Abstract
Objectives
To compare the practical use, safety and clinical outcomes associated with the TandemHeart (TH) versus Impella Recover 2.5 (IR2.5) devices when used for circulatory support during high-risk percutaneous coronary intervention (PCI).
Background
Small studies and registries suggest safety and efficacy for the TH and IR2.5 percutaneous-left ventricular assist devices (P-LVADs). However, these P-LVADs differ markedly in their insertion, operation and manner of circulatory augmentation. To date, no study has compared these devices.
Methods
We identified 68 patients (49 males, 19 females; age 71.1±12.1 years) from our single-center database that underwent ‘high-risk’ PCI with P-LVAD support from 04/2005–06/2010 (32 with TH, 36 with IR2.5). Relevant data were extracted and imputed for analysis.
Results
Baseline demographics were similar, including low LVEF (overall mean 31.0±13.7%) and elevated STS mortality risk score (4.2±3.7%). Angiographic characteristics were also similar, with a mean of 2.4±1.0 lesions treated per patient, and 29% undergoing left main PCI. PCI success rates were 99% in both groups, with similar in-hospital outcomes and a combined 7% major vascular access site complication rate. A single episode of left atrial perforation occurred during TH use. No patient required emergent CABG and no in-hospital deaths occurred. The 30 day MACE rate (death, myocardial infarction, target lesion revascularization) was 5.8%. There were no differences between the IR2.5 and TH groups with respect to short- or long-term clinical outcomes.
Conclusions
The IR2.5 and TH assist devices are safe, equally effective, and associated with acceptable short- and long-term clinical outcomes in patients undergoing ‘high-risk’ PCI.
Indexing words: left ventricular assist device, coronary disease, revascularization
Introduction
Precutaneous conronary intervention (PCI) is the predominant mode of revascularization for patients with clinically significant coronary artery disease. In the United States during 2006, while an estimated 448,000 coronary artery bypass graft (CABG) operations were performed, during the same period the number of PCIs (1,313,000) surpassed that of CABG by a ratio of almost 3:1 [1]. Inevitably, a small but definite proportion patients undergoing PCI will be at ‘high-risk’ for adverse procedural outcomes. While there are several algorithms that may be used to identify such patients [2–3], with respect to PCI, the combination of older age, extensive or complex coronary disease and low left ventricular ejection fraction (LVEF) identifies a sub-group that is at particularly high-risk for adverse procedural outcomes [4–6]. This combination of adverse risk factors may be further compounded in patients undergoing PCI for left main and/or proximal coronary artery disease [7], where poor ischemic tolerance during transient balloon and stent inflation may be associated with a catastrophic acute deterioration in left ventricular (LV) performance.
In the setting of circulatory collapse in the catheterization laboratory, or preferably as an ‘upfront’ strategy during PCI to avoid this occurrence [8–9], either an intra-aortic balloon pump (IABP) or percutaneous left ventricular assist device (P-LVAD) may be used to rapidly establish circulatory support without the need for extracorporeal membrane oxygenators or additional surgical procedures. Of the P-LVADs, the TandemHeart (TH) (CardiacAssist, Inc., Pittsburgh, PA) and Impella Recover 2.5 (IR2.5) (Abiomed, Inc., Danvers, MA) are the two that are commercially available and approved for use in the United States. In brief, the TH is a 15–17 Fr left atrial-to-iliac arterial system, driven by a low-speed centrifugal continuous flow pump, requiring both transseptal puncture and arterial cannulation, and which provides up to 4.5 L/min of circulatory support. In contrast, the IR2.5 is a 13 Fr impeller-driven system requiring only femoral arterial access, with the device placed retrogradely across the aortic valve and able to aspirate blood from the LV and expel it into the ascending aorta at a rate of up to 2.5 L/min.
Compared to the traditional IABP, P-LVADs afford true myocardial protection, because they actively unload the LV, lessen myocardial mechanical work, reduce myocardial oxygen demand and augment cardiac output [10–13]. However, the TH and IR2.5 differ significantly in their design, insertion and operation, and virtually no comparative data exists to assist operators in choosing between these devices. Furthermore, while preliminary studies have indicated provisional safety [14–16] and that the hemodynamic improvements associated with TH may be superior those of IABP [13,17], isolated studies have also reported a potentially high rate of vascular access and bleeding complications, and other adverse events, with P-LVAD use [15,17–18]. Given these uncertainties, we sought to evaluate and compare the safety and clinical outcomes associated with TH and IR2.5 use during high-risk PCI.
Materials and Methods
Patients undergoing PCI with either TH or IR2.5 hemodynamic support were prospectively identified and entered into our single center, institutional review-board approved interventional database. All patients undergoing PCI are entered into the database within 24 hours of their intervention. Initial data captured includes baseline clinical characteristics, procedural details, details of events occurring immediately post-procedure, subsequent in-hospital clinical course, laboratory data, and other test results associated with the index procedure. All patients undergo blood collection at least once at 6–8 hours post-procedure for CK-MB measurement. Peri-procedural myocardial infarction (MI) is defined by a normal pre-procedure CK-MB that increases to > 3 times the upper limit of normal post-procedure. The Society of Thoracic Surgeons (STS) risk score was calculated using the online STS risk calculator (http://209.220.160.181/STSWebRiskCalc261/). Subjects are routinely contacted at 30 days and > 1 year post-procedure. Primary data for all patients undergoing PCI with either TH or IR2.5 was accessed for the period of April 2005 to June 2010.
All patients underwent pre-procedure transthoracic echocardiography. At the operator’s discretion, patients were considered ‘high-risk’ and underwent PCI with P-LVAD support due to complex coronary anatomy, low LVEF, comorbid conditions and/or refusal for CABG by the patient or the operating surgeon due to prohibitive surgical risk (see results). Although it was considered a Class III indication (procedure contraindicated) throughout much of the study period, our institution performed occasional stenting of the left main coronary artery during this time using P-LVAD support on highly selected patients, which have been included in this analysis. Patients presenting with acute ST segment elevation MI and/or those in overt cardiogenic shock were excluded. Also, patients with mechanical aortic valves, severe aortic valve disease, significant peripheral arterial disease, mural LV thrombus, aneurysm/anomaly of the aorta, or bleeding diathesis were excluded due to their respective device contraindications.
P-LVAD device selection was performed on a temporal basis. TH was used as the exclusive P-LVAD from 4/1/2005 until 10/15/2007. After that date, IR2.5 was used almost exclusively, with only 2 patients undergoing PCI with TH from 10/15/2007 onwards. Therefore ‘(device) selection bias’, that may have favored the use of one P-LVAD over the other for certain situations/patients, was not a factor in our analysis.
All patients underwent ‘up-front’ P-LVAD insertion prior to the initiation of PCI. Duration of cardiac support and explantation time was at the discretion of the operator. PCI was performed according to contemporary best clinical practice with an activated clotting time maintained at >300 seconds throughout the procedure using unfractionated heparin or bivalirudin, or >250 seconds if a glycoprotein IIb/IIIa inhibitor was also used.
Femoral artery ‘preclose’
As previously reported [14], following aseptic prepping and draping, patients underwent femoral angiography via micro-catheter access. If the vascular anatomy was appropriate (absence of significant peripheral vascular disease, micro-access in common femoral artery, artery > 5 mm diameter), the micro-catheter was upsized to a 6 Fr sheath. If micro-access was in the superficial or profunda femoral arteries, where possible, the micropuncture needle was removed, manual compression applied for 5–10 minutes and higher access secured under fluoroscopic guidance. If appropriate common femoral arterial access was achieved, and by exchanging over a 0.035 inch J-wire, two Perclose devices (Abbott Vascular, Redwood City, CA) were sequentially deployed at the same arteriotomy site at a 90 degree angle to each other, with the Perclose sutures then harvested and secured (but not tightened). The 6 Fr sheath was then reinserted over the J-wire to be used as the arterial P-LVAD access site. Typically, the P-LVAD was placed in the left femoral artery (with ‘preclose’ technique), with the PCI performed via standard right femoral arterial access.
After the PCI and when cardiac support was no longer required, the arterial large-bore sheath or cannula was removed over a 0.035 or 0.038 inch J-wire and temporary manual compression applied. The suture limbs and knots were then advanced, the J-wire removed and the knots secured. Additional manual compression and/or a Femostop (Radi Medical Systems, Inc., Reading, MA) were applied as needed [14].
TandemHeart implantation technique
This technique has been previously described elsewhere [14]. In brief, the right femoral vein was accessed and transseptal puncture then performed using a Brockenbrough needle and Mullins sheath. Once in the left atrium, systemic anticoagulation was given and the Mullins sheath exchanged for a 14/21 Fr two-stage dilator over a J-tip guidewire, followed by the 21 Fr TH transseptal cannula. The position of the TH cannula in the left atrium was confirmed by dye injection and assessing oxygenation levels of left atrial blood. The peripheral end of the cannula was secured to the patient’s thigh and clamped. The ‘preclosed’ left femoral artery 6 Fr sheath was then exchanged for the 15 Fr TH arterial perfusion cannula, with the distal end of the cannula placed above the aortic bifurcation. The peripheral end of this cannula was similarly secured and clamped. After de-airing, the venous and arterial cannulae were connected to the TH device, and a heparinized saline infusate was initiated to provide hydrodynamic lubrication, anticoagulation and local cooling of the TH device. The pump was then connected to the TH controller and its speed adjusted to a flow rate of 2.5 to 3.0 L/minute. In all cases, the TH device was removed post-PCI and before the patient left the catheterization laboratory. All patients (both TH and IR2.5) underwent post-procedure transthoracic echocardiography prior to discharge.
Impella implantation technique
The ‘preclosed’ left femoral access was upsized to a 13 Fr sheath and systemic anticoagulation administered. A diagnostic Judkin’s right catheter (JR4) with a 0.014 inch guidewire was then placed across the aortic valve and into the LV, and the wire exchanged for the dedicated 0.018 inch Impella introduction wire. The JR4 catheter was then removed and the Impella device advanced retrogradely (via monorail technique) across the aortic valve under direct fluoroscopic guidance. After confirmation of proper placement by fluoroscopy and pressure assessment, the Impella performance levels were progressively increased (P1 to P9; lowest to highest) and titrated to a target of 2.5 L/min hemodynamic support prior to PCI initiation. In all cases, the Impella device was successfully weaned and removed post-PCI and before the patient left the catheterization laboratory.
During PCI, the use of TH or IR2.5 is not an isolated event, but rather, requires operator attention before, during and after the procedure. Therefore, we found the specific recording of an isolated ‘implantation’ or ‘explantation’ time to be impractical and highly arbitrary. Therefore, usage times for TH and IR2.5 are presented in the context of overall PCI time.
Statistical analyses
Parametric data were analyzed by 2-tailed Student’s t test and non-parametric data by 2-sided Fisher’s exact test or Chi-square test. Survival analysis was by Kaplan Meier analysis, with potential between group differences assessed by Log Rank (Mantel-Cox) and Breslow comparisons. Given the differing lengths of follow-up between groups and to permit unbiased comparisons, Kaplan Meier analyses for both the TH and IR2.5 (all) patients were arbitrarily censored at the longest day of follow-up for the IR2.5 group (827 days). All between group comparisons were unpaired. Differences were deemed significant if P < 0.05. Correction for multiple comparisons was not made due to the low number of subjects and likely low statistical power. Statistical analyses were performed using IBM SPSS/PASW Statistics 18 (SPSS Inc. Chicago, Il). Unless otherwise stated data are presented as mean ± SD.
Results
Baseline demographics of patients undergoing PCI with P-LVAD support were typical of those at high-risk for coronary revascularization, with a mean overall age of 71.1 ± 12.1 years (range 43 – 92 years), low LVEF (31.0 ± 13.7%), elevated left ventricular end diastolic pressure (19 ± 8 mmHg) and a high incidence of left main coronary artery disease and unstable presentation (Table I). This was reflected in the relatively high baseline STS estimated mortality risk scores (overall mean 4.2 ± 3.7%). While the majority of baseline clinical characteristics were similar between groups, patients undergoing PCI with IR2.5 support were perhaps of marginally higher risk for adverse outcomes due to lower mean LVEF, a corresponding increased use of diuretics, and more extensive coronary artery disease (Table I).
Table I.
Baseline clinical characteristics and demographics of patients undergoing PCI with TH versus IR2.5.
| TandemHeart (n = 32) | Impella 2.5 (n = 36) | All patients (n = 68) | P value for between group comparison | |
|---|---|---|---|---|
|
| ||||
| Age (y) 1 | 70.1 ± 12.1 | 71.9 ± 12.2 | 71.1 ± 12.1 | 0.55 |
|
| ||||
| Male sex2 | 24/32 (75%) | 25/36 (69%) | 49/68 (72%) | 0.79 |
|
| ||||
| Cardiovascular risk factors | ||||
|
| ||||
| Diabetes2 | 15/32 (47%) | 17/36 (47%) | 32/68 (47%) | 1.0 |
|
| ||||
| Hypertension2 | 27/32 (84%) | 33/36 (92%) | 60/68 (88%) | 0.46 |
|
| ||||
| Dyslipidemia2 | 27/32 (84%) | 34/36 (94%) | 61/68 (90%) | 0.24 |
|
| ||||
| Positive family history2 | 15/32 (47%) | 16/36 (44%) | 31/68 (46%) | 1.0 |
|
| ||||
| Current Smoking2 | 4/32 (13%) | 4/36 (11%) | 8/68 (12%) | 1.0 |
|
| ||||
| BMI (kg. m2)1 | 29.5 ± 6.8 | 27.1 ± 5.6 | 28.2 ± 6.2 | 0.12 |
|
| ||||
| Serum creatinine (mg/dL)1 | 1.4 ± 1.4 | 1.2 ± 0.6 | 1.3 ± 1.0 | 0.35 |
|
| ||||
| GFR (ml/min)1 | 73.8 ± 42.1 | 67.9 ± 31.6 | 70.6 ± 36.6 | 0.52 |
|
| ||||
| Cardiac Status | ||||
|
| ||||
| Baseline systolic BP (mmHg)1 | 121 ± 25 | 126 ± 27 | 124 ± 27 | 0.57 |
|
| ||||
| Baseline MAP (mmHg)1 | 87 ± 15 | 83 ± 18 | 85 ± 17 | 0.35 |
|
| ||||
| Baseline LVEDP (mmHg)1 | 19 ± 10 | 19 ± 7 | 19 ± 8 | 0.94 |
|
| ||||
| LVEF (%)1 | 35.7 ± 18.2 | 26.9 ± 6.0 | 31.0 ± 13.8 | 0.02 |
|
| ||||
| Prior CABG2 | 5/32 (16%) | 9/36 (25%) | 14/68 (21%) | 0.38 |
|
| ||||
| Left Main Disease2 | 7/32 (22%) | 11/36 (31%) | 18/68 (26%) | 0.58 |
|
| ||||
| Unstable presentation2,* | 15/32 (47%) | 19/36 (54%) | 34/68 (50%) | 0.79 |
|
| ||||
| Number of diseased coronary vessels3 | 1 = 4 patients | 1 = 2 patients | 1 = 6 patients | 0.031 |
| 2 = 13 patients | 2 = 6 patients | 2 = 19 patients | ||
| 3 = 15 patients | 3 = 28 patients | 3 = 43 patients | ||
| Mean: 2.3 ± 0.7 | Mean: 2.7 ± 0.6 | Mean: 2.5 ± 0.7 | ||
|
| ||||
| STS risk score | ||||
|
| ||||
| STS risk mortality | 3.5 ± 3.5 | 4.7 ± 3.8 | 4.2 ± 3.7 | 0.19 |
|
| ||||
| STS morbidity & mortality | 21.2 ± 13.8 | 25.1 ± 12.9 | 23.3 ± 13.4 | 0.24 |
|
| ||||
| Medication usage | ||||
|
| ||||
| Aspirin2 | 29/32 (91%) | 31/36 (86%) | 60/68 (88%) | 0.71 |
|
| ||||
| Plavix2 | 24/32 (75%) | 27/36 (75%) | 51/68 (75%) | 1.0 |
|
| ||||
| ACEI/ARB2 | 22/32 (69%) | 23/36 (64%) | 45/68 (66%) | 0.80 |
|
| ||||
| β-blocker2 | 25/32 (78%) | 33/36 (92%) | 58/68 (85%) | 0.17 |
|
| ||||
| Calcium Channel Blocker2 | 5/32 (16%) | 4/36 (11%) | 9/68 (13%) | 0.74 |
|
| ||||
| Nitrates (long acting)2 | 9/32 (28%) | 13/36 (36%) | 22/68 (32%) | 0.61 |
|
| ||||
| Diuretic2 | 9/32 (28%) | 24/36 (67%) | 33/68 (49%) | 0.02 |
|
| ||||
| Lipid Lowering Agent2 | 26/32 (81%) | 32/36 (89%) | 58/68 (85%) | 0.50 |
Data is presented as mean ± SD or number of subjects (%).
Abbreviations not previously defined: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; GFR, glomerular filtration rate (calculated by Cockcroft-Gault formula); LVEDP, left ventricular end diastolic pressure; MAP, mean arterial pressure.
Statistical analyses used:
2-sided non-paired Student’s t test,
2-sided Fisher’s exact test,
Chi-square test.
Presentation with unstable angina or non-ST segment elevation MI.
Angiographic and target lesion characteristics were similar between patients undergoing PCI with either TH or IR2.5 P-LVAD support, with an overall mean of 2.4 ± 1.0 lesions treated per patient, and 29% of patients undergoing left main coronary artery intervention (Table II). Indicating the complex nature of these procedures, > 85% of all lesions were American Heart Association class B2 or C lesion morphology (Table II).
Table II.
Baseline target lesion and angiographic details of patients undergoing PCI with TH versus IR2.5.
| TandemHeart (n = 32 patients; n = 74 lesions) | Impella 2.5 (n = 36 patients; n = 91 lesions) | All patients (n = 68 patients; n = 165 lesions) | P value for between group comparison | |
|---|---|---|---|---|
|
| ||||
| Number of lesions treated per patient1 | 2.3 ± 1.0 | 2.5 ± 1.0 | 2.4 ± 1.0 | 0.9 |
|
| ||||
| Vessels intervened upon per patient2,* | ||||
| -Left main | 10/32 (31%) | 10/36 (28%) | 20/68 (29%) | 0.79 |
| -Left anterior descending | 22/32 (69%) | 25/36 (69%) | 47/68 (69%) | 1.0 |
| -Circumflex | 14/32 (44%) | 20/36 (56%) | 34/68 (50%) | 0.47 |
| -Right coronary | 5/32 (16%) | 6/36 (17%) | 11/68 (16%) | 1.0 |
| -Saphenous Vein Graft | 2/32 (6%) | 2/36 (6%) | 4/68 (6%) | 1.0 |
| -Left Internal Mammary Graft | 0/32 (0%) | 1/36 (3%) | 1/68 (1%) | 1.0 |
|
| ||||
| Number of lesions intervened upon per vessel1,* | ||||
| -Left main | 0.31 ± 0.47 | 0.28 ± 0.45 | 0.29 ± 0.46 | 0.76 |
| -Left anterior descending | 1.09 ± 0.93 | 1.11 ± 1.04 | 1.10 ± 0.98 | 0.94 |
| -Circumflex | 0.50 ± 0.62 | 0.75 ± 0.81 | 0.63 ± 0.73 | 0.16 |
| -Right coronary | 0.28 ± 0.72 | 0.28 ± 0.78 | 0.28 ± 0.75 | 0.98 |
|
| ||||
| Lesion length (mm)1 | 16.5 ± 9.9 | 18.0 ± 16.3 | 17.3 ± 13.8 | 0.51 |
|
| ||||
| Reference vessel diameter (mm)1 | 3.2 ± 0.5 | 3.0 ± 0.5 | 3.1 ± 0.5 | 0.02 |
|
| ||||
| Pre PCI stenosis severity (%)1 | 83.3 ± 13.3 | 88.2 ± 11.2 | 86.0 ± 12.4 | 0.01 |
|
| ||||
| AHA class B2 or C lesion morphology2 | 63/74 (85%) | 79/91 (87%) | 142/165 (86%) | 0.82 |
Data is presented as mean ± SD or number of subjects/lesions (%).
Abbreviations not previously defined: AHA, American Heart Association.
Statistical analyses used:
2-sided non-paired Student’s t test,
2-sided Fisher’s exact test,
Chi-square test.
More than 1 lesion may have undergone PCI per vessel; more than 1 vessel may have undergone PCI per patient.
The use of IR2.5, as compared to TH, was associated with reduced overall procedural times (total procedural time including implant, PCI and explant time), from 67.0 ± 39.1 to 41.7 ± 38.7 min (P = 0.009). During PCI, although patients in the IR2.5 group had marginally fewer stents implanted per lesion, procedural details were otherwise generally similar between groups (Table III). From a hemodynamic perspective, the degree of support appeared very similar between the TH and IR2.5 devices, and there were no differences with respect to either the minimum recorded intra-procedural blood pressure or the need for neosynephrine administration (Table III). However, as an important difference, a single episode of left atrial perforation with cardiac tamponade was observed in the TH group; presumably as a direct and unique result of TH device usage (Table III).
Table III.
Procedural details of patients undergoing PCI with TH versus IR2.5. With the exception of rotablator use, minimum systolic BP and neosynephrine administration (presented per patient), data is presented on a per lesion basis.
| TandemHeart (n = 32 patients; n = 74 lesions) | Impella 2.5 (n = 36 patients; n = 91 lesions) | All patients (n = 68 patients; n = 165 lesions) | P value for between group comparison | |
|---|---|---|---|---|
|
| ||||
| Rotablator use2 | 9/32 (28%) | 16/36 (44%) | 25/68 (37%) | 0.21 |
|
| ||||
| Stent/PTCA strategy3 | 0.45 | |||
| -Angioplasty only | 5/74 (7%) | 8/91 (9%) | 13/165 (8%) | |
| -Bare metal stent | 12/74 (16%) | 9/91 (10%) | 21/165 (13%) | |
| -Drug eluting stent | 57/74 (77%) | 74/91 (81%) | 131/165 (79%) | |
|
| ||||
| Number of stents1 | 1.2 ± 0.4 | 1.0 ± 0.2 | 1.1 ± 0.3 | 0.02 |
|
| ||||
| Total stented length1 | 21.0 ± 11.0 | 19.5 ± 9.1 | 20.2 ± 10.0 | 0.33 |
|
| ||||
| Maximum stent diameter1 | 3.1 ± 0.5 | 3.0 ± 0.4 | 3.1 ± 0.4 | 0.15 |
|
| ||||
| Minimum systolic BP (mmHg)1 | 106 ± 22 | 108 ± 26 | 108 ± 24 | 0.83 |
|
| ||||
| Neosynephrine administration2 | 14/32 (44%) | 11/36 (31%) | 25/68 (37%) | 0.32 |
|
| ||||
| Procedural success2 | 73/74 (99%) | 90/91(99%) | 163/165 (99%) | 1.0 |
|
| ||||
| Intra-procedural complications2 | 0.20 | |||
| -side branch closure* | 1/74 (1%) | 0/91 (0%) | 1/165 (0.6%) | |
| -cardiac tamponade† | 1/74 (1%) | 0/91 (0%) | 1/165 (0.6%) | |
All data is presented as mean ± SD or number of subjects/lesions (%).
Statistical analyses used:
2-sided non-paired Student’s t test,
2-sided Fisher’s exact test,
Chi-square test.
Side-branch closure resulting in small peri-procedural MI with peak CK-MB 32.7ng/mL;
Cardiac tamponade requiring pericardiocentesis and subsequent emergent thoracotomy; found to have 7mm perforation in left atrial appendage requiring oversew repair.
In-hospital clinical outcomes were similar between groups (Table IV). Although a total of 3 peri-procedural MIs were observed, the extent of these events was modest (Table IV). While 1 patient required emergent thoracotomy and left atrial appendage oversew, no patient required emergent CABG and no in-hospital deaths occurred.
Table IV.
Clinical peri-procedural and in-hospital outcomes of patients undergoing PCI with TH versus IR2.5.
| TandemHeart (n = 32) | Impella 2.5 (n = 36) | All patients (n = 68) | P value for between group comparison | |
|---|---|---|---|---|
|
| ||||
| Death | 0/32 (0%) | 0/36 (0%) | 0/68 (0%) | - |
|
| ||||
| Myocardial infarction2,* | 1/32 (3%) | 2/36 (6%) | 3/68 (4%) | 1.0 |
|
| ||||
| TIA or Stroke | 0/32 (0%) | 0/36 (0%) | 0/68 (0%) | - |
|
| ||||
| Urgent/emergent CABG | 0/32 (0%) | 0/36 (0%) | 0/68 (0%) | - |
|
| ||||
| Renal failure3 | 0.18 | |||
| -not requiring dialysis | 0/32 (0%) | 2/36 (6%) | 2/68 (3%) | |
| -requiring dialysis | 0/32 (0%) | 0/36 (0%) | 0/68 (0%) | |
|
| ||||
| Vascular access site complications3,† | 0.48 1.0 |
|||
| Total major access complications‡ | 2/32 (6%) | 3/36 (8%) | 5/68 (7%) | |
| -Small hematoma (<4cm) | 1/32 (3%) | 2/36 (6%) | 3/68 (4%) | |
| -Large hematoma (>4cm) without transfusion | 0/32 (0%) | 1/36 (3%) | 1/68 (1%) | |
| -Large hematoma requiring transfusion | 2/32 (6%) | 1/36 (3%) | 3/68 (4%) | |
| -Pseudoaneurysm resolved with thrombin injection | 0/32 (0%) | 1/36 (3%) | 1/68 (1%) | |
| -Femoral artery occlusion§ | 0/32 (0%) | 1/36 (3%) | 1/68 (1%) | |
|
| ||||
| Thrombocytopenia or need for platelet transfusion | 0/32 (0%) | 0/36 (0%) | 0/68 (0%) | - |
|
| ||||
| Hemolysis | 0/32 (0%) | 0/36 (0%) | 0/68 (0%) | - |
|
| ||||
| Any in-hospital complication | 4/32 (12%) | 10/36 (28%) | 14/68 (21%) | 0.14 |
Data is presented as mean ± SD or number of subjects (%).
Abbreviations not previously defined: TIA, transient ischemic attack.
Statistical analyses used:
2-sided non-paired Student’s t test,
2-sided Fisher’s exact test,
Chi-square test.
Highest recorded CK-MB of any patient was 50.8 ng/mL.
Other vascular access site complications, such as retroperitoneal bleeding or arterio-venous fistula, were not observed.
Major vascular access complications were defined as hematoma or bleeding requiring transfusion, or the need for further procedure to treat the access site complication (included 1 patient with pseudoaneurysm and 1 with femoral artery occlusion).
Successfully treated percutaneously.
Vascular access site complication rates were similar between groups and consistent with the large-bore cannulas required for TH and IR2.5 usage. Of all patients, 3/68 (4%) suffered a large hematoma requiring transfusion, 1/68 (1%) a pseudoaneurysm requiring thrombin injection, and 1/68 (1%) a femoral artery occlusion successfully managed by percutaneous intervention. Therefore, the overall rate of major vascular access site complications was 7% (5/68) (Table IV).
One patient died at home 4 days after an uneventful procedure. However, there were no other additional instances of death, MI or target lesion revascularization within 30 days of the index PCI. Therefore, including the 3 peri-procedural MIs, the 30 day rate of major adverse cardiac events (MACE - death, MI and target lesion revascularization [TLR]) was 5.8% (4/68).
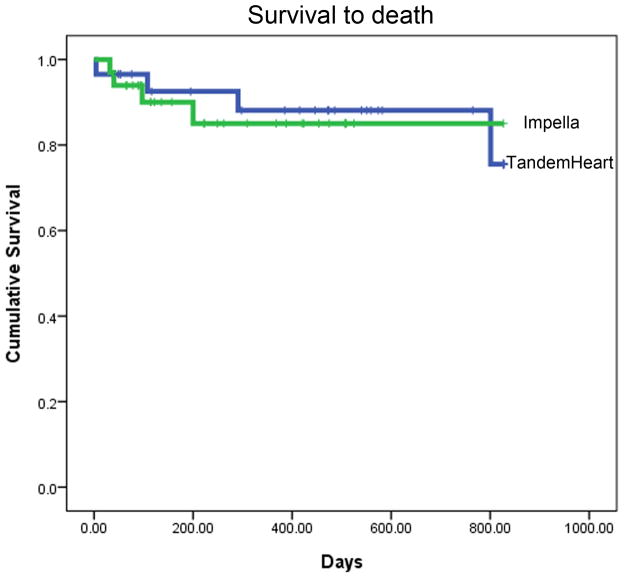
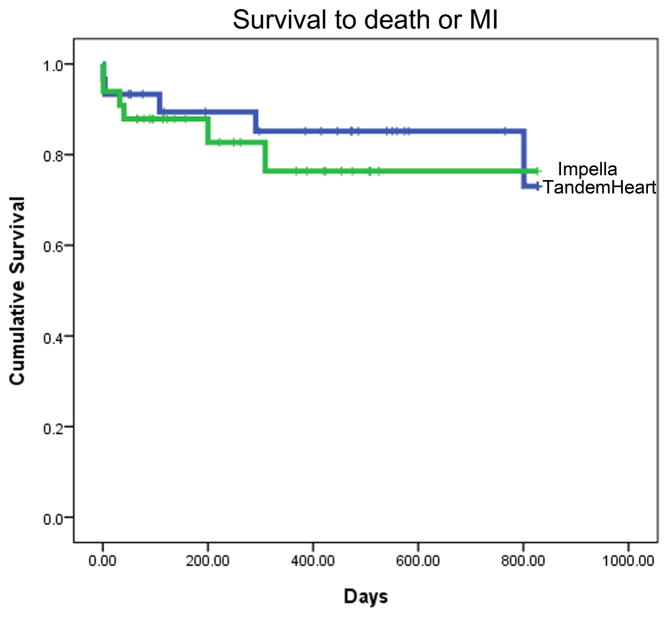
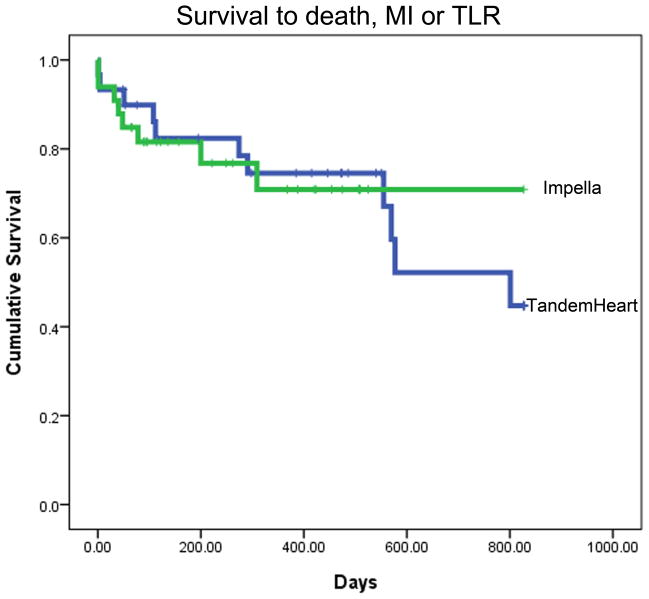
Intermediate- to long-term follow-up data were available for 30/32 (94%) TH and 33/36 (92%) IR2.5 patients. Due to the consecutive temporal pattern of P-LVAD device allocation, mean time to follow-up was significantly longer for the TH than the IR2.5 group (579 ± 464 versus 266 ± 197 days respectively, P = 0.001). Overall rates of death and MI were consistent with the high-risk nature of these patients. Kaplan-Meier analyses for estimated time to death; death and MI; and death, MI and TLR are presented in Fig. 1. There were no statistically significant differences between the TH and IR2.5 groups for these outcomes.
Fig. 1. Survival to (A) death, (B) death or MI, and (C) death, MI or TLR.
Kaplan Meier analyses showing mean event free survival times as indicated. Data includes peri-procedural events. Data for TH is shown in blue and for IR2.5 in green. There were no statistically significant differences between the TH and IR2.5 groups for these outcomes.
Discussion
Our single-center comparison between the IR2.5 and TH demonstrates that these P-LVADs are safe, feasible, and effective for cardiac support during elective high-risk PCI. Importantly, for both devices, procedural success rates were high and intermediate-term mortality rates were consistent with the significant co-morbidities of these patients (Table I, Fig. 1). There appeared to be little if any difference with respect to acute- or long-term outcomes between the IR2.5 and TH devices. To our knowledge, this is the first direct comparison of these two percutaneous cardiac support devices used at the time of high-risk PCI. Further, this study highlights several important issues regarding the current state and future application of cardiac support devices.
Since their introduction, numerous case studies and small series have demonstrated the efficacy of P-LVADs [10–18], and these devices have now supplanted IABPs as the primary cardiac support device in a variety of clinical scenarios. Importantly, likely driving this switch and as a key difference, unlike IABPs the short-term efficacy of P-LVADs does not intrinsically rely on significant residual cardiac function. Furthermore, cardiogenic shock trials have shown that compared to IABPs, P-LVADs provide improved cardiac output, cardiac index, and mean arterial pressures with lower pulmonary capillary wedge pressures [13,17]. However, perhaps due to low patient numbers, these superior hemodynamic parameters have not yet translated into improved clinical outcomes [13,17]. Nevertheless, growing enthusiasm exists for the use of P-LVADs both during high-risk PCI and in the immediate recovery period for those patients with severe LV impairment and/or cardiogenic shock. Indeed, the recent neutral findings of the BCIS-1 trial in ‘high-risk’ elective PCI patients, in which an ‘up-front’ IABP strategy conferred no benefit over no additional hemodynamic support, may serve to increase the use of P-LVADs for this indication [19].
Potentially, the implantation and setup of the TH appears more complex due to the need for multiple access sites, a transseptal puncture and extra-corporeal blood circulation. Therefore, the complexity of the TH device may require a greater level of operator and staff expertise. In addition, what was arguably the only serious and life-threatening intra-procedural adverse event to occur in this cohort, a case of atrial perforation, would appear attributable to the inherent design of the TH device. Nevertheless, we reiterate that the IR2.5 and TH devices were associated with very similar procedural, in-hospital and long-term outcomes, and that they appear to be equally efficacious for hemodynamic support during ‘high-risk’ PCI.
There were no differences in vascular access complications between the IR2.5 and TH groups; a finding likely attributable to the similar arterial sheath sizes required for these devices. We observed a 7% major access site complication rate, with no patient suffering permanent vascular sequelae. Although serious arterial access complications or limb ischemia have been observed in 20–30% of patients in certain small studies [12,17], our results are consistent with those of large contemporary publications in which, depending on the exact definition used, serious access complications arise in 4 – 8% of patients [20]. We suspect that our relatively low rate of vascular access site complications, particularly when the 13 – 15 Fr P-LVAD arterial sheaths are considered, may be attributable to our careful approach to arterial access and the use of the ‘preclose’ technique (see methods).
The respective potential of the TH versus IR2.5 to augment systemic arterial circulation is another key functional aspect of these devices that must be considered. Although the TH can provide greater support in terms of absolute flow rate (up to 4.5 L/min versus 2.5 L/min for IR2.5), the fact that blood is drawn from the left atrium but returned via the femoral artery means a non-physiologic retrograde aortic circulatory pattern is established during TH use that is in competition with any residual forward cardiac outflow. The degree of true cerebral and cardiac perfusion, at least in human patients during complex PCI, is unknown. On the other hand, the Impella device closely mimics the normal physiologic circulation and directly augments forward ventricular and coronary flow. Furthermore, an even newer version of Impella, the Impella 5.0, can now provide hemodynamic support at 5 L/min. However, this device requires a significantly larger sheath necessitating open femoral artery cut down (19 Fr for Impella 5.0 versus 13 Fr for IR2.5), which has effectively limited its use to the cardiothoracic operating room. Again, while only simple hemodynamic data were recorded in our study, we observed no differences with respect to hemodynamic support between the IR2.5 and TH devices.
Overall, our experience with the IR2.5 in high-risk PCI is consistent with recently published reports from other institutions [10–11,15,20]. Specifically, our patients were similar to those in the PROTECT I trial [15], although, the mean age of subjects in that study was 60 years (versus 71 years in our study). Overall 30 day rates of death and MI were 20% in the PROTECT I, versus 5.8% in our study. Interestingly, the PROTECT I study enrolled 20 patients from across 7 centers and in 2 countries. As a result, at least within the context of PROTECT I, the individual operators implanted very few IR2.5 devices. Potentially, this may have contributed to our markedly lower procedural times (mean procedure time in PROTECT 1 = 3.3 hours) [15]. Similarly, our results compare favorably with those of the Europella registry of 144 consecutive patients that underwent high-risk PCI with IR2.5 support [20]. In this relatively large study, the authors reported a 30 day death rate of 5.5%, whereas we experienced only a single death within this time (1.5%) [20].
Multiple practical challenges remain to be overcome in the use of percutaneous cardiac support devices during PCI. Apart from the technical issues and ‘learning curve’ associated with P-LVAD implantation and use, as this study highlights, there is little data to actually guide interventionalists as to which device is optimally suited to a particular high-risk patient. In addition, there is no consensus definition as to ‘who’ or ‘what’ constitutes ‘high-risk’ with respect to PCI. Adequately powered, multi-center randomized trials are clearly required that, ideally, will simultaneously address these complimentary issues. Nevertheless, the encouraging data presented in this and other studies [10–11,15,20] augers well for those patients afflicted with complex coronary artery disease and significant concurrent co-morbidities. We anticipate that with increasing operator familiarity and improved patient selection, the use of P-LVADs may facilitate the percutaneous revascularization of patients that might otherwise have been considered at prohibitively high-risk.
Limitations
This was a single-center, prospectively collected but largely retrospective and non-blinded analysis. The study was non-randomized and indeed, it is unclear if there can be an objective comparison made between the IR2.5 and TH devices given the different temporal periods that they were used. Further, our growing experience with these devices and at performing ‘high-risk’ PCI during the study period, along with evolving procedural techniques, further limits any conclusions that can be drawn. As a result of these differing temporal periods of P-LVAD use, the length of follow-up differed between the TH and IR2.5 groups. Cardiac output and right heart hemodynamic data were not routinely collected and are not presented.
Conclusions
The IR2.5 and TH assist devices appear safe, equally effective, and are associated with acceptable short- and long-term clinical outcomes in patients undergoing ‘high-risk’ PCI. Prospective, multi-center, randomized trials are now required to more adequately define the optimal patient and anatomic groups that will benefit from an ‘upfront’ P-LVAD strategy at the time of ‘high-risk’ PCI.
Acknowledgments
We gratefully acknowledge the work of our database management team and Roja Thapi. We thank the staff of the Mount Sinai Hospital catheterization laboratory.
Funding sources:
No specific funding or grant was used to fund this study.
Footnotes
Disclosures:
The following authors have no conflicts of interest to declare: Jason C. Kovacic, Huy T. Nguyen, Rucha Karajgikar, Annapoorna S. Kini.
Samin K. Sharma acknowledges speaker’s bureau from Boston Scientific, Abbott, The Medicines Company and Lilly/DSI.
References
- 1.American Heart Association. [Accessed July 20th 2010];Heart Disease and Stroke Statistics - 2010 Update. Available at: http://www.americanheart.org/presenter.jhtml?identifier=1200026.
- 2.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 3.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 4.Grayson AD, Moore RK, Jackson M, Rathore S, Sastry S, Gray TP, Schofield I, Chauhan A, Ordoubadi FF, Prendergast B, Stables RH. Multivariate prediction of major adverse cardiac events after 9914 percutaneous coronary interventions in the north west of England. Heart. 2006;92:658–663. doi: 10.1136/hrt.2005.066415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor GT, Malenka DJ, Quinton H, Robb JF, Kellett MA, Jr, Shubrooks S, Bradley WA, Hearne MJ, Watkins MW, Wennberg DE, Hettleman B, O’Rourke DJ, McGrath PD, Ryan T, Jr, VerLee P. Multivariate prediction of in-hospital mortality after percutaneous coronary interventions in 1994–1996. Northern New England Cardiovascular Disease Study Group. J Am Coll Cardiol. 1999;34:681–691. doi: 10.1016/s0735-1097(99)00267-3. [DOI] [PubMed] [Google Scholar]
- 6.Valgimigli M, Serruys PW, Tsuchida K, Vaina S, Morel MA, van den Brand MJ, Colombo A, Morice MC, Dawkins K, de Bruyne B, Kornowski R, de Servi S, Guagliumi G, Jukema JW, Mohr FW, Kappetein AP, Wittebols K, Stoll HP, Boersma E, Parrinello G. Cyphering the complexity of coronary artery disease using the syntax score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am J Cardiol. 2007;99:1072–1081. doi: 10.1016/j.amjcard.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 7.Capodanno D, Di Salvo ME, Cincotta G, Miano M, Tamburino C. Usefulness of the SYNTAX score for predicting clinical outcome after percutaneous coronary intervention of unprotected left main coronary artery disease. Circ Cardiovasc Interv. 2009;2:302–308. doi: 10.1161/CIRCINTERVENTIONS.108.847137. [DOI] [PubMed] [Google Scholar]
- 8.Briguori C, Airoldi F, Chieffo A, Montorfano M, Carlino M, Sangiorgi GM, Morici N, Michev I, Iakovou I, Biondi-Zoccai G, Colombo A. Elective versus provisional intraaortic balloon pumping in unprotected left main stenting. Am Heart J. 2006;152:565–572. doi: 10.1016/j.ahj.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 9.Mishra S, Chu WW, Torguson R, Wolfram R, Deible R, Suddath WO, Pichard AD, Satler LF, Kent KM, Waksman R. Role of prophylactic intra-aortic balloon pump in high-risk patients undergoing percutaneous coronary intervention. Am J Cardiol. 2006;98:608–612. doi: 10.1016/j.amjcard.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 10.Remmelink M, Sjauw KD, Henriques JP, de Winter RJ, Koch KT, van der Schaaf RJ, Vis MM, Tijssen JG, Piek JJ, Baan J., Jr Effects of left ventricular unloading by Impella recover LP2. 5 on coronary hemodynamics. Catheter Cardiovasc Interv. 2007;70:532–537. doi: 10.1002/ccd.21160. [DOI] [PubMed] [Google Scholar]
- 11.Valgimigli M, Steendijk P, Sianos G, Onderwater E, Serruys PW. Left ventricular unloading and concomitant total cardiac output increase by the use of percutaneous Impella Recover LP 2. 5 assist device during high-risk coronary intervention. Catheter Cardiovasc Interv. 2005;65:263–267. doi: 10.1002/ccd.20380. [DOI] [PubMed] [Google Scholar]
- 12.Vranckx P, Schultz CJ, Valgimigli M, Eindhoven JA, Kappetein AP, Regar ES, Van Domburg R, Serruys PW. Assisted circulation using the TandemHeart during very high-risk PCI of the unprotected left main coronary artery in patients declined for CABG. Catheter Cardiovasc Interv. 2009;74:302–310. doi: 10.1002/ccd.22011. [DOI] [PubMed] [Google Scholar]
- 13.Burkhoff D, Cohen H, Brunckhorst C, O’Neill WW. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J. 2006;152:469, e1–8. doi: 10.1016/j.ahj.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 14.Rajdev S, Krishnan P, Irani A, Kim MC, Moreno PR, Sharma SK, Kini AS. Clinical application of prophylactic percutaneous left ventricular assist device (TandemHeart) in high-risk percutaneous coronary intervention using an arterial preclosure technique: single-center experience. J Invasive Cardiol. 2008;20:67–72. [PubMed] [Google Scholar]
- 15.Dixon SR, Henriques JP, Mauri L, Sjauw K, Civitello A, Kar B, Loyalka P, Resnic FS, Teirstein P, Makkar R, Palacios IF, Collins M, Moses J, Benali K, O’Neill WW. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial U.S. experience. JACC Cardiovasc Interv. 2009;2:91–96. doi: 10.1016/j.jcin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Henriques JP, Remmelink M, Baan J, Jr, van der Schaaf RJ, Vis MM, Koch KT, Scholten EW, de Mol BA, Tijssen JG, Piek JJ, de Winter RJ. Safety and feasibility of elective high-risk percutaneous coronary intervention procedures with left ventricular support of the Impella Recover LP 2. 5. Am J Cardiol. 2006;97:990–992. doi: 10.1016/j.amjcard.2005.10.037. [DOI] [PubMed] [Google Scholar]
- 17.Thiele H, Sick P, Boudriot E, Diederich KW, Hambrecht R, Niebauer J, Schuler G. Randomized comparison of intra-aortic balloon support with a percutaneous left ventricular assist device in patients with revascularized acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2005;26:1276–1283. doi: 10.1093/eurheartj/ehi161. [DOI] [PubMed] [Google Scholar]
- 18.Vranckx P, Meliga E, De Jaegere PP, Van den Ent M, Regar ES, Serruys PW. The TandemHeart, percutaneous transseptal left ventricular assist device: a safeguard in high-risk percutaneous coronary interventions. The six-year Rotterdam experience. EuroIntervention. 2008;4:331–337. doi: 10.4244/eijv4i3a60. [DOI] [PubMed] [Google Scholar]
- 19.Perera D, Stables R, Thomas M, Booth J, Pitt M, Blackman D, de Belder A, Redwood S. Elective intra-aortic balloon counterpulsation during high-risk percutaneous coronary intervention. JAMA. 2010;304:867–74. doi: 10.1001/jama.2010.1190. [DOI] [PubMed] [Google Scholar]
- 20.Sjauw KD, Konorza T, Erbel R, Danna PL, Viecca M, Minden HH, Butter C, Engstrom T, Hassager C, Machado FP, Pedrazzini G, Wagner DR, Schamberger R, Kerber S, Mathey DG, Schofer J, Engstrom AE, Henriques JP. Supported high-risk percutaneous coronary intervention with the Impella 2. 5 device the Europella registry. J Am Coll Cardiol. 2009;54:2430–2434. doi: 10.1016/j.jacc.2009.09.018. [DOI] [PubMed] [Google Scholar]