Abstract
Maternal mood disorders such as depression and chronic anxiety can negatively affect the lives of both mothers and their adult offspring. An active focus of maternal depression and anxiety research has been the role of chronic social stress in the development of these disorders. Chronic exposure to social stress is common in humans, especially in lactating mothers, and postpartum mood disorders have been correlated with high levels of social conflict and low levels of social support. Recent studies have described an effective and ethologically relevant chronic social stress (CSS) based rodent model for postpartum depression and anxiety. Since CSS attenuates maternal behavior and impairs both dam and offspring growth, it was hypothesized that CSS is an ethologically relevant form of early life stress for the developing female offspring and may have effects on subsequent adult maternal behavior and neuroendocrinology Dams exposed to early life CSS as infants display substantial increases in pup retrieval and nursing behavior that are specifically associated with attenuated oxytocin, prolactin, and vasopressin gene expression in brain nuclei involved in the control of maternal behavior. Since the growth patterns of both groups were similar despite substantial increases in nursing duration, the early life CSS dams exhibited an attenuated nursing efficiency. It is concluded that early life CSS has long term effects on the neuroendocrinology of maternal care (oxytocin and prolactin) which results in decreased nursing efficiency in the adult dams. The data support the use of early life CSS as an effective model for stress-induced impairments in nursing, such as those associated with postpartum depression and anxiety.
INTRODUCTION
Exposure to stress is a known predictor of mood disorders, including postpartum depression (Heim et al. 1997; Hammen 2005; Ross and Dennis 2009; Murgatroyd et al. 2010). Despite evidence that depression in the mother has negative effects on both parenting (Lovejoy et al. 2000) and psychological development of the offspring (Goodman 2007) little is known, about the effects of postpartum depression as an early life stress on adult behavior Recent rodent studies have been focusing on the use of ethologically relevant stressors, such as chronic social stress (CSS), in the study of the effects of stress on mood disorders (Herzog et al. 2009; Brunton and Russell 2010; Nephew and Bridges 2011) It is postulated that CSS may be a relevant and effective form of early life stress to study the transgenerational effects of mood disorders on the offspring.
Chronic social stress (CSS) during lactation can affect maternal behavior and growth of the dam and offspring. The daily presentation of a novel male intruder during lactation decreases maternal care, increases maternal aggression, and inhibits growth in both the dam and her offspring (Nephew and Bridges 2011) These effects indicate that CSS is an ethologically relevant model for mood disorders that involve impairments in maternal care and attenuated offspring growth, such as postpartum depression. Both the maternal care and offspring growth data suggest that CSS may have enduring effects on the offspring, and research on the effects of chronic stress on maternal behavior support the hypothesis that the effects of chronic stress on maternal behavior are hormonally mediated.
Several neurohormones have been implicated in the etiology of stress-related mood disorders and/or the control of maternal behavior in both rodents and humans, including oxytocin (OXT), prolactin (PRL), arginine vasopressin (AVP), and corticosteroid releasing hormone (CRH). OXT mediates both maternal care (Pedersen and Prange Jr. 1979; Pedersen et al. 1982; Champagne et al. 2001; Pedersen and Boccia 2003) and maternal aggression (Giovenardi et al. 1998; Elliot et al. 2001; Bosch et al. 2004; Bosch et al. 2005; Nephew et al. 2009; Bosch and Neumann 2011) in rodents In humans, plasma OXT during pregnancy is negatively associated with postpartum depression, as low plasma OXT suggests an increased risk for PPD (Skrundz et al. 2011). Increased levels of OXT are associated with increased maternal attachment, and serum OXT in mothers with securely attached children is higher during mother-child interactions when compared to insecure mothers (Strathearn et al. 2009). Children raised by their biological parents have higher OXT levels compared to those that were exposed to early neglect (Fries et al. 2005), suggesting that early experience affects the development of central social behavior mechanisms. Several reports have also implicated PRL in the control of rodent maternal behavior (Bridges et al. 1990; Bridges and Ronsheim 1990; Bridges and Mann 1994; Bridges et al. 2001), and low plasma levels of PRL are associated with postpartum depression in humans (Abou-Saleh et al. 1998). Central AVP mediates rodent maternal care (Bosch and Neumann 2008; Nephew and Bridges 2008; Nephew and Bridges 2008) and maternal aggression (Nephew and Bridges 2008; Gutzler et al. 2010; Nephew et al. 2010), and is elevated in humans suffering from depression and animal models of depression (Goekoop et al. 2006; Surget and Belzung 2008; Rotzinger et al. 2010). Studies in male mice have identified an epigenetic mechanism for the effects of early life stress on central vasopressin and behavioral alterations associated with depression (Murgatroyd et al. 2009). Corticosteroid releasing hormone (CRH), which increases in response to stress, inhibits maternal care in rats (Pedersen et al. 1991) and primates (Saltzman et al. 2011) and suppresses maternal aggression in mice (Gammie et al. 2004). It has also been a primary target for the development of treatments for stress-induced mood disorders (Heim et al. 1997).
The current investigation compares the maternal behavior and neuroendocrinology of adult female offspring of dams exposed to CSS during infancy to the adult offspring of control dams. Similar studies focusing on the exposure of male rodents to early maternal separation have reported significant depression-associated behavioral and neuroendocrine effects that are mediated by epigenetic mechanisms (Murgatroyd et al. 2009; Der-Avakian and Markou 2010). Since the pups are left in the cage during the CSS protocol and there were significant effects on dam growth and behavior and offspring growth (Nephew and Bridges 2011), it was hypothesized that CSS is an ethologically relevant form of early life stress for the female offspring and may have long term effects on subsequent adult maternal behavior and central OXT, PRL, AVP and/or CRH mRNA expression.
METHODS
Animals
Animals in this study were maintained in accordance with the guidelines of the Committee of the Care and Use of Laboratory Animals Resources, National Research Council, and the research protocol was approved by the Tufts Institutional Animal Care and Use Committee.
The mothers of the Sprague Dawley rat dams in the current study (Charles River, Wilmington, MA) were subjected to a chronic social stress protocol from days 2 to 16 of lactation as reported in Nephew and Bridges 2011 This procedure consisted of placing a similarly sized (220–300g) novel male intruder into a lactating female’s home cage for one hour from day 2 to 16 of lactation. The pups were left in the cage during the intruder presentation, and the CSS exposure resulted in decreased maternal care and growth of the both the dam and her pups, and increased maternal aggression (Nephew and Bridges 2011) “CSS dams” refers to the adult females exposed to chronic social stress during lactation, and “early life CCS dams” refers to the adult female offspring of the CSS dams from Nephew and Bridges 2011 who were exposed to CSS when they were 2–16 days old; the focus of the present study.
Three females from eight control and eight chronic social stress dams were housed in groups of three until 70 days of age when they were mated (24 females each for the control and early life CSS groups). Mating consisted of placing a male in each cage for eight days. Three of the control females and five of the early life CSS females failed to give birth. One stressed dam died during parturition, and another early life CSS dam failed to nurse. Two animals from each group did not give birth in time to be included in the study. The final sample sizes were 18 for the control group and 16 for the stressed group. Total pup number and litter weights were recorded on the day of parturition, and litters were then culled to four females and four males. Brains were extracted from the dams on day 21 of lactation, frozen at −80C and then micropunched to obtain samples of the paraventricular nucleus (PVN), supraoptic nucleus (SON), lateral septum (LS), central amygdala (CeA), and medial amygdala (MeA) for PCR analysis of relative mRNA levels.
Behavioral Testing
Maternal care and maternal aggression were assessed on days 2, 9, and 16 of lactation (early, mid, and late lactation) between 0900 and 1200 h in all dams to assess the effects of early life CSS at different time points during lactation A digital video camera (Panasonic PV-. GS180) allowed for behavioral observation without human interference. Maternal care testing consisted of the re-introduction of all eight pups to the home cage after a 30-minute removal, and behavior was then video recorded for 30 minutes The pup removal allowed for the assessment of pup retrieval and also partially controlled for variations in daily nursing patterns. These 30 minute behavior observations produce consistent and substantial behavioral data that is similar to observations of undisturbed maternal care over 30 and 60 minutes (Byrnes et al. 2000; Johnson et al. 2011) The pups were returned to the home cage in groups of 2–3 in the three corners distal to the nest. The latencies to retrieve pups back to the nest and initiate nursing, and frequencies and durations of pup retrieval, pup grooming, nursing, nesting, self-grooming, and general locomotor activity were scored by an observer who was blind to the treatment using ODLog behavioral analysis software (Macropod Inc., USA). The ODLog software records continuous data in 5 second bins, and also generates frequency and duration summaries for all behavioral measures over the 30-minute observation period. Frequency data represent the total number of bouts for a specific behavior during the observation period. Nursing behavior scoring was started when the dam had been motionless over the litter for longer than 10 seconds, and stopped whenever she moved off the pups. Nursing latency is defined as the period of time from the start of the maternal care observation to the initial expression of nursing behavior, and nursing duration is the cumulative nursing behavior during the 30 minute observation. Nesting was defined as manipulation of the nesting material with mouth or paws. Total maternal care included the combined durations of pup grooming, nursing and nesting. Due to the dam being in her home cage with bedding and nesting material and the location of the pups under the dam it was not possible to determine the specific nature of the interactions between the dam and individual pups (nutritive suckling, non-nutritive suckling, sleeping pups).
Following the maternal care testing, the pups were left in the cage, and a similarly sized (225–300g) novel male Sprague Dawley intruder was introduced for a 30 min maternal aggression test Males were used on average once every third day, and the dams were never exposed to the same male twice. The latency to initiate aggression and the frequency and durations of attacking (boxing or tackling), biting, kicking, pinning to the bottom of the cage, self-grooming, and locomotor activity were scored. Total aggression included attacks, bites, kicks, and pins. The mean duration of an aggressive bout was calculated by dividing the total aggression time by the total number of aggressive interactions. In addition to maternal aggression, maternal care was observed during the aggression tests and recorded as previously described.
RNA expression analyses
Total RNA and DNA were simultaneously extracted (Bettscheider et al. 2011) and reverse transcription (RT) reactions were performed on 200 ng RNA using RevertAid Premium Reverse Transcriptase (Fermentas) and oligo(dT)primer to analyze transcript levels. Quantitative PCR (qPCR) was performed on a LightCycler (Roche) using LightCycler FastStart DNA Master SYBR Green (Roche). Primer sequences and conditions for qPCR reactions are listed in Table 2. Expression levels for OXT, OXT receptor (OXT R), AVP, CRH, and the long form of the PRL receptor (PRL R)were normalized against three combined housekeeping genes, β-actin, hypoxanthine phosphoribosyltransferase (HPRT) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
TABLE 2.
Primer sequences and conditions for qPCR reactions.
| Gene | Sequence | |
|---|---|---|
| cDNA | OXT | F, tctgacctccgcctgctacatc; R, aagcagcgcctttgccgcc |
| OXT receptor | F, gtactggccttcatcgtgtgc; R, tgcagcagctgttgaggctg | |
| PRL receptor (long form) | F, gtagatggagccaggagagttc; R, accagagtcactgtcgggatct | |
| AVP | F, cagatgctcggcccgaag; R, ttccagaactgcccaagag | |
| CRH | F, cagaacaacagtgcgggctca; R, aaggcagacagggcgacagag | |
| β-actin | F, ttgctgacaggatgcagaa; R, accaatccacacagagtactt | |
| HPRT | F, tggtcaagcagtacagcccc; R, tactggccacatcaacagga | |
| GAPDH | F, catcaccatcttccaggagc; R, taagcagttggtggtgcagg |
Statistics
Mean pup bodyweight across lactation was tested using a 2-way repeated measures ANOVA with lactation days 2, 9, and 16 as the repeated factor. Maternal bodyweight on day 2 of lactation and behaviors of the control and CSS dams were compared on each lactation day with one-tailed t-tests. Relative mRNA expression levels were compared with individual t-tests on each peptide or receptor for each brain region. Pearson correlations were used to test for significant gene-gene (21 tests on the data in fig. 3+4) and gene-behavior associations (14 tests on day 9 retrieval and nursing durations and the data in fig. 3+4) using data from the adult offspring of both the control and CSS dams. Corrections for multiple comparisons were not performed due to the low number of comparisons and the focus on significant differences in gene expression and behavior. The sample sizes for the correlations were 30–34. All graphical results are presented as mean + SEM, and the level of statistical significance was p ≤ 0.05.
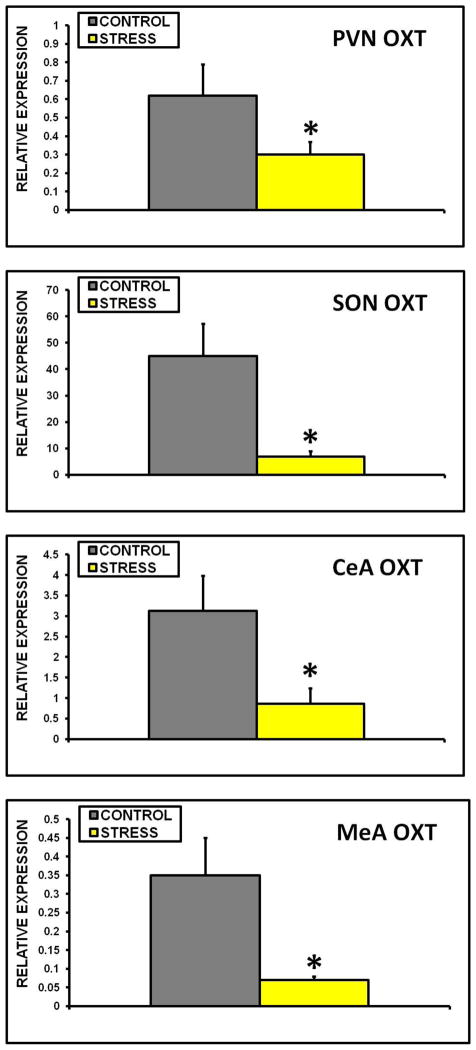
Figure 3.
Mean + SEM relative expression of OXT mRNA in the PVN (A), SON (B), CeA (C), and MeA(D) of control and early life CSS (STRESS) dams. * indicates a significant effect of treatment (t-test, p≤0.05).
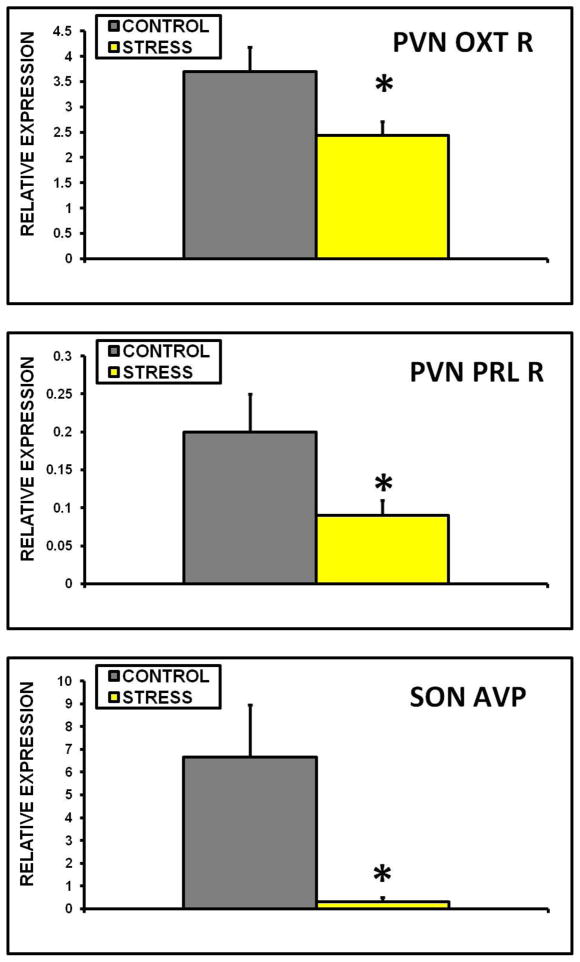
Figure 4.
Mean + SEM relative expression of OXT receptor mRNA in the PVN (A), PRL receptor (long form) mRNA in the PVN (B), and AVP mRNA in the SON (C) of control and early life CSS (STRESS) dams. * indicates a significant effect of treatment (t-test, p≤0.05).
RESULTS
Growth
Mean bodyweights of the dams on day 2 of lactation were similar (control = 304.1 ± 7.3, stress = 301.2 ± 8.7g). There were no differences between control and CSS group litters in number of pups at birth (13.85 ± 0.8 vs. 13.75 ± 0.7) or mean pup weight on days 2, 9, and 16 (table 1).
TABLE 1.
Mean pup weights on days 2, 9, and 16.
| Day 2 | Day 9 | Day 16 | |
|---|---|---|---|
| CONTROL | 6.9±0.2g | 22.4±0.4g | 44.0±0.8g |
| STRESS | 6.8±0.2g | 21.6±0.6g | 42.4±0.8g |
Mean pup weights on days 2, 9, and 16 of lactation.
Maternal Care Testing
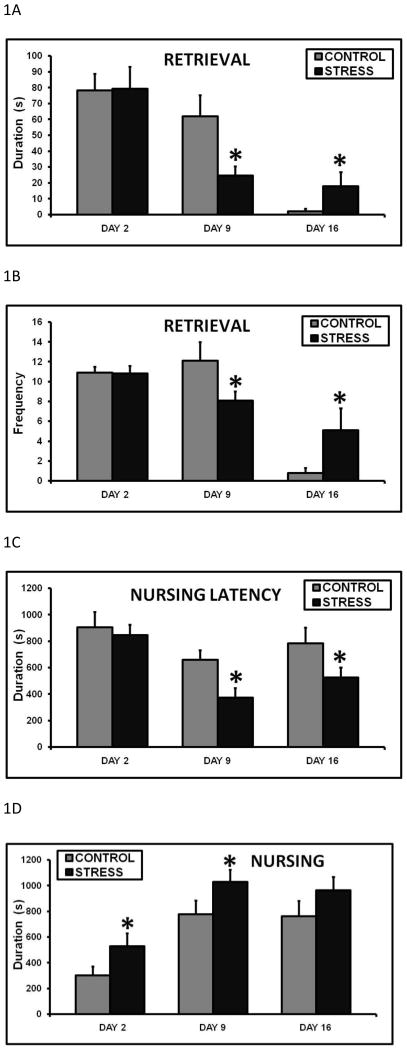
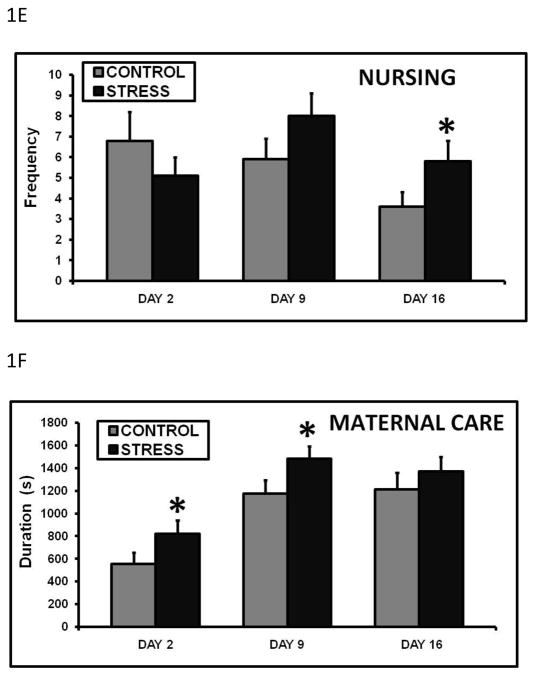
Maternal care was observed at birth, and all animals that had successful parturitions retrieved their pups to the nest, cleaned them, and began nursing. On day 9 of lactation, the early life stressed dams spent significantly less time retrieving all pups to the nest (fig. 1A). The opposite effect was observed on day 16, where the CSS treatment increased retrieval duration (fig. 1A). Due to the size and mobility of the pups at this stage, pup retrieval during late lactation is very rare (Nephew and Bridges 2011), as pups will typically pursue the dam to initiate nursing on day 16. Similar to the effects of early life CSS on retrieval duration, early life stressed dams had a significantly lower mean retrieval frequency on day 9 and an increased frequency on day 16 (fig. 1B). These effects on retrieval resulted in decreased nursing latencies on days 9 and 16 in the stressed dams (fig. 1C). It should be noted that pup retrieval is not always directly correlated to latency to nurse. Dams retrieve the pups, groom them, and then nurse. On day 9, all pups are retrieved back to the nest at the beginning of the maternal care test. A decrease in duration and frequency of retrieval on day 9 indicates that the stress dams took less time and attempts to gather the pups to the nest. This faster retrieval resulted in the pups being together in the nest sooner, which allowed the dam to initiate nursing earlier. In contrast, on day 16 retrieval is typically rare due to the mobility of the pups. There is no full retrieval at this stage as the pups do not need to be retrieved; they pursue the dam to nurse. The increased retrieval by CSS dams on day 16 also resulted in the pups being in the nest together sooner, and facilitated the decrease in nursing latency. Over the 30 minute maternal care observation, the stressed dams spent more time nursing on days 2 and 9 (fig. 1D), and nursing frequency was elevated on day 16 (fig. 1E). There was a 76% (3:48) increase in nursing duration on day 2, and a 32% (4:12) increase on day 9 compared to same day controls When the duration from days. 2, 9, and 16 are combined, the early life CSS dams spent significantly more time nursing across lactation compared to controls (2474.9 ± 224.6 vs. 1709.3 ± 210.2s, p=0.03). These changes in nursing contributed to the increase in total maternal care on days 2 and 9 (fig. 1F). Since the mean growth rates of the pups in both groups were similar, the early life CSS dams displayed an attenuated nursing efficiency; they spent more time nursing to produce pups the same size as the control dams. There were no effects of early life CSS on pup grooming duration (day 2 control = 160.6 ± 36.0, stress = 160.1 ± 43.1; day 9 control = 363.0 ± 37.0, stress = 428.1 ± 38.2; day 16 control = 447.8 ± 49.2, stress = 401.3 ± 38.8) or frequency (data not shown, all p’s > 0.2).
Figure 1.
Mean + SEM of duration of pup retrieval (A), frequency of pup retrieval (B), duration of the latency to initiate nursing (C), duration of nursing (D), frequency of nursing (E) and duration of total maternal care (pup grooming, nursing, nesting) (F) on days 2, 9, and 16 of lactation during a 30 minute maternal care observation in control and early life CSS (STRESS) dams. * indicates a significant effect of treatment (t-test, p≤0.05).
Maternal Aggression Testing
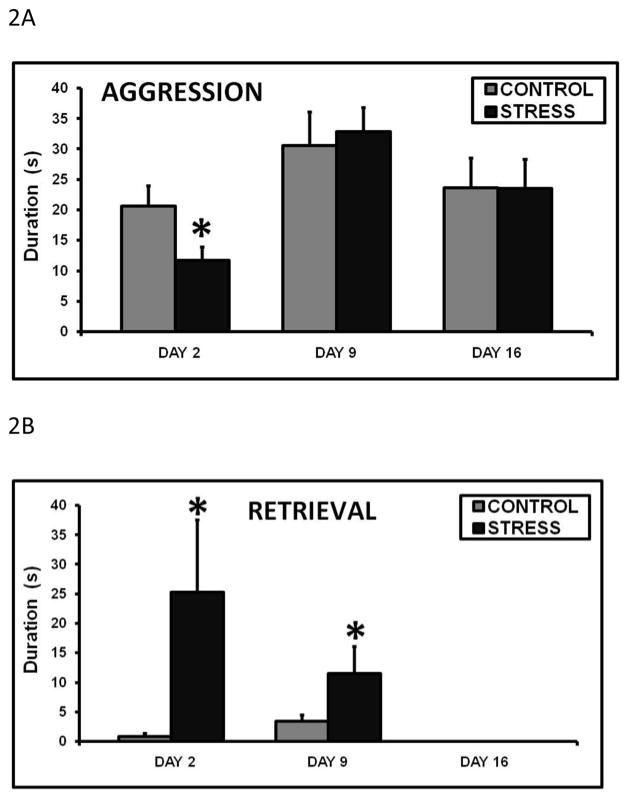
Early life CSS attenuated total aggression duration on day 2 of lactation, but had no effects on days 9 or 16 (fig. 2A). Similar to observations during the maternal care test, retrieval duration was significantly elevated in the early life CSS dams during the maternal aggression tests on days 2 and 9 (fig. 2B), which is also not commonly observed during aggression tests due to the fact that pups are either already in the nest area, or the dam is focusing her attention on the intruder male (Nephew and Bridges 2011).
Figure 2.
Mean + SEM frequency of total aggression (attacks, bites, kicks, pins) (A) and duration of pup retrieval (B) on days 2, 9, and 16 of lactation during a 30 minute maternal aggression observation in control and early life CSS (STRESS) dams. * indicates a significant effect of treatment (t-test, p≤0.05).
Gene Expression
Exposure to early life CSS depressed OXT, OXT receptor (OXT R), AVP, and PRL receptor long form (PRL R) mRNA expression in several brain regions. OXT expression was lower in the PVN, SON, CeA, and MeA of the adult dams exposed to CSS as an early life stressor (fig. 3A–D). OXT R and PRL R mRNA levels were also lower in the PVN of the stressed dams (fig. 4A+B). AVP levels were significantly lower in the SON of stressed dams (fig. 4C). There were no differences between groups in the LS, and CRH mRNA levels were similar in the control and early life CSS groups in the PVN, SON, CeA, MeA, and LS. PRL R mRNA levels were only measured in the PVN.
Correlations
There were significant gene-gene correlations between PVN OXT and PVN OXT R and PVN PRL R (negative correlation), PVN OXT R and PVN PRL R and SON OXT, and SON OXT was correlated with SON AVP and MeA OXT (table 3). All gene-behavior correlations involve the mean duration of the behavior When including both the control and early life CSS dams, there were significant gene-behavior correlations between SON OXT with day 9 retrieval and nursing (negative correlation), and MeA OXT was negatively correlated with day 9 nursing (table 4). PVN OXTR, PRL R, and SON AVP were also negatively correlated with day 9 nursing (table 4). When only the early life CSS dams were included, SON OXT, MeA OXT, PVN OXT R, and PVN PRL R were negatively correlated with day 9 nursing (table 5).
TABLE 3.
Pearson correlation results for gene-gene associations in both control and early life CSS dams.
| GENE 1 | GENE 2 | r | R2 | p |
|---|---|---|---|---|
| PVN OXT | PVN OXT R | −0.33 | 0.11 | 0.03 |
| PVN OXT | PVN PRL R | −0.42 | 0.18 | 0.01 |
| PVN OXT R | PVN PRL R | 0.71 | 0.51 | <0.01 |
| PVN OXT R | SON OXT | 0.30 | 0.09 | 0.04 |
| SON OXT | SON AVP | 0.87 | 0.76 | <0.01 |
| SON OXT | MeA OXT | 0.61 | 0.38 | <0.01 |
TABLE 4.
Pearson correlation results for gene-behavior associations in both control and early life CSS dams. All gene-behavior correlations use the mean duration of the behavior.
| GENE | BEHAVIOR | r | R2 | p |
|---|---|---|---|---|
| SON OXT | DAY 9 RETRIEVAL | 0.42 | 0.18 | 0.02 |
| SON OXT | DAY 9 NURSING | −0.52 | 0.27 | <0.01 |
| MeA OXT | DAY 9 NURSING | −0.39 | 0.15 | 0.04 |
| PVN OXT R | DAY 9 NURSING | −0.43 | 0.19 | 0.01 |
| PVN PRL R | DAY 9 NURSING | −0.48 | 0.23 | 0.01 |
| SON AVP | DAY 9 NURSING | −0.52 | 0.27 | <0.01 |
TABLE 5.
Pearson correlation results for gene behavior associations in early life CSS dams. All gene behavior correlations use the mean duration of the behavior.
| GENE | BEHAVIOR | r | R2 | p |
|---|---|---|---|---|
| SON OXT | STRESS DAY 9 RETRIEVAL | −0.16 | 0.03 | 0.31 |
| SON OXT | STRESS DAY 9 NURSING | −0.57 | 0.34 | 0.02 |
| MeA OXT | STRESS DAY 9 NURSING | −0.69 | 0.47 | <0.01 |
| PVN OXT R | STRESS DAY 9 NURSING | −0.65 | 0.43 | 0.01 |
| PVN PRL R | STRESS DAY 9 NURSING | −0.56 | 0.31 | 0.03 |
| SON AVP | STRESS DAY 9 NURSING | 0.27 | 0.07 | 0.20 |
DISCUSSION
Dams exposed to early life CSS display substantial decreases in nursing latency and increases in nursing behavior that are associated with attenuated OXT, OXT R, PRL R and AVP gene expression in brain nuclei involved in the control of maternal care. Since the growth patterns of both groups were similar despite the increased nursing, the result of these behavioral changes is that early life CSS dams displayed an attenuated nursing efficiency; CSS dams had to spend more time nursing to produce pups similar in size to the control dams. The significant and specific associations between nursing behaviors and neuroendocrine targets involve hormones and nuclei that are primary mediators of milk production and release. It is concluded that early life CSS has long term effects on the neuroendocrinology of maternal care which result in decreased nursing efficiency (poorer maternal care) in the adult dams. While the dams in the current study were able to compensate for this decrease in nursing efficiency in a laboratory setting with no exposure to social stress and unlimited supplies of food, water, and time for nursing, this may not be likely or possible for mothers in more challenging environments. The relative effect of early life CSS on dam-pup interactions versus the potential direct effect of the male intruder is currently unknown. The data support the use of early life CSS as an effective model for stress-induced impairments in nursing behavior, such as those associated with postpartum depression and anxiety (Henderson et al. 2003; Pippins et al. 2006; Dennis and McQueen 2007; Goodman 2007; Adewuya et al. 2008; Dennis and McQueen 2009).
The enhanced pup retrieval may have facilitated the increase in overall nursing duration through shorter nursing latencies during mid and late lactation. On day 9, the offspring from stressed dams required less time and fewer retrievals to gather all pups to the nest and initiate nursing. However, the increases in nursing were not dependent on enhanced retrieval, as CSS dam nursing was also elevated on day 2 when retrieval and nursing latencies were similar in both groups Although retrieval duration was increased on day 16, this increase was also associated with an attenuated nursing latency. Increased retrieval efficiency on days 9 and 16 allowed the CSS dams to initiate nursing faster than the control dams. The similar effects of early life CSS on retrieval during maternal aggression testing, when retrieval is rare (Nephew and Bridges 2011), underscores the strength of this behavioral change. Our retrieval data support and expand on studies of the effects of prenatal psycho-social stress on retrieval in rats selected for high anxiety-like behavior which reported that stressed high anxiety dams spent less time retrieving pups on day 8 of lactation (Neumann et al. 2005). However, the data contrast with the behavioral observations from OXT knockout mice which demonstrate specific impairments in pup retrieval (Pedersen et al. 2006), but those effects may be mediated by changes in OXT in brain regions other than those examined in the current study, or by developmental effects. An important question for future studies pertaining to the effects on pup retrieval is whether the signal for this behavioral change originates from the dam, the pup, or both.
The specific changes in retrieval and nursing behaviors, but not pup grooming, indicate that the dams retrieved the pups faster to specifically initiate nursing during the maternal care test. The absolute sizes of the increases in nursing duration due to early life social stress were substantial, especially when compared to the Nephew and Bridges 2011 study which used identical behavioral testing protocols. In that study, moderate effects of CSS on maternal care were associated with substantial and persistent impairments in pup growth. In the current study, we report larger effects of early life CSS on nursing, yet the growth profiles of the control and early life CSS offspring are almost identical. Based on the maternal care data during early, mid, and late lactation, the early life CSS dams were less efficient at nursing compared to the control dams. Furthermore, the increased nursing in the early life CSS mothers during the maternal care test was not followed by an increase in nursing by the control dams during the aggression test, indicating that the effects of CSS on nursing are not due to differences in the temporal expression of nursing behavior Although nursing was observed during the aggression tests, it is postulated that the lack of treatment effect was due to the timing of the aggression test immediately following the maternal care test and the focus of the resident dam on the male intruder. Taken together, the retrieval and nursing data suggest that the CSS mothers were more motivated to gather and nurse their pups throughout lactation. Since the effects of early life CSS on nursing and oxytocin expression were unexpected, there were no additional measures of lactation in the current study. Therefore, the mechanism for the inefficient nursing could involve several factors, including decreased milk release, consumption, and/or energy content.
In addition to altered maternal care, the stressed dams displayed altered maternal aggression. Exposure to CSS decreased maternal aggression, but this effect was only recorded on day 2 of lactation and there were no differences on days 9 or 16. Despite the substantial differences in PVN OXT and OXT R and SON AVP levels at the end of lactation, the differences in maternal aggression were limited to early lactation. Furthermore, the PVN and SON are not typically associated with OXT or AVP’s role in maternal aggression (Lonstein and Gammie 2002). Due to the lack of temporal and anatomical correlation between the aggression and neuroendocrine data, the current results do not support the hypothesis that the effects of early life CSS on central OXT and AVP activity are involved in the change in maternal aggression during early lactation. It is also possible that the decrease in maternal aggression may be indicative of an increased motivation to nurse, rather than a direct effect of social stress on aggression.
The neuroendocrine data and the correlations between the neuroendocrine and behavioral effects support the hypothesis that early life CSS affects retrieval and nursing through a centrally mediated nursing specific mechanism. Given evidence indicating that OXT and AVP mRNA and their respective receptor mRNA levels (Nephew et al. 2009) and OXT receptor and AVP V1a receptor binding (Caughey et al. 2011) are relatively consistent across lactation in the PVN, SON, MeA, and CeA of primiparous animals, the differences reported in gene expression in the current study at day 21 are relevant to the reported behavioral effects on days 2, 9 and 16. The majority of the effects on gene expression involved OXT and PRL activity in the hypothalamus. Both OXT and PRL R gene expression were attenuated in the hypothalamus, and these attenuations were consistently correlated with decreased nursing efficiency (OXT, OXT R, and PRL R were negatively correlated with nursing duration). PRL stimulates OXT mRNA production and release in the PVN during late pregnancy (Popeski et al. 2003) and PRL has stimulatory actions on central OXT mRNA and OXT release during early lactation (Sarkar 1989; Ghosh and Sladek 1995). Women with a history of child abuse have lower CSF OXT concentrations, and it is suggested that alterations in central OXT mediate the negative effects of early life stress, such as increased incidence of depression and anxiety disorders (Heim et al. 2008) This relationship between early life stress and OXT has also been reported in males (Opacka-Juffry and Mohiyeddini 2012) In the current investigation, the increase in nursing was negatively correlated with OXT, OXT R, and PRL R mRNA. It is postulated that the negative feedback control of PRL is still functional in the CSS dams, resulting in depressed OXT activity. Another related hypothesis is that OXT positive feedback is impaired in the stressed dams. The strong correlation of OXT R and PRL R mRNA expression in the PVN suggests that early life CSS affected both neuroendocrine systems. Further study is needed to determine if the central changes in gene expression are associated with alterations in peripheral hormone concentrations and measures of lactation, such as milk output, intake, and quality, in addition to nursing behavior.
In contrast to a nursing specific mechanism for the effects of early life CSS on pup retrieval and nursing, it is possible that the neuroendocrine changes are having direct effects on overall maternal care. OXT and PRL are directly implicated in the control of maternal care, which often involves effects on pup retrieval and grooming, but the present results do not support the hypothesis that early life CSS increases nursing through a direct behavioral effect, as decreases in the these hormones are typically associated with decreases in maternal care (Bridges et al. 1990; Bridges and Ronsheim 1990; Pedersen and Boccia 2003; Pedersen et al. 2006; Gordon et al. 2010) Dams that express high levels of pup grooming show increases in. OXT expression in the MPOA and PVN (Shahrokh et al. 2010), and also have elevated OXT R levels in the MPOA, LS, CeA, PVN, and BNST (Champagne et al. 2001) Despite the significant difference in PVN OXT R, there were no differences in pup grooming in the present study. The current data do not support a direct behavioral effect of CSS on general maternal care through an OXT mediated mechanism.
Exposure of the mothers of the current dams to CSS resulted in decreased maternal care and suppressed growth in female offspring, which are often features of postpartum depression (Goodman 2007; Adewuya et al. 2008; Black et al. 2009; Nephew and Bridges 2011) Several recent reviews have highlighted the strong associations between maternal depression, oxytocin, nursing, and infant growth in humans (Gress-Smith et al. 2011; Parsons et al. 2011; Surkan et al. 2011) and suggest that there are similar neuroendocrine mechanisms mediating early cessation of nursing and maternal depression (Stuebe et al. 2011) Although the adult offspring of CSS dams (which exhibited decreases in maternal care) did not display decreased maternal care, another negative effect of postpartum depression that has long term consequences is impaired nursing Women with depressive symptoms in the early postpartum period are at an increased risk for impaired nursing, including a decreased likelihood of initiating breastfeeding, increased difficulties with breast feeding, and reduced breastfeeding duration (Dennis and McQueen 2009). It is suggested that postpartum depression symptoms are negatively associated with exclusive breastfeeding, and the effect of depression on nursing may exacerbate symptoms of depression, as the act of breastfeeding is associated with a decrease in negative mood (Mezzacappa 2004; Groër 2005). Although lactation of the dams in the initial study of CSS on maternal behavior was not measured, a similar study by Lau and Simpson concluded that chronic exposure to a male intruder significantly decreased milk release (Lau and Simpson 2004), and considering the substantial effects on growth, it is likely that the early life CSS dams in the current study were exposed to impaired milk production, release, and/or intake in addition to the depressed maternal care when they were infants It is argued that the compensation in nursing observed in the present study may not be observed in humans, who may be unable or unlikely to compensate, as reasons for the early cessation of breastfeeding include inadequate milk supply and the perception that the baby was not satiated (Ahluwalia et al. 2005) Two future questions to pursue are whether the adult offspring of CSS dams would decrease maternal care if exposed to CSS during the postpartum period, and whether the decrease in nursing efficiency would negatively affect infant growth if time for nursing was limited It is postulated that maternal care and/or offspring growth in early life CSS dams would be attenuated in a more challenging environment, as has been reported in a study of male rats where maternally separated rats are more susceptible to anhedonia in response to chronic social defeat (Der-Avakian and Markou 2010).
While further study is needed to elucidate the mechanisms for the elevated retrieval and decreased nursing efficiency, the present data indicate that exposure to CSS during early development has substantial long term effects on adult maternal behavior which are associated with decreased central OXT and PRL activity. Early life CSS is an ethologically relevant stressor that can be used as a model to study the persistent effects of stress on maternal behavior.
Acknowledgments
The authors would like to thank Marc Bettscheider for assistance with the gene expression analyses and Dietmar Spengler for suggestions and comments on the manuscript.
Role of the Funding Source
The studies in this manuscript were funded by NIH K99 HD059943 to BCN.
Footnotes
The authors have no conflicts of interest to report.
Contributors
Dr. Nephew completed the animal work and behavioral analysis, and Dr. Murgatroyd completed the gene expression analyses. Both authors contributed to the writing and revision of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Saleh MT, Ghubash R, Karim L, Krymski M, Bhai I. Hormonal aspects of postpartum depression. Psychoneuroendocrinology. 1998;23(5):465–475. doi: 10.1016/s0306-4530(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Adewuya AO, Ola BO, Aloba OO, Mapayi BM, Okeniyi JAO. Impact of postnatal depression on infants’ growth in Nigeria. Journal of affective disorders. 2008;108(1–2):191–193. doi: 10.1016/j.jad.2007.09.013. [DOI] [PubMed] [Google Scholar]
- Ahluwalia IB, Morrow B, Hsia J. Why Do Women Stop Breastfeeding? Findings From the Pregnancy Risk Assessment and Monitoring System. Pediatrics. 2005;116(6):1408–1412. doi: 10.1542/peds.2005-0013. [DOI] [PubMed] [Google Scholar]
- Black MM, Baqui AH, Zaman K, Arifeen SE, Black RE. Maternal depressive symptoms and infant growth in rural Bangladesh. The American Journal of Clinical Nutrition. 2009;89(3):951S–957S. doi: 10.3945/ajcn.2008.26692E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. Release of Oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defense. Neuroscience. 2004;124:439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain Oxytocin Correlates with Maternal Aggression: Link to Anxiety. J Neurosci. 2005;25(29):6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. PNAS. 2008;105:17139–17144. doi: 10.1073/pnas.0807412105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: From central release to sites of action. Hormones and Behavior. 2011;61(3):293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Mann PE. Prolactin-brain interactions in the induction of maternal behavior in rat. Psychoneuroendocrinology. 1994;19(5–7):611–622. doi: 10.1016/0306-4530(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central Prolactin Infusions Stimulate Maternal Behavior in Steroid-Treated, Nulliparous Female Rats. Proc Natl Acad Sci. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Rigero BA, Byrnes EM, Yang L, Walker AM. Central infusions of the recombinant human prolactin receptor antagonist, S179D-PRL, delay the onset of maternal behavior in steroid-primed nulliparous female rats. Endocrinology. 2001;142(2):730–739. doi: 10.1210/endo.142.2.7931. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Ronsheim PM. Prolactin (PRL) regulation of maternal behavior in rats: bromocriptine treatment delays and PRL promotes the rapid onset of behavior. Endocrinology. 1990;126:837–848. doi: 10.1210/endo-126-2-837. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Prenatal Social Stress in the Rat Programmes Neuroendocrine and Behavioural Responses to Stress in the Adult Offspring: Sex-Specific Effects. Journal of Neuroendocrinology. 2010;22(4):258–271. doi: 10.1111/j.1365-2826.2010.01969.x. [DOI] [PubMed] [Google Scholar]
- Byrnes EM, Rigero BA, Bridges RS. Opioid receptor antagonism during early lactation results in the increased duration of nursing bouts. Physiology and Behavior. 2000;70(1–2):211–216. doi: 10.1016/s0031-9384(00)00231-6. [DOI] [PubMed] [Google Scholar]
- Caughey SD, Klampfl SM, Bishop VR, Pfoertsch J, Neumann ID, Bosch OJ, Meddle SL. Changes in the Intensity of Maternal Aggression and Central Oxytocin and Vasopressin V1a Receptors Across the Peripartum Period in the Rat. Journal of Neuroendocrinology. 2011;23(11):1113–1124. doi: 10.1111/j.1365-2826.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behaivor in the rat are associated with differences in estrogen-inducible central oxytocin receptors. PNAS. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Pædiatrica. 2007;96(4):590–594. doi: 10.1111/j.1651-2227.2007.00184.x. [DOI] [PubMed] [Google Scholar]
- Dennis CL, McQueen K. The Relationship Between Infant-Feeding Outcomes and Postpartum Depression: A Qualitative Systematic Review. Pediatrics. 2009;123(4):e736–e751. doi: 10.1542/peds.2008-1629. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Markou A. Neonatal maternal separation exacerbates the reward-enhancing effect of acute amphetamine administration and the anhedonic effect of repeated social defeat in adult rats. Neuroscience. 2010;170(4):1189–1198. doi: 10.1016/j.neuroscience.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot JC, Lubin DA, Walker CH, Johns JM. Acute cocaine alters oxytocin levels in the medial preoptic area and amygdala in lactating rat dams: implications for cocaine-induced changes in maternal behavior and maternal aggression. Neuropeptides. 2001;35(2):127–134. doi: 10.1054/npep.2001.0854. [DOI] [PubMed] [Google Scholar]
- Fries ABW, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(47):17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Negron A, Newman SM, Rhodes JS. Corticotropin-releasing factor inhibits maternal aggression in mice. Behav Neurosci. 2004;118(4):105–114. doi: 10.1037/0735-7044.118.4.805. [DOI] [PubMed] [Google Scholar]
- Ghosh R, Sladek CD. Prolactin modulates oxytocin mRNA during lactation by its action on the hypothalamo-neurohypophyseal axis. Brain Research. 1995;672(1–2):24–28. doi: 10.1016/0006-8993(94)01340-n. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: Effects of Ibotenic acid lesion and oxytocin antisense. Physiol and Behav. 1998;63(3):351–359. doi: 10.1016/s0031-9384(97)00434-4. [DOI] [PubMed] [Google Scholar]
- Goekoop JG, de Winter RPF, de Rijk R, Zwinderman KH, Frankhuijzen-Sierevogel A, Wiegant VM. Depression with above-normal plasma vasopressin: Validation by relations with family history of depression and mixed anxiety and retardation. Psychiatry Research. 2006;141(2):201–211. doi: 10.1016/j.psychres.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Goodman SH. Depression in Mothers. Annu Rev Clin Psychol. 2007;3:107–35. doi: 10.1146/annurev.clinpsy.3.022806.091401. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biol Psychiatry. 2010;68:377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gress-Smith J, Luecken L, Lemery-Chalfant K, Howe R. Postpartum Depression Prevalence and Impact on Infant Health, Weight, and Sleep in Low-Income and Ethnic Minority Women and Infants. Maternal and Child Health Journal. 2011:1–7. doi: 10.1007/s10995-011-0812-y. [DOI] [PubMed] [Google Scholar]
- Groër MW. Differences Between Exclusive Breastfeeders, Formula-Feeders, and Controls: A Study of Stress, Mood, and Endocrine Variables. Biological Research For Nursing. 2005;7(2):106–117. doi: 10.1177/1099800405280936. [DOI] [PubMed] [Google Scholar]
- Gutzler SJ, Karom M, Erwin WD, Albers HE. Arginine-vasopressin and the regulation of aggression in female Syrian hamsters (Mesocricetus auratus) European Journal of Neuroscience. 2010;31(9):1655–1663. doi: 10.1111/j.1460-9568.2010.07190.x. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. The Role of Early Adverse Life Events in the Etiology of Depression and Posttraumatic Stress Disorder. Annals of the New York Academy of Sciences. 1997;821(1):194–207. doi: 10.1111/j.1749-6632.1997.tb48279.x. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Mol Psychiatry. 2008;14(10):954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Henderson JJ, Evans SF, Straton JAY, Priest SR, Hagan R. Impact of Postnatal Depression on Breastfeeding Duration. Birth. 2003;30(3):175–180. doi: 10.1046/j.1523-536x.2003.00242.x. [DOI] [PubMed] [Google Scholar]
- Herzog CJ, Czéh B, Corbach S, Wuttke W, Schulte-Herbrüggen O, Hellweg R, Flügge G, Fuchs E. Chronic social instability stress in female rats: A potential animal model for female depression. Neuroscience. 2009;159(3):982–992. doi: 10.1016/j.neuroscience.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM. Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Frontiers in Psychiatry. 2011;2:1–10. doi: 10.3389/fpsyt.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C, Simpson C. Animal models for the study of the effect of prolonged stress on lactation in rats. Physiology & Behavior. 2004;82(2–3):193–197. doi: 10.1016/j.physbeh.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Gammie SC. Sensory, hormonal, and neural controls of maternal aggression in laboratory rodents. Neuroscience and Biobehav Rev. 2002;26:869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G. Maternal depression and parenting behavior: A meta-analytic review. Clinical Psychology Review. 2000;20(5):561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES. Breastfeeding and Maternal Stress Response and Health. Nutrition Reviews. 2004;62(7):261–268. doi: 10.1111/j.1753-4887.2004.tb00050.x. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Patchev AV, Wu Y, Micale V, Bockmuhl Y, Fischer D, Holsboer F, Wotjak CT, Almeida OFX, Spengler D. Dynamic DNA methylation programs persistent adverse effects of early-life stress. Nat Neurosci. 2009;12(12):1559–1566. doi: 10.1038/nn.2436. [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Wu Y, Bockmuhl YSD. Genes learn from stress: How infantile trauma programs us for depression. Epigenetics. 2010;5(3):194–199. doi: 10.4161/epi.5.3.11375. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiology & Behavior. 2008;95(1–2):182–186. doi: 10.1016/j.physbeh.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacology Biochemistry and Behavior. 2008;91(1):77–83. doi: 10.1016/j.pbb.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011;14(6):677–684. doi: 10.3109/10253890.2011.605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS. Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress. 2011;0(0):null. doi: 10.3109/10253890.2011.605487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, Lovelock DF, Byrnes EM. Enhanced maternal aggression and associated changes in neuropeptide gene expression in reproductively experienced rats. Behavioral Neuroscience. 2009;123(5):949–957. doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Byrnes EM, Bridges RS. Vasopressin mediates enhanced offspring protection in multiparous rats. Neuropharmacology. 2010;58(1):102–106. doi: 10.1016/j.neuropharm.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Kromer SA, Bosch OJ. Effects of psycho-social stress during pregnancy on neuroendocrine and behavioural parameters in lactation depend on the genetically determined stress vulnerability. Psychoneuroendocrinology. 2005;30(8):791–806. doi: 10.1016/j.psyneuen.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Opacka-Juffry J, Mohiyeddini C. Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress. 2012;15(1):1–10. doi: 10.3109/10253890.2011.560309. [DOI] [PubMed] [Google Scholar]
- Parsons CE, Young KS, Rochat TJ, Kringelbach ML, Stein A. Postnatal depression and its effects on child development: a review of evidence from low- and middle-income countries. British Medical Bulletin. 2011:1–23. doi: 10.1093/bmb/ldr047. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML. Oxytocin antagonism alters rat dams’ oral grooming and upright posturing over pups. Physiology and Behavior. 2003;80:233–241. doi: 10.1016/j.physbeh.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, McGuire M, Evans DL. Corticotronpin-releasing hormone inhibits maternal behavior and induces pup-killing. Life Sciences. 1991;48(16):1537–1546. doi: 10.1016/0024-3205(91)90278-j. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Prange AJ., Jr Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. PNAS. 1979;76:6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes, Brain and Behavior. 2006;5(3):274–281. doi: 10.1111/j.1601-183X.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Pippins JR, Brawarsky P, Jackson RA, Fuentes-Afflick E, Haas JS. Association of Breastfeeding with Maternal Depressive Symptoms. Journal of Women’s Health. 2006;15(6):754–762. doi: 10.1089/jwh.2006.15.754. [DOI] [PubMed] [Google Scholar]
- Popeski N, Amir S, Diorio J, Woodside B. Prolactin and Oxytocin Interaction in the Paraventricular and Supraoptic Nuclei: Effects on Oxytocin mRNA and Nitric Oxide Synthase. Journal of Neuroendocrinology. 2003;15(7):687–696. doi: 10.1046/j.1365-2826.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- Ross LE, Dennis CL. The Prevalence of Postpartum Depression among Women with Substance Use, an Abuse History, or Chronic Illness: A Systematic Review. Journal of Women’s Health. 2009;18(4):475–486. doi: 10.1089/jwh.2008.0953. [DOI] [PubMed] [Google Scholar]
- Rotzinger S, Lovejoy DA, Tan LA. Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides. 2010;31(4):736–756. doi: 10.1016/j.peptides.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Boettcher CA, Post JL, Abbott DH. Inhibition of Maternal Behaviour by Central Infusion of Corticotrophin-releasing Hormone in Marmoset Monkeys. Journal of Neuroendocrinology. 2011:no–no. doi: 10.1111/j.1365-2826.2011.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK. Evidence for Prolactin Feedback Actions on Hypothalamic Oxytocin, Vasoactive Intestinal Peptide and Dopamine Secretion. Neuroendocrinology. 1989;49(5):520–524. doi: 10.1159/000125161. [DOI] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-Dopamine Interactions Mediate Variations in Maternal Behavior in the Rat. Endocrinology. 2010;151(5):2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma Oxytocin Concentration during Pregnancy is associated with Development of Postpartum Depression. Neuropsychopharmacology. 2011;36(9):1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult Attachment Predicts Maternal Brain and Oxytocin Response to Infant Cues. Neuropsychopharmacology. 2009;34(13):2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, Pedersen CA, Propper C, Meltzer-Brody S. Failed Lactation and Perinatal Depression: Common Problems with Shared Neuroendocrine Mechanisms? Journal of Women’s Health. 2011;21:264–272. doi: 10.1089/jwh.2011.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget A, Belzung C. Involvement of vasopressin in affective disorders. European Journal of Pharmacology. 2008;583(2–3):340–349. doi: 10.1016/j.ejphar.2007.11.065. [DOI] [PubMed] [Google Scholar]
- Surkan PJ, Kennedy CE, Hurley KM, Black MM. Maternal depression and early childhood growth in developing countries: systematic review and meta-analysis. Bulletin of the World Health Organization. 2011;89:607–615. doi: 10.2471/BLT.11.088187. [DOI] [PMC free article] [PubMed] [Google Scholar]