Abstract
Physical and genetic maps have been used for chromosomal localization of genes in vectors of infectious diseases. The availability of polytene chromosomes in malaria mosquitoes provides a unique opportunity to precisely map genes of interest. We report physical mapping of two actin genes on polytene chromosomes of the major malaria vector in Amazon Anopheles darlingi. The clones with the actin genes sequences were obtained from a cDNA library constructed from RNA isolated from adult females and males of An. darlingi. Each of the two clones was mapped to a unique site on the chromosomal arm 2L in subdivisions 21A (clone pl05-A04) and 23B (clone pl17-G06). The obtained results together with previous mapping data provide a suitable basis for comparative genomics and for establishing chromosomal homologies among major malaria vectors.
Keywords: physical mapping, chromosomal homologies, FISH, malaria vector
Anopheles (Nyssorhynchus) darlingi Root, 1926 is a major human malaria vector and the most anthropophillic and endophagous species of Anopheles in the neotropical region and especially in the Brazilian Amazon Basin (Tadei et al., 1998). The relevance of An. darlingi as vector of malaria, its geographic distribution and population structure have been demonstrated in several studies (dos Santos et al., 1999; Gilman et al., 2006; Schlichting et al., 2003; Tadei et al., 1998). However, crucial genetic and genomic studies on An. darlingi have been lagged behind because of the lack a laboratory colony for this vector. Knowledge of the chromosomal location of genes has important applications for comparative genomics, map-based cloning, and genetic manipulations. Anopheles darlingi has been a neglected species in cytogenetic research, because the source of polytene chromosomes is limited to the wild-caught larvae, and because of the lack of the high-resolution chromosomal map. The first photo map of polytene chromosomes from salivary glands of the An. darlingi larvae was developed by Kreutzer et al. (1975). However, this map was not divided into numbered and lettered regions, and, therefore, was not useful for detailed physical mapping. More recently, we created a new cytogenetic map with positions of inversion breakpoints for this species and with numbered and lettered regions (Rafael et al., 2010). The new photomap can serve as a tool to perform evolutionary genetic studies, to localize genes of interest on chromosomes, and to guide a genome assembly effort for An. darlingi.
Recently, we developed a cDNA library from total RNA isolated from adult females and males pool of An. darlingi collected in Coari, Amazonas state (M. Rafael, unpublished observations). This library is a suitable source of gene sequences that can be directly mapped to chromosomes of this species. In this study, we identified two actin genes sequences in the An. darlingi Contig 167, ADLSDA03021A01.g00 (GenBank accession number: Actin_Ad1 JQ307420) and Contig 152, ADELSDA03017G06.g00 (GenBank accession number: Actin_Ad2 JQ307421). Both actin genes sequences were mapped on polytene chromosomes of An. darlingi to determine their chromosomal locations and to establish chromosomal homologies between major malaria vectors. Actin is a highly conserved gene in eukaryotes (Hennessey et al., 1993), which functions include the determination of cell shape, cell motility, cytokinesis, intracellular transport and construction of microfibrils in muscle cells. It was recently demonstrated that engineering of late-acting, repressible, tissue-specific, and female-specific transgene expression to cause a flightless phenotype in female Ae. aegypti is possible (Fu et al., 2010). This system was based on the promoter derived from the Ae. aegypti actin-4 gene, which leads to the expression of tTA in a stage-, tissue-, and sex-specific manner.
We used Anopheles darlingi collected in Coari, (4o06’S, 63o03’W), Amazonas state, Brazil for the physical mapping. We dissected salivary glands of fourth instar larvae in Fixative I (Carnoy’s solution and water), Fixative II (Carnoy’s solution and water) and Fixative III (95% lactic acid, P.A., acetic acid and water). We analyzed the bandingpattern of 10 chromosome preparations of An. darlingi under a Zeiss Axioplan phase contrast Microscope 100x objective and 10x/25 objective (Carl Zeiss MicroImaging, Inc., USA). For in situ hybridization, we used two clones from a cDNA library constructed in pCMVsport6.0 plasmid vector using total RNA isolated from adult females and males pool of An. darlingi collected in Coari, Amazonas state, Brazil (GenBank accession numbers: Actin_Ad1 JQ307420 and Actin_Ad2 JQ307421). We labeled the isolated DNA with Cy5-AP3-dUTP (GE Healthcare UK Ltd., Buckinghamshire, England) or with Biotin-16-dUTP using a modified Nick-Translation Mix protocol (Roche Applied Science) and hybridized to the chromosomes at 39°C overnight in hybridization solution (Invitrogen Corporation, Carlsbad, CA, USA). We detected fluorescent signals using an ACCORD Automatic Fluorescent Imaging System (BioView (USA), Inc., Billerica, MA, USA) and determined localization within a subdivision, using a standard cytogenetic map for An. darlingi (Rafael et al., 2010).
In this study, two cDNA clones were mapped to polytene chromosomes of An. darlingi using fluorescence (FISH) and non-fluorescence in situ hybridization. The new cytogenetic photomap of An. darlingi (Rafael et al., 2010) allowed, for the first time, the identification of chromosomal positions of the probes within subdivisions. The probe pl05-A04 (GenBank accession number: Actin_Ad1 JQ307420) was mapped to section 21A on the left arm of chromosome 2 of An. darlingi (Fig. 1A). The clone pl17-G06 (GenBank accession number: Actin_Ad2 JQ307421) was hybridized to section 23B on 2L (Fig. 1B). We used TBLASTX to search against transcripts of the AgamP3.6 Gene Build, which is available at VectorBase (Lawson et al., 2009), to identify homologous sequences in An. gambiae. Accordingly, the An. darlingi cDNA clone pl05-A04 (1378 bp) (GenBank accession number: Actin_Ad1 JQ307420) had the highest similarity to the An. gambiae actin gene AGAP011514 (e-value=8e-15). The An. darlingi cDNA clone pl17-G06 (779 bp) (GenBank accession number: Actin_Ad2 JQ307421) had the highest similarity to the An. gambiae actin genes AGAP011516 and AGAP005095 (e-value=1e-146). Gene AGAP005095 is located in subdivision 21D of the 2L arm. Genes AGAP011516 and AGAP011514 are located close to each other in the An. gambiae genome, in subdivision 43C of 3L arm (Lawson et al., 2009). However, the homologous sequences of pl05-A04 and pl17-G06 are separated by four cytological subdivisions on the An. darlingi 2L chromosome (Fig. 2). These results suggest that tandem organization of the actin genes was disrupted by inversions or transpositions in the An. darlingi lineage. A previous study demonstrated that paracentric inversions and whole-arm translocations are the major types of chromosome rearrangements in Anopheles (Xia et al., 2010).
Fig. 1.
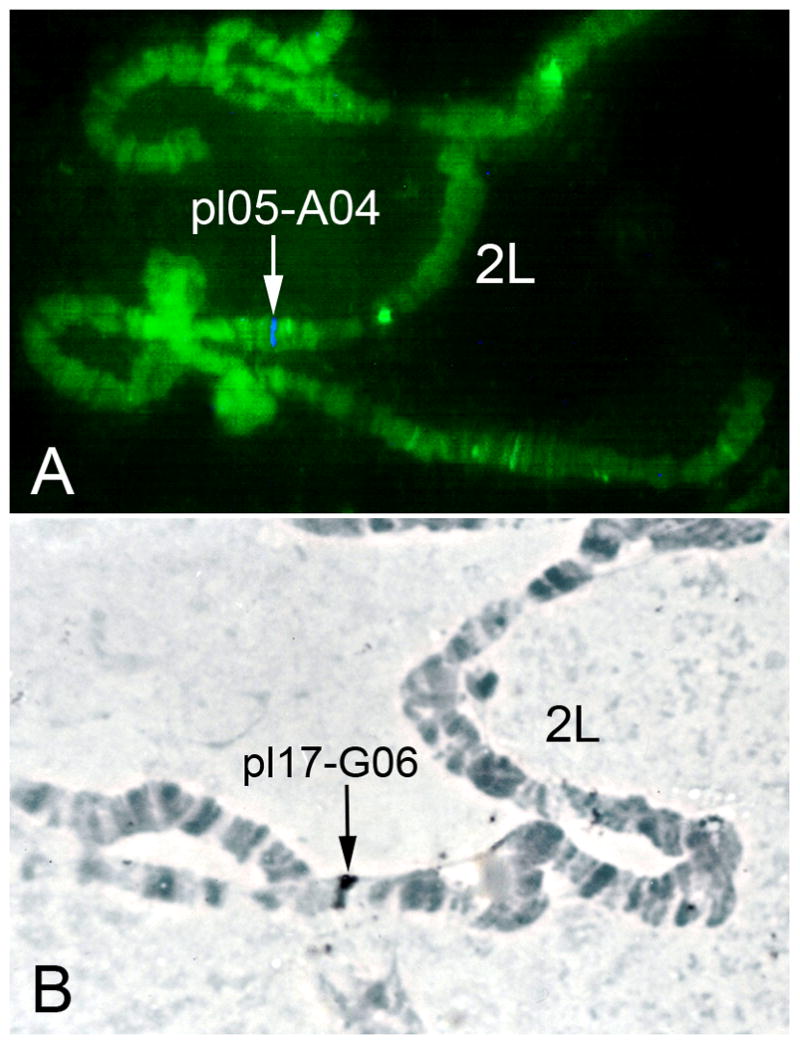
Mapping of actin genes to polytene chromosomes of the malaria mosquito An. darlingi. A) FISH of An. darlingi cDNA pl05-A04 labeled with Cy5. B) Non-fluorescent in situ hybridization of An. darlingi cDNA pl17-G06 labeled with biotin. Arrows indicate the signal of hybridization in subdivision 21A, 2L (A) and subdivision 23B, 2L (B).
Fig. 2.
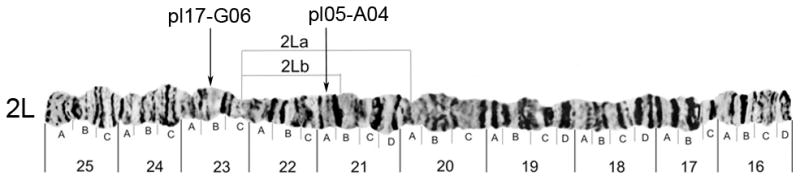
The photomap of chromosome arm 2L of An. darlingi (Rafael et al., 2010) showing the positions of pl05-A04 and pl17-G06 in relation to the polymorphic inversions 2La and 2Lb.
On the 2L chromosome photomap of An. darlingi, breakpoints of the inversion 2La are located in subdivisions 20A and 23C, and breakpoints of a complex inversion 2Lab are located in subdivisions 21B and 23C. Of the two clones containing actin genes, pl05-A04 was mapped inside both inversions and close to the 2Lb proximal breakpoint (Fig. 2). Clone pl17-G06 was mapped outside these inversions. Reduced recombination and selection can influence loci within inversions or near inversion breakpoints, resulting in estimates of gene flow that may depart significantly from loci located outside inversions (Lanzaro et al., 1998; Tripet et al., 2005). Therefore, we can expect higher differentiation between naturally occurring alleles of actin gene of pl05-A04 than that of actin gene of pl17-G06.
According to the mapping of actin genes, arm 2L of An. darlingi is homologous to arm 3L of An. gambiae, 2L in An. stephensi, 3L in An. funestus, and 3R in An. albimanus (Cornel and Collins 2000; Xia et al., 2010). The results indicate that whole-arm translocations are common not only in subgenus Cellia (An. gambiae, An. stephensi, An. funestus) (Xia et al., 2010) but also in subgenus Nyssorhynchus (An. albimanus, An. darlingi). Previously we mapped rDNA to the proximal end (5C region) of the X chromosome of An. darlingi (Rafael et al., 2003). rDNA is also found in the X heterochromatin of An. gambiae and other mosquitoes (Collins et al., 1987; Rafael et al., 2006). The physical location of the Hsp70-12A and Hsp70-14A genes on 2R chromosome (subdivisions 12A and 14A) of An. darlingi was also useful for establishing chromosome homology (Rafael et al., 2004). Hsp70 has been also mapped to two locations on 2R in An. albimanus (11C and 13C) (Benedict et al., 1993) and to three locations on 2R in An. gambiae indicating that 2R arms are homologous in multiple mosquito species. Thus, physical maps are a useful tool for establishing chromosome arm homology and evolutionary genomics studies among Anopheles species.
Acknowledgments
We thank BSP 7E Fellowhip - Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, PPP / CT-INFRA / Fundação de Amparo à Pesquisa do Estado do Amazonas – FAPEAM / Ministério da Ciência e Tecnologia (MCT)/CNPq, PROCAD Amazônia / CAPES and Projeto Institucional Prj-14 – INPA/MCT, Manaus, AM, Brazil to M. Rafael as well as grant from National Institute of Health grant 1R21AI094289 to Igor V. Sharakhov, and Pronex – Rede Malaria / FAPEAM, CT-Petro to W. P. Tadei. Further, we thank Dr. Renato Vicentini from Bioinformatica e Biologia de Sistemas – CBMEG from Universidade Estadual de Campinas – UNICAMP, Brazil, Dr. Felipe Rodrigues da Silva from Embrapa Bioinformática Agropecuária, UNICAMP, Dr. Jacqueline da Silva Batista from Laboratório Temático de Biologia Molecular and PP-G GCBEv / INPA, Brazil, Dr. Vera Lúcia da Silva Valente Gaiesky from Universidade Federal do Rio Grande do Sul, Brazil and MS.c Mauro de Freitas Ortiz from PP-G GCBEv, INPA for technical support.
References
- Benedict MQ, Cockburn AF, Seawright JAN. The Hsp70 heat-shock gene family of the mosquito Anopheles albimanus. Insect Molecular Biology. 1993;2:93–102. doi: 10.1111/j.1365-2583.1993.tb00130.x. [DOI] [PubMed] [Google Scholar]
- Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. American Journal Tropical Medicine and Hygiene. 1987;37:37–41. doi: 10.4269/ajtmh.1987.37.37. [DOI] [PubMed] [Google Scholar]
- Cornel AJ, Collins FH. Maintenance of chromosome arm integrity between two Anopheles mosquito subgenera. Journal of Heredity. 2000;91:364–370. doi: 10.1093/jhered/91.5.364. [DOI] [PubMed] [Google Scholar]
- dos Santos JMM, Lobo JA, Tadei WP, Contel EPB. Intrapopulational genetic differentiation in Anopheles (N.) darlingi Root, 1926 (Diptera : Culicidae) in the Amazon region. Genetics and Molecular Biology. 1999;22:325–331. [Google Scholar]
- Fu G, Rosemary SL, Derric N, et al. Female-specific flightless phenotype for mosquito control. Proceedings of the National Academy of Sciences USA. 2010;107:4550–4554. doi: 10.1073/pnas.1000251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyrberg C, Ryan L, Kenton M, Fyrberg E. Genes encoding actin-related proteins of Drosophila melanogaster. Journal of Molecular Biology. 1994;241:498–503. doi: 10.1006/jmbi.1994.1526. [DOI] [PubMed] [Google Scholar]
- Gilman RH, Pinedo-Cancino V, Sheen P, Tarazona-Santos E, Oswald WE, Jeri C, Vittor AY, Patz JAN. Limited diversity of Anopheles darlingi in the peruvian Amazon region of Iquitos. American Journal of Tropical Medicine and Hygiene. 2006;75:238–245. [PMC free article] [PubMed] [Google Scholar]
- He M, Haymer DS. The actin gene family in the oriental fruit fly Bactrocera dorsalis. Muscle specific actins. Insect Biochemical Molecular Biology. 1994;24:891–906. doi: 10.1016/0965-1748(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Hennessey ES, Drummond DR, Sparrow JC. Molecular genetics of actin function. Biochemical Journal. 1993;291 ( Pt 3):657–671. doi: 10.1042/bj2910657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzer RD, Kitzmiller JB, Rabbani MG. The salivary gland chromosomes of Anopheles argyritarsis compared with those of certain other species in the subgenus Nyssorhynchus. Mosquito News. 1975:35. [Google Scholar]
- Lanzaro GC, Toure YT, Carnahan, et al. Complexities in the genetic structure of Anopheles gambiae populations in west Africa as revealed by microsatellite DNA analysis. Proceedings of the National Academy of Sciences U S A. 1998;95:14260–14265. doi: 10.1073/pnas.95.24.14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D, Peter A, Peter A, et al. VectorBase: a data resource for invertebrate vector genomics. Nucleic Acids Research. 2009;37:D583–587. doi: 10.1093/nar/gkn857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Marinotti O, Krzywinski J, Tadei WP, James AA, Achee NL, Conn JE. Complete mtDNA genomes of Anopheles darlingi and an approach to anopheline divergence time. Malaria Journal. 2010;9:127. doi: 10.1186/1475-2875-9-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD, Selden SC, Maupin P. Interaction of actin filaments with microtubules. Journal of Cell Biology. 1984;99:33s–37s. doi: 10.1083/jcb.99.1.33s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafael MS, Tadei WP, Recco-Pimentel SM. Location of ribosomal genes in the chromosomes of Anopheles darlingi and Anopheles nuneztovari (Diptera, Culicidae) from the Brazilian Amazon. Memorias Instituto Oswaldo Cruz. 2003;98:629–635. doi: 10.1590/s0074-02762003000500008. [DOI] [PubMed] [Google Scholar]
- Rafael MS, Tadei WP, Hunter FF. The physical gene Hsp70 map on polytene chromosomes of Anopheles darlingi from the Brazilian Amazon. Genetica. 2004;121:89–94. doi: 10.1023/b:gene.0000019959.45267.d7. [DOI] [PubMed] [Google Scholar]
- Rafael MS, Santos IP, Jr, Tadei WP, Carvalho KA, Recco-Pimentel SM, Sallum MA, Forattini OP. Cytogenetic study of Anopheles albitarsis (Diptera: Culicidae) by C-banding and in situ hybridization. Hereditas. 2006;143:62–67. doi: 10.1111/j.2006.0018-0661.01926.x. [DOI] [PubMed] [Google Scholar]
- Rafael MS, Rohde C, Bridi LC, Valente Gaiesky VL, Tadei WP. Salivary polytene chromosome map of Anopheles darlingi, the main vector of neotropical malarian. American Journal of Tropical Medicine and Hygiene. 2010;83:241–249. doi: 10.4269/ajtmh.2010.09-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar CE, Hamm DM, Wesson DM, Beard CB, Kumar V, Collins FH. A cytoskeletal actin gene in the mosquito Anopheles gambiae. Insect Molecular Biology. 1994;3:1–13. doi: 10.1111/j.1365-2583.1994.tb00145.x. [DOI] [PubMed] [Google Scholar]
- Schevzov G, Lloyd C, Gunning P. High level expression of transfected beta- and gamma-actin genes differentially impacts on myoblast cytoarchitecture. Journal of Cell Biology. 1992;117:775–785. doi: 10.1083/jcb.117.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Póvoa MM, Conn JE, Schlichting CD, et al. Malaria vectors, epidemiology, and the re-emergence of Anopheles darlingi in Belem, Para, Brazil. Journal of Medical Entomology. 2003;40:379–386. doi: 10.1603/0022-2585-40.4.379. [DOI] [PubMed] [Google Scholar]
- Sharakhov IV, Serazin AC, Grushko OG, et al. Inversions and gene order shuffling in Anopheles gambiae and An. funestus. Science. 2002;298:182–185. doi: 10.1126/science.1076803. [DOI] [PubMed] [Google Scholar]
- Tadei WP, Thatcher BD, Santos JM, Scarpassa VM, Rodrigues IB, Rafael MS. Ecologic observations on anopheline vectors of malaria in the Brazilian Amazon. American Journal Tropical Medicine and Hygiene. 1998;59:325–335. doi: 10.4269/ajtmh.1998.59.325. [DOI] [PubMed] [Google Scholar]
- Tripet F, Dolo G, Lanzaro GC. Multilevel analyses of genetic differentiation in Anopheles gambiae s.s. reveal patterns of gene flow important for malaria-fighting mosquito projects. Genetics. 2005;169:313–324. doi: 10.1534/genetics.104.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyazunova I, Lan Q. Stage-specific expression of two actin genes in the yellow fever mosquito, Aedes aegypti. Insect Molecular Biology. 2004;13:241–249. doi: 10.1111/j.0962-1075.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- Xia A, Sharakhova M, Leman S, Tu Z, Bailey J, Smith C, Sharakhov IV. Genome landscape and evolutionary plasticity of chromosomes in malaria mosquitoes. PLoS ONE. 2010;5:e10592. doi: 10.1371/journal.pone.0010592. [DOI] [PMC free article] [PubMed] [Google Scholar]


