Abstract
A quantitative long-term fluid consumption and fluid licking assay was performed in two mouse models with either an ~ 2Mb genomic deletion, Df(11)17, or the reciprocal duplication CNV, Dp(11)17, analogous to the human genomic rearrangements causing either Smith-Magenis syndrome [SMS; OMIM #182290] or Potocki-Lupski syndrome [PTLS; OMIM #610883], respectively. Both mouse strains display distinct quantitative alteration in fluid consumption compared to their wild-type littermates; several of these changes are diametrically opposing between the two chromosome engineered mouse models. Mice with duplication vs. deletion showed longer vs. shorter intervals between visits to the waterspout, generated more vs. less licks per visit and had higher vs. lower variability in the number of licks per lick-burst as compared to their respective wild-type littermates. These findings suggest that copy number variation can affect long-term fluid consumption behavior in mice. Other behavior differences were unique for either the duplication or deletion mutants; the deletion CNV resulted in increased variability of the licking rhythm, and the duplication CNV resulted in a significant slowing of the licking rhythm. Our findings document a readily quantitated complex behavioral response that can be directly and reciprocally influenced by a gene dosage effect.
Keywords: Copy number variation (CNV), fluid consumption behavior, gene dosage effect Smith-Magenis syndrome (SMS), Potocki-Lupski syndrome (PTLS)
INTRODUCTION
Potocki-Lupski syndrome (PTLS, OMIM#610883) is an intellectual disability and multiple congenital anomalies (ID/MCA) syndrome caused by an interstitial duplication of chromosome 17p11.2 [Potocki et al., 2000, 2007]. The reciprocal deletion of the same genomic interval causes another ID/MCA disorder known as Smith-Magenis syndrome (SMS, OMIM#182290) [Bi and Lupski, 2008; Edelman et al., 2007; Elsea and Girirajan, 2008]. Speech and language impairment and oropharyngeal dysphagia are common to both syndromes; however, individuals with PTLS are at a higher risk for failure to thrive (FTT) during infancy and early childhood whereas individuals with SMS are prone to obesity [Edelman et al., 2007; Potocki et al., 2007; Soler-Alfonso et al., 2011].
Via chromosomal engineering, we previously constructed mouse models of PTLS and SMS carrying an ~2 Mb genomic duplication [Dp(11)17] or deletion [Df(11)17] that is syntenic to the PTLS/SMS common recurrent copy number variation (CNV) interval [Walz et al., 2003]. Both Dp(11)17/+ and Df(11)17/+ mice partially recapitulate the respective human phenotypes of PTLS and SMS including phenotypic clinical manifestations of craniofacial abnormalities [Yan et al., 2007; Yan et al., 2004], energy metabolism anomalies [Lacaria et al., 2012a], altered learning, memory and social interaction [Lacaria et al., 2012b; Molina et al., 2008; Ricard et al., 2010; Walz et al., 2003; Walz et al., 2004], and display a transcriptome that is distinct from their wild-type (WT) littermates [Ricard et al., 2010].
Deficits and abnormalities in the rhythmic fluid-licking behavior of mice can be assessed quantitatively in a recently developed long-term fluid-licking assay [Heck et al., 2008; Roy et al., 2011]. These deficits may reflect oral sensorimotor defects in patients and likely have predominant cerebellar origins [Bryant et al., 2010; Heck et al., 2008]. To better characterize and further investigate the oromotor dysfunction of PTLS and SMS, and to assess this specific, novel behavioral aspect in the genetic context of a CNV, we applied the long-term fluid-licking assay to both Dp(11)17/+ and Df(11)17/+ mice (Fig. 1).
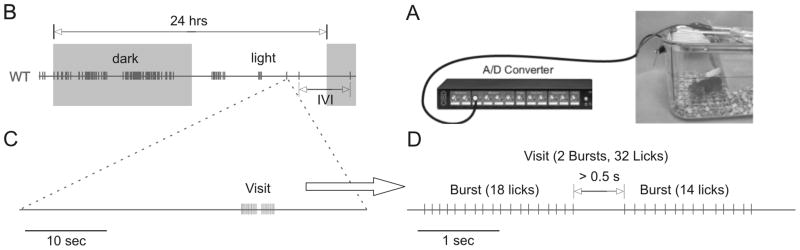
Figure 1.
Long-term licking assay and data example: A) Mice were housed singly in a standard home cage environment. A metal grid placed underneath the waters spout and a stainless steel wire inserted into the water bottle were connected to an analogue-digital converter as described in the methods section. B) Data example from a wild-type (Dup) mouse, showing a ca. 28 hr observation time with dark periods marked in grey. Each vertical line represents a visit to the water spout, which typically consists of multiple licks. Double arrow above the trace marks a 24 hrs period. The double arrow below the trace marks an inter-visit-interval (IVI). C) Enlarged lick pattern of a single visit to the water spout. D) Further expansion of a single visit lick pattern in this example consisting of two bursts.
MATERIALS AND METHODS
Mouse Genetics
Mutant mouse strains Dp(11)17 and Df(11)17 were originally described in [Walz et al., 2003]. Heterozygous Dp(11)17/+ mice were maintained on a congenic C57BL/6Tyrc-Brd background (>N10), and heterozygous Df(11)17/+ mice were maintained on a congenic 129S5 background (N>10). All mice were bred and housed 2–5 animals per cage in a 12 h light: 12 h dark cycle and provided standard mouse chow and water ad libitum. All research and animal care procedures were approved by the Baylor College of Medicine or University of Tennessee Health Science Center’s Institutional Animal Care and Use Committee.
Long-Term Licking Behavior
All mice were bred at Baylor College of Medicine and shipped to the University of Tennessee Health Science Center for testing. At the time of testing all mice were between two and five months old. We tested 16 Dp(11)17/+ mice and 17 of their wild-type littermates, and 16 Df(11)17/+ mice, and 24 of their wild-type littermates. Water consumption and licking behavior were monitored continuously and unsupervised for 72 hours, typically over weekends to minimize disturbances due to noise from animal facility maintenance. Access to food and water was unlimited during the entire test period. Mice were placed singly in standard housing cages, which were modified to allow detection of contact between the mouse’s tongue and the water spout [Bryant et al., 2010; Hayar et al., 2006].
Briefly, a metal wire mesh (~10 × 15 cm) was placed under the waterspout to serve as an electrical reference. The spout and the wire mesh were connected to the input and ground of an analogue to digital converter channel via USB connector (CED 1401, Cambridge Electronic Design, Cambridge, UK). Each tongue-to-water spout contact (while the mouse stood on the metal grid) resulted in a voltage signal of 0.1 – 1 Volt that reflects the junction potential between the water and the metal spout [Hayar et al., 2006]. Voltage signals were digitized at 2 kHz and stored on hard disk. Lick events were analyzed off line using Spike 2 software (Cambridge Electronic Design, Cambridge, UK). Statistical comparison of behavioral variables was performed using either Student’s t-test for variables with normal distributions and equal variance or using the Mann-Whitney rank sum test (SigmaStat software, Systat, San Jose, CA). Which test was used is stated in each case. The coefficient of variation (the standard deviation divided by the mean) and the Fano factor (the variance divided by the mean) were calculated as measures of variability for normally distributed variables.
Table I lists the variables extracted from the long-term lick recordings and analyzed for differences between mutant and wild-type mice of the same strain. Only variables with significant differences in at least one strain are discussed further.
Table 1.
List of quantitative behavioral variables derived from long-term observations of fluid licking behavior evaluated in this study
| Number of inter-lick-intervals (ILIs) | Intervals within a range from 40 – 180 ms. This range included the primary ILI distribution. All longer intervals were excluded from all analyses based on ILI counts (Boughter and others 2007). |
| Mean ILI duration | Mean of the ILI distribution |
| Coefficient of variation of the ILI distribution | A measure of the variability. The distribution standard deviation divided by the mean. |
| Fano Factor of the ILI distribution | A measure of variability. The distribution variance divided by the mean. |
| Number of visits to the water spout | A visit consisted of two or more licks with an ILI within the 40 – 180 ms range. By definition visits had to be preceded by a 1 min interval without licks. |
| Mean log(inter visit interval duration), log(IVI) | Mean of the log10(IVI) distribution |
| Coef. of Var. of the log(IVI) distribution | A measure of the variability. The distribution standard deviation divided by the mean. |
| Fano Factor of the log(IVI) distribution | A measure of variability. The distribution variance divided by the mean. |
| Mean number of licks per visit | Average of the counts of all licks in all visit |
| Coef. of Var. of licks per vist | A measure of the variability. The distribution standard deviation divided by the mean. |
| Fano Factor of licks per visit | A measure of variability. The distribution variance divided by the mean. |
| Mean number of licks per burst | A burst is defined as an uninterrupted sequence of licks with no interval exceeding 500 ms duration. This allows for “missed” licks which occur occasionally when the mouse extends the tongue but does not touch the water spout. This results in a double-long interval which shows up in the ILI distribution as a small second peak centered at twice the mean ILI (Boughter and others 2007). |
| Coef. of Var. of licks per burst | A measure of variability. The distribution standard deviation divided by the mean. |
| Fano Factor of licks per burst | A measure of variability. The distribution variance divided by the mean. |
| Mean number of bursts per visit | Average count of the number of bursts in all visits. |
| Coef. of Var. of bursts per vist | A measure of the variability. The distribution standard deviation divided by the mean. |
| Fano Factor of bursts per visit | A measure of variability. The distribution variance divided by the mean. |
RESULTS
Fluid Licking Rhythm
Fluid licking in mice is a highly rhythmic oromotor behavior. The average beat of the fluid licking rhythm in mice is approximately 10 Hz, but the mean frequency can differ significantly between strains [Boughter et al., 2007]. Evidence suggests that this motor pattern is generated by brainstem central pattern generating circuits and modulated by higher motor structures [Travers et al., 1997] such as the cerebellum [Bryant et al., 2010; Welsh et al., 1995]. We have previously shown that gene mutations associated with two autism spectrum disorders in humans, Angelman [OMIM#105830] and fragile X syndromes [OMIM#300624], cause a slower fluid licking rhythm in the mouse models of both disorders [Heck et al., 2008; Roy et al., 2011].
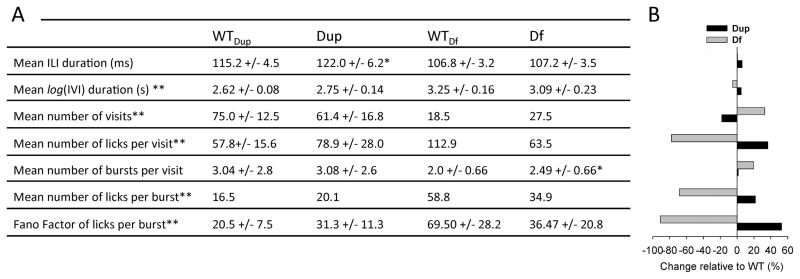
The mean licking rhythm is inversely proportional to the mean inter-lick-interval (ILI) duration, which is the behavioral variable utilized here to compare licking rhythms. The mean ILI duration of Dp(11)17/+ mice was significantly longer than that of their wild-type littermates (mean ILI duration +/− SD.: WT, 115.2 +/− 4.5 ms; Dp(11)17/+, 122.0 +/− 6.2 ms; Student’s t-test, t = 3.636, p ≤ 0.001). No differences in the mean ILI durations were observed between Df(11)17/+ mice and their wild-type littermates. Results from all variables that showed significant phenotypic differences in this assay system between WT and duplication or deletion mice are summarized as Fig. 2.
Figure 2.
Summary of differences in fluid licking behavior. A) List of variables with their mean +/− standard deviation or median values that showed significant differences associated with a deletion, Df(11)17, duplication CNV, Dp(11)17, or both. Variable names in the left column marked with double asterisks showed opposing phenotypic differences in deletion vs. duplication mice. Variables without asterisks showed differences in only one of the strains and an asterisk next to the mutant’s value in the table marks the strain with the significant difference. B) Graphic representation of phenotypic differences between wild-type mice and their duplication or deletion litter mates expressed in percent of the wild-type value.
Variables of water intake behavior
The long-term fluid licking assay also provides quantitative measurements of slow or long term behavioral patterns, i.e. those acting over prolonged durations of time, such as the homeostatic regulation of water intake during the course of a day. Water intake patterns were assessed by measuring the duration of intervals between drinking events or “visits” to the waterspout, with inter-visit-interval (IVI) durations ranging from minutes to hours. Because of the broad time scale, we used the logarithmic values (base 10) for statistical analyses of IVI distributions between genotypes.
Compared to their respective wild-type littermates, mean log(IVI) durations significantly increased in Dp(11)17/+ mice (mean log(IVI) durations: +/− SD: WT, 2.62 +/− 0.08 s; Dp(11)17/+, 2.75 +/− 0.14 s; Student’s t-test, t = 3.264, p = 0.003), but were significantly reduced in Df(11)17/+ mice (mean log(IVI) durations: +/− SD: WT, 3.25 +/− 0.16 s; Df(11)17/+, 3.09 +/− 0.23 s; Student’s t-test, t = 2.582, p = 0.014). Consistent with these differences in average IVI durations, Dp(11)17/+ mice made fewer visits during the observation period than their wild-type littermates (mean number of visits +/− SD: WT, 75.0 +/− 12.5; Dp(11)17/+, 61.4 +/− 16.8; Student’s t-test, t = 3.264, p = 0.012) and Df(11)17/+ mice made more (median number of visits: WT, 18.5; Df(11)17/+, 27.5; Mann–Whitney U = 94.5, p = 0.007).
Interestingly, the background strains for the Dp(11)17 and Df(11)17 CNV mutations differ substantially in their long-term fluid intake behavior. The wild-type congenic (N > 10) 129S5 mice (littermates of the Df(11)17 mice) made much fewer visits in each 24 hr observation period than the wild-type mice of the C57BL/6 strain (littermates of the Dp(11)17/+ mice (129S5, 18.5 vs. C57BL/6, 75.0). Strain differences have been observed for most established behavioral tests [Crawley et al., 1997; Paylor et al., 2006] and underscore the sensitivity and stability of the long-term fluid licking assay in this context. Although interesting in themselves, the observed inter-strain differences are of little relevance to this study since all phenotypes were defined by within-strain comparisons made between animals of each genotype and their littermates of the same strain background.
Compared to their littermates, neither of the genotypes, Dp(11)17/+ nor Df(11)17/+, displayed difference in the total number of licks generated during the 24 hr observation period. Thus, the opposing phenotypic differences in the number of visits in Dp(11)17/+ and Df(11)17/+ mice must be compensated for by a respectively higher and lower number of licks generated during each visit. Consistent with expectations, Dp(11)17/+ mutants generated on average a higher number of licks per visit (Mean number of licks/visit +/− SD: WT, 57.8+/− 15.6; Dp(11)17/+, 78.9 +/− 28.0; Student’s t-test, t = 2.704, p < 0.011) and Df(11)17/+ mice generated a lower number (Median number of licks/visits: WT, 112.9; Df(11)17/+, 63.5; Mann–Whitney U = 213.0, p = 0.002) than their respective wild-type littermates.
Fluid licking typically occurs in brief, uninterrupted trains of licks or lick bursts (Fig. 1B). An average visit to the water spout consists of two or more bursts. The organization of visits into bursts was quantified by counting the number of bursts per visit and the number of licks per burst. Df(11)17/+ mice had a higher number of burst per visit than their wild-type littermates (Mean number of bursts/visit +/− SD: WT, 2.0 +/− 0.66; Df(11)17/+, 2.49 +/− 0.66; Student’s t-test, t = 2.284, p = 0.028). Dp(11)17/+ mice had the same average number of burst per visit as their wild-type littermates.
Analysis of the number of licks per burst revealed opposing phenotypes with increased numbers in Dp(11)17/+ (Median number of licks/burst: WT, 16.5; Dp(11)17/+, 20.1; Mann–Whitney U = 72.0, p ≤ 0.022) and decreased numbers in Df(11)17/+ mice (median number of licks/burst: WT, 58.8; Df(11)17/+, 34.9; Mann–Whitney U = 319.0, p ≤ 0.001), compared to their corresponding wild-type littermates.
The average Fano Factors, a measure of variability, of the numbers of licks per burst was also altered in opposite directions in Dp(11)17/+ vs. Df(11)17/+ mice. The licks per burst Fano Factor was higher in Dp(11)17/+ (average Fano Factor licks/burst +/− SD: WT, 20.5 +/− 7.5; Dp(11)17/+, 31.3 +/− 11.3; Student’s t-test, t = 3.248, p ≤ 0.003) and lower in Df(11)17/+ mice (average Fano Factor licks/burst +/− SD: WT, 69.50 +/− 28.2; Df(11)17/+, 36.47 +/− 20.8; Student’s t-test, t = 3.999, p ≤ 0.001) than their corresponding wild-type littermates.
DISCUSSION
SMS and PTLS present clear oral sensorimotor deficits
Early feeding problems (including poor suckling) have been reported for 92% of infants with PTLS and FTT continues to be present in 71% of PTLS children under the age of five [Potocki et al., 2007; Soler-Alfonso et al., 2011]. Infantile hypotonia, mild micrognathia, palate abnormalities, as well as cardiac abnormalities and sinus arrhythmia, may impair oral sensorimotor functions of patients with PTLS. Indeed, when a cohort or 24 subjects with PTLS were evaluated by comprehensive swallow function studies, the results revealed sensorimotor defects including abnormal lingual function (67%), reduced/immature chewing (39%), mild delay in initiation of swallowing (61%), residue after swallowing (72%) and laryngeal penetration (39%) [Soler-Alfonso et al., 2011].
Although SMS patients tend to have higher BMIs than the population norm after 2–5 years of age [Lacaria et al., 2012a], almost all patients are characterized by feeding difficulties and possibly FTT in their early infancy [Elsea and Girirajan, 2008; Gropman et al., 2006]. SMS infants present marked oral sensorimotor dysfunction, with poor suckling reflex, gastroesophageal reflux and hypotonia [Elsea and Girirajan, 2008; Gropman et al., 2006]. The presence of oromotor dysfunction is estimated to be 75–100% among SMS patients with a genomic deletion of 17p11.2 [Edelman et al., 2007].
Besides swallowing/feeding problems, language difficulties can also have contributory oromotor elements in their etiology [Ercan-Sencicek et al., 2011; McFarland and Tremblay 2006; Mizuno and Ueda 2005]. Both PTLS and SMS manifest clear oral sensorimotor deficits that likely contribute to both feeding/swallowing defects and impaired language ability. Feeding and language crucially impact the quality of life for PTLS and SMS patients and their families and could potentially be mitigated or possibly ameliorated through oral-motor therapy.
The oral sensorimotor deficits in SMS/PTLS can be effectively modeled by the fluid licking behavior of Df (11)17 and Dp(11)17 mice
The rhythmic and highly coordinated procedure of fluid licking in rodents that can be measured and quantitated provides a valuable and currently the only tool to assess oral sensorimotor functions in an experimental setting in rodents.
The Dp(11)17 mouse model for PTLS has recently been found to have many behaviors consistent with those observed in patients with autism and autism spectrum disorders (ASD) and has been proposed as a potential animal model of CNV associated autism [Lacaria et al., 2012b]. Indeed, many patients with PTLS manifest neurobehavioral abnormalities and many of those characterized also presented with autistic symptoms; 10 of 15 met diagnostic criteria for an autistic spectrum disorder. Interestingly, in our previous licking assay studies of two mouse models for single gene disorders that manifest ASD as part of their complex neurobehavioral phenotype (Angelman syndrome and Fragile X syndrome) licking abnormalities were also observed [Heck et al., 2008; Roy et al., 2011]. Thus, three animal models for human syndromes having autism spectrum neurobehavior have now been found to have abnormalities in licking rhythm.
Strikingly, both chromosome engineered mutant mouse strains tested here displayed clearly altered licking behavior; the alterations of both strains were different (licking rhythm, bursts per visit) or diametrically opposing (total number of visits, length of IVI, number of licks per visit and per burst). These data underline the sensitivity of this quantitative assay as a model to assess oral sensorimotor phenotypes in subjects, to perceive gene dosage changes, and to evaluate potential therapeutic efforts to improve the oral sensorimotor function. The different or opposing phenotypes between the two mouse models further indicate that there may be significant mechanistic difference in the etiology of the apparent FTT and language difficulty displayed by both PTLS and SMS.
As with the opposing licks-per-visit phenotypes in mouse models of SMS vs. PTLS tested here, the number of licks per visit showed a significant gene dosage effect in the Ube3a deficient mouse model of Angelman syndrome [Heck et al., 2008]. The genetic and neuronal mechanisms involved in controlling this phenotype and whether it is specific to genetic conditions associated with autism in humans has yet to be determined.
New insights about the licking-assay
Diagnostic neuroimaging revealed potential neuroanatomic anomalies in SMS patients, including a reduced volume of the cerebellar vermis [Greenberg et al., 1996]. No neuroimaging data of PTLS patients has been reported. Studies in rodents strongly implicate the cerebellum in the control of fluid consumption behavior [Bryant et al., 2010; Heck et al., 2008; Roy et al., 2011; Welsh et al., 1995], suggesting that cerebellar neuropathologies might be at least partially responsible for oromotor deficits associated with SMS and PTLS subjects. Interestingly, cerebellar deficits are increasingly recognized in animal models for human ASD [Critchley et al., 2000; Fatemi et al., 2012; Stanfield et al., 2008].
Fluid licking is a dosage–sensitive behavior directly affected by CNV
Human genetic data indicated that, just like traditional single nucleotide variations (SNV), copy number variations (CNV) of a genomic segment can result in a broad spectrum of clinical manifestations including neurobehavioral traits [Lee and Scherer 2010; Lupski 2011; Stankiewicz and Lupski 2010; Zhang et al., 2009]. These observations were affirmed by studies in animal models which experimentally proved that CNV can be directly causative for phenotypes of cognitive ability, level of anxiety, social behavior as well as reaction to a new environment [Horev et al., 2011; Molina et al., 2008; Ricard et al., 2010; Walz et al., 2004]. Interestingly, these functional phenotypes are accompanied by different levels of CNV – caused alterations in gene expression [Horev et al., 2011; Ricard et al., 2010] and brain anatomy [Horev et al., 2011]. Further, phenotypes resulting from a reciprocal dosage change of CNV (copy number gain vs. copy number loss) are often diametrically opposing [Horev et al., 2011; Ricard et al., 2010], an observation that echoes accumulating findings in human genetics, where reciprocal phenotypes associated with opposing gene/genome dosage alterations (i.e. copy number loss versus copy number gain) have been described for the complex neuropsychiatric traits of schizophrenia and autism, as well as microcephaly and macrocephaly, associated with, respectively, duplication/deletion CNV at 16p11.2 [McCarthy et al., 2009; Shinawi et al., 2010; Weiss et al., 2008] and deletion/duplication of 1q21.1 [Brunetti-Pierri et al., 2008; Consortium 2008; Lupski 2008; Stefansson et al., 2008].
This current neurobehavioral licking assay study in mouse chromosome engineered models of two human genomic disorders demonstrated that the rhythmic and highly coordinated complex oral sensorimotor behavior of liquid licking in mice can also be directly affected by CNV. Moreover, a number of parameters including number of visits, inter-visit time, licks per burst and licks per visit at increased (Dp(11)17) and reduced gene dosage (Df(11)17) show diametrically opposing trends of alteration. Different from the other known CNV – caused phenotypes, fluid licking is a predominantly cerebellum – regulated behavior. These findings thus demonstrate that, in addition to amygdala-regulated anxiety, dopamine pathway regulated activity and serotonin pathway regulated social preference, cerebellum-regulated behavior can also be sensitive to dosage change and react to mirroring changes in diametrically opposing ways.
Acknowledgments
Support: This work was supported by grants from the National Institute of Health (NIH).
This research was supported by grants from the National Institutes of Health to DHH (R01NS060887, R01NS067201 and R01NS063009) and from the National Institutes of Neurological Diseases and Stroke (R01NS058529) to JRL. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest Statement. None declared.
Disclosure: J.R.L. is a consultant for Athena Diagnostics, holds stock ownership in 23andMe and Ion Torrent Systems, and is a coinventor on multiple United States and European patents for DNA diagnostics. The Department of Molecular and Human Genetics at the Baylor College of Medicine derives revenue from clinical testing using genetic and genomic assays.
References
- Bi W, Lupski JR. RAI1, the Smith–Magenis, and Potocki–Lupski syndromes. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn Errors of Development. New York: Oxford university press; 2008. [Google Scholar]
- Boddaert N, De Leersnyder H, Bourgeois M, Munnich A, Brunelle F, Zilbovicius M. Anatomical and functional brain imaging evidence of lenticulo-insular anomalies in Smith Magenis syndrome. Neuroimage. 2004;21:1021–1025. doi: 10.1016/j.neuroimage.2003.10.040. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr, Baird JP, Bryant J, St John SJ, Heck D. C57BL/6J and DBA/2J mice vary in lick rate and ingestive microstructure. Genes Brain Behav. 2007;6:619–627. doi: 10.1111/j.1601-183X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Berg JS, Scaglia F, Belmont J, Bacino CA, Sahoo T, Lalani SR, Graham B, Lee B, Shinawi M, Shen J, Kang SH, Pursley A, Lotze T, Kennedy G, Lansky-Shafer S, Weaver C, Roeder ER, Grebe TA, Arnold GL, Hutchison T, Reimschisel T, Amato S, Geragthy MT, Innis JW, Obersztyn E, Nowakowska B, Rosengren SS, Bader PI, Grange DK, Naqvi S, Garnica AD, Bernes SM, Fong CT, Summers A, Walters WD, Lupski JR, Stankiewicz P, Cheung SW, Patel A. Recurrent reciprocal 1q21.1 deletions and duplications associated with microcephaly or macrocephaly and developmental and behavioral abnormalities. Nat Genet. 2008;40:1466–1471. doi: 10.1038/ng.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant JL, Boughter JD, Gong S, LeDoux MS, Heck DH. Cerebellar cortical output encodes temporal aspects of rhythmic licking movements and is necessary for normal licking frequency. Eur J Neurosci. 2010;32:41–52. doi: 10.1111/j.1460-9568.2010.07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium IS. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455(7210):237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DG. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain: a journal of neurology. 2000;123 (Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Di Cicco M, Padoan R, Felisati G, Dilani D, Moretti E, Guerneri S, Selicorni A. Otorhinolaringologic manifestation of Smith-Magenis syndrome. Int J Pediatr Otorhinolaryngol. 2001;59:147–150. doi: 10.1016/s0165-5876(01)00475-x. [DOI] [PubMed] [Google Scholar]
- Edelman EA, Girirajan S, Finucane B, Patel PI, Lupski JR, Smith AC, Elsea SH. Gender, genotype, and phenotype differences in Smith-Magenis syndrome: a meta-analysis of 105 cases. Clin Genet. 2007;71:540–550. doi: 10.1111/j.1399-0004.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- Elsea SH, Girirajan S. Smith-Magenis syndrome. Eur J Hum Genet. 2008;16:412–421. doi: 10.1038/sj.ejhg.5202009. [DOI] [PubMed] [Google Scholar]
- Ercan-Sencicek A, Wright NRD, Frost SJ, Fulbright RK, Felsenfeld S, Hart L, Landl N, Mencl EW, Sanders SJ, Pugh KR, State MW, Grigorenko EL. Searching for Potocki Lupski syndrome phenotype: A patient with language impairment and no autism. Brain and Development. 2011 doi: 10.1016/j.braindev.2011.11.003. [Epub ahread of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Aldinger KA, Ashwood P, Bauman ML, Blaha CD, Blatt GJ, Chauhan A, Chauhan V, Dager SR, Dickson PE, Estes AM, Goldowitz D, Heck DH, Kemper TL, King BH, Martin LA, Millen KJ, Mittleman G, Mosconi MW, Persico AM, Sweeney JA, Webb SJ, Welsh JP. Consensus Paper: Pathological Role of the Cerebellum in Autism. Cerebellum. 2012 Feb 28;2012 doi: 10.1007/s12311-012-0355-9. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco D, Romano C, Reitano S, Barone C, Benedetto DD, Castiglia L, Fichera M, Galesi O, Zingale M, Buono S, Uliana V, Caselli R, Canitano R, Hayek G, Renieri A. Three new patients with dup(17)(p11.2p11.2) without autism. Clin Genet. 2008;73:294–296. doi: 10.1111/j.1399-0004.2007.00959.x. [DOI] [PubMed] [Google Scholar]
- Greenberg F, Lewis RA, Potocki L, Glaze D, Parke J, Killian J, Murphy MA, Williamson D, Brown F, Dutton R, McCluggage C, Friedman E, Sulek M, Lupski JR. Multi-disciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2) Am J Med Genet. 1996;62:247–254. doi: 10.1002/(SICI)1096-8628(19960329)62:3<247::AID-AJMG9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gropman AL, Duncan WC, Smith AC. Neurologic and developmental features of the Smith-Magenis syndrome (del 17p11.2) Pediatr Neurol. 2006;34:337–350. doi: 10.1016/j.pediatrneurol.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Hayar A, Bryant JL, Boughter JD, Heck DH. A low-cost solution to measure mouse licking in an electrophysiological setup with a standard analog-to-digital converter. Journal of neuroscience methods. 2006;153:203–207. doi: 10.1016/j.jneumeth.2005.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck DH, Zhao Y, Roy S, LeDoux MS, Reiter LT. Analysis of cerebellar function in Ube3a-deficient mice reveals novel genotype-specific behaviors. Human molecular genetics. 2008;17:2181–2189. doi: 10.1093/hmg/ddn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horev G, Ellegood J, Lerch JP, Son YE, Muthuswamy L, Vogel H, Krieger AM, Buja A, Henkelman RM, Wigler M, Mills AA. Dosage-dependent phenotypes in models of 16p11.2 lesions found in autism. Proc Natl Acad Sci U S A. 2011;108:17076–17081. doi: 10.1073/pnas.1114042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaria M, Saha P, Potocki L, Bi W, Yan J, Girirajan S, Burns B, Walz K, Elsea S, Chan L, Lupski J, Gu W. A duplication CNV that conveys traits reciprocal to metabolic syndrome and protects against diet induced obesity. Plos Genetics. 2012a;8(5):e1002713. doi: 10.1371/journal.pgen.1002713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaria M, Spencer C, Gu W, Paylor R, Lupski J. Enriched rearing improves behavioral responses of an animal model for CNV-based autistic-like trait. Hum Mol Gen. 2012b doi: 10.1093/hmg/dds124. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Scherer SW. The clinical context of copy number variation in the human genome. Expert Rev Mol Med. 2010;12:e8. doi: 10.1017/S1462399410001390. [DOI] [PubMed] [Google Scholar]
- Lupski JR. Schizophrenia: Incriminating genomic evidence. Nature. 2008;455(7210):178–179. doi: 10.1038/455178a. [DOI] [PubMed] [Google Scholar]
- Lupski JR. Insights for autism from Charcot-Marie-Tooth disease. Simons foundation autism research initiative. Viewpoint. 2011 Sep 27;2011 [ http://sfari.org/news-and-opinion/viewpoint/2011/insights-for-autism-from-charcot-marie-tooth-disease] [Google Scholar]
- McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, Perkins DO, Dickel DE, Kusenda M, Krastoshevsky O, Krause V, Kumar RA, Grozeva D, Malhotra D, Walsh T, Zackai EH, Kaplan P, Ganesh J, Krantz ID, Spinner NB, Roccanova P, Bhandari A, Pavon K, Lakshmi B, Leotta A, Kendall J, Lee YH, Vacic V, Gary S, Iakoucheva LM, Crow TJ, Christian SL, Lieberman JA, Stroup TS, Lehtimaki T, Puura K, Haldeman-Englert C, Pearl J, Goodell M, Willour VL, Derosse P, Steele J, Kassem L, Wolff J, Chitkara N, McMahon FJ, Malhotra AK, Potash JB, Schulze TG, Nothen MM, Cichon S, Rietschel M, Leibenluft E, Kustanovich V, Lajonchere CM, Sutcliffe JS, Skuse D, Gill M, Gallagher L, Mendell NR, Craddock N, Owen MJ, O’Donovan MC, Shaikh TH, Susser E, Delisi LE, Sullivan PF, Deutsch CK, Rapoport J, Levy DL, King MC, Sebat J. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41:1223–1227. doi: 10.1038/ng.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DH, Tremblay P. Clinical implications of cross-system interactions. Semin Speech Lang. 2006;27:300–309. doi: 10.1055/s-2006-955119. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Ueda A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Dev Med Child Neurol. 2005;47:299–304. doi: 10.1017/s0012162205000587. [DOI] [PubMed] [Google Scholar]
- Molina J, Carmona-Mora P, Chrast J, Krall PM, Canales CP, Lupski JR, Reymond A, Walz K. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Hum Mol Genet. 2008;17:2486–2495. doi: 10.1093/hmg/ddn148. [DOI] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Potocki L, Bi W, Treadwell-Deering D, Carvalho CM, Eifert A, Friedman EM, Glaze D, Krull K, Lee JA, Lewis RA, Mendoza-Londono R, Robbins-Furman P, Shaw C, Shi X, Weissenberger G, Withers M, Yatsenko SA, Zackai EH, Stankiewicz P, Lupski JR. Characterization of Potocki-Lupski syndrome (dup(17)(p11.2p11.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am J Hum Genet. 2007;80:633–649. doi: 10.1086/512864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocki L, Chen KS, Park SS, Osterholm DE, Withers MA, Kimonis V, Summers AM, Meschino WS, Anyane-Yeboa K, Kashork CD, Shaffer LG, Lupski JR. Molecular mechanism for duplication 17p11.2- the homologous recombination reciprocal of the Smith-Magenis microdeletion. Nat Genet. 2000;24:84–87. doi: 10.1038/71743. [DOI] [PubMed] [Google Scholar]
- Ricard G, Molina J, Chrast J, Gu W, Gheldof N, Pradervand S, Schutz F, Young JI, Lupski JR, Reymond A, Walz K. Phenotypic consequences of copy number variation: insights from Smith-Magenis and Potocki-Lupski syndrome mouse models. PLoS Biol. 2010;8(11):e1000543. doi: 10.1371/journal.pbio.1000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Zhao Y, Allensworth M, Farook MF, LeDoux MS, Reiter LT, Heck DH. Comprehensive motor testing in Fmr1-KO mice exposes temporal defects in oromotor coordination. Behavioral neuroscience. 2011;125:962–969. doi: 10.1037/a0025920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinawi M, Liu P, Kang SH, Shen J, Belmont JW, Scott DA, Probst FJ, Craigen WJ, Graham BH, Pursley A, Clark G, Lee J, Proud M, Stocco A, Rodriguez DL, Kozel BA, Sparagana S, Roeder ER, McGrew SG, Kurczynski TW, Allison LJ, Amato S, Savage S, Patel A, Stankiewicz P, Beaudet AL, Cheung SW, Lupski JR. Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size. J Med Genet. 2010;47:332–341. doi: 10.1136/jmg.2009.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Dykens E, Greenberg F. Behavioral phenotype of Smith-Magenis syndrome (del 17p11.2) Am J Med Genet. 1998;81:179–185. doi: 10.1002/(sici)1096-8628(19980328)81:2<179::aid-ajmg10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Smith AC, McGavran L, Robinson J, Waldstein G, Macfarlane J, Zonona J, Reiss J, Lahr M, Allen L, Magenis E. Interstitial deletion of (17)(p11.2p11.2) in nine patients. Am J Med Genet. 1986;24:393–414. doi: 10.1002/ajmg.1320240303. [DOI] [PubMed] [Google Scholar]
- Soler-Alfonso C, Motil KJ, Turk CL, Robbins-Furman P, Friedman EM, Zhang F, Lupski JR, Fraley JK, Potocki L. Potocki-Lupski syndrome: A microduplication syndrome associated with oropharyngeal dysphagia and failure to thrive. J Pediatr. 2011;158:655–659. doi: 10.1016/j.jpeds.2010.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. European psychiatry: J Assoc Europ Psychiatrists. 2008;23:289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Stankiewicz P, Lupski JR. Structural variation in the human genome and its role in disease. Annual review of medicine. 2010;61:437–455. doi: 10.1146/annurev-med-100708-204735. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, Steinberg S, Fossdal R, Sigurdsson E, Sigmundsson T, Buizer-Voskamp JE, Hansen T, Jakobsen KD, Muglia P, Francks C, Matthews PM, Gylfason A, Halldorsson BV, Gudbjartsson D, Thorgeirsson TE, Sigurdsson A, Jonasdottir A, Jonasdottir A, Bjornsson A, Mattiasdottir S, Blondal T, Haraldsson M, Magnusdottir BB, Giegling I, Moller HJ, Hartmann A, Shianna KV, Ge D, Need AC, Crombie C, Fraser G, Walker N, Lonnqvist J, Suvisaari J, Tuulio-Henriksson A, Paunio T, Toulopoulou T, Bramon E, Di Forti M, Murray R, Ruggeri M, Vassos E, Tosato S, Walshe M, Li T, Vasilescu C, Muhleisen TW, Wang AG, Ullum H, Djurovic S, Melle I, Olesen J, Kiemeney LA, Franke B, Sabatti C, Freimer NB, Gulcher JR, Thorsteinsdottir U, Kong A, Andreassen OA, Ophoff RA, Georgi A, Rietschel M, Werge T, Petursson H, Goldstein DB, Nothen MM, Peltonen L, Collier DA, St Clair D, Stefansson K. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455(7210):232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers JB, Dinardo LA, Karimnamazi H. Motor and premotor mechanisms of licking. Neuroscience and biobehavioral reviews. 1997;21:631–647. doi: 10.1016/s0149-7634(96)00045-0. [DOI] [PubMed] [Google Scholar]
- Treadwell-Deering DE, Powell MP, Potocki L. Cognitive and behavioral characterization of the Potocki-Lupski syndrome (duplication 17p11.2) J Dev Behav Pediatr. 2010;31(2):137–43. doi: 10.1097/DBP.0b013e3181cda67e. [DOI] [PubMed] [Google Scholar]
- Walz K, Caratini-Rivera S, Bi W, Fonseca P, Mansouri DL, Lynch J, Vogel H, Noebels JL, Bradley A, Lupski JR. Modeling del(17)(p11.2p11.2) and dup(17)(p11.2p11.2) contiguous gene syndromes by chromosome engineering in mice: phenotypic consequences of gene dosage imbalance. Mol Cell Biol. 2003;23:3646–3655. doi: 10.1128/MCB.23.10.3646-3655.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz K, Spencer C, Kaasik K, Lee CC, Lupski JR, Paylor R. Behavioral characterization of mouse models for Smith-Magenis syndrome and dup(17)(p11.2p11.2) Hum Mol Genet. 2004;13:367–378. doi: 10.1093/hmg/ddh044. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, Saemundsen E, Stefansson H, Ferreira MA, Green T, Platt OS, Ruderfer DM, Walsh CA, Altshuler D, Chakravarti A, Tanzi RE, Stefansson K, Santangelo SL, Gusella JF, Sklar P, Wu BL, Daly MJ. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Lang EJ, Suglhara I, Llinas R. Dynamic organization of motor control within the olivocerebellar system. Nature. 1995;374(6521):453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- Yan J, Bi W, Lupski JR. Penetrance of craniofacial anomalies in mouse models of Smith-Magenis syndrome is modified by genomic sequence surrounding Rai1: not all null alleles are alike. Am J Hum Genet. 2007;80:518–525. doi: 10.1086/512043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Keener VW, Bi W, Walz K, Bradley A, Justice MJ, Lupski JR. Reduced penetrance of craniofacial anomalies as a function of deletion size and genetic background in a chromosome engineered partial mouse model for Smith-Magenis syndrome. Hum Mol Genet. 2004;13:2613–2624. doi: 10.1093/hmg/ddh288. [DOI] [PubMed] [Google Scholar]
- Zhang F, Gu W, Hurles ME, Lupski JR. Copy number variation in human health, disease, and evolution. Annu Rev Genomics Hum Genet. 2009;10:451–481. doi: 10.1146/annurev.genom.9.081307.164217. [DOI] [PMC free article] [PubMed] [Google Scholar]