Abstract
The immune response triggers a complicated sequence of events, one of which is release of the cytokine tumor necrosis factor-α (TNF-α) from stromal cells such as monocytes and macrophages. In this work we explored the biophysical effects of TNF-α on endothelial cells (ECs), including changes in cell morphology, biomechanics, migration, and cytoskeletal dynamics. We found that TNF-α induces a wide distribution of cell area and aspect ratio, with these properties increasing on average during treatment. Interestingly, aspect ratio peaks around 10 hours of exposure to TNF-α, corresponding also to a peak in exerted traction forces. Meanwhile, ECs treated with TNF-α soften, and we associate this with significant increases in estimated cellular volume. In addition, our evaluation of migratory dynamics demonstrates an inverse correlation between cell aspect ratio and migration speed after TNF-α treatment, suggesting that cell shape may be an important functional regulator of EC migration during an inflammatory response. Finally, we address the basic mechanics of how the reorganization of F-actin filaments occurs during TNF-α treatment, and we demonstrate a dynamic shift of existing actin filaments. Together, our results suggest a functional link between EC morphology, biomechanics, migration, and cytoskeletal dynamics during an inflammatory response.
Keywords: cell stiffness, cell mechanics, immune response, cell migration, actin, traction forces
INTRODUCTION
Tumor necrosis factor-alpha (TNF-α) is a cytokine produced by a variety of stromal cells, primarily monocytes and macrophages, as a result of an immune or inflammatory response. The vascular endothelium, which normally serves as a protective barrier between the blood vessel lumen and nearby tissues, undergoes a series of biological changes after binding TNF-α, resulting in increased permeability to both macromolecules (Goldblum et al. 1989) and immune cells (Stroka and Aranda-Espinoza 2011b). One of the most prominent biological changes is the increased expression of selectins, as well as adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1), on the surface of the endothelium (Dustin and Springer 1988). These molecules assist in the leukocyte adhesion cascade (Dustin and Springer 1988), which includes leukocyte migration along and transmigration through the vascular endothelium. At the same time, junctional molecules such as occludin and VE-cadherin dissociate away from the junctions, leading to decreased barrier function (Mckenzie and Ridley 2007).
There is also evidence that TNF-α affects endothelial cell (EC) morphological and biomechanical properties. For example, cells within a confluent endothelium become larger and more elongated in response to TNF-α (Stroka and Aranda-Espinoza 2011b). F-actin undergoes significant reorganization and aligns along the major axis of the cell (Mckenzie and Ridley 2007; Stroka and Aranda-Espinoza 2011b; Wojciak-Stothard et al. 1998), events which are generally linked to enhanced cell contractility and stiffness. In seemingly contradictory data, cells soften after exposure to TNF-α (Kang et al. 2008; Stroka and Aranda-Espinoza 2011b). Here, we utilized a variety of biophysical techniques, including live cell imaging, atomic force microscopy, traction force microscopy, total internal reflection fluorescence microscopy, confocal microscopy, and transfection, to understand the links between EC morphology, biomechanics, and migratory dynamics after exposure of ECs to TNF-α. We chose to evaluate single cells in order to simplify the system, since, for example, cell-cell adhesion affects EC stiffness (Stroka and Aranda-Espinoza 2011a). These changes in EC properties are key components of the inflammatory response, yet are often overshadowed by the biological changes that occur. Together, our results suggest a functional link between EC morphology, biomechanics, migration, and cytoskeletal dynamics during an inflammatory response.
METHODS
Cell culture
Human umbilical vein ECs (HUVECs) were purchased from Lifeline Cell Technology (Walkersville, MD) and cultured as previously described (Stroka and Aranda-Espinoza 2011a; Stroka and Aranda-Espinoza 2011b). Glass coverslips (22×22 mm, Fisher Scientific, Pittsburgh, PA) were coated with 0.1 mg/mL fibronectin (Sigma, St. Louis, MO) for 2 hours at room temperature, and HUVECs (1×104 total; passages 2-6) were plated onto the fibronectin-coated glass coverslips. Cells were incubated at 37°C, 5% CO2, and 55% humidity overnight for approximately 16 hours and were subsequently treated with 25 ng/mL TNF-α or no treatment (control).
Cell staining
HUVECs were fixed in 0.5% glutaraldehyde (Ted Pella, Inc., Redding, CA) for 10 minutes and washed twice in phosphate buffered saline (PBS). Cells were then permeabilized and blocked for nonspecific binding as previously described (Stroka and Aranda-Espinoza 2011a). F-actin, nuclear DNA, and vinculin were stained as previously described (Stroka and Aranda-Espinoza 2011a).
Cell transfection
Cells were transfected for GFP-actin using an Amaxa Nucleofector (Lonza, Walkersville, MD). Primary mammalian endothelial cell solutions (Lonza) were used in combination with program A-034 on the Nucleofector, and the manufacturer's optimized protocol for HUVECs was followed. After transfection, cells were plated onto fibronectin-coated glass coverslips and allowed to attach overnight. Cells were then rinsed and subsequently used in fluorescence timelapse microscopy experiments.
Atomic force microscopy
Young's moduli of live HUVECs were measured using an atomic force microscope (AFM; Agilent Technologies, Santa Clara, CA) as previously described in detail (Stroka and Aranda-Espinoza 2011a; Stroka and Aranda-Espinoza 2011b). Briefly, a silicon nitride cantilever (Novascan, Ames, IA) with a spherical glass SiO2 probe of diameter 5 μm was used to obtain typical force curve measurements on live HUVECs. Twenty-five force curves were taken on at least 10 cells for each condition (control and TNF-α), for a total of at least 250 force curves for each condition. Using a custom-written Matlab (The Mathworks, Natick, MA) program, data were fit to the Hertz-Sneddon model (Sneddon 1965) for a paraboloid indenter (Weisenhorn et al. 1993), as previously described (Stroka and Aranda-Espinoza 2011a; Stroka and Aranda-Espinoza 2011b). Cells were probed at both the “cell body” (raised portion) and “periphery” (near the base of the cell body, but not at the flattened lamellipodia). Indentations were much smaller (<20%) than the total cell height, indicating that the stiff glass substrate below the cell did not affect the force curves.
To estimate cell volume, the free and open source software Gwyddion was used. Cross-sectional plot profiles from AFM topograph images were taken across the major and minor axes of the cell (determined by eye). The plot profile was used to determine an approximate height of the cell body and periphery regions, as well as the lengths of the major and minor axes. Then, the cell volume was approximated by summing the volume of two elliptical cylindrical disks, for the flattened portion of the cell and cell body, using the following equation:
where A1 is half the length of the minor axis of the flattened region, B1 is half the length of the major axis of the flattened region, C1 is an estimate of the height of the flattened region, A2 is half the length of the minor axis of the cell body, B2 is half the length of the major axis of the cell body, and C2 is an estimate of the height of the cell body (Figure 2d).
Figure 2.
TNF-α induces changes in EC mechanical properties. (a) Box and whisker plot indicates EC stiffness decreases with 24-hour TNF-α treatment, both at the cell body and periphery regions, as measured by atomic force microscopy (AFM). *** indicates P<0.001 using a Student's t-test. AFM deflection images of (b) control and (c) 24-hour TNF-α-treated ECs display the drastic change in morphology that occurs in most cells upon TNF-α exposure. AFM images are 90 μm by 90 μm. (d) Using AFM topographic information, cellular volume was estimated by summing the volume of two ellipsoids, for the flattened portion of the cell and cell body, using the equation and procedure outlined in the Methods section. Top and side views of the relevant dimensions are shown. (f) Estimates of cellular volume for control and 24-hour TNF-α-treated ECs. * indicates P<0.05 using a Student's t-test.
Traction force microscopy
Cellular traction forces were measured using the well-established technique of traction force microscopy, as previously described in detail (Dembo and Wang 1999; Norman et al. 2010). Briefly, 5 kPa flexible polyacrylamide gels, composed of 8% acrylamide and 0.07% bisacrylamide, were embedded with 0.5 μm fluorescent marker beads and coated with 0.1mg/mL fibronectin (Norman et al. 2010; Stroka and Aranda-Espinoza 2009). Cells were plated onto the substrates and given ~16 hours to adhere before adding TNF-α. Phase contrast and fluorescence images were captured of the spread cells and embedded beads, and tryspin-EDTA was added to remove the cell. An image of the relaxed marker beads was captured. Based on the mechanical properties of the gel and the displacements of the marker beads, the cellular traction forces were computed using the technique described by Dembo and Wang (Dembo and Wang 1999). The overall force exerted by the cell, |F|, is an integral of the traction field magnitude over the area of the cell, where T(x,y) = [Tx(x,y),Ty(x,y)] is the continuous field of traction vectors defined at the spatial position (x,y) within the cell, as previously described (Smith et al. 2007).
Phase and fluorescence microscopy
Phase contrast image timelapse sequences were taken in HUVEC culture media at 37°C, 5% CO2, and 55% humidity using an Olympus IX71 (Olympus, Center Valley, PA) inverted microscope and a 10×/0.3 NA Ph1 objective. Images were captured using a QImaging Retiga-SRV charge-coupled device (CCD) digital camera (QImaging Corporation, Surrey, British Columbia, Canada) and IPLab software as previously described (Stroka and Aranda-Espinoza 2011b). Fluorescence images of GFP-actin-transfected cells were taken in HUVEC culture media at 37°C, 5% CO2, and 55% humidity using an Olympus IX81 inverted microscope and a 60×/1.42 NA oil objective. Images were captured using a QImaging Rolera-MGi CCD digital camera (QImaging Corporation) and Slidebook software. Confocal images of cells stained for tubulin and F-actin were taken at room conditions using an Olympus IX81 scanning disk confocal microscope and a 60×/1.42 NA oil objective. Images were captured using a Hammamatsu ORCA-ET CCD camera (Leeds Precision Instruments, Minneapolis, MN) using Slidebook software. Cell morphology (area and aspect ratio) were measured using ImageJ (NIH, Bethesda, MD) as previously described (Stroka and Aranda-Espinoza 2009, 2011a). Cell speeds were calculated for each frame of the movie by dividing the cell's displacement by the time step (20 minutes). Then, a moving average of the previous 5 timepoints was plotted (as in Figures 3 and 4) to smooth the data. Total internal reflection fluorescence (TIRF) microscopy was completed on cells immunostained for vinculin, as previously described in detail (Stroka and Aranda-Espinoza 2011a). Focal adhesion (FA) size and density were measured using Image J for the TIRF microscopy images (Stroka and Aranda-Espinoza 2011a).
Figure 3.
TNF-α exposure increases traction force generation in ECs and decreases focal adhesion density. (a) Average traction force exerted by control and 24-hour TNF-α-activated ECs. Error bars indicate average of 7 and 10 experiments for control and TNF-α-activated ECs, respectively. * indicates P<0.05 using a Student's t-test. (b) Change in traction force generation for a representative cell with respect to untreated condition, over the course of TNF-α treatment. TNF-α was added at T=0 as indicated. Also shown are total internal reflection fluorescence (TIRF) microscopy images of vinculin-stained (c) control ECs and (d) ECs treated with TNF-α for 24 hours. In panel c, the scale bar is 20 μm and also applies to panel d. Focal adhesion size (e) and density (f) were quantified using image analysis. *** indicates P<0.001 using a Student's t-test. Bars are mean ± SEM.
Figure 4.
Cell speed is not necessarily correlated with aspect ratio in migrating control ECs. (a) Cell aspect ratio (primary axis) and speed (secondary axis) are plotted on the same axis for a representative migrating control cell. (b) Cell area is plotted as a function of time. In panels a and b, time=0 corresponds to ~16 hours after plating cells. In panel a, letters C, D, E, and F correspond to timepoints relevant to the images shown in panels c, d, e, and f, respectively. Scale bar is 20 μm for all images.
RESULTS
TNF-α induces changes in cell morphology
HUVECs were plated onto glass coverslips and given approximately 16 hours to spread, though maximal spreading occurred after only one hour. Subsequently, phase contrast images were taken, and then the cells were treated with a physiological concentration of TNF-α (25 ng/mL) in culture medium. Phase contrast images were taken at 8 hours and 24 hours following treatment. Prior to TNF-α treatment, HUVECs displayed a distribution of areas (Fig. 1a), while addition of TNF-α resulted in a shift of this distribution to the right after 8 hours of treatment (Fig. 1b), and further to the right after 24 hours of treatment (Fig. 1c). Importantly, at 24 hours, untreated (control) cell area was not different from cell area at 0 hours. While increases in cell area and aspect ratio have previously been reported for TNF-α (Mckenzie and Ridley 2007; Stroka and Aranda-Espinoza 2011b; Yang et al. 2005), here we additionally noted that the width of the distribution of areas became noticeably wider after the 24-hour treatment (Fig. 1c). The increase in cell area throughout the treatment was also reflected in the average areas of untreated cells versus cells at 8 hours and 24 hours after addition of TNF-α (P<0.001) (Fig. 1d).
Figure 1.
TNF-α induces changes in endothelial cell (EC) morphology. Shown are histograms of cellular area for (a) control, (b) 8-hour TNF-α-treated, and (c) 24-hour TNF-α-treated ECs. (d) Box and whisker plot indicates that average cellular area increases with TNF-α treatment. Also shown are histograms of cellular aspect ratio, calculated by dividing the length of the major axis by the minor axis, for (e) control, (f) 8-hour TNF-α-treated, and (g) 24-hour TNF-α-treated ECs. Insets in panels e, f, and g are representative images of cells for each condition. Scale bars for all insets are 20 μm. (h) Box and whisker plot indicates that average cellular aspect ratio increases with TNF-α treatment, with aspect ratios at 8 hours being significantly higher than those at 24 hours. *** indicates P<0.001 using a Student's t-test.
HUVECs also underwent a change in aspect ratio due to TNF-α treatment. In comparison with the distribution of aspect ratios prior to treatment (Fig. 1e), at 8 hours after addition of TNF-α, HUVECs were more elongated, as evidenced by the shift in the aspect ratio distribution peak to the right (Fig. 1f). At 24 hours, the distribution shifted back to the left, though the distribution peak was still at a significantly larger aspect ratio than control cells (Fig. 1g). The degree of elongation varied from the box-like morphology of untreated cells (Fig. 1e, inset), to an extremely extended morphology (Fig. 1f, inset), with aspect ratio around 10. The average aspect ratios of control cells versus cells at 8 hours and 24 hours after addition of TNF-α followed the trend of the distribution peaks and were statistically different from each other (P<0.001) (Fig. 1h).
TNF-α induces changes in cell biomechanical properties
Using AFM, we measured the stiffness (Young's modulus) of HUVECs, both at the periphery and cell body regions. As we previously reported, the cell body of control cells was softer than the periphery region (Stroka and Aranda-Espinoza 2011a) (Fig. 2a). The same trend was measured for cells after 24-hour exposure to TNF-α (Fig. 2a). In addition, cells treated with TNF-α were softer than control cells at both the cell body and periphery regions (P<0.001) (Fig. 2a). AFM deflection images of control (Fig. 2b) and TNF-α-treated (Fig. 2c) cells indicate the drastic changes in morphology that occurred in most cells after exposure to TNF-α. We also estimated cellular volume from the AFM topographic images. Average estimated cell volume was larger for cells treated with TNF-α for 24 hours, in comparison with untreated control cells (P<0.05) (Fig. 2e).
Using traction force microscopy, we also measured traction forces generated by HUVECs during TNF-α treatment. Average traction forces increased approximately two-fold after 24 hours of exposure to TNF-α (Fig. 3a). Interestingly, traction force generation was dynamic, reaching a peak in magnitude around 10 hours after addition of TNF- α (Fig. 3b). Meanwhile, TNF-α treatment did not affect FA size (P>0.05), but decreased FA density (P<0.001) and caused the FAs to elongate and align along the major length of the cell (Figs. 3c-f), an effect that might contribute to the increase in traction forces exerted by the cells.
TNF-α induces an inverse correlation between cell aspect ratio and migration speed
Using phase contrast timelapse microscopy we also evaluated the migratory behavior of HUVECs in response to TNF-α. In untreated control cells, aspect ratio and migration speed were not necessarily correlated. For example, in the representative cell shown in Figure 4a, as the time reached point D on the plot, there was first a peak in speed that correlated with a peak in aspect ratio; however, the aspect ratio then dropped off as the migration speed continued to increase. There was an additional peak in speed at point F, but this was not correlated with any changes in aspect ratio. Over the 24-hour period (of no treatment), the area of the control cell fluctuated and finally rose slightly. However, on average, control cells were not larger after 24 hours. The aspect ratio data at points C, D, E, and F on Figure 4a correspond to the phase contrast images shown in Figures 4c, 4d, 4e, and 4f, respectively.
In agreement with the average aspect ratio trends at 8 hours and 24 hours after addition of TNF-α (Fig. h), most cells tracked individually experienced a peak in aspect ratio within the first 15 hours of TNF-α treatment, as in the representative cell shown in Figure 5a. Interestingly, in TNF-α-treated cells, aspect ratio was usually inversely correlated with cell migration speed (Fig. 5a). For example, migration speed fell to the lowest value just before the aspect ratio peaked around point E in Figure 5a. Later, at point F, aspect ratio decreased rapidly as migration speed increased to a peak value. In addition, cell area increased steadily over 24 hours of TNF-α treatment (Fig. 5b), which is in agreement with the average cell areas at 8 and 24 hours after addition of TNF-α (Fig. 1d). The aspect ratio data at points C, D, E, and F on Figure 5a correspond to the phase contrast images shown in Figures 5c, 5d, 5e, and 5f, respectively. This same cell is featured in Movie 1.
Figure 5.
Cell speed is inversely correlated with aspect ratio in TNF-α-activated ECs. (a) Cell aspect ratio (primary axis) and speed (secondary axis) are plotted on the same axis for a representative migrating TNF-α-activated cell. (b) Cell area is plotted as a function of time. In panels a and b, time=0 corresponds to ~16 hours after plating cells, which is the time when TNF-α was added to the cell media. In panel a, letters C, D, E, and F correspond to timepoints relevant to the images shown in panels c, d, e, and f, respectively. Scale bar is 20 μm for all images.
TNF-α induces alignment of actin filaments along the major axis of cells
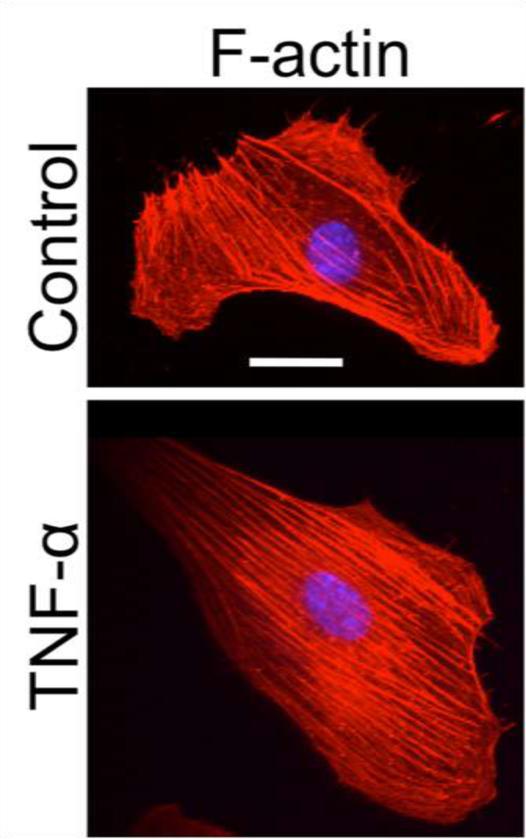
To evaluate the effects of TNF-α on the HUVEC cytoskeletal architecture, we stained for F-actin and captured confocal images of the cells. In control ECs, mature stress fibers formed and were arranged in parallel groups along the basal surface of the cells (Fig. 6). Activation of the cells with TNF-α induced significant changes in the F-actin organization of ECs. After 24-hour treatment with TNF-α, most cells’ F-actin was arranged parallel to the major (long) axis of the cell (Fig. 6).
Figure 6.
TNF-α exposure induces F-actin along cell major axis. ECs stained for F-actin (red) or DNA (blue) are shown for representative control and 24-hour TNF-α-treated cells. Scale bar is 20 μm and applies to all images.
TNF-α induces dynamic actin rearrangement
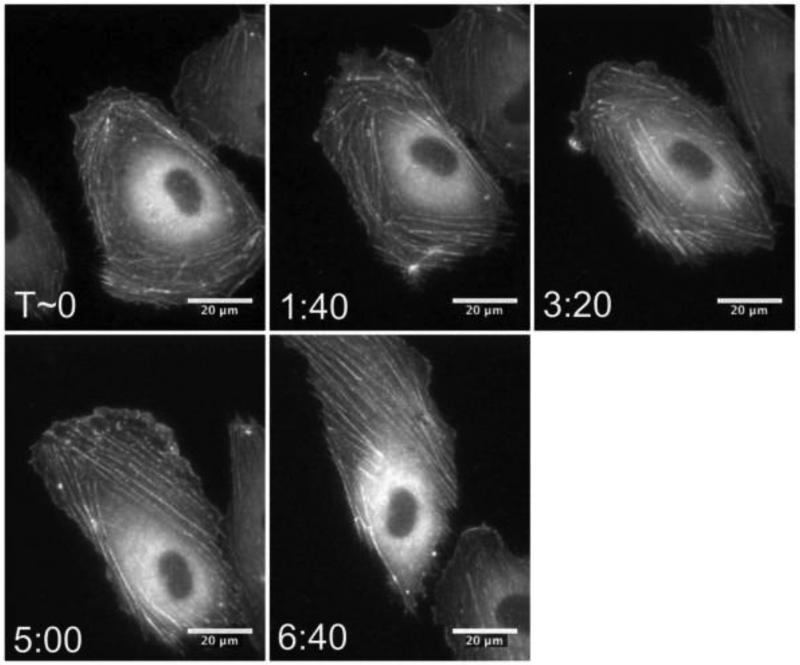
In addition to a static assessment of the effects of TNF-α on cytoskeletal architecture, we also evaluated how the transition occurred dynamically. Specifically, we aimed to address the question of whether the F-actin reorganization involved depolymerization and repolymerization of actin, or whether it was simply a shift of existing actin fibers. Thus, we transfected HUVECs for GFP-actin and captured fluorescence images over the entire 24-hour period of TNF-α treatment. The timelapse sequence in Figure 7, (also see Movie 2), indicates the dynamic shift of F-actin inward towards the nucleus as the polymers aligned in parallel along the major axis of the cell.
Figure 7.
TNF-α-induced changes in the F-actin cytoskeleton involve a dynamic shift of existing actin filaments. ECs were transfected with GFP-actin and fluorescence images were captured after addition of TNF-α. A time sequence for a representative cell is shown. Scale bar is 20 μm and applies to all images. T indicates the time (in hours:minutes format) after which TNF-α was added to the cell media.
DISCUSSION
The immune response triggers a complicated sequence of events, one of which is release of the cytokine TNF-α from stromal cells such as monocytes and macrophages. While the biological effects of TNF-α have been studied, here we focus on less-studied components of the inflammatory response, including EC morphology, biomechanics, migration, and cytoskeletal dynamics. These properties are important because, for example, neutrophils are mechanosensitive (Oakes et al. 2009; Stroka and Aranda-Espinoza 2009), and the biophysical properties of ECs are beginning to be understood as key regulators of the inflammatory response (Stroka and Aranda-Espinoza 2010).
One of the most striking results of our work is the relationship between cell aspect ratio, traction forces, and migration speed. We report, for the first time, an interesting trend where aspect ratio peaks around T=8-10 hours, and then drops off as time approaches 24 hours (Fig. 1e-h). This morphology behavior is also related to the migratory dynamics of the cell, as cell speed and aspect ratio are inversely correlated in TNF-α-treated cells (Fig. 5), but not in control cells (Fig. 4). Further, a peak in traction forces around 10 hours of treatment (Fig. 3b) is nearly concurrent with the maximum in aspect ratio. We suggest that this relationship between aspect ratio, migration, and traction forces arises because a high cellular aspect ratio does not promote efficient migration, and thus an elongated cell must first detach at the rear in order to migrate forward. Detachment requires contractile forces, which could explain the peak in traction forces that we observe around T=10 hours of TNF-α treatment (Fig. 3b).
We also suggest that these changes in cell shape, biomechanics, and migration are related to cytoskeletal dynamics. The shift of the F-actin over the first several hours of TNF-α treatment (Fig. 7) indicated that changes in aspect ratio involve or are even caused by alterations in the cell's cytoskeleton. Thus, the changes in aspect ratio induced by TNF-α exposure can likely be explained by F-actin arrangement.
While decreases in cell stiffness after 24 hours of TNF-α exposure (Fig. 2) are consistent with previous reports (Kang et al. 2008; Stroka and Aranda-Espinoza 2011b), these results are somewhat counterintuitive with respect to the morphological changes we observed. Specifically, increased F-actin (Fig. 6), and traction forces (Fig. 3b) have been associated with increases in cell stiffness (Wang et al. 2002). In addition, TNF-α exposure has been found to initiate Rho, ROCK, and myosin light chain kinase (MLCK) signaling pathways which are associated with cell contraction (Mckenzie and Ridley 2007). However, an important consideration here is that force measurements made by AFM utilize an indentation that is very small compared to the total height of the cell, and thus the Young's modulus measurements that we report are not necessarily reflective of the basal surface of the cell, where the stress fibers are localized. Rather, our measurements are reflective of cell cytoplasmic or cortical actin stiffness. Thus, the significant increase in estimated cellular volume with TNF-α treatment (Fig. 2e) could explain the decrease in cell stiffness, despite changes in F-actin and traction forces that might suggest opposite effects on stiffness. To our knowledge, we are the first to report an increase in EC volume after 24 hours of TNF-α treatment, though TNF-α has previously been implicated in regulation of ion channels, which play a significant role in cell volume regulation. It has been reported that in smooth muscle cells, TNF-α treatment activates swelling-activated chloride channels (Matsuda et al. 2010) and also enhances the peak and plateau levels of intracellular Ca2+ levels in response to acetylcholine and bradykinin (White et al. 2006). Further, an increase in stress fiber formation is a key characteristic of TNF-α treatment (Mckenzie and Ridley 2007); we expect that this event is accompanied by an influx of water and ions, thus increasing cell volume. Therefore, the overall increase in cellular volume that we measure via AFM is likely caused by TNF-α-induced activation of volume-regulating ion channels.
CONCLUSIONS
Here, we demonstrated the relationships between cellular morphology, biomechanics, migration, and cytoskeletal dynamics during an inflammatory response. Previously, much attention has been placed on the biological signaling due to cytokine exposure, though little work has focused on the biomechanics and dynamics of ECs in response to TNF-α. These biophysical properties of the vascular endothelium are key aspects of the immune response and are therefore likely to affect the efficiency of the leukocyte adhesion cascade.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Micah Dembo for providing the Fortran code to analyze traction force data, and for engaging in useful discussions on traction forces. This work was supported by a National Institutes of Health National Research Service Award F31NS068028 (K.M.S.) and NSF Award CMMI-0643783 (H.A.E.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health.
REFERENCES
- Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophysical Journal. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Springer TA. Lymphocyte function-associated antigen-1 (LFA-1) interaction with intercellular adhesion molecule-1 (ICAM-1) is one of at least three mechanisms for lymphocyte adhesion to cultured endothelial cells. J Cell Biol. 1988;107:321–331. doi: 10.1083/jcb.107.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldblum SE, Hennig B, Jay M, Yoneda K, McClain CJ. Tumor necrosis factor alpha-induced pulmonary vascular endothelial injury. Infection and immunity. 1989;57:1218–1226. doi: 10.1128/iai.57.4.1218-1226.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang I, Panneerselvam D, Panoskaltsis VP, Eppell SJ, Marchant RE, Doerschuk CM. Changes in the hyperelastic properties of endothelial cells induced by tumor necrosis factor-alpha. Biophys J. 2008;94:3273–3285. doi: 10.1529/biophysj.106.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JJ, Filali MS, Moreland JG, Miller FJ, Lamb FS. Activation of Swelling-activated Chloride Current by Tumor Necrosis Factor-alpha Requires ClC-3-dependent Endosomal Reactive Oxygen Production. J Biol Chem. 2010;285:22862–22871. doi: 10.1074/jbc.M109.099838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckenzie JAG, Ridley AJ. Roles of Rho/ROCK and MLCK in TNF-alpha-induced changes in endothelial morphology and permeability. J Cell Physiol. 2007;213:221–228. doi: 10.1002/jcp.21114. [DOI] [PubMed] [Google Scholar]
- Norman LL, Oetama RJ, Dembo M, Byfield F, Hammer DA, Levitan I, Aranda-Espinoza H. Modification of Cellular Cholesterol Content Affects Traction Force, Adhesion and Cell Spreading. Cellular and Molecular Bioengineering. 2010;3:151–162. doi: 10.1007/s12195-010-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes PW, Patel DC, Morin NA, Zitterbart DP, Fabry B, Reichner JS, Tang JX. Neutrophil morphology and migration are affected by substrate elasticity. Blood. 2009;114:1387–1395. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LA, Aranda-Espinoza H, Haun JB, Dembo M, Hammer DA. Neutrophil traction stresses are concentrated in the uropod during migration. Biophys J. 2007;92:L58–60. doi: 10.1529/biophysj.106.102822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon IN. The relation between load and penetration in the axisymmetric boussinesq problem for a punch of arbitrary profile. Int J Eng Sci. 1965;3:47–57. [Google Scholar]
- Stroka KM, Aranda-Espinoza H. Neutrophils display biphasic relationship between migration and substrate stiffness. Cell Motil Cytoskeleton. 2009;66:328–341. doi: 10.1002/cm.20363. [DOI] [PubMed] [Google Scholar]
- Stroka KM, Aranda-Espinoza H. A biophysical view of the interplay between mechanical forces and signaling pathways during transendothelial cell migration. FEBS Journal. 2010;277:1145–1158. doi: 10.1111/j.1742-4658.2009.07545.x. [DOI] [PubMed] [Google Scholar]
- Stroka KM, Aranda-Espinoza H. Effects of Morphology vs. Cell-Cell Interactions on Endothelial Cell Stiffness. Cellular and Molecular Bioengineering. 2011a;4:9–27. doi: 10.1007/s12195-010-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroka KM, Aranda-Espinoza H. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood. 2011b;118:1632–1640. doi: 10.1182/blood-2010-11-321125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Tolic-Norrelykke IM, Chen J, Mijailovich SM, Butler JP, Fredberg JJ, Stamenovic D. Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. Am J Physiol Cell Physiol. 2002;282:C606–616. doi: 10.1152/ajpcell.00269.2001. [DOI] [PubMed] [Google Scholar]
- Weisenhorn AL, Khorsandit M, Kasast S, Gotzost V, Butt H-J. Deformation and height anomaly of soft surfaces studied with an AFM. Nanotechnology. 1993;4:106–113. [Google Scholar]
- White TA, Xue AL, Chini EN, Thompson M, Sieck GC, Wylam ME. Role of transient receptor potential C3 in TNF-alpha-enhanced calcium influx in human airway myocytes. Am J Resp Cell Mol. 2006;35:243–251. doi: 10.1165/rcmb.2006-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.