was applied to the L6 spinous, and the indentation load applied to determine spinal stiffness. Specifically, the indentation load was applied at a rate of 0.5 mm/sec to a maximal depth of 4mm. Continuous values of applied force and resultant displacement were recorded (Kawchuk and Herzog 1996; Shirley et al. 2002). In addition to pre-conditioning the spine, these 5 trials were also use to assess the reliability of the stiffness measure (below).
SMT parameters
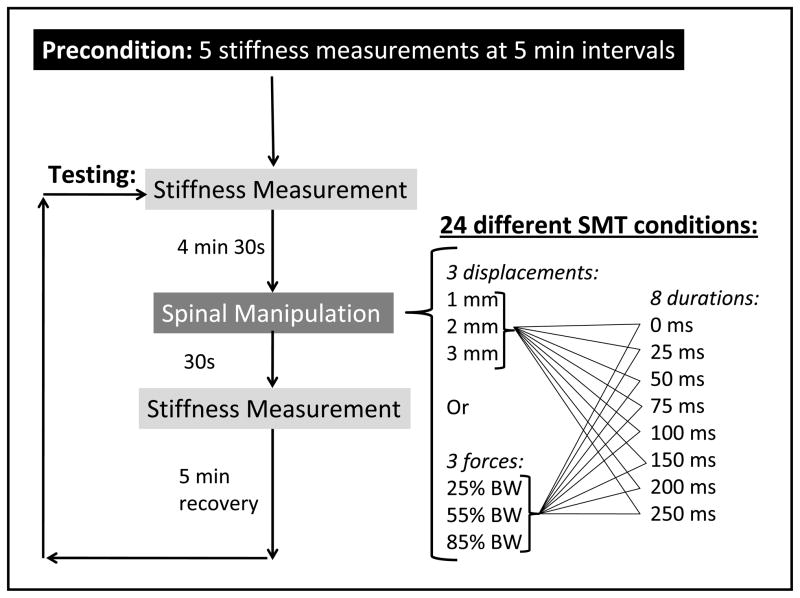
Two SMT parameters (duration and amplitude) were studied while controlling either force or displacement during SMT. These parameters were apportioned between 4 experimental groups. Eight SMT durations were applied: 0 (control), 25, 50, 75, 100, 150, 200 and 250 ms. Each duration was performed using three displacement amplitudes (1, 2, and 3mm) in two of the four experimental groups. In the remaining two groups, three force amplitudes were employed (25, 55, and 85% body weight). The force and displacement amplitudes selected for the study were scaled-down values taken from human trials (Herzog 2000; Pickar, and Kang 2006; Ianuzzi et al. 2009) and are similar to values used in previous animal studies (Pickar and Kang, 2006; Pickar et al., 2007). To further simulate clinical conditions, and in addition to the contact load, two of the four experimental groups received a preload of 10% body weight (BW) for 4.31 s just prior to SMT application.
As a result of the above, each of the four experimental groups contained 8 SMT durations and 3 amplitudes (force or displacement) resulting in 24 unique combinations of SMT application parameters. In each subject, these 24 SMT parameter combinations were performed in order of increasing displacement or force amplitude and increasing duration. Figure 2 illustrates the timing and order of SMT application for each group. A pre-SMT stiffness measure was taken 4.5 min prior to each SMT application and a post-SMT measure taken 0.5 min following SMT application. A 5 min recovery period separated the next block of pre-SMT, SMT, post-SMT procedures (Figure 2). Following experimental procedures, each animal was euthanized in accordance with standard operating procedures of the Animal Care and Use Committee of the University of Alberta.
Figure 2.
Diagram showing an overview of the protocol timing.
Determining Stiffness
Force-displacement data collected during indentation testing were plotted and the ascending portion of the curve cropped at the starting (immediately after contact load) and ending points (when the maximal indentation of 4mm was achieved). The resulting curve was then fit using a 5th order polynomial and the resulting function normalized by subtracting the first value of the displacement signal to all remaining displacement values (Kawchuk, and Fauvel 2001).
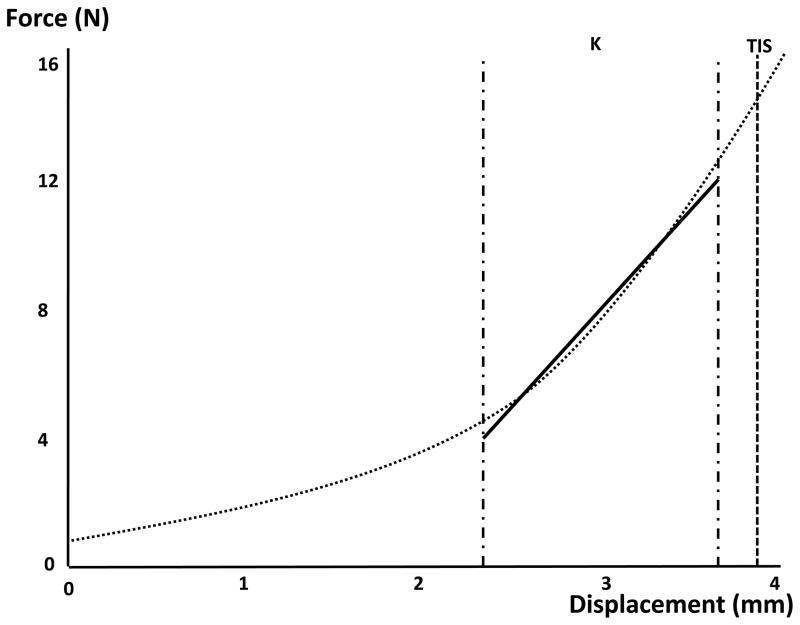
The region of the FD curve displaying the greatest variability was identified by plotting the variance of the force between post-SMT stiffness tests against the displacement for each subject. An inflection point in the variance measures near 60% of the total displacement was chosen as the most sensitive region of the FD curve. As a result, a stiffness coefficient (k) was calculated for the region spanning 60% to 90% (2.35 to 3.6mm) of total displacement. Specifically, a linear regression line was fit to this region, and the slope of this line was used to describe the stiffness coefficient, k (Figure 3). A second measurement, terminal instantaneous stiffness (TIS) was defined as the stiffness (i.e. force/displacement) just prior to maximal indentation (3.8 mm) (Figure 3). Maximal indentation (4.mm) was not selected as the final point on the curve to prevent measurement artifacts (+/− <0.01V) arising from changes in motor direction.
Figure 3.
Force-displacement (FD) curve with normalized displacement values. The stiffness coefficient (k) and the terminal instantaneous stiffness (TIS) are calculated from the FD curve.
Data Analysis
Repeatability
An Intraclass Correlation Coefficient (ICC (3,1)) was calculated for k and for TIS. A two-way mixed model ANOVA was used to determine the reliability of a single measurement (PASW Statistics 17.0, SPSS, IBM, Chicago, Illinois). Five values were used as input for this analysis: the last four of the five pre-conditioning trials and the first pre-SMT indentation trial (Shirley et al. 2002).
Primary Analyses
Linear mixed effects models were fit for post-SMT k and post-SMT TIS variables with adjustments made for the respective pre-SMT value to account for between-subject heterogeneity (SAS version 9.1, SAS Institute, Inc., Cary, North Carolina). Direct product compound symmetry covariance structures were used in each model to account for within-subject correlation over the repeated measures of amplitude and duration. When a significant interaction was found, post-hoc tests of each SMT duration were compared to the no duration control (0 ms) using Dunnett’s test.
RESULTS
Descriptive statistics
Cats tested with SMT using displacement control without preload (DispPre−, protocol 1), had a mean mass of 3.74 (±0.60) kg. In these animals, the mean contact load was 2.03 (±0.30) N yielding stiffness values of k= 12.14 (±2.53) N/mm and TIS = 9.32 (±1.72) N/mm. Similarly, cats tested with SMT using displacement control and preload (DispPre+, protocol 2) were on average 3.58 (±0.59)kg with a mean contact load of 1.26 (±0.31). The average stiffen values of these animals was k = 4.39 (±0.51) N/mm and TIS = 3.96 (±0.54)N/mm.
In cats receiving load-control SMT without preload (ForcePre−, protocol 3) the mean mass was 2.97 (±0.60) kg with a mean contact load of 1.08 (±0.36) N. The average stiffness outcomes in these animals were k = 6.07 (±2.60) N/mm and TIS = 5.18 (±2.31) N/mm. For animals given load-control SMT and preload (ForcePre+, protocol 4), the mean mass of the animals was 3.73 (±0.61) kg with a contact load of 1.42 (±0.51) N. The average stiffness of these animals was 9.18 k = (±3.08) N/mm and TIS = 7.01 (±2.30) N/mm.
Repeatability
The repeatability analysis for k and TIS yielded ICC values of 0.99 (95%CI 0.989–0.997) for k and 0.99 (95%CI 0.985–0.996) for TIS. The mean within-subject change in k and TIS for the 5 preconditioning measures was 0.05 (±0.10) and 0.03 (±0.07) N/mm respectively. As seen in Table 1, these mean values fall outside the confidence intervals of k and TIS values obtained following SMT.
Table 1.
Confidence intervals (95%) for each SMT condition.
| Duration Adjusted Mean (95% CI) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Var. | Amp | 0 | 25 | 50 | 75 | 100 | 150 | 200 | 250 |
| 1 Displacement, no preload | K | 1 | 13.05 (10.99, 15.12) | 12.67 (10.60, 14.74) | 12.71 (10.65, 14.78) | 12.87 (10.80, 14.94) | 12.67 (10.60, 14.74) | 12.13 (10.06, 14.20) | 12.86 (10.80, 14.93) | 12.92 (10.86, 14.99) |
| 2 | 12.71 (11.87, 13.55) | 12.67 (11.83, 13.51) | 13.01 (12.17, 13.85) | 12.83 (11.99, 13.67) | 12.88 (12.04, 13.72) | 12.73 (11.89, 13.56) | 12.72 (11.89, 13.56) | 12.59 (11.75, 13.43) | ||
| 3 | 12.68 (11.87, 13.49) | 12.93 (12.13, 13.74) | 12.78 (11.97, 13.58) | 12.85 (12.04, 13.65) | 12.77 (11.96, 13.58) | 12.53 (11.72, 13.34) | 12.84 (12.04, 13.65) | 12.75 (11.93, 13.56) | ||
| TIS | 1 | 9.96 (7.97, 11.94) | 9.89 (7.90, 11.87) | 9.77 (7.78, 11.75) | 9.82 (7.83, 11.80) | 9.60 (7.61, 11.59) | 9.17 (7.19, 11.16) | 9.72 (7.73, 11.70) | 9.76 (7.77, 11.74) | |
| 2 | 9.59 (8.94, 10.24) | 9.64 (9.00, 10.29) | 9.90 (9.26, 10.55) | 9.75 (9.10, 10.40) | 9.72 (9.07, 10.37) | 9.57 (8.92, 10.22) | 9.50 (8.86, 10.15) | 9.48 (8.83, 10.12) | ||
| 3 | 9.36 (8.73, 9.98) | 9.90 (9.28, 10.53) | 9.69 (9.07, 10.32) | 9.62 (9.00, 10.24) | 9.63 (9.01, 10.260) | 9.58 (8.96, 10.21) | 9.72 (9.09, 10.34) | 9.60 (8.97, 10.23) | ||
| 2 Displacement, with preload | K | 1 | 4.40 (4.22, 4.57) | 4.44 (4.27, 4.62) | 4.57 (4.40, 4.75) | 4.69 (4.51, 4.86) | 4.67 (4.50, 4.84) | 4.68 (4.50, 4.85) | 4.68 (4.50, 4.85) | 4.73 (4.56, 4.91) |
| 2 | 4.73 (4.60, 4.85) | 4.75 (4.62, 4.87) | 4.76 (4.63, 4.88) | 4.81 (4.69, 4.94) | 4.76 (4.64, 4.88) | 4.78 (4.66, 4.90) | 4.82 (4.69, 4.94) | 4.80 (4.68, 4.92) | ||
| 3 | 4.75 (4.64, 4.86) | 4.71 (4.60, 4.82) | 4.81 (4.69, 4.92) | 4.81 (4.69, 4.92) | 4.83 (4.72, 4.94) | 4.81 (4.69, 4.92) | 4.85 (4.74, 4.96) | 4.86 (4.74, 4.97) | ||
| TIS | 1 | 4.07 (3.89, 4.25) | 4.10 (3.92, 4.28) | 4.20 (4.02, 4.37) | 4.27 (4.09, 4.45) | 4.24 (4.06, 4.41) | 4.22 (4.04, 4.40) | 4.18 (4.01, 4.36) | 4.18 (4.00, 4.36) | |
| 2 | 4.17 (4.05, 4.29) | 4.20 (4.07, 4.32) | 4.20 (4.08, 4.33) | 4.20 (4.07, 4.32) | 4.10 (3.98, 4.23) | 4.15 (4.03, 4.28) | 4.16 (4.03, 4.28) | 4.11 (3.99, 4.24) | ||
| 3 | 4.10 (4.00, 4.19) | 4.09 (3.99, 4.18) | 4.13 (4.03, 4.22) | 4.13 (4.03, 4.23) | 4.15 (4.05, 4.24) | 4.11 (4.01, 4.20) | 4.13 (4.03, 4.22) | 4.13 (4.03, 4.22) | ||
| 3 Force no preload | K | 25% | 6.20 (5.81, 6.59) | 6.60 (5.67, 6.45) | 6.15 (5.76, 6.540 | 6.18 (5.79, 6.57) | 6.40 (6.01, 6.79) | 6.22 (5.84, 6.61) | 6.26 (5.87, 6.65) | 6.53 (6.14, 6.91) |
| 55% | 6.55 (6.23, 6.86) | 6.71 (6.40, 7.03) | 6.73 (6.42, 7.040 | 6.66 (6.35, 6.97) | 6.67 (6.36, 6.98) | 6.55 (6.24, 6.87) | 6.71 (6.40, 7.02) | 6.62 (6.31, 6.93) | ||
| 85% | 6.40 (5.87, 6.93) | 6.81 (6.28, 7.34) | 7.04 (6.51, 7.56) | 6.79 (6.26, 7.32) | 6.90 (6.37, 7.43) | 6.69 (6.16, 7.22) | 6.59 (6.06, 7.12) | 6.85 (6.32, 7.38) | ||
| TIS | 25% | 5.10 (4.84, 5.35) | 5.01 (4.76, 5.27) | 5.00 (4.74, 5.26) | 5.07 (4.81, 5.32) | 5.22 (4.96, 5.48) | 5.12 (4.86, 5.38) | 5.13 (4.87, 5.39) | 5.23 (4.97, 5.49) | |
| 55% | 5.14 (4.96, 5.33) | 5.41 (5.22, 5.59) | 5.32 (5.13, 5.50) | 5.34 (5.16, 5.53) | 5.41 (5.22, 5.59) | 5.36 (5.17, 5.54) | 5.31 (5.12, 5.49) | 5.29 (5.11, 5.47) | ||
| 85% | 4.96 (4.66, 5.26) | 5.43 (5.13, 5.73) | 5.54 (5.24, 5.84) | 5.39 (5.09, 5.69) | 5.42 (5.12, 5.72) | 5.19 (4.89, 5.49) | 5.12 (4.82, 5.420 | 5.40 (5.10, 5.70) | ||
| 4 Force, with preload | K | 25% | 9.19 (8.74, 9.64) | 9.47 (9.02, 9.92) | 9.25 (8.80, 9.70) | 9.38 (8.93, 9.83) | 9.19 (8.74, 9.64) | 9.34 (8.89, 9.79) | 9.34 (8.89, 9.79) | 9.15 (8.70, 9.60) |
| 55% | 9.38 (8.94, 9.82) | 9.41 (8.97, 9.85) | 9.32 (8.88, 9.77) | 9.55 (9.11, 9.99) | 9.40 (8.95, 9.84) | 9.40 (8.96, 9.85) | 9.29 (8.85, 9.73) | 9.45 (9.01, 9.90) | ||
| 85% | 9.24 (8.71, 9.78) | 9.57 (9.03, 10.10) | 9.68 (9.15, 10.22) | 9.62 (9.09, 10.16) | 9.81 (9.27, 10.34) | 9.38 (8.84, 9.91) | 9.39 (8.86, 9.93) | 9.63 (9.09, 10.16) | ||
| TIS | 25% | 7.19 (6.86, 7.53) | 7.37 (7.03, 7.70) | 7.30 (6.97, 7.64) | 7.28 (6.95, 7.61) | 7.24 (6.90, 7.57) | 7.21 (6.88, 7.55) | 7.30 (6.96, 7.63) | 7.02 (6.69, 7.35) | |
| 55% | 7.16 (6.76, 7.56) | 7.35 (6.95, 7.75) | 7.19 (6.78, 7.59) | 7.39 (6.98, 7.79) | 7.26 (6.86, 7.66) | 7.21 (6.81, 7.62) | 7.07 (6.66, 7.47) | 7.21 (6.81, 7.61) | ||
| 85% | 7.11 (6.68, 7.54) | 7.42 (6.99, 7.84) | 7.32 (6.89, 7.75) | 7.30 (6.87, 7.73) | 7.48 (7.05, 7.91) | 7.19 (6.76, 7.62) | 7.18 (6.75, 7.61) | 7.17 (6.74, 7.60) | ||
Primary Analysis
Effect of SMT Force Amplitude and Duration on k
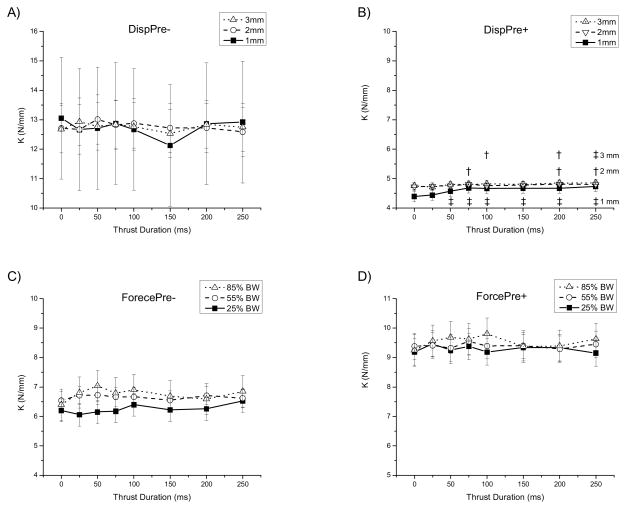
Figure 4 displays the adjusted means and 95% confidence intervals for the 8 force durations and 3 levels of amplitude including the no-force control (0 ms). Changing SMT force duration and force amplitude had neither interactive nor main effects when the manipulation was applied under displacement control and was not preceded by a preload (Figure 4A (DispPre−): amplitude: F(2,10)=0.00, p=1.00; duration: F(7,28)=1.37, p=0.26; interaction: F(14,55)=1.24, p =0.27) nor when it was applied under force control with a preload (Figure 4D (ForcePre+): amplitude: F(2,10)=0.71, p=0.52; duration: F(7,35)=0.48, p=0.84; interaction: F(14,70)=0.79, p =0.67).
Figure 4.
Effect of SMT force magnitude and force duration on lumbar spinal stiffness (k). Symbols represent adjusted means bounded by their 95% confidence intervals. † p ≤0.04, ‡ p<0.01 compared with 0 ms force duration. Animals given SMT under displacement control without preload = DispPre−. Animals given SMT under displacement control with preload = DispPre+. Animals given SMT under force control without preload = ForcePre−. Animals given SMT under force control with preload = forcePre+.
When SMT was applied under displacement control and preceded by a preload, a significant interactive effect occurred between amplitude and duration (Figure 4B (DispPre+): amplitude: F(2,10)=2.08, p=0.18; duration: F(7,35)=14.06, p<0.001; interaction: F(14,70)=2.77, p=0.003). Inspection of Figure 4B shows the smallest force amplitude employed in the study (1mm) increased k as force durations increased from 50 to approximately 100ms. At longer force durations, and with larger force displacements regardless of force duration, k remained relatively constant. Post-hoc comparisons shown in Figure 4B indicate that the 1mm force amplitude increased k significantly in comparison to preload alone (0 ms duration) for force durations greater than 25ms. The 2mm force amplitude significantly increased k when force durations lasted 75, 200, and 250ms. Similarly the 3mm manipulative displacement significantly increased stiffness at force durations of 100, 200, and 250ms.
When manipulation was given under force control without a preload, no interaction occurred. There was the suggestion of a main effect for force duration (Figure 4C (ForcePre−): amplitude: F(2,8)=2.20, p=0.17; duration: F(7,28)=2.03, p=0.08; interaction: F(14,56)=1.25, p=0.27).
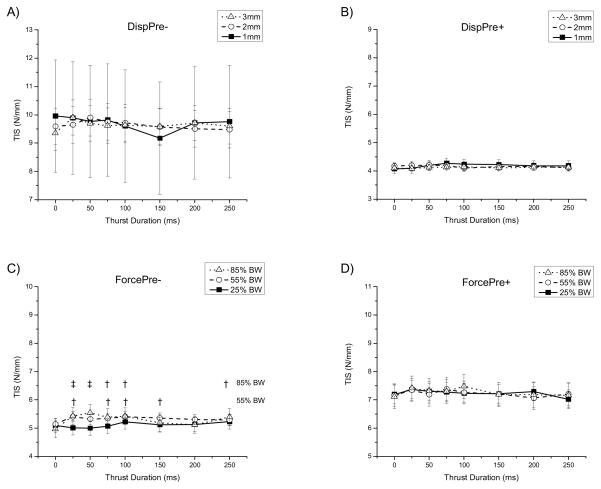
Effect of SMT Force Amplitude and Duration on TIS
Figure 5 shows the adjusted means and 95% confidence intervals for TIS of 8 force durations and 3 levels of force amplitude including a no-force control (0ms). With manipulation given under displacement control without and with a preceding preload, there was the suggestion of an interaction effect (Figure 5A (DispPre−) amplitude: F(2,8)=0.00, p=1.00; duration: F(7,28)=1.57, p=0.19; interaction: F(14,55) =1.75, p=0.07; Figure 5B (DispPre+): amplitude: F(2,10)=0.45, p=0.65; duration: F(7,35)=1.38, p=0.24; interaction: F(14,70)=1.77, p=0.06)).
Figure 5.
Effect of SMT force magnitude and force duration on the terminal instantaneous stiffness (TIS). Symbols represent adjusted means bounded by their 95% confidence intervals.† p ≤0.04, ‡ p<0.01 compared with 0 ms force duration. Animals given SMT under displacement control without preload = DispPre−. Animals given SMT under displacement control with preload = DispPre+. Animals given SMT under force control without preload = ForcePre−. Animals given SMT under force control with preload = forcePre+.
When manipulation was given under force control without a preload, no interaction occurred although a main effect for force duration was observed (Figure 5C (ForcePre−): amplitude: F(2,8)=1.75, p=0.23; duration: F(7,28) =2.62, p=0.03; interaction: F(14,56)=1.55, p=0.12). Post-hoc comparisons demonstrated that the 55% and 85% BW force amplitudes significantly increased stiffness compared to the control (0 ms duration) for the majority of force durations of 150ms or less.
There were neither an interaction or main effect when SMT was given under force control with a preload (Figure 5BD (ForcePre+): amplitude: F(2,10)=0.03, p=0.97; duration: F(7,35)=0.99, p=0.45; interaction: F(14,70)=0.60, p=0.86).
DISCUSSION
Recently, it has been shown that spinal stiffness decreases significantly and immediately following SMT application in human subjects classified as SMT responders (Fritz et al. 2011). To better understand the way in which SMT may influence spinal mechanics, the current study was designed to investigate the effect of specific application parameters of SMT (displacement or force) on spinal stiffness in a feline model. Our results suggest a complex relation between these variables. Compared to the recent human trial described above, this study did not identify any combination of SMT force duration and SMT amplitude that resulted in an immediate decrease in spinal stiffness. Conversely, we observed an small increase in spinal stiffness over the course of multiple SMT applications with some SMT displacement amplitudes but not others. While this may suggest a differential stiffness response between SMT amplitudes, the resulting stiffness increase is not a preferred clinical outcome although several explanations for this observation can be given.
First, several human studies have now shown that stiffness does not change significantly in all subjects following SMT and in particular, those subjects who are classified as non-responders (Fritz et al. 2011) or those who are asymptomatic (Tuttle et al. 2008; Campbell, and Snodgrass 2010). Specifically, stiffness also appears to change most readily in subjects identified as having segmental hypomobility (Fritz et al. 2005; Brennan et al. 2006) and segmental pain (Tuttle et al. 2008). In the preparation used in this experiment, there was no attempt to create hypomobilities or back pain. As a result, the subjects in this study may have been unresponsive to SMT due to a floor effect; their spinal stiffness was near its most minimal value. Accordingly, the small increase observed in stiffness at some SMT amplitudes and durations is most likely a viscoelastic phenomenon in that fluid recovery in the target tissues was not sufficient given the viscosity of the tissues, the rate of indentation and the number of repeated indentations and SMTs performed in this experiment (Lee, and Evans 1992; Latimer et al. 1996; Shirley et al. 2002). Alternatively, the observed changes may reflect variation within our measurement, however, the observed changes in k and TIS following SMT were greater in magnitude (Table 1) than those observed in our preconditioning trials where no SMT was provided. Second, the experimental preparation employs a series of supports for the spine which may limit the mechanical response of the spine to SMT and therefore affect the ability of SMT to alter spinal stiffness. Third, the use of general anesthesia in the preparation may influence neurological mechanisms which could facilitate decreases in stiffness following SMT (Pickar 2002; Colloca et al. 2006). Fourth, several studies in this field have used different representations of “k” to quantify stiffness (Edmondston et al. 1998; Kawchuk, and Fauvel 2001; Owens et al. 2007). While one version of k has been observed to decrease following SMT in human responders, these variables may not best-reflect post-SMT changes in spinal stiffness should they exist in a feline model. As pointed out by others, separate regions of the cervical spine’s force-displacement curve differ in their stiffness responses to spinal mobilizations (Tuttle et al. 2008). This variability in how stiffness may change across different regions of the force-displacement curve is consistent with our observation of divergent changes when comparing k and TIS results in the DispPre+ group: significant changes occurred in k but not TIS. This comparison suggests that TIS may represent a different stiffness characteristic compared to k. Fifth, the SMT technique used in this study is substantially different from those techniques which have generated post-SMT decreases in human spinal stiffness (Fritz et al. 2011; Cleland et al. 2007; Cramer et al. 2002). As has been pointed out in the literature, not every SMT technique should be expected to produce the same clinical effect (Cleland et al. 2007) because subject positioning and the direction of SMT force application are thought to differentially affect the forces placed on the spinal tissues (Colloca et al. 2004).
Significance
While the application of SMT can be changed readily through numerous parameters, there is little research that describes the resolution with which a human can control a given SMT parameter. While there is some evidence to suggest that clinicians can control force magnitude during slower applications of force (posteroanterior challenge of the spine) (Chiradejnant et al. 2003), the ability of clinicians to control force magnitude over a smaller time duration (e.g. SMT) remains unknown but can become more consistent with experience (Triano et al. 2011). Should other studies demonstrate that these (Cambridge et al. 2012), or other SMT application parameters may influence clinical outcome (e.g. application site, application angle), there would be an interesting opportunity to train clinicians to reproduce these specific parameters and then evaluate their ability to do so (Harvey et al. 2011).
Conclusion
The findings from this experiment demonstrate that spinal stiffness in a feline model was affected by the interaction of the amplitude and duration parameters of SMT but not in the expected direction. The exact nature of this interaction remains unclear but warrants further investigation given that other possible SMT parameters not tested here may facilitate the ability of SMT to decrease spinal stiffness.
Acknowledgments
The authors would like to thank the staff of the HSLAS for their assistance in this project. This project was supported by NIH Grant U19 AAT004137 to JG Pickar and GN Kawchuk. Support for GN Kawchuk is provided by the Canada Research Chairs Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Assendelft WJ, et al. Spinal Manipulative Therapy for Low Back Pain. a Meta-analysis of Effectiveness Relative to Other Therapies. Ann Intern Med. 2003;138(11):871–81. doi: 10.7326/0003-4819-138-11-200306030-00008. [DOI] [PubMed] [Google Scholar]
- Brennan GP, et al. Identifying Subgroups of Patients with Acute/subacute “nonspecific” Low Back Pain: Results of a Randomized Clinical Trial. Spine. 2006;31(6):623–31. doi: 10.1097/01.brs.0000202807.72292.a8. [DOI] [PubMed] [Google Scholar]
- Bronfort G, et al. Efficacy of Spinal Manipulation and Mobilization for Low Back Pain and Neck Pain: a Systematic Review and Best Evidence Synthesis. The Spine J. 2004;4(3):335–56. doi: 10.1016/j.spinee.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Cambridge Edward DJ, et al. Comparison of Force Development Strategies of Spinal Manipulation Used for Thoracic Pain. Man Ther. 2012 doi: 10.1016/j.math.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Campbell Brad D, Snodgrass Suzanne J. The Effects of Thoracic Manipulation on Posteroanterior Spinal Stiffness. J Orthop Sports Phys Ther. 2010;40(11):685–93. doi: 10.2519/jospt.2010.3271. [DOI] [PubMed] [Google Scholar]
- Chiradejnant A, et al. Objective Manual Assessment of Lumbar Posteroanterior Stiffness Is Now Possible. J Manipulative Physiol Ther. 2003;26(1):34–39. doi: 10.1067/mmt.2003.3. [DOI] [PubMed] [Google Scholar]
- Cleland JA, et al. Short-term Effects of Thrust Versus Nonthrust Mobilization/manipulation Directed at the Thoracic Spine in Patients with Neck Pain: a Randomized Clinical Trial. Phys Ther. 2007;87(4):431–40. doi: 10.2522/ptj.20060217. [DOI] [PubMed] [Google Scholar]
- Cleland Joshua A, et al. Comparison of the Effectiveness of Three Manual Physical Therapy Techniques in a Subgroup of Patients with Low Back Pain Who Satisfy a Clinical Prediction Rule: a Randomized Clinical Trial. Spine. 2009;34(25):2720–29. doi: 10.1097/BRS.0b013e3181b48809. [DOI] [PubMed] [Google Scholar]
- Colloca CJ, et al. Biomechanical and Neurophysiological Responses to Spinal Manipulation in Patients with Lumbar Radiculopathy. J Manipulative Physiol Ther. 2004;27(1):1–15. doi: 10.1016/j.jmpt.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Colloca CJ, et al. Spinal Manipulation Force and Duration Affect Vertebral Movement and Neuromuscular Responses. Clin Biomech. 2006;21(3):254–62. doi: 10.1016/j.clinbiomech.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Cramer Gregory D, et al. The Effects of Side-posture Positioning and Spinal Adjusting on the Lumbar Z Joints: a Randomized Controlled Trial with Sixty-four Subjects. Spine. 2002;27(22):2459–66. doi: 10.1097/00007632-200211150-00008. [DOI] [PubMed] [Google Scholar]
- Edmondston SJ, et al. Effect of Position on the Posteroanterior Stiffness of the Lumbar Spine. Man Ther. 1998;3(1):21–26. doi: 10.1054/math.1998.0312. [DOI] [PubMed] [Google Scholar]
- Fritz Julie M, et al. Preliminary Investigation of the Mechanisms Underlying the Effects of Manipulation: Exploration of a Multivariate Model Including Spinal Stiffness, Multifidus Recruitment, and Clinical Findings. Spine. 2011;36(21):1772–81. doi: 10.1097/BRS.0b013e318216337d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz Julie M, et al. Lumbar Spine Segmental Mobility Assessment: an Examination of Validity for Determining Intervention Strategies in Patients with Low Back Pain. Arch Phys Med Rehabil. 2005;86(9):1745–52. doi: 10.1016/j.apmr.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Harvey Marie-Pierre, et al. Learning Spinal Manipulation: a Comparison of Two Teaching Models. The Journal of chiropractic education. 2011;25(2):125–31. doi: 10.7899/1042-5055-25.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog W, et al. Forces Exerted During Spinal Manipulative Therapy. Spine. 1993;18(9):1206–12. doi: 10.1097/00007632-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Herzog W, et al. Forces Exerted During Spinal Manipulative Therapy. Spine. 1993;18(9):1206–12. doi: 10.1097/00007632-199307000-00014. [DOI] [PubMed] [Google Scholar]
- Herzog Walter. Clinical biomechanics of spinal manipulation. 2000. [DOI] [PubMed] [Google Scholar]
- Ianuzzi Allyson, et al. Determination of Torque-limits for Human and Cat Lumbar Spine Specimens During Displacement-controlled Physiological Motions. Spine J. 2009;9(1):77–86. doi: 10.1016/j.spinee.2007.07.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawchuk G, Herzog W. A New Technique of Tissue Stiffness (compliance) Assessment: Its Reliability, Accuracy and Comparison with an Existing Method. J Manipulative Physiol Ther. 1996;19(1):13–18. [PubMed] [Google Scholar]
- Kawchuk GN, Fauvel OR. Sources of Variation in Spinal Indentation Testing: Indentation Site Relocation, Intraabdominal Pressure, Subject Movement, Muscular Response, and Stiffness Estimation. J Manipulative Physiol Ther. 2001;24(2):84–91. doi: 10.1067/mmt.2001.112566. [DOI] [PubMed] [Google Scholar]
- Kawchuk GN, Herzog W. Biomechanical Characterization (fingerprinting) of Five Novel Methods of Cervical Spine Manipulation. J Manipulative Physiol Ther. 1993;16(9):573–77. [PubMed] [Google Scholar]
- Kawchuk Gregory N, et al. Identification of Spinal Tissues Loaded by Manual Therapy: a Robot-based Serial Dissection Technique Applied in Porcine Motion Segments. Spine. 2010;35(22):1983–90. doi: 10.1097/BRS.0b013e3181ddd0a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latimer J, et al. Evaluation of a New Device for Measuring Responses to Posteroanterior Forces in a Patient Population, Part 1: Reliability Testing. Phys Ther. 1996;76(2):158–65. doi: 10.1093/ptj/76.2.158. [DOI] [PubMed] [Google Scholar]
- Lee R, Evans J. Load-displacement-time Characteristics of the Spine Under Posteroanterior Mobilisation. Australian Journal of Physiotherapy. 1992;38:115–23. doi: 10.1016/S0004-9514(14)60556-0. [DOI] [PubMed] [Google Scholar]
- Owens EF, et al. The Reliability of a Posterior-to-anterior Spinal Stiffness Measuring System in a Population of Patients with Low Back Pain. J Manipulative Physiol Ther. 2007;30(2):116–23. doi: 10.1016/j.jmpt.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Pickar JG. An in Vivo Preparation for Investigating Neural Responses to Controlled Loading of a Lumbar Vertebra in the Anesthetized Cat. J Neurosci Methods. 1999;89(2):87–96. doi: 10.1016/s0165-0270(99)00060-6. [DOI] [PubMed] [Google Scholar]
- Pickar JG. Neurophysiological Effects of Spinal Manipulation. The Spine J. 2002;2(5):357–71. doi: 10.1016/s1529-9430(02)00400-x. [DOI] [PubMed] [Google Scholar]
- Pickar JG, Kang YM. Paraspinal Muscle Spindle Responses to the Duration of a Spinal Manipulation Under Force Control. J Manipulative Physiol Ther. 2006;29(1):22–31. doi: 10.1016/j.jmpt.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Shirley D, et al. The Response of Posteroanterior Lumbar Stiffness to Repeated Loading. Man Ther. 2002;7(1):19–25. doi: 10.1054/math.2001.0432. [DOI] [PubMed] [Google Scholar]
- Triano JJ. Biomechanics of Spinal Manipulative Therapy. The Spine J. 2001;1(2):121–30. doi: 10.1016/s1529-9430(01)00007-9. [DOI] [PubMed] [Google Scholar]
- Triano J, Schultz AB. Loads Transmitted During Lumbosacral Spinal Manipulative Therapy. Spine. 1997;22(17):1955–64. doi: 10.1097/00007632-199709010-00003. [DOI] [PubMed] [Google Scholar]
- Triano John J, et al. Maturation in Rate of High-velocity, Low-amplitude Force Development. J Manipulative Physiol Ther. 2011;34(3):173–80. doi: 10.1016/j.jmpt.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Tuttle Neil, PhD, et al. Relation Between Changes in Posteroanterior Stiffness and Active Range of Movement of the Cervical Spine Following Manual Therapy Treatment. Spine. 2008;33:19–55. doi: 10.1097/BRS.0b013e31817f93f9. [DOI] [PubMed] [Google Scholar]
- Tuttle Neil, et al. Postero-anterior Movements of the Cervical Spine: Repeatability of Force Displacement Curves. Man Ther. 2008;13(4):341–48. doi: 10.1016/j.math.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Vaillant Michele, et al. Performance and Reliability of a Variable Rate, Force/displacement Application System. J Manipulative Physiol Ther. 2010;33(8):585–93. doi: 10.1016/j.jmpt.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]