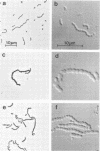
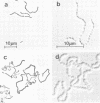
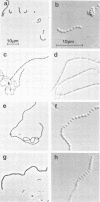
Abstract
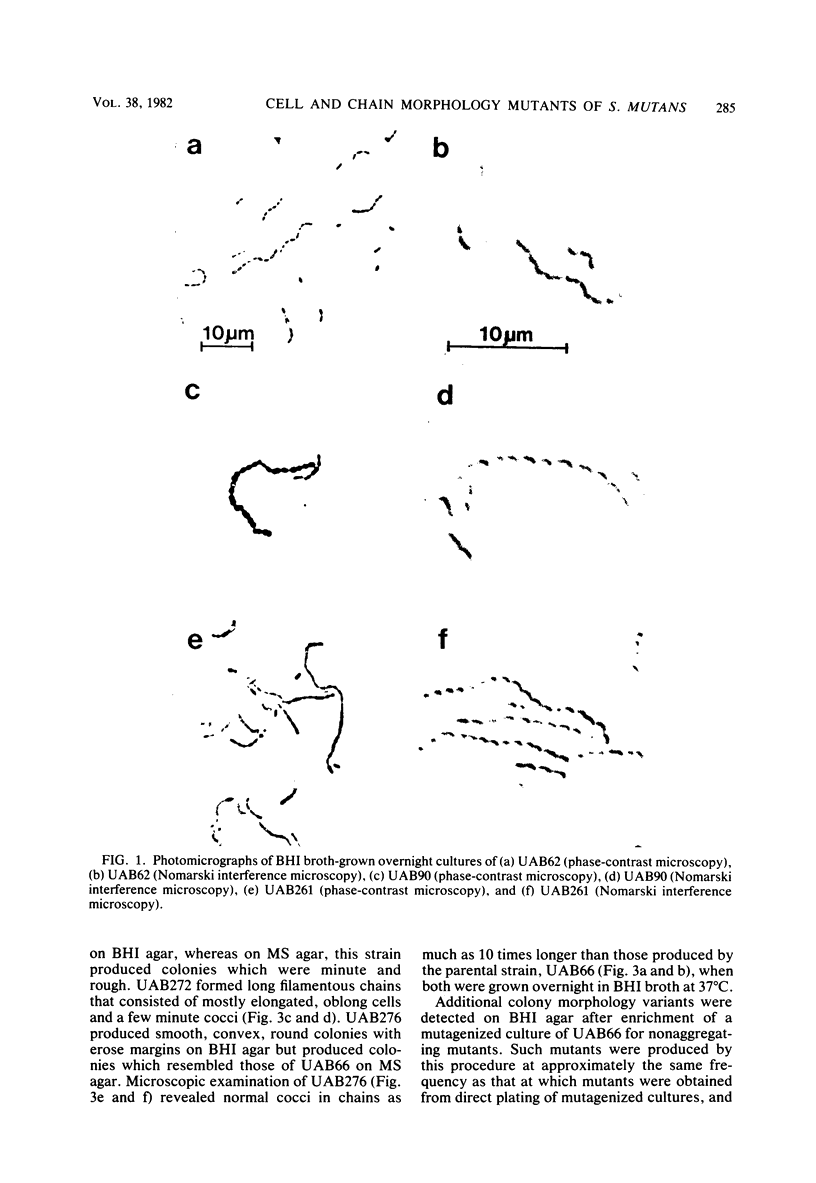
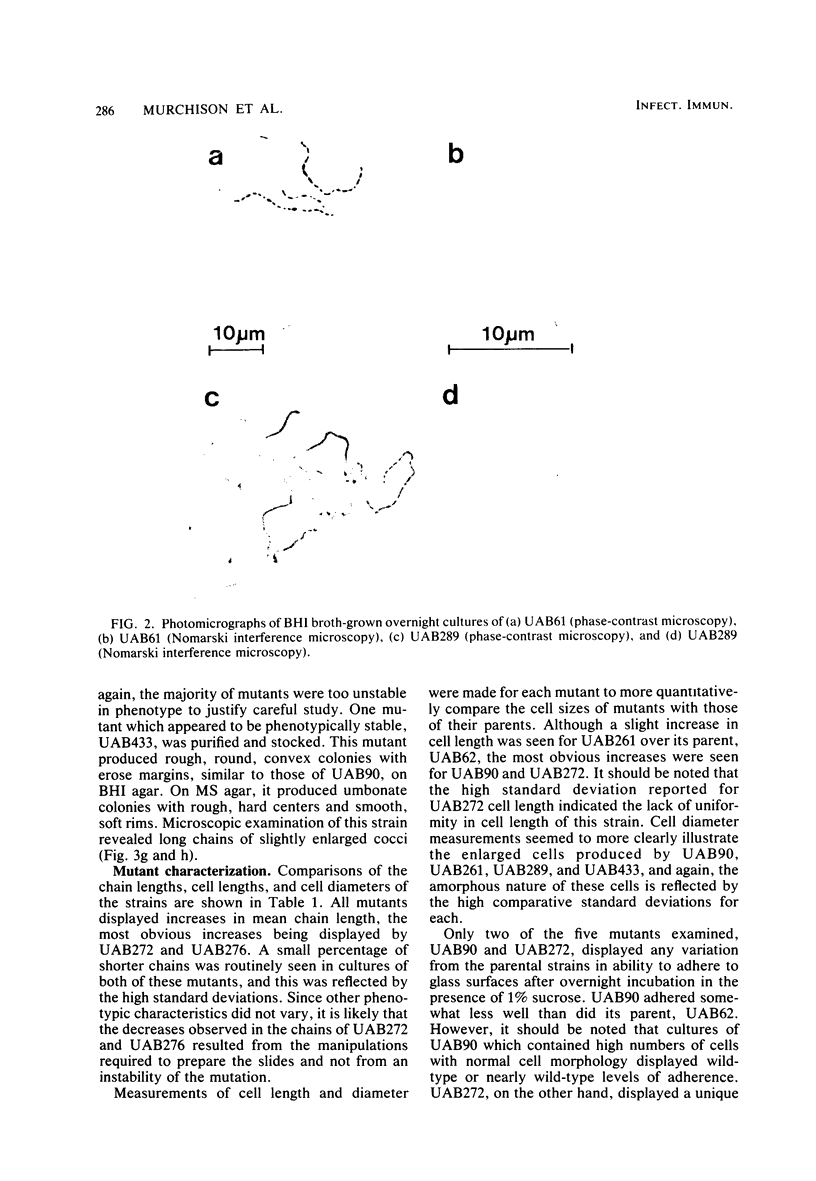
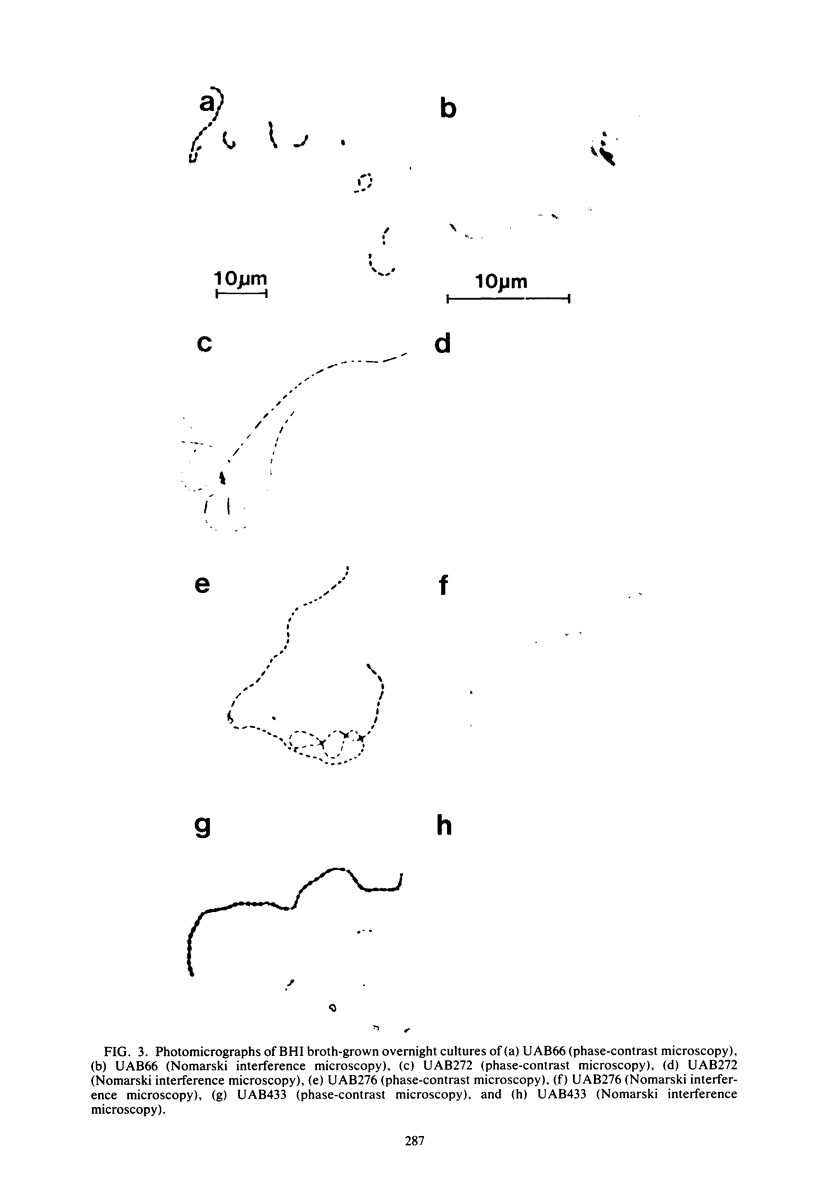
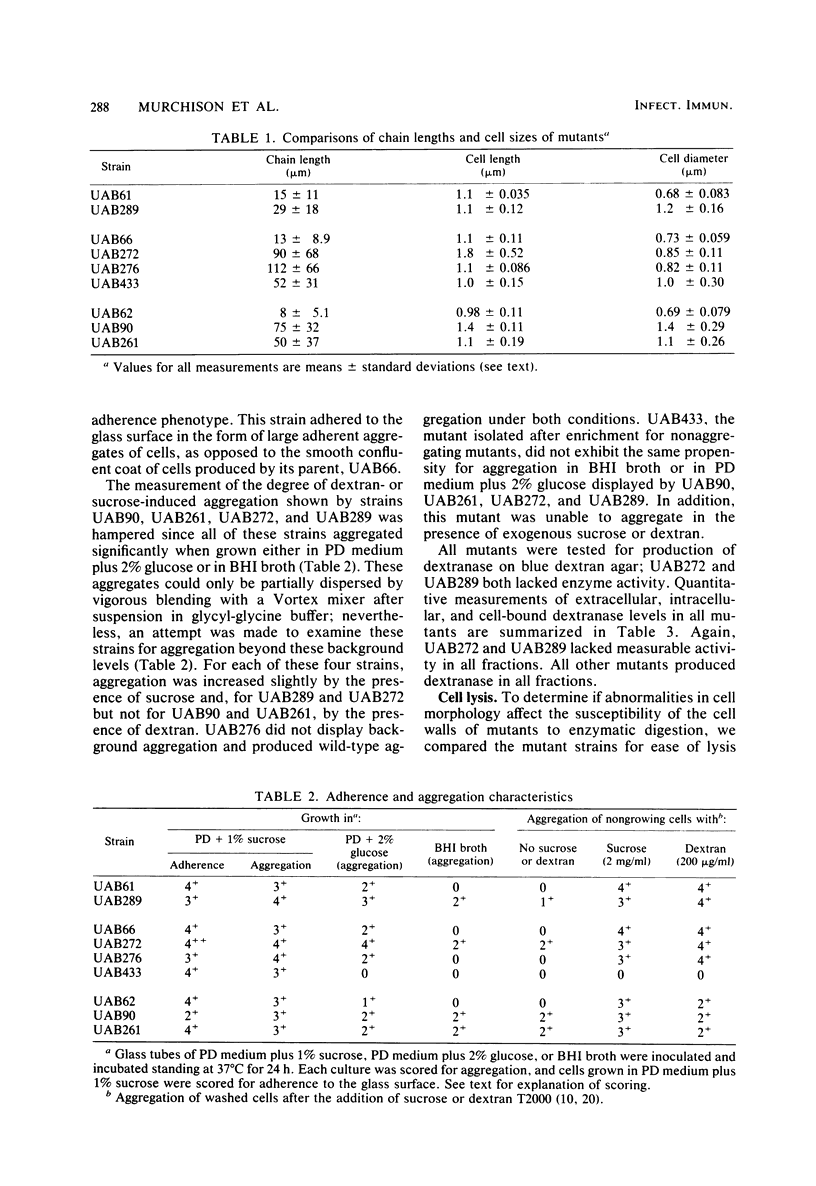
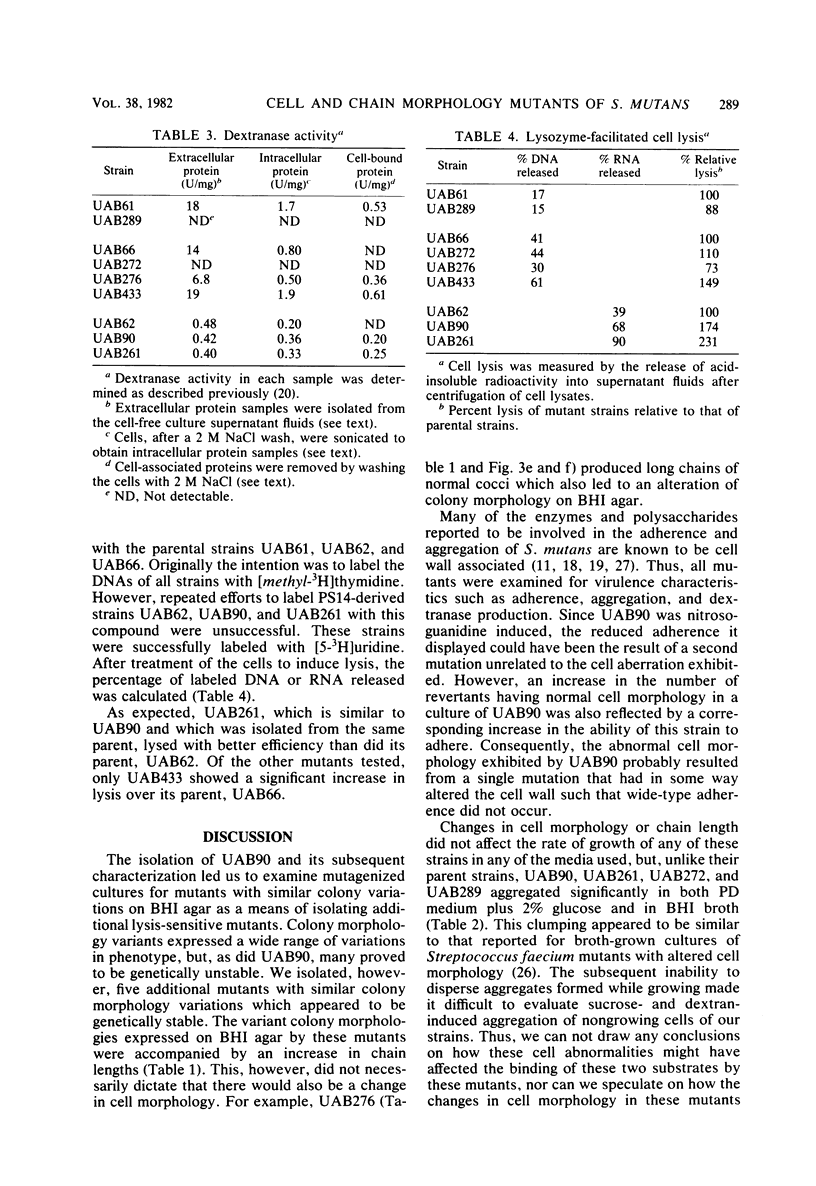
A nitrosoguanidine-induced mutant, designated UAB90, of Streptococcus mutans PS14 (serotype c) strain UAB62, was identified on the basis of its unique colony morphology and isolated on brain heart infusion agar. Other mutants displaying similar colony morphologies on brain heart infusion agar were isolated after ethyl methane sulfonate mutagenesis of UAB62 and S. mutans 6715 (serotype g) strains UAB61 and UAB66, and these were found to exhibit abnormalities in cell morphology, chain length, or both. All mutants were examined further for (i) adherence and aggregation after overnight growth in medium containing sucrose, (ii) growth and aggregation in brain heart infusion broth and medium containing glucose, (iii) aggregation of nongrowing cells in the presence of 2 mg of sucrose per ml or 200 μg of dextran per ml, (iv) dextranase activity, and (v) ease of cell lysis. Mutants isolated included several with long chains of enlarged cocci, and two of these strains, UAB261 and UAB433, along with UAB90, were more susceptible to cell lysis than were their parents. UAB261, isolated from UAB62, maintained other parental characteristics, whereas UAB433, isolated from UAB66, lost its ability to aggregate in the presence of either sucrose or dextran. The “fragile” mutant UAB90 was particularly useful in the isolation of high-molecular-weight DNA for early gene cloning experiments by our laboratory. Two other cell morphology mutants, UAB272 from UAB66 and UAB289 from UAB61, did not lyse better than their parents, but both lacked measurable dextranase activity. A final mutant, UAB276 from UAB66, displayed only increased chain length without apparent cell morphology variations. Chains produced by this mutant were up to 10 times longer than those produced by UAB66. UAB276 lysed slightly less well than its parent but retained all other wild-type characteristics examined.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Chassy B. M., Giuffrida A. Method for the lysis of Gram-positive, asporogenous bacteria with lysozyme. Appl Environ Microbiol. 1980 Jan;39(1):153–158. doi: 10.1128/aem.39.1.153-158.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman S. E., van de Rijn I., Bleiweis A. S. Lysis of grouped and ungrouped streptococci by lysozyme. Infect Immun. 1970 Nov;2(5):563–569. doi: 10.1128/iai.2.5.563-569.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. J., Lillmars K. A method for gentle lysis of Streptococcus sanguis and Streptococcus mutans. Biochem Biophys Res Commun. 1975 Jul 8;65(1):378–384. doi: 10.1016/s0006-291x(75)80104-5. [DOI] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M. Use of bacteriolytic enzymes in determination of wall structure and their role in cell metabolism. Bacteriol Rev. 1968 Dec;32(4 Pt 2):425–464. [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheim B., Burckhardt J. J. Isolation and properties of a dextranase from streptococcus mutans OMZ 176. Helv Odontol Acta. 1974 Oct;18(2):101–113. [PubMed] [Google Scholar]
- Ikeda T., Shiota T., McGhee J. R., Otake S., Michalek S. M., Ochiai K., Hirasawa M., Sugimoto K. Virulence of Streptococcus mutans: comparison of the effects of a coupling sugar and sucrose on certain metabolic activities and cariogenicity. Infect Immun. 1978 Feb;19(2):477–480. doi: 10.1128/iai.19.2.477-480.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Hamada S., Kotani S., Kato K. Enzymic lysis and structure of the cell walls of the oral Streptococcus mutans BHT. Arch Oral Biol. 1979;24(7):529–537. doi: 10.1016/0003-9969(79)90132-8. [DOI] [PubMed] [Google Scholar]
- Knox K. W., Jacques N. A., Campbell L. K., Wicken A. J., Hurst S. F., Bleiweis A. S. Phenotypic stability of the cell wall of Streptococcus mutans Ingbritt grown under various conditions. Infect Immun. 1979 Dec;26(3):1071–1078. doi: 10.1128/iai.26.3.1071-1078.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of cell-associated dextransucrase activity from glucose-grown cells of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):227–235. doi: 10.1128/iai.10.1.227-235.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchison H., Larrimore S., Curtiss R., 3rd Isolation and characterization of Streptococcus mutans mutants defective in adherence and aggregation. Infect Immun. 1981 Dec;34(3):1044–1055. doi: 10.1128/iai.34.3.1044-1055.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock J. J., Katona L. I., Goodman H., Cho M. I., Iacono V. J. Bacteriolysis of Streptococcus mutans BHT by lysozyme and inorganic anions normally present in human saliva. Arch Oral Biol. 1981;26(9):711–716. doi: 10.1016/0003-9969(81)90187-4. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Higgins M. L. Problems of cell wall and membrane growth, enlargement, and division. Ann N Y Acad Sci. 1974 May 10;235(0):161–197. doi: 10.1111/j.1749-6632.1974.tb43265.x. [DOI] [PubMed] [Google Scholar]
- Shungu D. L., Cornett J. B., Shockman G. D. Morphological and physiological study of autolytic-defective Streptococcus faecium strains. J Bacteriol. 1979 May;138(2):598–608. doi: 10.1128/jb.138.2.598-608.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Schachtele C. F. Evaluation of dextranase production by the cariogenic bacterium Streptococcus mutans. Infect Immun. 1974 Feb;9(2):467–469. doi: 10.1128/iai.9.2.467-469.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]