Abstract
PURPOSE
Triapine (Vion Pharmaceuticals), a novel inhibitor of the M2 subunit of ribonucleotide reductase (RR), is a potent radiosensitizer. This NCI/CTEP-sponsored phase I study assessed the safety/tolerability of triapine in combination with radiation (RT) in patients with locally advanced pancreas cancer (LAPCA).
METHODS AND MATERIALS
We evaluated 3 dose levels of triapine (24 mg/m2, 48 mg/m2, 72 mg/m2) administered with 50.4 Gy of RT in 28 fractions. Patients with LAPCA received triapine thrice weekly, every other week during the course of RT. Dose-limiting toxicity (DLT) was assessed during and for 4 weeks following completion of RT. Dynamic contrast enhanced (DCE)-MRI and serum RR levels were evaluated as potential predictors for early response.
RESULTS
Twelve patients were treated. Four patients (1 non-evaluable [NE]) were enrolled at dose level 1 (DL1), three patients at DL2, and five patients (2NE) at DL3. No DLTs were observed and the MTD was not reached. Two patients (17%) achieved PR and 6 patients (50%) had SD. One patient underwent R0 resection following therapy. 92% of patients (100% on DL3) experienced freedom from local tumor progression. 75% of patients who eventually progressed developed metastases without local progression. RR levels did not appear to predict outcome. In 4 patients with available data, DCE-MRI may predict early response or resistance to therapy.
CONCLUSION
The combination of triapine at 72 mg/m2 three times weekly every other week and standard RT is tolerable with interesting activity in patients with LAPCA.
Keywords: Triapine, radiation, pancreas cancer
INTRODUCTION
Pancreas cancer remains a leading cause of cancer death (1). Less than 15% of patients have resectable disease at diagnosis. Another 25% of patients will present with locally advanced, unresectable tumors with a median survival of less than 1 year (2). Treatment with gemcitabine or 5FU-based chemoradiotherapy (CRT) is a preferred treatment for locally advanced pancreas cancer (LAPCA), however less than 10% of patients with LAPCA will undergo curative-intent resection following CRT (3).
Repair of radiation-induced DNA damage is a mechanism of tumor resistance to radiation therapy (RT). Ribonucleotide reductase (RR) is the rate-limiting step in de novo synthesis of deoxyribonucleotide triphosphates (dNTPs) required for DNA repair, and its activity is associated with malignant behavior (4). The RR enzyme unit is composed of a regulatory subunit, M1, and a catalytic subunit, M2. The M2 subunit has 2 isoforms: hRRM2 or its homologue, p53R2.
The M2 subunit is up-regulated in cancer cells exposed to RT, leading to increased RR activity and decreased radiosensitivity of cancer cells. Overexpression of the M1 subunit has no effect on radiosensitivity, implicating the M2 subunit as the more promising therapeutic target (5). Inactivation of RR results in decreased intracellular concentrations of dNTPs, inhibition of DNA synthesis and repair, cell cycle arrest and apoptosis (6) This suggests that administering RR enzyme inhibitors, particularly M2 inhibitors, with RT can potentially enhance RT-mediated cytotoxicity. Thus, RR, and particularly its M2 subunit is considered a good therapeutic target.
Triapine (3-aminopyridine-2-carboxaldehyde thiosemicarbazone, 3AP) is a potent derivative of α-heterocyclic carboxaldehyde thiosemicarbazone (HCT) that inhibits hRRM2 and p53R2 isoforms of the M2 subunit (7). In preclinical studies, Triapine increased radiosensitivity in pancreas cancer cells in vitro (8, 9) and xenograft mouse model of pancreas cancer in-vivo (8). Triapine is active in cancer cells resistant to RR inhibitors hydroxyurea and gemcitabine (10). Preclinical data suggest that RR inhibitors optimally enhance RT-mediated cytoxicity when administered immediately following RT (11). In phase I studies of patients with advanced solid tumors, intravenous Triapine was well-tolerated as a single agent at doses of 96 to 120 mg/m2 with various dosing schedules (12, 13). Triapine was well-tolerated at a dose of 25 mg/m2 three times weekly in combination with cisplatin and RT in advanced cervical cancer patients with evidence of promising radiosensitizing activity (14).
We conducted a dose escalation study of triapine in combination with RT in patients with LAPCA. We also evaluated dynamic contrast-enhanced MRI (DCE-MRI) and serum hRRM1 and hRRM2 levels as predictors of response to therapy.
PATIENTS AND METHODS
Eligibility Criteria
Patients aged ≥ 18 years old were required to have untreated, pathologically confirmed adenocarcinoma of the pancreas that was determined to be locally advanced/unresectable and non-metastatic (stage III disease). Patients had Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, life expectancy of ≥ 12 weeks, adequate bone marrow (leukocytes ≥ 3,000/µL, absolute neutrophil count ≥ 1,500/µL, platelets ≥ 100,000/µL), liver (total bilirubin ≤ 2× and AST/ALT ≤ 3× institutional upper limit of normal), and kidney (creatinine within institutional normal limit or GFR ≥ 60 for patients with creatinine above institutional upper limit of normal) function. Pregnant women, patients with uncontrolled pulmonary, cardiac, or psychiatric disease or active infections, and those receiving other investigational agents were excluded. As triapine has the potential to cause severe methemoglobinemia in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency, patients with known G6PD deficiency were excluded, and patients considered high-risk for G6PD deficiency were screened prior to enrollment.
The study protocol was approved by the National Cancer Institute (NCI) Cancer Therapeutics Evaluation Treatment (CTEP) committee, and the institutional review board of the institution, and monitored by the data safety monitoring board. Signed informed consent was required from all patients.
Study design
This was a standard 3×3 design dose-escalation study of daily radiation with Triapine administered 3 times weekly, every other week within 30 minutes of radiation. Doses of Triapine evaluated were 24 mg/m2, 48 mg/m2, and 72 mg/m2. Dose limiting toxicity (DLT) was defined as ≥ grade 3 non-hematologic toxicity (excluding grade 3 nausea, ≥ grade 3 vomiting or grade 3 diarrhea that are controlled with appropriate medical therapy) or ≥ grade 4 hematologic toxicity (excluding lymphopenia). If one DLT was observed in a dosing cohort, an additional 3 patients were enrolled at that dose level. Dose-escalation was continued if no additional DLTs were seen at that dose level. If 2 DLTs were seen in 6 patients at a dose level, dose escalation was stopped and the maximum tolerated dose (MTD) was defined as the maximum dose below this dose level at which 6 patients had been treated with ≤1 DLT.
Treatment administration
Triapine was supplied by Vion Pharmaceuticals and distributed by NCI-CTEP in 10 mL vials containing 50 mg of Triapine which was diluted in 0.9% sodium chloride or 5% dextrose in water to a final concentration of 0.01 to 2 mg/mL. Patients received Triapine via 2-hour intravenous infusion, within 30 minutes of radiation, every Monday, Wednesday and Friday of weeks 1, 3 and 5 (Total = 9 doses). Supportive measures including anti-emetics and anti-diarrheals were administered according to institutional guidelines.
Radiation therapy was delivered with 3-dimensional conformal technique with a total dose of 50.4 Gy in 28 fractions (45 Gy + 5.4 Gy boost). Radiation machinery consisted of a linear accelerator capable of producing ≤ 6MV and a source to axis distance of 100 cm. Radiation volumes were defined as follows: gross tumor volume (GTV) included the tumor visualized on CT or MRI, including pancreas and lymph nodes measuring >1.5 cm in any dimension; clinical target volume (CTV) included areas of gross tumor as well as areas of possible microscopic disease (for tumors of the pancreatic head, this volume included porta-hepatic, celiac, and pancreaticoduodenal nodes; for body/tail tumors, celiac nodes were included); in addition, a margin of 1.0 – 1.5 cm was added to GTV in directions with no anatomic barrier to microscopic spread; and planning target volume (PTV) included an additional margin of 1 cm to the CTV to account for setup error and patient movement. Patients received the initial 45 Gy in 25 fractions to the PTV; a boost of 5.4 Gy in 3 fractions was delivered to the GTV + 1cm. For purposes of quality assurance, independent review of the treatment plan, port films and calculations was performed within 1 week of treatment.
Safety Assessments
Complete history, physical examination, baseline laboratory values, computed tomography scan and/or magnetic resonance imaging and electrocardiogram were obtained within 28 days of treatment initiation. Patients underwent weekly history, physical examination and toxicity assessment during weeks 1–6, week 10, and every 3 months thereafter.
Dose delays and modifications
Adverse events were evaluated using National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Triapine and radiation were held for neutrophils <500/µL and platelets <50,000/µL and were resumed with a 25% dose-reduction in triapine upon resolution of toxicity to ≤ grade 1. For grade 3–4 nausea, vomiting, and diarrhea, treatment was interrupted until toxicity decreased to grade 0–1 and then was resumed at a 25% dose-reduction of triapine.
Up to a 15% rise in methemoglobin levels were expected. If patients developed hypoxia (oxygen saturation ≤ 92%), dyspnea, or methemoglobin level >15% with rapid resolution treatment was continued at the next lowest dose level. If not, patients were removed from study.
Evaluation of response
Response was assessed at week 10 by the same radiographic method used for baseline measurements. Responses were graded using Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 (15).
Correlative Studies
Dynamic contrast-enhanced (DCE) MRI
DCE-MRI was evaluated as a potential predictor of early response to therapy. Images were obtained at weeks 0, 2, and 10.. A 3 Tesla MR system (Achieva; Philips, Cleveland, OH) using a surface coil was used. DCE-MRI was performed using a 3D T1-weighted fast field echo (3D-FFE) imaging sequence in the axial or coronal plane. The T1W-3D-FFE sequence (TR/TE = 3.7/2.3 ms; flip angle = 20°; FOV = 370 mm; matrix = 128 × 128; 10 slices; 12-mm slice thickness; 4.4 sec per volume; 60 time points) was applied. The extracellular Gd-based contrast agent (Magnevist, Schering, Berlin, Germany) was intravenously injected by a power injector (Spectris; MedRad, Warrendale, PA; injection rate = 0.5 mL/sec) followed by a 20 cc saline flush at a rate of 2 mL/sec.
Data analysis was performed using software based on the IDL environment (Interactive Data Language; ITT, Boulder, CO). Regions of interest (ROIs) were drawn on dynamic imaging data sets and were co-registered to compensate for subject motion during scans. ROI placement was performed by an experienced reader and confirmed by a radiologist. The tumor time-enhancement curves were quantitatively analyzed. The adjusted Brix’s model-based pharmacokinetic parameters (Amp, kep, kel) (16) were determined by using the MINPACK-1 method for fitting the tracer kinetics equation to the tissue time-signal intensity curves
Measurement of hRRM2 and hRRM1 levels
Patients underwent blood draw immediately before and after triapine administration on days 1 and 33 with quantitative assessment of serum hRRM1 and hRRM2 performed by ELISA (17). Polystyrene 96-well plates were coated with hRRM1 or hRRM2 immunogen at a concentration of 1 g/mL in pyrogen-free PBS at pH 7. Unbound sites were incubated with 2% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 1 hour at 37°C. Serum or supernatant from tissue culture plates were added and incubated at 37°C for 1 hour. After several washes, plates were incubated with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) antibody for 1 hour, then washed and incubated with the substrate solution. Spectrophotometric measurements were taken and reported as 50% titer. All samples were measured in 2 separate studies. All washings were performed 3 times with phosphate buffered saline using a Nunc Immuno-washer (Thermo Scientific).
Statistical Methods
The primary endpoint was to determine the maximum tolerated dose (MTD) of triapine in combination with radiation in LAPCA. Secondary endpoints included toxicity, objective response rate (ORR) defined as the proportion of patients achieving complete or partial response (CR or PR) as assessed by RECIST 1.0, overall survival (OS), defined as time from enrollment to death from any cause, time to first progression, defined as time from enrollment until first progression or death from any cause. Toxicity data, time-to-event outcomes, serum hRRM1 and hRRM2 levels, and DCE-MRI results were reported descriptively.
RESULTS
Patient characteristics
Patient characteristics are outlined in Table 1. Twelve patients (7 male, 5 female) with untreated LAPCA (stage III disease) were enrolled from May 2007 through March 2011. The median patient age was 65 (range 53–80).. Only one patient had biliary stent placement prior to treatment initiation.
Table 1.
Patient Characteristics (N=12)
| Age (years) | |
| Median | 65 |
| Range | 53–80 |
| Sex | |
| Male | 7 |
| Female | 5 |
| Race/Ethnicity | |
| Caucasian | 12 |
| ECOG performance status | |
| 0 | 5 |
| 1 | 6 |
| 2 | 1 |
| Disease stage | |
| III | 12 |
| Staging modality | |
| CT scan | 12 |
| MRI | 3 |
| EUSa | 6 |
| Laparoscopy/laparotomy | 2 |
| CA 19-9 (N=11)b | |
| Normalc | 1 |
| Elevatedd | 10 |
| Median CA19-9 (U/mL) | 401 |
EUS= endoscopic ultrasound
Pretreatment CA 19-9 was unavailable for 1 patient.
Normal: ≤ 37 U/mL
Elevated: > 37 U/mL
Dose Escalation and Toxicity
Four patients were treated at dose level 1 (24 mg/m2) with no DLTs. One patient was replaced with PD after 5 doses of Triapine and 19 fractions (34.2 Gy) of radiation. Three patients were treated at dose level 2 (48 mg/m2). No dose delays or modifications were required and no DLTs were observed. Five patients were treated at dose level 3 (72 mg/m2) with no DLTs observed; 3 patients completed treatment. Two patients were replaced at dose level 3: one patient had PD after 6 doses of triapine, and a second patient received the full 50.4 Gy of radiation, but triapine was discontinued after 5 doses due to grade 2 fever which was attributed to a triapine infusion reaction, but did not qualify as a DLT. One patient required a delay in therapy while hospitalized for unrelated cholangitis. Toxicities are outlined in Table 2. Treatment was generally well-tolerated and toxicities were similar across dose levels. The most common toxicities observed were lymphopenia (92%), nausea (56%), anemia (56%), vomiting (44%), and fatigue (44%). Grade 3–4 uncomplicated lymphopenia was observed in all patients at dose level 3. Other grade 3–4 toxicities were uncommon including anemia (8%), thrombocytopenia (8%). There were no incidences of methemoglobinemia >15% or persistent hypoxia requiring hospitalization.
Table 2.
Toxicities Observed According to NCI-CTCAE v. 3.0
| All (N=12) | Dose level 1(N=4) | Dose level 2 (N=3) | Dose level 3 (N=5) | ||||
|---|---|---|---|---|---|---|---|
| Gr 1–2 | Gr 3–4 | Gr 1–2 | Gr 3–4 | Gr 1–2 | Gr 3–4 | ||
| Hematologic | |||||||
| Anemia | 6 (50) | 1 (25) | 1 (25) | 3 (100) | 0 (0) | 1 (33) | 0 (0) |
| Leukopenia | 3 (25) | 1 (25) | 0 (0) | 1 (33) | 0 (0) | 1 (33) | 0 (0) |
| Lymphopenia | 11(92) | 1(25) | 2 (50) | 2(67) | 1 (33) | 0 (0) | 5 (100)* |
| Thrombocytopenia | 3 (25) | 0 (0) | 0 (0) | 1 (33) | 1 (33) | 1 (33) | 0 (0) |
| Non-Hematologic | |||||||
| Nausea | 5 (56) | 2 (50) | 0 (0) | 2 (67) | 0 (0) | 1 (33) | 0 (0) |
| Vomiting | 4(44) | 2 (50) | 0 (0) | 2 (67) | 0 (0) | 1 (33) | 0 (0) |
| Fatigue | 4 (44) | 0 (0) | 0 (0) | 2 (67) | 0 (0) | 1 (33) | 0 (0) |
| Diarrhea | 3 (33) | 1 (25) | 0 (0) | 1 (33) | 0 (0) | 1 (33) | 0 (0) |
| Increased ALT | 2 (22) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 1 (33) | 0 (0) |
| Anorexia | 2 (22) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 1 (33) | 0 (0) |
| Increased AST | 1 (11) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0 (0) | 0 (0) |
| Altered taste | 1 (11) | 1 (25) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
2 patients (40%) experienced grade 4 lymphopenia at dose level 3.
Efficacy
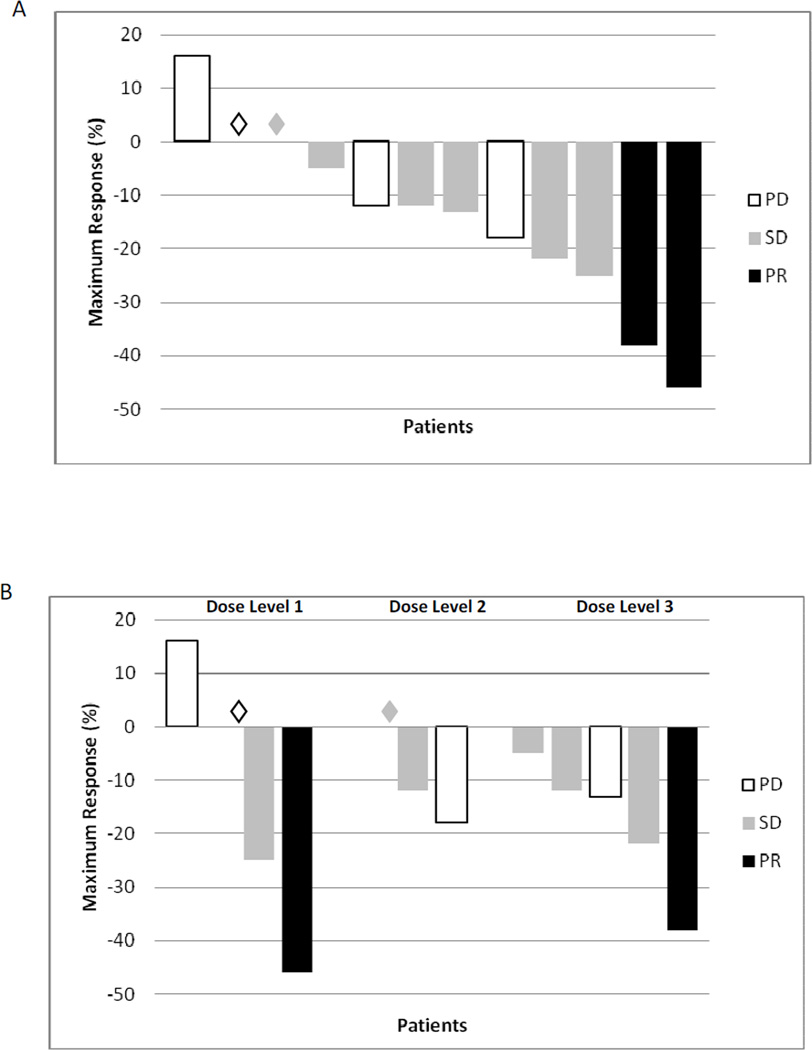
Clinical efficacy data by dose level are summarized in Table 3. Eleven patients showed evidence of freedom from local tumor progression (Figure 1A). Response in the primary tumor by dose level is depicted in Figure 1B. By RECIST, two patients (17%) had PR and 6 patients (50%) had SD for a disease control rate of 67%. One patient underwent complete (R0) resection after treatment. Time to first documented progression ranged from 0.8–10.2 months, and overall survival ranged from 1–18.6 months. Six patients (75%) had a decrease in CA 19-9 levels with 2 patients having >50% decline.
Table 3.
Treatment Efficacy by Dose Level
| Patient | Dose Level |
Best RECIST Response on study |
Baseline primary tumor diameter (cm) * |
Max % change in primary tumor |
Best CA19-9 response on study |
hRRM1 Max Change |
hRRM2 Max Change |
Time to 1st progression (months)a |
OS (months) |
Post-study therapy (Yes/No) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | PR | 2.8 | 46% ↓ | NEb | NAc | NAc | 4.9 | 18.6 | Yes |
| 2 | 1 | PD | 4.5 | No change | NE | NAc | 12% ↓e | 2 | 6.1 | Yes |
| 3 | 1 | PD | 5.6 | 16% ↑ | NE | No change | 6% ↓ | 0.8 | 2.8 | Yes |
| 4 | 1 | SDd | 2.2 | 25% ↓ | 56% ↓ | 12% ↑e | 8% ↑e | 8.4 | 14.6 | Yesf |
| 5 | 2 | SD | 2.1 | No change | 21% ↓ | 13% ↑e | 7% ↑e | 7.5 | 9.1 | Yes |
| 6 | 2 | PD | 5.1 | 18% ↓ | NE | 13% ↑ | No change | 2.1 | 6.7 | Yes |
| 7 | 2 | SD | 3.4 | 12% ↓ | 68% ↓ | 3% ↓e | 14% ↓e | 10.2 | 11.6 | Yes |
| 8 | 3 | PR | 6.1 | 38% ↓ | 62% ↓ | 9% ↓e | 4% ↓e | 6.8 | 6.8 | Yes |
| 9 | 3 | PD | 4.3 | 13% ↓ | NE | 3% ↓ | 3% ↓ | 0.8 | 1 | No |
| 10 | 3 | SD | 3.8 | 5% ↓ | 147% ↑ | 4% ↓ | 15% ↑ | 6.3 | 6.3 | No |
| 11 | 3 | SD | 3.8 | 12% ↓ | 47% ↓ | 7% ↓e | 9% ↓e | 6.1 | 6.1 | Yes |
| 12 | 3 | SD | 4.6 | 22% ↓ | 360% ↑ | 16% ↓e | 15% ↓e | 4.4 | 9.4 | Yes |
Denotes longest cross-sectional diameter
Includes post-study therapy
NE: not evaluable- data not available (N=3) or CA 19-9 not elevated (N=1)
Data not available
Patient underwent pancreaticoduodenectomy post-study
Indicates complete (Day 1 and Day 33) data available
Patient received adjuvant therapy and subsequent treatment for metastatic disease
Figure 1.
Waterfall plot of response in the primary tumor (A) and RECIST response in all patients and by dose level (B). PD: progressive disease. SD: stable disease. PR: partial response. (◊) Denotes patients with 0% change as maximum response.
hRRM2 and hRRM1 measurements
Data are presented in Table 3. Complete data (obtained pre-and post treatment on days 1 and 33) were available on 7 patients (Patients 2, 4, 5, 7, 8, 11 and 12). All patients treated on dose level 3 had a decline in hRRM1 levels and all but one also had decline in hRRM2 levels. All patients at this dose level experienced freedom from local tumor progression.
DCE-MRI
DCE-MRI data were available on 4 patients (Table 4). DCE-MRI findings by individual patient were compared with RECIST responses. Patient 1 experienced a partial response by RECIST and DCE-MRI findings suggested increased apoptosis and reduction in neoangiogenesis in the primary tumor. DCE-MRI findings for Patient 2 suggested a lack of response in the primary tumor, consistent with lack of change in the primary tumor observed by RECIST. Findings from DCE-MRI on patient 3 suggest lack of response, consistent with clinical progressive disease. Patient 4 experienced stable disease by RECIST, with DCE-MRI indicating a modest biologic response in the primary tumor without significant reduction in tumor vascularity. These findings suggested a potential predictive value for DCE-MRI in the evaluation of the primary tumor.
Table 4.
DCE MRI Parameters and Tumor Response by RECIST 1.0
| Time point | Ampa | kepb | kelc | % Change in primary tumord |
Radiographic responsee by RESIST |
|
|---|---|---|---|---|---|---|
| Patient 1g | Week 0 | 1.90 | 1.53 | 0.07 | 46% ↓ | PR |
| Week 2 | 1.71 | 1.19 | 0.01 | |||
| Patient 2h | Week 0 | 1.44 | 1.89 | 0.28 | 0% | PDe |
| Week 2 | 1.58 | 0.93 | 0.04 | |||
| Patient 3i | Week 0 | 1.56 | 1.30 | −0.11 | 16% ↑ | |
| Week 2 | 1.71 | 0.76 | 0.02 | |||
| Patient 4j | Week 0 | 1.88 | 1.10 | −0.13 | 12% ↓ | SD |
| Week 2 | 2.99 | 0.62 | −0.06 | |||
Amp= amplitude [automatic unit]
kep=exchange rate [/min]
kel=elimination rate [/min]
According to RECIST 1.0
Progressive disease at week 10 (liver metastases)
Progressive disease at week 5 (liver metastases)
Patient 1 in Table 3
Patient 2 in table 3
Patient 3 in table 3
Patient 11 in Table 3
DISCUSSION
The mainstay of treatment for LAPCA has been CRT. Patients able to undergo surgical resection following treatment appear to have similar prognosis to those with resectable disease at diagnosis (18).
Triapine was well tolerated at a three times weekly every other week schedule with dosage up to 72 mg/m2 combined with RT, with no grade 3–4 nonhematologic toxicities observed across all dose levels. With the exception of uncomplicated lymphopenia, grade 3–4 hematologic toxicities occurred in less than 10% of patients. There were no DLTs observed and the MTD was not reached. In general, the rates of toxicities observed on our study were similar to previously reported phase I studies of single agent triapine (12, 13), as well as triapine combined with cisplatin and radiation (14) in cervical cancer patients. It is therefore possible that higher dosage or a more aggressive schedule may be able to be achieved with single agent triapine combined with RT.
While assessment of clinical outcome was not the primary aim of this study, our results support preliminary radiosensitizing activity of triapine. Our results also suggest that the combination of triapine and RT at the doses studied may not provide sufficient systemic disease control. Data suggest that CRT may be best administered in LAPCA following induction chemotherapy (19). This allows for early systemic disease control and selection of patients who are most likely to benefit from local therapy. Future CRT studies in LAPCA should consider incorporating triapine with other radiosensitizers that inhibit RR such as gemcitabine. This may promote incorporation of gemcitabine into DNA with combined inhibition of RRM1 and RRM2, enhancing radiosensitization (20).
DCE-MRI is a promising modality for early detection of response to anticancer therapy. In our study, DCE-MRI was potentially predictive of local response to therapy as early as 2 weeks in patients with LAPCA receiving triapine and RT. Although our sample size was limited to four patients, it is representative of the spectrum of responses. Interestingly, both patients who did not experience shrinkage of their primary tumors developed PD. The interpretation of these findings is of course limited by the small sample size.
We found no clear correlation between hRRM1 and/or hRRM2 levels and clinical outcome, suggesting limited utility for these biomarkers. Our data do suggest that more effective inhibition of hRRM1 and hRRM2 may occur more consistently with higher dose levels of triapine where tumor responses were more predictable.
In conclusion, triapine was well tolerated at a three times weekly every other week schedule with dosage up to 72 mg/m2 combined with RT in patients with LAPCA. Future studies should confirm the efficacy and tolerability of this regimen in LAPCA following systemic induction chemotherapy or consider incorporating triapine with other chemotherapy such as gemcitabine and RT. DCE-MRI may document clinical benefit as early as 2 weeks from initiation of therapy and these findings deserve further investigation in prospective trials. Future prospective studies should incorporate patient-reported outcomes in order to assess quality of life measures.
SUMMARY.
Ribonucleotide reductase M2 subunit (RRM2) plays a key role in repairing radiation-induced DNA damage. Preclinical data suggests radiosensitizing properties for Triapine, an RRM2 inhibitor. We performed a phase I study of triapine and radiation in locally advanced pancreas cancer. In this study, 92% of patients experienced freedom from local tumor progression, confirming the clinical significance of the radiosensitizing effect of triapine. Correlative analyses suggest dynamic contrast-enhanced MRI may predict early response to therapy.
Acknowledgments
Funding was provided by the National Cancer Institute- Cancer Therapy Evaluation Program U01CA 76576-03
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS NOTIFICATION
No conflicts of interest to disclose.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012 Jan;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Cohen SJ, Pinover WH, Watson JC, Meropol NJ. Pancreatic cancer. Curr Treat Options Oncol. 2000 Dec;1(5):375–386. doi: 10.1007/s11864-000-0065-2. [DOI] [PubMed] [Google Scholar]
- 3.Kim HJ, Czischke K, Brennan MF, Conlon KC. Does neoadjuvant chemoradiation downstage locally advanced pancreatic cancer? J Gastrointest Surg. 2002 Sep-Oct;6(5):763–769. doi: 10.1016/s1091-255x(02)00017-3. [DOI] [PubMed] [Google Scholar]
- 4.Souglakos J, Boukovinas I, Taron M, Mendez P, Mavroudis D, Tripaki M, et al. Ribonucleotide reductase subunits M1 and M2 mRNA expression levels and clinical outcome of lung adenocarcinoma patients treated with docetaxel/gemcitabine. Br J Cancer. 2008 May 20;98(10):1710–1715. doi: 10.1038/sj.bjc.6604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuo ML, Hwang HS, Sosnay PR, Kunugi KA, Kinsella TJ. Overexpression of the R2 subunit of ribonucleotide reductase in human nasopharyngeal cancer cells reduces radiosensitivity. Cancer J. 2003 Jul-Aug;9(4):277–285. doi: 10.1097/00130404-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Cory JG. Ribonucleotide reductase as a chemotherapeutic target. Adv Enzyme Regul. 1988;27:437–455. doi: 10.1016/0065-2571(88)90030-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhu L, Zhou B, Chen X, Jiang H, Shao J, Yen Y. Inhibitory mechanisms of heterocyclic carboxaldehyde thiosemicabazones for two forms of human ribonucleotide reductase. Biochem Pharmacol. 2009 Nov 1;78(9):1178–1185. doi: 10.1016/j.bcp.2009.06.103. [DOI] [PubMed] [Google Scholar]
- 8.Barker CA, Burgan WE, Carter DJ, Cerna D, Gius D, Hollingshead MG, et al. In vitro and in vivo radiosensitization induced by the ribonucleotide reductase inhibitor triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone) Clin Cancer Res. 2006 May 1;12(9):2912–2918. doi: 10.1158/1078-0432.CCR-05-2860. [DOI] [PubMed] [Google Scholar]
- 9.Kunos CA, Radivoyevitch T, Pink J, Chiu SM, Stefan T, Jacobberger J, et al. Ribonucleotide reductase inhibition enhances chemoradiosensitivity of human cervical cancers. Radiat Res. 2010 Nov;174(5):574–581. doi: 10.1667/RR2273.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feun L, Modiano M, Lee K, Mao J, Marini A, Savaraj N, et al. Phase I and pharmacokinetic study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) using a single intravenous dose schedule. Cancer Chemother Pharmacol. 2002 Sep;50(3):223–229. doi: 10.1007/s00280-002-0480-0. [DOI] [PubMed] [Google Scholar]
- 11.Kunos CA, Chiu SM, Pink J, Kinsella TJ. Modulating radiation resistance by inhibiting ribonucleotide reductase in cancers with virally or mutationally silenced p53 protein. Radiat Res. 2009 Dec;172(6):666–676. doi: 10.1667/RR1858.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murren J, Modiano M, Clairmont C, Lambert P, Savaraj N, Doyle T, et al. Phase I and pharmacokinetic study of triapine, a potent ribonucleotide reductase inhibitor, administered daily for five days in patients with advanced solid tumors. Clin Cancer Res. 2003 Sep 15;9(11):4092–4100. [PubMed] [Google Scholar]
- 13.Wadler S, Makower D, Clairmont C, Lambert P, Fehn K, Sznol M. Phase I and pharmacokinetic study of the ribonucleotide reductase inhibitor, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, administered by 96-hour intravenous continuous infusion. J Clin Oncol. 2004 May 1;22(9):1553–1563. doi: 10.1200/JCO.2004.07.158. [DOI] [PubMed] [Google Scholar]
- 14.Kunos CA, Waggoner S, von Gruenigen V, Eldermire E, Pink J, Dowlati A, et al. Phase I trial of pelvic radiation, weekly cisplatin, and 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) for locally advanced cervical cancer. Clin Cancer Res. 2010 Feb 15;16(4):1298–1306. doi: 10.1158/1078-0432.CCR-09-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. european organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of canada. J Natl Cancer Inst. 2000 Feb 2;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Liang J, Heverhagen JT, Jia G, Schmalbrock P, Sammet S, et al. Improving the pharmacokinetic parameter measurement in dynamic contrast-enhanced MRI by use of the arterial input function: Theory and clinical application. Magn Reson Med. 2008 Jun;59(6):1448–1456. doi: 10.1002/mrm.21608. [DOI] [PubMed] [Google Scholar]
- 17.Zhou B, Phan V, Liu X, Juhasz A, Chu PG, Yen Y. Production of a monoclonal antibody against the hRRM2 subunit of ribonucleotide reductase and immunohistochemistry study of human cancer tissues. Hybridoma (Larchmt) 2006 Oct;25(5):264–270. doi: 10.1089/hyb.2006.25.264. [DOI] [PubMed] [Google Scholar]
- 18.Gillen S, Schuster T, Meyer Zum Buschenfelde C, Friess H, Kleeff J. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010 Apr 20;7(4) doi: 10.1371/journal.pmed.1000267. e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huguet F, Andre T, Hammel P, Artru P, Balosso J, Selle F, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007 Jan 20;25(3):326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 20.Sigmond J, Kamphuis JA, Laan AC, Hoebe EK, Bergman AM, Peters GJ. The synergistic interaction of gemcitabine and cytosine arabinoside with the ribonucleotide reductase inhibitor triapine is schedule dependent. Biochem Pharmacol. 2007 May 15;73(10):1548–1557. doi: 10.1016/j.bcp.2007.01.025. [DOI] [PubMed] [Google Scholar]