Abstract
Endovascular treatment of very small aneurysms is technically difficult, although recent advances with coils, microcatheters and adjunctive techniques such as balloon- or stent-assisted coiling have improved the outcomes. The microangiographic fluoroscope (MAF) is a new high-resolution x-ray detector developed for neurointerventional procedures in which superior resolution is required within a small field of view. We report the successful coil embolization of a very small ruptured anterior communicating artery aneurysm using the MAF technique. The use of the MAF facilitated the precision of the coiling procedure and was helpful in preventing catheter- and coil-related intraprocedural complications.
INTRODUCTION
Endovascular treatment of very small aneurysms (≤3 mm) is technically challenging and high rates of intraprocedural rupture have been reported.1-3 Recent advances with coils and microcatheters and the use of adjunctive techniques such as balloon- or stent-assisted coiling have improved the outcomes of endovascular treatment of these aneurysms.4-6 The microangiographic fluoroscope (MAF) is a new ultrahigh resolution x-ray detector developed for neurointerventional procedures in which superior resolution (compared with that afforded by standard x-ray image intensifiers) is required within a small field of view (FOV).7-9 We report the results of an initial clinical application of the MAF during coil embolization of a very small ruptured anterior communicating artery (AcomA) aneurysm.
CASE DESCRIPTION
An individual in their early 30s presented with fever, photophobia, meningismus and altered level of consciousness 10 days after experiencing a sudden severe headache. A cranial CT scan was negative for subarachnoid hemorrhage; however, a lumbar puncture revealed xanthochromia in the cerebrospinal fluid without evidence of meningitis. On examination the patient was somnolent but arousable with no focal deficits. The Glasgow Coma Scale score was 13. A magnetic resonance (MR) angiogram showed severe vasospasm bilaterally in the anterior cerebral arteries without an obvious aneurysm. Catheter-based angiography confirmed severe vasospasm in the bilateral internal carotid artery (ICA) termini, A1 segments of the anterior cerebral arteries and M1 segments of the middle cerebral artery. Treatment with intra-arterial verapamil and angioplasty resulted in significant improvement in the patient’s vasospasm. With lessening of the vasospasm, a 2 mm AcomA aneurysm was subsequently identified (figure 1). In light of the severe spasm, the patient was considered a poor candidate for surgical clipping. The aneurysm was successfully coiled with the use of the novel institutional review board-approved MAF (see online video). The MAF was instrumental in evaluating the relationship of the microcatheter and extruding coil to provide superior control and details of intra-aneurysmal microcatheter and coil behavior (figure 2). Two Galaxy coils (2.5 mm32.5 cm and 2 mm31.5 cm; DePuy/Codman & Shurtleff, Johnson & Johnson, Warsaw, Indiana, USA) were placed through a Prowler select LP ES microcatheter (DePuy/Codman & Shurtleff, Johnson & Johnson). The patient did well and made a full neurologic recovery. Follow-up angiography at 6 months showed complete occlusion of the aneurysm.
Figure 1.
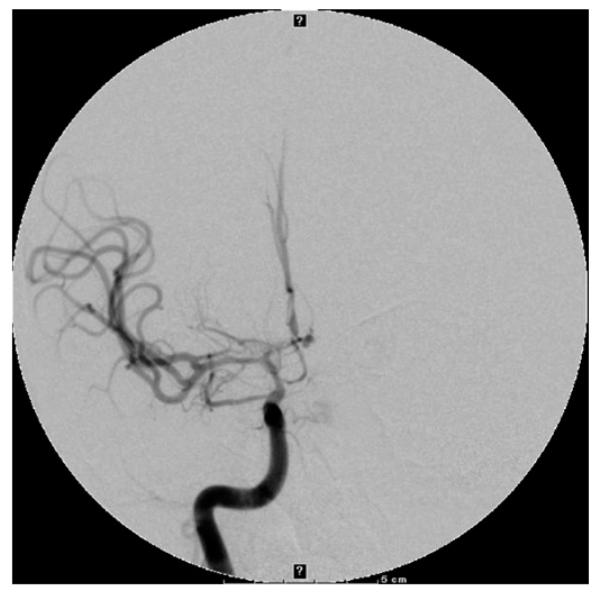
Digital subtraction angiogram, right internal carotid artery injection, anteroposterior view, showing a 2 mm anterior communicating artery aneurysm and evidence of vasospasm of the internal carotid artery terminus, A1 and M1.
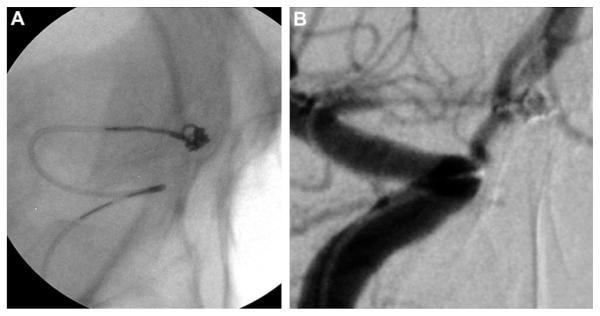
Figure 2.
(A) Still image during coil embolization with use of microangiographic fluoroscope detailing coil, the microcatheter and its distal marker, and the interface between the microcatheter and the coil. (B) Working anteroposterior view during coil embolization with standard fluoroscopy. Compared with (A), the resolution of the microcatheter and the coil is much lower.
MICROANGIOGRAPHIC FLUOROSCOPE AND IMAGING TECHNIQUES
The MAF detector has four major components arranged as shown in figure 3: a 300 mm cesium iodide input phosphor, a dual-stage second-generation microchannel plate light image intensifier (LII) attached to a minifying fiberoptic taper coupled to a charged-coupled device chip (CCD). The phosphor, which is thinner than that used in conventional detectors, has less light spreading, hence higher resolution. The light from the phosphor is converted in the initial photocathode of the LII into electron beams that are accelerated through a high voltage applied across the length of each parallel microchannel, thereby enabling the electrons to collide with the walls of the microchannels with enough energy to knock off additional electrons, which are in turn accelerated. The multiple collisions amplify the electron beam which collides with the thin high-resolution output phosphor of the LII resulting in an amplified optical image. The amplification in the LII is up to three orders of magnitude, easily making up for any phosphor light losses in the subsequent fiberoptic taper and CCD, and thus allowing the use of the detector in fluoroscopic as well as angiographic exposureranges. The fiberoptic taper enlarges the FOVof the CCD as well as the 12 μm CCD pixel size to a 35 μm effective pixel size, which is substantially smaller than the 150–200 μm size of a conventional x-ray image intensifier or flat panel imager, thus enabling higher resolution for the MAF, although over a smaller total FOV of 3.5 cm diameter. The MAF detector is attached to the anteroposterior C-arm of an x-ray image intensifier biplane angiographic unit and is controlled by custom-designed LabVIEW-based software designated CAPIDS (Control, Acquisition, Processing, and Image Display System).9 The MAF was brought into place over the fluoroscope tube only when superior resolution over a small FOV was necessary, such as during catheterization and coil embolization of the aneurysm. The C-arm system automatically collimates the x-ray beam to the detector active area where the catheterization and coil embolization took place to reduce the dose to the patient. Optimal image quality is obtained when the detector was used with a small x-ray focal spot and minimum geometric magnification. Despite the increase in exposure parameters (about twice those selected by the x-ray system) due to the smaller pixels and the reduced x-ray absorption of the thinner phosphor, the effective dose to the patient is actually reduced by more than 10-fold compared with the dose from a standard commercial x-ray neuroimaging system as a result of the small irradiation FOV of the MAF.10 Road mapping, digital angiography and digital subtraction angiography are also available for clinical use with the MAF system.
Figure 3.
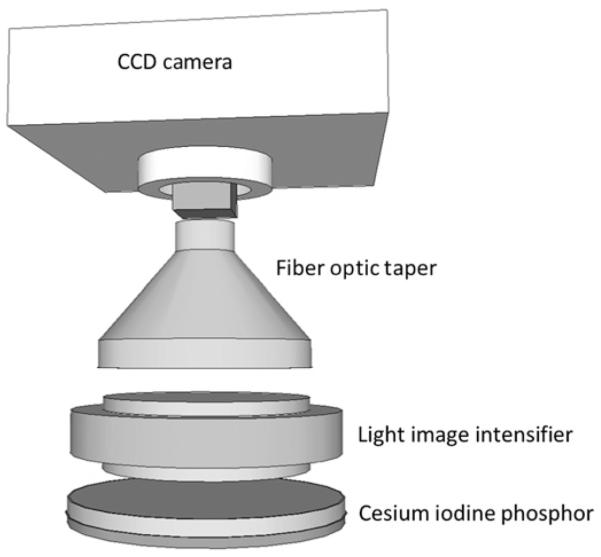
The four major components of the microangiographic fluoroscope and their coupling. CCD, charged-coupled device chip.
DISCUSSION
Endovascular treatment of very small aneurysms is technically difficult. Small aneurysms tend to have a thin friable wall and a limited space for microcatheter placement and coil embolization, leading to difficult coiling and higher rates of procedural rupture than for larger aneurysms.1,11 Doerfler et al1 reported the procedural rupture rate to be five times higher with aneurysms <3 mm. Recent advances in endovascular tools and techniques have allowed endovascular treatment of very small aneurysms with improved results.4-6 In a large series of 196 patients with aneurysms of ≤3 mm, van Rooij et al6 reported a procedure-related morbidity rate of 3.2%, which was not significantly different from the rate for larger aneurysms. Softer and complex coils, softer microcatheter tips and shorter stiff zones for detachment of coils are thought to reduce the risk of microcatheter- or coil-related rupture associated with the treatment of small aneurysms.12 Adjunctive techniques, such as balloon- and stent-assisted coiling, help to prevent coil herniation from the small aneurysm dome and the use of the jailing technique can provide additional stability to the microcatheter.4-6,13
However, as the tools and techniques for neurointervention continue to improve, we should not forget the parallel need for improvement in imaging techniques as a synergistic means to enhance our treatment ability. Microangiography represents one of the newest tools in our imaging armamentarium. Compared with a standard fluoroscope, the MAF provides a much higher image resolution in a small field, enabling superior road map navigation, and is ideal for aneurysm catheterization and coiling.7 In the case presented here, the resolution achieved allowed us to see the coils and the microcatheter tip in extreme detail, allowing a much better appreciation of microcatheter and coil behavior in and around the aneurysm for safe deployment of coils in a very small space. In our case, even the slightest movements of the microcatheter and coils were seen under microangiography, allowing us to reduce the risk of inadvertent perforation of the aneurysm dome by the microcatheter or coils. With the higher image resolution afforded by the MAF, we could also visualize any kickback of the microcatheter and make appropriate adjustments before the catheter actually dislodged out of the aneurysm, which occurs more frequently in the treatment of small aneurysms. Moreover, the superior visualization of the microcatheter under microangiography allowed us to position and reposition the microcatheter with greater precision, which is especially important because we tried to select the separate compartments of this small aneurysm with the microcatheter to achieve an optimal embolization result.
The generally accepted way to describe the effects of dose on living tissue is to use the concept of ‘effective dose’,14 which is calculated by multiplying actual organ doses by risk-weighting factors (which give each organ’s relative radiosensitivity to developing pathology such as cancer) and summing the values to provide a total whole body effective dose. Reduction of the irradiation field when using the MAF results in a substantial decrease in the effective dose to the patient.
As previously stated, the MAF is able to offer equivalent or superior images at fluoroscopic levels; however, if even finer detail such as intracranial stent structures and improved guidance is needed, the x-ray exposures could be increased, resulting in increased skin entrance exposure over a small localized region. In these specific cases, if the procedure is lengthy or overlapping the eye, frame rate reduction or C-arm angle repositioning for dose spreading may be recommended.
Despite its utility in aneurysm embolization, micro-angiography is still in its infancy. We hope future iterations of the system will enhance and broaden its applications, extending its use to procedures that require a larger FOV such as arterio-venous malformation embolizations. Our anticipation is that MAF will revolutionize procedural safety and effectiveness in a similar way to the introduction of the operative microscope to open neurosurgery (particularly vascular neurosurgery), while at the same time actually reducing total x-ray doses that are delivered intraprocedurally to the patient.
CONCLUSIONS
Endovascular embolization of very small aneurysms is challenging, although recent advances in endovascular materials and techniques have improved its safety and efficacy. The MAF is a high-resolution x-ray detector developed to provide a superior resolution over a small FOV compared with the standard biplane fluoroscope. In the case presented here, use of the MAF facilitated the precision of the coiling procedure and was helpful in preventing catheter- and coil-related intraprocedural rupture.
Supplementary Material
Acknowledgments
The authors thank Paul H Dressel for videography and preparation of the illustrations and Debra J Zimmer for editorial assistance. The microangiographic fluoroscope was developed at the University at Buffalo-Toshiba Stroke Research Center.
Funding National Institutes of Health (NIH) grants R01NS43924 and R01EB002873 provided for this and other related research.
Competing interests CNI and AJ receive research support from NIH grants R01NS43924 and R01EB002873. EIL receives research grant support, other research support (devices) and honoraria from Boston Scientific (Boston Scientific’s neurovascular business has been acquired by Stryker) and research support from Codman & Shurtleff and ev3/Covidien Vascular Therapies; has ownership interests in Intratech Medical Ltd and Mynx/Access Closure; serves as a consultant on the board of Scientific Advisors to Codman & Shurtleff; serves as a consultant per project and/or per hour for Codman & Shurtleff, ev3/Covidien Vascular Therapies and TheraSyn Sensors; and receives fees for carotid stent training from Abbott Vascular and ev3/Covidien Vascular Therapies. EIL receives no consulting salary arrangements; all consulting is per project and/or per hour. SR receives research support from NIH grants R01NS43924 and R01EB002873 (principal investigator) and equipment from Toshiba Corporation. AHS receives research support from NIH grants R01NS43924 and R01EB002873 (co-investigator), research grants from the National Institutes of Health (co-principal investigator: NINDS 1R01NS064592-01A1) and the University at Buffalo (Research Development Award); holds financial interests in Hotspur, Intratech Medical, StimSox and Valor Medical; serves as a consultant to Codman & Shurtleff, Concentric Medical, ev3/Covidien Vascular Therapies, GuidePoint Global Consulting and Penumbra; belongs to the speakers’ bureaus of Codman & Shurtleff and Genentech; serves on an advisory board for Codman & Shurtleff; and has received honoraria from American Association of Neurological Surgeons’ courses, an Emergency Medicine Conference, Genentech, Neocure Group and from Abbott Vascular and Codman & Shurtleff for training other neurointerventionists in carotid stenting and for training physicians in endovascular stenting for aneurysms. AHS receives no consulting salary arrangements; all consulting is per project and/or per hour. PK and PY have no financial relationships.
Footnotes
Ethics approval The Health Sciences Institutional Review Board, University at Buffalo, State University of New York (Buffalo, New York, USA) does not require approval in conjunction with a case report/technical note. However, the use of microangiographic fluoroscopy was approved by the board. The principles of the Declaration of Helsinki were followed.
Contributors PK, SR, AHS: concept and design; PK, AHS, CNI, AJ: acquired the data; PK, AHS, CNI, AJ: analyzed and interpreted the data; PK: drafted the manuscript; all authors made critical revisions of the manuscript for important intellectual content, reviewed the revisions and approved the final version of the manuscript.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Doerfler A, Wanke I, Egelhof T, et al. Aneurysmal rupture during embolization with Guglielmi detachable coils: causes, management, and outcome. AJNR Am J Neuroradiol. 2001;22:1825–32. [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen TN, Raymond J, Guilbert F, et al. Association of endovascular therapy of very small ruptured aneurysms with higher rates of procedure-related rupture. J Neurosurg. 2008;108:1088–92. doi: 10.3171/JNS/2008/108/6/1088. [DOI] [PubMed] [Google Scholar]
- 3.Sluzewski M, Bosch JA, van Rooij WJ, et al. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg. 2001;94:238–40. doi: 10.3171/jns.2001.94.2.0238. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Feng H, Tang W, et al. Endovascular treatment of very small intracranial aneurysms. Surg Neurol. 2008;70:30–5. doi: 10.1016/j.surneu.2007.05.059. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki S, Kurata A, Ohmomo T, et al. Endovascular surgery for very small ruptured intracranial aneurysms. Technical note. J Neurosurg. 2006;105:777–80. doi: 10.3171/jns.2006.105.5.777. [DOI] [PubMed] [Google Scholar]
- 6.van Rooij WJ, Keeren GJ, Peluso JP, et al. Clinical and angiographic results of coiling of 196 very small (< or = 3 mm) intracranial aneurysms. AJNR Am J Neuroradiol. 2009;30:835–9. doi: 10.3174/ajnr.A1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binning MJ, Orion D, Yashar P, et al. Use of the microangiographic fluoroscope for coiling of intracranial aneurysms. Neurosurgery. 2011;69:1131–8. doi: 10.1227/NEU.0b013e3182299814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel V, Hoffmann KR, Ionita CN, et al. Rotational micro-CT using a clinical C-arm angiography gantry. Med Phys. 2008;35:4757–64. doi: 10.1118/1.2989989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Ionita CN, Keleshis C, et al. Progress in the development of a new angiography suite including the high resolution micro-angiographic fluoroscope (MAF), a control, acquisition, processing, and image display system (CAPIDS), and a new detector changer integrated into a commercial C-arm angiography unit to enable clinical use. Proc SPIE. 2010;7622:76225I–cit. doi: 10.1117/12.844909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gill K, Bednarek DR, Rudin S. Evaluation of effective dose in region-of-interest neuroimaging (abstract SS-GG-I-74) Med Phys. 2010;37:3118. [Google Scholar]
- 11.Brinjikji W, Lanzino G, Cloft HJ, et al. Endovascular treatment of very small (3 mm or smaller) intracranial aneurysms: report of a consecutive series and a meta-analysis. Stroke. 2010;41:116–21. doi: 10.1161/STROKEAHA.109.566356. [DOI] [PubMed] [Google Scholar]
- 12.Lim YC, Kim BM, Shin YS, et al. Structural limitations of currently available microcatheters and coils for endovascular coiling of very small aneurysms. Neuroradiology. 2008;50:423–7. doi: 10.1007/s00234-008-0365-y. [DOI] [PubMed] [Google Scholar]
- 13.Fang C, Li MH, Zhu YQ, et al. The effectiveness and feasibility of endovascular coil embolization for very small cerebral aneurysms: mid- and long-term follow-up. Ann Vasc Surg. 2010;24:400–7. doi: 10.1016/j.avsg.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 14.International Commission on Radiological Protection . Annals of the ICRP. Vol. 21. Oxford: Pergamon Press (now Elsevier); New York: 1991. IRCP Publication 60: 1990 Recommendations of the International Commission on Radiological Protection; pp. 1–3. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.