Abstract
Anxiety is a key consequence of ethanol withdrawal and important risk factor for relapse. The neuropeptide nociceptin/orphanin FQ (N/OFQ), or agonists at this peptide’s receptor (NOP), exert anxiolytic-like and anti-stress actions. N/OFQ dysfunction has been linked to both a high-anxiety behavioral phenotype and excessive ethanol intake. Recent studies suggest a possible link between genetic polymorphisms of the NOP transcript and alcoholism. Thus, in the present study, the effects of intracerebroventricularly (ICV) administered N/OFQ were tested for modification of anxiety-like behaviors, using the shock-probe defensive burying and elevated plus maze tests, in ethanol-dependent vs. nondependent rats, one and three weeks following termination of ethanol exposure. Additionally, Prepro-N/OFQ (ppN/OFQ) and NOP receptor gene expression was measured in the central nucleus of the amygdala, in the bed nucleus of the stria terminalis, and in the lateral hypothalamus at the same time points in separate subjects. One week post-ethanol, N/OFQ dose-dependently attenuated elevated anxiety-like behavior in ethanol-dependent rats and produced anxiolytic-like effects in nondependent controls in both behavioral tests. However, three weeks post-ethanol, N/OFQ altered behavior consistent with anxiogenic-like actions in ethanol-dependent rats, but continued to exert anxiolytic-like actions in nondependent controls. These findings were paralleled by ethanol history-dependent changes of ppN/OFQ and NOP gene expression that showed a distinctive time-course in the examined brain structures. The results demonstrate that ethanol dependence and withdrawal are associated with neuroadaptive changes in the N/OFQ-NOP system suggesting a role of this neuropeptidergic pathway as a therapeutic target for the treatment of alcohol abuse.
Keywords: Nociceptin, stress, alcohol, mRNA, elevated-plus maze, defensive burying
Introduction
Withdrawal from chronic ethanol is characterized by a pronounced state of negative affect that persists long after discontinuation of ethanol use and represents a major factor in relapse risk (Heinz et al., 2009; Koob 2009). Abnormal stress sensitivity and elevated anxiety in ethanol-dependent subjects exists during protracted abstinence from alcohol and is associated with dysregulation of multiple neurotransmitter systems (Koob and Kreek, 2007). Similarly, animal models of ethanol dependence have revealed perturbations in stress-regulatory systems, reflected behaviorally in elevated anxiety-like responses during protracted withdrawal (Valdez et al., 2002; Zorrilla et al., 2001; Zhao et al., 2007a, 2007b).
A neuropeptide recently implicated in regulating stress-related behavior is nociceptin/orphanin FQ (N/OFQ) (Ciccocioppo et al., 2002; Ciccocioppo et al., 2003; Martin-Fardon et al., 2010). N/OFQ is structurally related to the opioid peptide dynorphin A, but binds with high affinity at the opioid receptor-like 1 (ORL-1), now included in the opioid receptor family and renamed N/OFQ-opioid peptide receptor, hereafter referred as NOP (Reinscheid et al., 1998; Reinscheid et al., 2001). N/OFQ and NOP are widely distributed throughout the brain, but are expressed at particularly high levels in limbic and limbic-related regions, including regions implicated in stress-regulatory functions such as the bed nucleus of the stria terminalis (BNST), the central nucleus of the amygdala (CeA), cortex, septum, and subnuclei of the hypotalamus (Neal et al., 1999a, b). Administration of N/OFQ in the BNST attenuates stress-associated anorexia produced by administration of corticotropin-releasing-factor (CRF) into the BNST (Ciccocioppo et al., 2004a; Ciccocioppo et al 2003). CRF is known to play a key role in physiological responses to stress; therefore, the anti-anorectic effects of N/OFQ have also been attributed to anti-anxiety actions resulting from functional antagonism of CRF neurotransmission in the BNST (Vale et al., 1981; Ciccocioppo et al., 2003). N/OFQ and its receptor NOP are also distributed in structures such as the amygdala and the BNST that have been implicated in behavioral effects triggered by various drugs of abuse, especially alcohol (Ciccocioppo et al., 1999; 2004b; Roberto and Siggins, 2006).
N/OFQ may have some specificity for attenuating behavior motivated by ethanol as suggested by findings that N/OFQ attenuates stress-induced ethanol but not cocaine-seeking in rats (Martin-Fardon et al., 2000). Lastly, whereas N/OFQ does not decrease ethanol consumption in nondependent Wistar rats (Economidou et al., 2008), it does so in post-dependent Wistar rats that are known to also show elevated anxiety-like behavior (Martin-Fardon et al., 2010). Thus, a link appears to exist between the regulation of anxiety by the N/OFQ-NOP system and its role in ethanol seeking and reinforcement. Similarly, recent association studies suggest a link between polymorphisms of the NOP transcript and alcoholism (Huang et al., 2008; Xuei et al., 2008). Therefore, the NOP receptor represents a potential pharmacotherapeutic target for relapse prevention as well as attenuation of ongoing ethanol abuse (for review, see Martin-Fardon et al., 2010).
The present study was designed to further investigate the role of the N/OFQ-NOP system in regulating withdrawal-associated anxiety and to detect changes in N/OFQ-NOP-related gene expression at different stages of ethanol abstinence. For this purpose, anti-anxiety actions of N/OFQ in rats with or without a history of ethanol dependence were investigated, combined with identification of the post-ethanol time dependence of these actions. Moreover, the study of Prepro-N/OFQ (ppN/OFQ) and NOP gene expression was performed in selected structures of the central nervous system (CNS) known to be involved in ethanol-related behavioral changes – the CeA, the BNST, and the lateral hypothalamus (LH) (Kauer and Malenka, 2007; Koob, 2008) and are also sites of action of N/OFQ.
Methods and Materials
Subjects
Male Wistar rats (Charles River, Wilmington, MA), weighing 200–250g on arrival, were used. Rats were pair-housed on a 12-h (Lights off at 2100) light/dark cycle in a temperature and humidity controlled vivarium with ad libitum availability of food and water. Behavioral tests were conducted between 1200–1900. All procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs
N/OFQ (Phe-Gly-Gly-Phe-Thr-Gly-Ala-Arg-Lys-Ser-Ala-Arg-Lys-Leu-Ala-Asp-Glu) was dissolved in sterile isotonic saline and administered intracerebroventricularly (ICV) in a volume of 1µl. Aliquots containing concentrations of 0, 0.5 µg (0.28 nmol), 1.0 µg (0.55 nmol), and 2.0 µl (1.11 nmol) were prepared via serial dilution of stock solution stored at −20° C. Ethanol solution (25% w/v) was prepared by diluting 95% ethanol in a vehicle solution consisting of a dietary supplement (Boost®, Nestle).
Surgery
Ten days before ethanol (or vehicle) treatments, rats were stereotaxically implanted with a stainless steel guide cannula (0.5mm external diameter) aimed at the lateral cerebral ventricle (AP −1.0; L 1.8; V −3.0; with reference to bregma; Paxinos & Watson, 2007). N/OFQ or vehicle was administered through a stainless steel injector that extended 1.0 mm beyond the cannula.
Drug administration
N/OFQ was administered 10 min before behavioral test sessions. Dose selection was counterbalanced between the elevated plus maze and shock-probe defensive burying tests to control for potential order effects.
Procedures
Ethanol intoxication by intragastric intubation
Ethanol dependence (Dependent, N = 74) was induced using an intragastric intubation procedure in which ethanol was administered four times daily for six consecutive days. On the first day, rats were intubated with a total of 11.0 g/kg ethanol in 4 fractional doses of 3.0, 3.0, 2.5, and 2.5 g/kg ethanol, administered at 4-hour intervals. During days 2 to 6, 12 hours after the last intubation on the preceding day, rats received a total of 10.0 g/kg ethanol in 4 fractional doses of 3.0, 2.5, 2.5, and 2.0 g/kg, again separated by 4-hour intervals. Rats serving as nondependent controls (Nondependent, N=79) were intubated with vehicle solution for ethanol (Boost®, Nestle) at time intervals and relative volumes identical to those in ethanol-dependent rats (22 ml/kg/day).
Measurement of blood ethanol levels
Tail blood (approximately 200 µl) was collected on day 5 immediately before the first daily dose of ethanol and 1 h after the second and third daily doses. Samples were collected and subsequently centrifuged (10 min, 5000 rpm). Ethanol content was assayed from 5 µl plasma aliquots using an oxygen-rate alcohol analyzer (Analox Instruments, Lunenburg, MA).
Measurement of ethanol withdrawal signs
Rats were examined daily for physical signs of withdrawal between 10–12 h after the last ethanol intubation. Using a withdrawal rating scale adapted from Macey and colleagues (1996), ethanol withdrawal signs including ventromedial limb retraction (VLR), vocalization when handled, tail rigidity, and body tremors were scored. Each withdrawal measure was assigned a score of 0–2, reflecting increasing severity. The sum of the four observation scores (0–8) was used as a quantitative measure of withdrawal severity.
Behavioral test procedures
One or three weeks following the last day of intragastric intubation rats were tested in the elevated plus-maze and defensive burying tests, using separate groups of rats for the two post-ethanol time points.
Elevated plus-maze
The elevated plus-maze apparatus was constructed of Plexiglas and consisted of four arms (10 X 50 cm) positioned at right angles and elevated 50 cm above the floor. Two arms were fitted with 40 cm high dark walls (enclosed arms). The other two arms had 0.5 cm high ledges (open arms). The elevated plus-maze was located in a quiet room that provided 1.5–2.0 lux of illumination for the open arms and <1 lux for the enclosed arms. On test day, rats were habituated for at least 2 h in the presence of white noise (70 dB; Realistic sound amplifier, model SA-10). Rats were then placed individually onto the center of the maze facing a closed arm, with white noise (70 dB) present. During the 5-min test, behavior was recorded by video camera and later scored under blind conditions for time spent in the open and closed arms.
Shock-probe defensive burying
This procedure was conducted in a standard polycarbonate cage (43 X 20 X 20 cm) containing a 5 cm layer of bedding material. During testing, a 6.5-cm long electrical probe (Staco Energy Products, Dayton, OH) was inserted through a 2-cm diameter opening located 2.5 cm above the bedding material in the center of the chamber’s long side. The probe was connected to a Coulborn precision shocker (model E13-01, Allentown, PA), calibrated to deliver 1.5 mA of current upon contact.
Rats were tested the day following the elevated plus-maze test. Animals had previously been habituated to the defensive burying apparatus (without the shock probe) on 2 consecutive days for a total of 5 h in the presence of white noise (70 dB). On the third day, rats were subjected to a test session with the electrified probe. Upon contact with the probe and shock delivery, verified by a startle response, the probe was deactivated and subsequent burying behavior was recorded for the next 10 min by video camera for later scoring. Bedding was changed and the cage washed with water between tests. Video recordings of each animal were scored for the duration of (1) burying, (2) freezing, defined as rigid immobile posture, (3) ambulation, and (4) rearing. In addition, shock reactivity was scored on a four-point scale (Pesold and Treit, 1992), based on the startle response exhibited by the rat upon first contact with the electrified probe. The shock reactivity scale measured: (1) flinch involving only the forepaw or head; (2) whole body flinch without ambulation away from the shock probe; (3) whole body flinch and/or jumping with ambulation away from the shock probe; and (4) whole body flinch and/or jumping followed by running to the opposite side of the test cage. This scale provides an index of the amount of pain produced by contact with the probe, a parameter that may influence burying behavior.
Quantitative Real-Time (RT)-PCR
Rats were subjected to the same ethanol dependence induction (and control) procedures as for the behavioral studies above and euthanized one or three weeks post-ethanol. For tissue collection, rats were deeply anesthetized and decapitated, their brains rapidly extracted, frozen in methylbutane, and stored at –80 °C. Brains were subsequently dissected into coronal sections, and brain regions of interest were collected by 15-gauge tissue punches. Sampled regions included the BNST (0.12 to 0.96 mm; with reference to bregma; Paxinos & Watson, 2007), CeA (1.44 to – 2.70 mm), and LH (2.80 to −4.16 mm). Brain punches were frozen on dry ice and stored at −80 °C. Total RNA was extracted using RNeasy Mini kit from Qiagen followed by DNase treatment. Integrity of RNA and absence of genomic DNA were checked by gel electrophoresis. RNA concentrations were measured with a Nanodrop. RNA with an OD260/OD280 ratio above 1.8 were used, subjected to DNase treatment and converted to cDNA (SuperScript II Reverse Transcriptase, Invitrogen, Oligo(dT)12–18, 300ng RNA). Relative abundance of each mRNA species was assessed by real-time PCR using 5ng cDNA template, lightCycler 480SYBR Green I Master reaction mix (Roche Applied Science) on a Mastercycler eprealplex (Eppendorf). Relative expression of mRNA was calculated using the comparative Ct method. All data were standardized with GAPDH as an endogenous reference gene, since all treatments did not change its levels in our experimental conditions (data not shown). RNA data from ethanol dependent and nondependent rats were normalized relative to RNA data from an additional group of experimentally naïve rats, age and weight-matched to rats in the ethanol (or vehicle) intubation group. Relative expression of different gene transcripts was calculated by the Delta-Delta Ct method and converted to a relative expression ratio (2-ΔΔCt) for statistical analysis (Livak and Schmittgen 2001). The melting curve (60°C to 95°C) confirmed that the amplification product was specific. The primers used for PCR amplification are shown in table 1 and were designed using Primer3 software.
Table 1.
Primer sequences for real-time (RT)-polymerase chain reaction
| Forward (5´- 3´) | Reverse (5´- 3´) | Size | |
|---|---|---|---|
| GAPDH | AGACAGCCGCATCTTCTTGT | CTTGCCGTGGGTAGAGTCAT | 207 |
| ppN/OFQ | TGCAGCACCTGAAGAGAATG | CAACTTCCGGGCTGACTTC | 170 |
| NOP | AGCTTCTGAAGAGGCTGTGT | GACCTCCCAGTATGGAGCAG | 101 |
Statistical Analyses
Differences in time spent on the open arms of the elevated plus-maze or the duration of burying in the shock-probe defensive burying test were analyzed separately for each post-ethanol time point by two-way analysis of variance (ANOVA) with ethanol history (dependent, nondependent) and N/OFQ dose as between subjects variables. The same statistical approach was employed to analyze total arm entries on the elevated plus maze, duration of freezing and ambulation, as well as shock reactivity in the SPDB test. Somatic ethanol withdrawal signs were analyzed for differences between ethanol-dependent and nondependent rats by independent t-test. Differences in RNA expression between groups within each brain region were analyzed by two-way ANOVA with ethanol history (dependent, nondependent) and post-ethanol time (one or three weeks) as between-subjects variables, followed by Newman-Keuls post-hoc tests or planned comparisons between experimentally naïve rats and ethanol history conditions.
Results
Blood alcohol concentrations
Mean (± SEM) blood alcohol levels on Day 5 of the intragastric intubation procedure, reached 183.2 ± 14.5 mg % following the second dose of ethanol and 252 ± 12.7 mg % following the third daily dose.
Ethanol withdrawal ratings
The overall score (mean ± SEM) for signs of somatic withdrawal in ethanol dependent rats was 5.2 ± 0.3 and significantly higher (t151 = 21.9, p < 0.0001) than in vehicle controls (0.6 ± 0.1).
Behavioral measures of anxiety
One-week withdrawal time point
Elevated plus maze
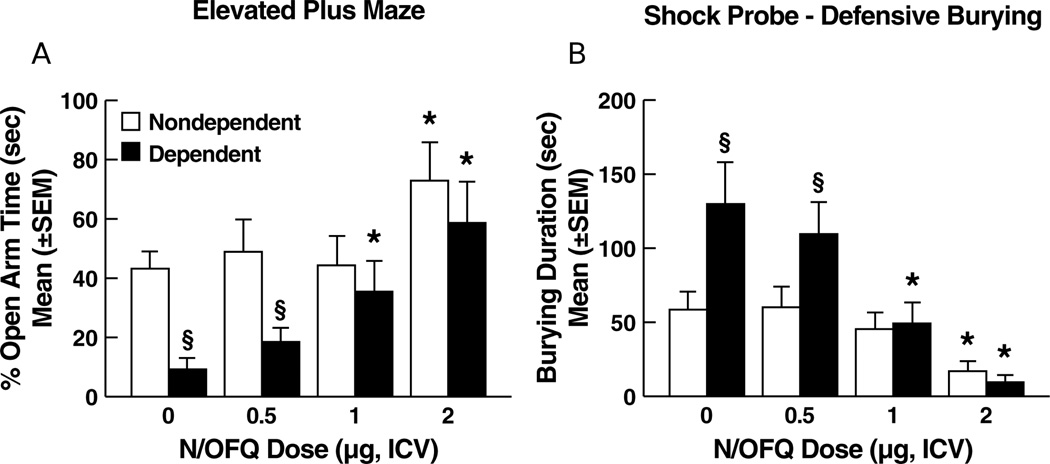
Ethanol-dependent rats treated with N/OFQ vehicle showed suppressed open arm exploration vs. nondependent controls. N/OFQ dose-dependently reversed this suppression in dependent rats and increased open arm exploration in vehicle controls at the highest N/OFQ dose (Figure 1A). Data were expressed as percent open arm time, calculated as [seconds in open arms / (seconds in open arms + seconds in closed arms)]×100. ANOVA revealed a main effect of ethanol history (F1,68 = 9.27; p < 0.01), N/OFQ dose (F3,68 = 6.22; p < 0.001) and an ethanol history × N/OFQ dose interaction (F3,68 = 2.97, p < 0.05). Newman-Keuls tests (p < 0.05) revealed decreased percent open arm time for ethanol-dependent animals vs. nondependent controls at the vehicle dose of N/OFQ. Increased percent open arm time was observed in both groups at 2.0 µg of N/OFQ while in ethanol-dependent rats N/OFQ also increased percent open arm time at 1.0 µg (p < 0.05). No differences were observed between dependent and nondependent controls with respect to the number of total arm entries, indicating that motor activity was not significantly altered by either ethanol history or administration of N/OFQ (see Table S1).
Figure 1.
Effects of N/OFQ on anxiety-like behavior as determined 1 week following termination of chronic ethanol (ethanol-dependent) or vehicle (nondependent) administration in (A) the elevated plus maze and (B) defensive burying tests. Ethanol-dependent rats showed significantly increased behavioral manifestations of anxiety in both the elevated plus maze (reduced open arm time) and defensive burying (longer burying duration) models. N/OFQ dose-dependently reversed anxiety-like behavior in ethanol-dependent rats. In nondependent rats, N/OFQ produced anxiolytic-like effects, but only at the highest (2 µg) dose. * p < 0.05 vs. vehicle (0 dose) within the same group; § p < 0.05 vs. nondependent.
Shock-probe defensive burying
Ethanol-dependent rats exhibited an overall increase in burying duration. N/OFQ produced differential effects as a function of ethanol treatment history (Figure 1B). ANOVA revealed main effects of ethanol history (F1,68 = 6.70, p < 0.05), N/OFQ dose (F3,68 = 11.82, p < 0.0001) and a significant ethanol history × N/OFQ dose interaction (F3,68 = 3.01, p < 0.05). In the ethanol-dependent group, Newman-Keuls tests confirmed significant decreases in burying duration at the 1.0 µg and 2.0 µg doses of N/OFQ. Newman-Keuls tests also confirmed a significant effect of N/OFQ in nondependent controls but only at the 2.0 µg dose (p < 0.05). A significant increase in burying duration in ethanol-dependent vs. vehicle control rats receiving N/OFQ vehicle was confirmed by Newman-Keuls test (p < 0.05). No differences were observed between ethanol-dependent and nondependent rats treated with N/OFQ vehicle with respect to freezing duration, ambulation, or shock reactivity (see Table S1).
Three-week withdrawal time point
Elevated plus maze
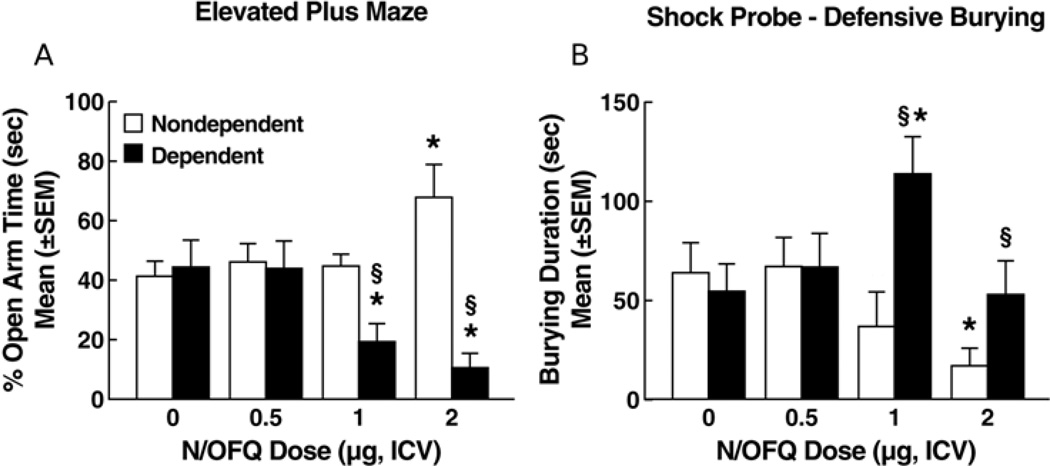
Ethanol dependent and nondependent rats treated with N/OFQ vehicle no longer showed differences in open arm exploration at the 3-week withdrawal time point (Figure 2A). However, with increasing doses of N/OFQ, open arm exploration decreased in ethanol-dependent rats whereas, in nondependent rats, N/OFQ produced a dose response profile similar to that at the one-week test point (Figure 2A). These divergent effects of N/OFQ were confirmed by a main effect of ethanol history (F1,69 = 15.68, p < 0.001) and a significant ethanol history × N/OFQ dose interaction (F3,69 = 7.58, p < 0.001). Newman-Keuls tests did not reveal any significant differences between ethanol-dependent rats and nondependent controls at the vehicle dose of N/OFQ. The N/OFQ dose response profile in nondependent controls remained essentially identical to that recorded one week post-ethanol where N/OFQ increased percent open arm time only at the 2.0-µg dose. In ethanol-dependent rats, however, N/OFQ dose-dependently decreased percent open arm time at both the 1.0 µg and 2.0 µg doses vs. 0 µg (p < 0.05; Newman-Keuls, see Fig. 1A and 2A). No differences were observed between dependent and nondependent controls with respect to the number of total arm entries, indicating that motor activity was not significantly altered by either treatment history or administration of N/OFQ (see Table S2).
Figure 2.
Effects of N/OFQ on anxiety-like behavior measured 3 weeks following termination of chronic ethanol (ethanol-dependent) or vehicle (nondependent) administration in (A) the elevated plus maze and (B) defensive burying tests. Overt anxiety-like behavior in ethanol-dependent rats no longer differed from that of nondependent rats in terms of either elevated plus maze or defensive burying performance (see zero dose, vehicle condition). As during the 1-week post-withdrawal test (Figure 1), N/OFQ produced anxiolytic-like effects at the high (2µg) dose in nondependent rats. In contrast, N/OFQ produced anxiogenic-like effects at doses of 1.0 µg (elevated plus maze, defensive burying) and 2 µg (elevated plus maze) in ethanol-dependent rats. * p < 0.05 vs. vehicle (0 dose) within the same group; § p < 0.05 vs. nondependent.
Shock-probe defensive burying
As in the elevated plus maze test, differences were no longer observed in defensive burying between ethanol dependent and nondependent rats treated with N/OFQ vehicle. However, in contrast to its suppressant effects on burying behavior at the 1-week withdrawal time point, N/OFQ failed to decrease burying behavior in ethanol dependent rats at any dose and even significantly increased this behavior at the 1-µg dose (Figure 2B). ANOVA revealed a significant main effect of ethanol history (F1,69 = 5.37, p < 0.05) and a significant ethanol history × N/OFQ dose interaction (F3,69 = 3.61, p < 0.05) for burying duration. In ethanol-dependent animals, Newman-Keuls tests confirmed a significant increase in burying at the 1.0 vs. 0-µg dose of N/OFQ (p < 0.05). Similar to the 1 week abstinence time, Newman-Keuls tests confirmed a significant decrease in burying duration as a result of N/OFQ in nondependent rats but only for the 2.0 vs. 0-µg dose (p < 0.05). The highest dose of N/OFQ produced increases in the duration of freezing behavior in ethanol dependent rats but not in nondependent controls (Table S2). With respect to duration of freezing, ANOVA revealed a significant ethanol history × N/OFQ dose interaction (F3,69 = 3.43, p < 0.05). Newman-Keuls tests confirmed a significant increase in freezing duration at the 2.0 vs. 0-µg dose of N/OFQ (p < 0.05). No differences were observed between dependent and nondependent controls in shock reactivity or ambulation (Table S2).
Real Time (RT)-PCR
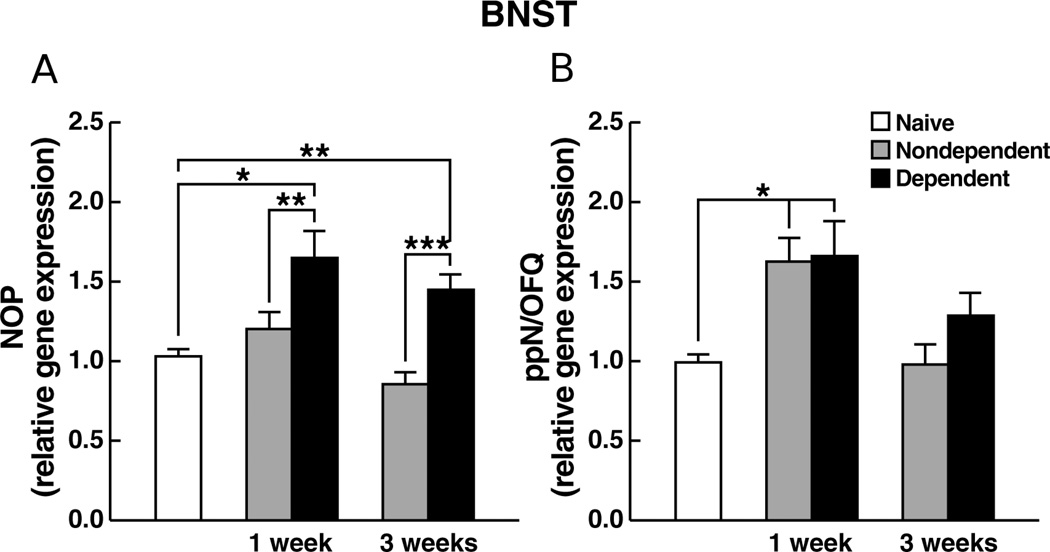
NOP gene expression in the BNST
An overall increase in NOP gene expression in ethanol-dependent animals vs. controls (Figure 3A) was confirmed by ANOVA revealing a significant main effect for ethanol history (F1,38 = 23.48, p < 0.0001). Newman-Keuls tests confirmed a significant increase in expression in ethanol-dependent animals vs. vehicle-controls at both 1 and 3 weeks of abstinence (p < 0.05). Planned comparisons revealed significant differences between ethanol-dependent and experimentally naïve rats at both 1 week (t18 = 9.32, p < 0.0001) and 3 weeks (t19 = 15.67, p < 0.0001) of abstinence.
Figure 3.
Relative gene expression levels of (A) NOP or (B) ppN/OFQ, in the BNST of rats measured 1 week or 3 weeks following termination of chronic ethanol (ethanol-dependent) or vehicle (nondependent) administration. Data are expressed as mean (±SEM) values relative to mRNA levels in experimentally-naïve age-matched controls. * p < 0.05, ** p < 0.01, *** p < 0.001.
ppN/OFQ gene expression in the BNST
Increased ppN/OFQ gene expression was observed for both ethanol- and nondependent animals at 1 vs. 3 weeks of abstinence (Figure 3B). These observations were confirmed by ANOVA showing a significant main effect for post-ethanol time (F1,38 = 32.67, p < 0.0001) followed by Newman-Keuls tests that revealed significant differences for both ethanol-dependent and nondependent rats at 1 vs. 3 weeks (p < 0.05). Planned contrasts also revealed that both ethanol- (t19 = 7.65, p < 0.0001) and nondependent (t19 = 13.41, p < 0.0001) animals differed from naïve controls at 1 but not 3 weeks of abstinence.
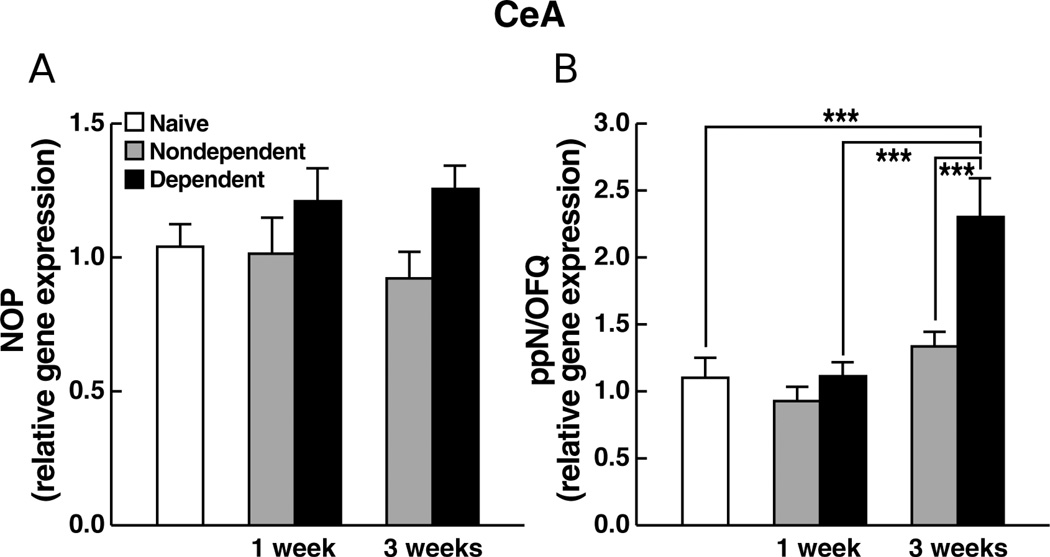
NOP gene expression in the CeA
No significant differences or interactions in NOP gene expression were found between the experimentally naïve, ethanol-dependent and nondependent animals at 1 vs. 3 weeks of abstinence (F1,38 = 0.87; NS). As well, differences between ethanol-dependent and nondependent rats were not statistically different at either 1 or 3 weeks of abstinence (F1,38 = 2.31; NS) (Figure 4A).
Figure 4.
Relative gene expression levels of (A) NOP or (B) ppN/OFQ, in the CeA of rats measured 1 week or 3 weeks following termination of chronic ethanol (ethanol-dependent) or vehicle (nondependent) administration. Data are expressed as mean (±SEM) values relative to mRNA levels in experimentally-naïve age-matched controls. *** p < 0.001.
ppN/OFQ gene expression in the CeA
Ethanol dependent rats showed increased ppN/OFQ mRNA levels compared to both nondependent rats and vehicle controls at the 3-week abstinence point as well as a significant increase vs. ethanol-dependent animals at the 1-week abstinence point (Figure 4B) as reflected by a significant ethanol history × post-ethanol time interaction (F1,38 = 24.45, p < 0.0001). Significant differences between ethanol dependent and nondependent rats at the 3-week abstinence time point, as well as between ethanol-dependent animals at 1 vs. 3 weeks, were confirmed by Newman-Keuls post hoc tests (p < 0.05). Planned contrasts revealed that ethanol dependent rats also differed significantly from experimentally naïve controls at 3 weeks of abstinence (t19 = 6.4, p < 0.0001).
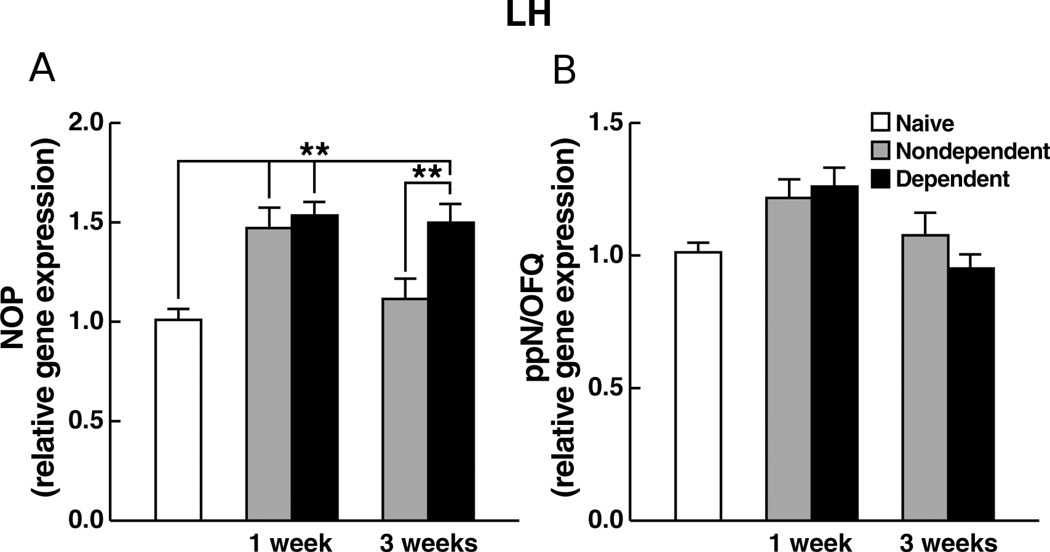
NOP gene expression in the LH
NOP mRNA levels were increased in both ethanol-dependent and nondependent rats at 1 week of abstinence and remained elevated in ethanol-dependent rats at 3 weeks of abstinence (Figure 5A), reflected by a significant main effect of time (F1,44 = 12.34, p < 0.01) and ethanol history × post-ethanol time interaction (F1,44 = 9.60, p < 0.01). Planned comparisons confirmed elevated mRNA levels in both ethanol-dependent (t23 = 11.21, p < 0.0001) and nondependent (t23 = 13.55, p < 0.0001) animals at 1 week vs. naïve controls as well as increased expression levels in ethanol-dependent animals (t23 = 14.5, p < 0.0001) vs. naïve controls at 3 weeks of abstinence. Additionally, Newman-Keuls tests confirmed the increased expression levels in ethanol-dependent over nondependent rats at 3 weeks of abstinence (p < 0.05).
Figure 5.
Relative gene expression levels of (A) NOP or (B) ppN/OFQ, in the LH of rats measured 1 week or 3 weeks following termination of chronic ethanol (ethanol-dependent) or vehicle (nondependent) administration. Data are expressed as mean (±SEM) values relative to mRNA levels in experimentally-naïve age-matched controls. ** p < 0.01.
ppN/OFQ gene expression in the LH
There were no significant differences or interactions among the three experimental conditions in ppN/OFQ gene expression at either 1 or 3 weeks of abstinence (Figure 5B). ANOVA revealed no significant main effects or interaction (F1,44 = 0.77; NS).
Discussion
One week following withdrawal, ethanol dependent rats exhibited increased behavioral manifestations of anxiety in both the elevated plus maze and defensive burying tests, consistent with dysregulation of stress regulatory function (e.g., Martin-Fardon et al., 2010 for review). N/OFQ dose-dependently reversed the exacerbated anxiety-like behaviour observed in ethanol-dependent rats and produced anxiolytic-like effects in nondependent rats at a high (2 µg) dose, similar to the previously reported anxiety-reducing actions of this peptide (Ciccocioppo et al., 2004; Jenck et al., 1997, Kamei et al., 2004; Economidou et al., 2011).
Three weeks following withdrawal, anxiety-like behavior in dependent rats no longer differed from nondependent rats in either the elevated plus maze or defensive burying tests (Fig. 2), suggesting that chronic ethanol- or withdrawal-induced dysregulation of N/OFQ-NOP neurotransmission is transient. However, altered levels of anxiety during protracted abstinence often are not manifest behaviorally until introduction of external stressors (e.g., Zhao et al 2007b; Valdez et al., 2003) or pharmacological manipulations (e.g., Aujla et al., 2008; Sidhpura et al., 2010). This was evident also in the present data where, despite identical “baseline” anxiety-like behavior, N/OFQ produced a markedly divergent dose-response profile in ethanol-dependent vs. nondependent rats, with anxiogenic-like or anxiolytic-like actions depending on ethanol history. In ethanol dependent rats, N/OFQ decreased open arm exploration and increased defensive burying at 1.0 µg, consistent with increased anxiety-like behavior, whereas behavior in nondependent controls was characterized by the same dose-response profile as in the one-week post-ethanol tests, continuing to be consistent with anti-anxiety actions of N/OFQ. While the duration of burying in post-dependent rats decreased to vehicle levels at the 2.0 µg N/OFQ dose, this effect is unlikely to reflect an anxiolytic action because, at this dose, N/OFQ also produced significant freezing behavior that interferes with the burying response. A similar inverse relationship between burying and freezing has been reported in rats treated with the CRF agonist stressin1-A (Zhao et al., 2007b). At a low dose, this anxiogenic agent increased burying without inducing freezing responses, but induced significant freezing at a high dose, with burying behavior returning to vehicle levels (Zhao et al., 2007b). The increased freezing response produced by the 2.0-µg N/OFQ dose in ethanol-dependent rats does not reflect alterations in pain sensitivity since no differences were observed in shock reactivity at this dose compared to animals receiving N/OFQ vehicle. As well, freezing responses associated with the 2.0-µg dose cannot be attributed to general motor impairment, given the lack of group differences in either ambulation in the shock-probe burying test or total number of arm entries in the elevated plus maze test.
The behavioral findings in ethanol-dependent rats at the three-week abstinence time point may reflect neuroplasticity resulting in a distinct “switch” from an anxiolytic to anxiogenic action of N/OFQ or, alternatively, an interaction between time-dependent post-ethanol alterations in N/OFQ-NOP function and biphasic dose-response effects of N/OFQ previously reported in mice (Kamei et al., 2004) and rats (Sandin et al., 2004). For example, in the hole-board test, an animal model of anxiety in mice, a low N/OFQ dose (0.01 nmol) increased head dips, indicative of anxiolytic action, whereas a high dose (5.0 nmol) decreased this behavior, reflective of anxiogenic action (Kamei et al. 2004). Both the anxiolytic- and anxiogenic-like actions of the low vs. high N/OFQ doses were sensitive to reversal by the NOP-functional antagonist nocistatin, confirming that both actions are mediated by NOP (Kamei et al., 2004). Such biphasic dose-response effects may also account for reports of anxiogenic-like effects of N/OFQ in the EPM in rats (Fernandez et al., 2004). However, while biphasic actions of N/OFQ have been observed with respect to motor output (Devine et al., 1996; Florin et al., 1996) and learning (Sandin et al., 2004; Liu et al., 2007), studies examining broad dose ranges that may reveal biphasic effects on anxiety-like performance in rats are lacking. Considering the evidence that N/OFQ can exert anxiety-like actions in EPM tests in rats and produce a biphasic dose-response profile in mice, the emergence of anxiogenic effects at previously anxiolytic N/OFQ doses between the one and three-week post-ethanol period may reflect a leftward shift of a biphasic N/OFQ dose-response profile. However, support for this hypothesis would require documentation of anxiolytic effects at N/OFQ doses of less than 0.5 µg at the three-week post-ethanol time point and this hypothesis remains for further evaluation.
A more likely alternative explanation for the failure to observe a reduction in anxiety-like behavior at the 0.5-µg dose in ethanol-dependent rats is that neuroplasticity associated with a history of ethanol dependence, rather than producing a shift in the dose response profile, leads to a “switch” from anxiolytic to anxiogenic actions of N/OFQ. Indeed, the differential actions of N/OFQ on anxiety-like behavior in ethanol nondependent and dependent rats one week post-ethanol suggest that adaptations in the N/OFQ-NOP system had occurred as a result of dependence induction, followed by further adaptations over the three-week withdrawal period. This hypothesis is tentatively supported by alterations in N/OFQ-NOP function quantified in terms of Prepro-N/OFQ (ppN/OFQ) and NOP gene expression at the two stages of ethanol withdrawal. These findings revealed changes in both ppN/OFQ and NOP gene expression in the BNST, CeA, and LH of ethanol-dependent rats. More specifically, in the CeA, ppN/OFQ gene expression in ethanol-dependent animals was markedly higher three weeks post-withdrawal compared to both nondependent controls and experimentally naïve rats while there were no differences at the one-week abstinence time point (Fig. 4B). The differential ppN/OFQ gene expression time-course in the CeA correlates with the differential behavioral response to N/OFQ at the one and three-week abstinence time points, although the “baseline” anxiety-like behavior of ethanol-dependent animals had returned to levels of nondependent controls at three week post-withdrawal. Thus, at the one-week abstinence time point the status of the N/OFQ-NOP system in CeA was unmodified as revealed by the absence of ppN/OFQ and NOP gene expression alterations in this brain area. Conversely, at three weeks, the increase in ppN/OFQ gene expression in ethanol-dependent rats possibly reflects the presumptive “switch” from anxiolytic to anxiogenic actions of N/OFQ.
It cannot be ruled out that these time-dependent differential N/OFQ actions depend on interactions with stress-regulatory systems. For example, the functionally related extrahypothalamic CRF stress system changes during the transition from acute to protracted abstinence in a manner that parallels changes in anxiety-like behavior (Zorilla et al., 2001; Zhao et al., 2007). Given that a functional antagonist relationship exists between CRF and N/OFQ, with N/OFQ serving an “anti-CRF” role (Ciccocioppo et al., 2002; 2003; 2004), the differential anxiolytic vs. anxiogenic actions of N/OFQ in ethanol-dependent vs. control rats three weeks post-withdrawal possibly reflects the consequences of a concurrent dysregulation in CRF and N/OFQ-NOP neurotransmission. Indeed, CRF release in the CeA is a factor in alcohol withdrawal-related anxiety since blockade of CRF receptor in the CeA attenuate the anxiogenic effects of alcohol withdrawal (Rassnick et al., 1993). Moreover, N/OFQ prevents ethanol-induced increases in GABAergic transmission in the CeA (Roberto and Siggins, 2006). In this structure, GABA- and CRF-mediated mechanisms play a role in motivational effects of ethanol, in anxiety associated with ethanol dependence, and ethanol seeking during abstinence (Rassnick et al., 1993). Therefore, it is plausible that anti-CRF actions of N/OFQ occur in the CeA and that the increased ppN/OFQ gene expression in the CeA at the three week withdrawal time point may represent a neuroadaptive response, possibly CRF-induced, opposing increased post-ethanol anxiety apparent at the one-week withdrawal time point. In other words, at the level of the CeA, the N/OFQ-NOP system may respond to the withdrawal-related increase in anxiety, experienced the first week of abstinence, with an overproduction of ppN/OFQ, which is revealed at 3 weeks of abstinence. This adaptation would increase N/OFQ production to counteract the increase in anxiety occurring at one week withdrawal time point.
The NOP and ppN/OFQ gene expression findings in the CeA are consistent with a recent report that pronociceptin gene expression is significantly increased on the first day of alcohol abstinence, and returns to control levels one-week post withdrawal (D’Addario et al., 2011). This suggests that the N/OFQ-NOP system in the CeA does not play a significant role in withdrawal-related anxiety at this time point of the withdrawal progression. However, the present finding that three weeks post-ethanol ppN/OFQ gene expression was significantly increased in ethanol-dependent rats compared to nondependent and control animals suggest that the ppN/OFQ gene expression changes at one day and three week withdrawal time point may reflect a cascade of neuroadaptive changes triggered by the ethanol abstinence. However, unlike the alterations observed in ppN/OFQ expression, the present study, consistent with D’Addario et al. (2011), revealed no changes of NOP gene expression in the CeA, at these time points (Fig. 4A). While we did not observe any alteration of CeA NOP as a function of ethanol history or withdrawal duration, increased NOP expression has been demonstrated in other studies, including that of Green and Devine (2009) which demonstrated increased NOP mRNA in the CeA in response to acute social defeat. Thus, the lack of increased NOP gene expression in our study, even when the anxiety levels were elevated suggests that these alterations are, at least in part, stimulus-dependent (that is social defeat stress versus ethanol withdrawal triggered anxiety). Also, it is quite possible that brain regions apart from those examined in our paper may play an important role in open arm exploration or defensive burying. Further work in future studies will be required to establish exactly which adaptations contribute to the observed behaviours.
In the LH, ppN/OFQ gene expression was unchanged during abstinence in both experimental groups. However, NOP gene expression was elevated compared to naïve controls at the one-week time point in both ethanol-dependent and nondependent rats. Thus this finding remains inconclusive with respect to providing a mechanism for our behavioral obvservations. While the observed decrease in NOP expression would, at face value, be at odds with the idea of increased sensitivity to N/OFQ, it is not entirely clear that the decreased expression in receptors reflects decreased efficiency at these sites. Gene expression for NOP allows for quantification of the receptors in a steady state situation, but it does not necessarily indicate other types of changes that may occur within the system that may mediate, or contribute to, the observed behavioural alterations (e.g. changes in receptor efficiency as a function of g-protein coupling, alterations in trafficking, etc). Also, it is quite possible that brain regions apart from those examined in our paper may play an important role or that the changes are stimulus specific, as discussed earlier.
However, unlike in nondependent controls, NOP gene expression in the LH remained significantly elevated three weeks post-ethanol in ethanol-dependent compared to experimentally naïve rats (Fig. 5). These changes in LH gene expression can perhaps be explained in the context of the role of CRF in this brain region. CRF-immunopositive terminals in the LH establish excitatory synapses with neurons synthesizing hypocretins (de Lecea et al., 1998; Winsky-Sommerer et al., 2004). Hypocretins participate in regulating ethanol reward and re-establishment of ethanol seeking through actions in the LH and VTA (Dayas et al., 2008; Lawrence et al., 2006). It is plausible that withdrawal associated CRF activation in the LH elevates hypocretin release (Winsky-Sommerer et al., 2004) altering the activity of N/OFQ neurons that have recently been discovered to make synaptic contacts with hypocretin neurons in the LH (Gerashchenko et al., 2011). Indeed, in the LH, N/OFQ blocks stress-induced analgesia via direct inhibition of hypocretin neurons (Gerashchenko et al., 2011). It is therefore reasonable to speculate that an analogous mechanism contribute to anxiety triggered by the ethanol abstinence and that the increased NOP gene expression in the LH may represent an up-regulation to counteract CRF-induced hyperactivity of hypocretin neurons during withdrawal. Thus, the differential elevation of ppN/OFQ gene expression in the CeA at three, but not one week post-withdrawal parallels the differential effects of N/OFQ on anxiety-like behaviour. This alteration, along with the differential upregulation of NOP gene expression in the LH, may possibly represent a factor in the transition of N/OFQ’s actions from anxiolytic to anxiogenic.
In the BNST, ethanol-dependent rats showed increased NOP gene expression at both the one- and three-week week abstinence time points relative to nondependent controls and experimentally naïve rats. The increased NOP gene expression one week post-withdrawal is likely to also be linked in part to CRF-dependent regulation of stress and anxiety responses in the BNST. Stress responses induced by intra-BNST administration of CRF are attenuated by coadministration of N/OFQ (Ciccocioppo et al., 2004a; Ciccocioppo et al 2003). More importantly, intracerebroventricular CRF administration increases NOP receptor expression in the BNST suggesting that stressful stimulation results in elevated NOP expression (Rodi et al., 2008). Thus, the increased NOP gene expression in the BNST of ethanol-dependent rats one week post ethanol, a time point associated with high anxiety-like behavior, may reflect elevated CRF transmission. Inconsistent with this interpretation, however, is the disappearance of anxiety-like behavior in ethanol-dependent animals three weeks post-ethanol in the presence of increased NOP gene expression. Understanding of this discrepancy remains for future research.
What represents a challenge for interpretation is increased ppN/OFQ gene expression in the BNST, as well as increased expression of NOP in the LH, at the one-week time point in nondependent animals vs. naïve controls. At first glance, these alterations appear to be linked to the ethanol administration procedure per se. However, a previous experiment examining this issue failed to find effects of the intubation procedure per se on anxiety-like behavior on the EPM (Braconi et al., 2010). Thus, the increased expression of ppN/OFQ gene expression in the BNST and NOP gene expression in the LH appears to be uncoupled from overt behavioral changes.
In summary, among the many changes in ppN/OFQ and NOP gene expression, only the differential levels of ppN/OFQ transcript in the CeA and of NOP transcript in the LH at the one- and three-weeks post-ethanol paralleled the transition of N/OFQ’s actions from anxiolytic to anxiogenic. Other changes, although substantial, are not clearly conclusive at present with respect to their significance for the differential behavioral effects of N/OFQ at the two post-ethanol time points.
Overall, the results confirm that N/OFQ is effective in attenuating anxiety-like behavior in ethanol nondependent rats, but reveal that N/OFQ produces stronger anxiolytic effects in rats with a history of ethanol dependence during the early postdependence phase, reflective of a heightened sensitivity to N/OFQ. However, during a later post-ethanol stage N/OFQ produces anxiogenic effects. This effect is may be the result of a switch in N/OFQ’s anxiety regulatory function from anxiolytic to anxiogenic irrespective of dose. Changes in ppN/OFQ and NOP gene expression show a different time-course in the CeA, BNST, and LH, providing evidence of a time-related functional role of the N/OFQ-NOP system in these brain areas during ethanol withdrawal. Differential ppN/OFQ gene expression in the CeA paired with differential upregulation of NOP gene expression in the LH may represent a factor in the transition of N/OFQ’s actions from anxiolytic to anxiogenic, although the exact mechanism underlying this switch remains to be clarified. Understanding of these issues will be essential for determining the suitability of the N/OFQ-NOP system as a target for the treatment of alcohol abuse. Several lines of evidence have implicated the NOP receptor as a promising pharmacotherapeutic target for various aspects of the ethanol addiction cycle. These include reduction of ongoing ethanol use (Ciccocioppo et al. 2004b), alleviation of withdrawal symptoms (Economidou et al., 2011), and interference with craving and relapse (Martin-Fardon et al., 2000; Ciccocioppo et al. 2004b) associated with both exposure to stress and ethanol cues (for review, see Martin-Fardon et al., 2010). While the present findings are consistent with this evidence in terms of treatment target promise of the N/OFQ system during early stages of ethanol withdrawal and abstinence, the role and the significance of this system as potential treatment target in long-term craving and relapse prevention requires further clarification, particularly with respect to appropriate dosing. Future studies examining the actions of NOP antagonists on anxiety-related performance in alcohol-dependent animals would be invaluable in differentiating between the alternative adaptations in the NOP-N/OFQ system that underlie the observed behavioural alterations.
Supplementary Material
Table S1. Mean (±SEM) of motor output during the elevated plus maze and shock probe defensive burying tests 1 week following the termination of chronic ethanol (ethanol-dependent) or vehicle (nondependent) administration.
Table S2. Mean (±SEM) of motor output during the elevated plus maze and shock probe defensive burying tests 3 weeks following the termination of chronic ethanol (ethanol-dependent) or vehicle (nondependent) administration. N/OFQ (2 µg) increased freezing in dependent but not in nondependent controls. * p < 0.05 vs. vehicle (0 dose).
Acknowledgements
This research was supported by National Institutes of Health Grant AA014351 from the National Institute on Alcohol Abuse and Alcoholism. We thank D.F. Aschauer for technical support and D. Carretta, MD for significant contribution to the development of the discussion. This is publication 21229 from The Scripps Research Institute.
Footnotes
Disclosure/Conflict of Interest
None of the authors have any conflicts of interest relating to the work described in this manuscript.
Author contributions
HA, R Cannarsa, RM-F and FW were responsible for the study concept and design. HA and RM-F contributed to the acquisition of animal data. R Cannarsa performed the gene expression analysis. HA and R Cannarsa, performed the data analysis and interpretation of findings. HA drafted the manuscript. RM-F, FW, PR, R Cannarsa and R Ciccocioppo provided critical intellectual input. All authors critically reviewed content and approved final version for publication.
References
- Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- Braconi S, Sidhpura N, Aujla H, Martin-Fardon R, Weiss F, Ciccocioppo R. Revisiting Intragastric Ethanol Intubation as a Dependence Induction Method for Studies of Ethanol Reward and Motivation in Rats. Alcohol Clin Exp Res. 2009;34:538–544. doi: 10.1111/j.1530-0277.2009.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calo' G, Rizzi A, Bigoni R, Guerrini R, Salvadori S, Regoli D. Pharmacological profile of nociceptin/orphanin FQ receptors. Clin Exp Pharmacol Physiol. 2002;29:223–228. doi: 10.1046/j.1440-1681.2002.03633.x. [DOI] [PubMed] [Google Scholar]
- Carboni E, Silvagni A, Rolando MT, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci. 2000;20:RC102. doi: 10.1523/JNEUROSCI.20-20-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Biondini M, Antonelli L, Wichmann J, Jenck F, Massi M. Reversal of stress- and CRF-induced anorexia in rats by the synthetic nociceptin/orphanin FQ receptor agonist, Ro 64-6198. Psychopharmacology. 2002;161:113–119. doi: 10.1007/s00213-002-1020-7. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Cippitelli A, Economidou D, Fedeli A, Massi M. Nociceptin/orphanin FQ acts as a functional antagonist of corticotropin-releasing factor to inhibit its anorectic effect. Physiol Behav. 2004a;82:63–68. doi: 10.1016/j.physbeh.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology. 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology. 2004b;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario C, Caputi FF, Rimondini R, Gandolfi O, Del Borrello E, Candeletti S, Romualdi P. Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brain. Addict Biol. 2011 Apr 20; doi: 10.1111/j.1369-1600.2011.00326.x. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Levin N, Walker CD, Bradbury MJ, Suemaru S, Scribner KS. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann. N. Y. Acad. Sci. 1995;746:22–31. doi: 10.1111/j.1749-6632.1994.tb39206.x. [DOI] [PubMed] [Google Scholar]
- Dayas C, McGranahan T, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff T, Peyron C, Gao X, Foye P, Danielson P, Fukuhara C, Battenberg E, Gautvik V, Bartlett Fn, Frankel W, van den Pol A, Bloom F, Gautvik K, Sutcliffe J. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. PNAS. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devine DP, Taylor L, Reinscheid RK, Monsma FJ, Jr, Civelli O, Akil H. Rats rapidly develop tolerance to the locomotor-inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem Res. 1996;21:1387–1396. doi: 10.1007/BF02532380. [DOI] [PubMed] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, et al. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal. Alcohol Clin Exp Res. 2011;35:747–755. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, et al. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez F, Misilmeri MA, Felger JC, Devine DP. Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology. 2004;29:59–71. doi: 10.1038/sj.npp.1300308. [DOI] [PubMed] [Google Scholar]
- Florin S, Suaudeau C, Meunier JC, Costentin J. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur J Pharmacol. 1996;317:9–13. doi: 10.1016/s0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Horvath TL, Xie X. Direct inhibition of hypocretin/orexin neurons in the lateral hypothalamus by nociceptin/orphanin FQ blocks stress-induced analgesia in rats. Neuropharmacology. 2011;60:543–549. doi: 10.1016/j.neuropharm.2010.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MK, Devine DP. Nociceptin/orphanin FQ and NOP receptor gene regulation after acute or repeated social defeat stress. Neuropeptides. 2009;43:507–514. doi: 10.1016/j.npep.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson AC, Cippitelli A, Sommer WH, Fedeli A, Björk K, Soverchia L, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. PNAS. 2006;103:15236–15241. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Beck A, Wrase J, Mohr J, Obermayer K, Gallinat J, et al. Neurotransmitter systems in alcohol dependence. Pharmacopsychiatry. 2009;42(Suppl 1):S95–S101. doi: 10.1055/s-0029-1214395. [DOI] [PubMed] [Google Scholar]
- Huang J, Young B, Pletcher MT, Heilig M, Wahlestedt C. Association between the nociceptin receptor gene (OPRL1) single nucleotide polymorphisms and alcohol dependence. Addict Biol. 2008;13:88–94. doi: 10.1111/j.1369-1600.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- Jenck F, Moreau J-L, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ. Orphanin FQ acts as an anxiolytic to attenuate behavioral responses to stress. PNAS. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei J, Matsunawa Y, Miyata S, Tanaka S, Saitoh A. Effects of nociceptin on the exploratory behavior of mice in the hole-board test. Eur J Pharmacol. 2004;489:77–87. doi: 10.1016/j.ejphar.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology. 2009;56(Suppl 1):18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J Pharmacol Exp Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br. J. Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu EH, Lee TL, Nishiuchi Y, Kimura T, Tachibana S. Nocistatin and its derivatives antagonize the impairment of short-term acquisition induced by nociceptin. Neurosci Lett. 2007;416:155–159. doi: 10.1016/j.neulet.2007.01.066. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Macey DJ, Schulteis G, Heinrichs SC, Koob GF. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol. 1996;13:163–170. doi: 10.1016/0741-8329(95)02030-6. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, N/OFQ/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard JL, Champagne DL, Meaney MJP. Variations of maternal care differentially influence “fear” reactivity and regional patterns of cFos immunoreactivity in response to the shock-probe burying test. Neuroscience. 2004;129:297–308. doi: 10.1016/j.neuroscience.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999a;406:503–547. [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid RK, Nothacker H-P, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J Comp Neurol. 1999b;406:503–547. [PubMed] [Google Scholar]
- Okuda-Ashitaka E, Ito S. Nocistatin: a novel neuropeptide encoded by the gene for the nociceptin/orphanin FQ precursor. Peptides. 2000;21:1101–1109. doi: 10.1016/s0196-9781(00)00247-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th Ed. NY: Academic Press; 2007. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Heinrichs SC, Britton KT, Koob GF. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993;605:25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ, Jr, Civelli O. Structures that delineate orphanin FQ and dynorphin A pharmacological selectivities. J Biol Chem. 1998;273:1490–1495. doi: 10.1074/jbc.273.3.1490. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker H, Civelli O. The orphanin FQ/nociceptin gene: structure, tissue distribution of expression and functional implications obtained from knockout mice. Peptides. 2001;21:901–906. doi: 10.1016/s0196-9781(00)00226-6. [DOI] [PubMed] [Google Scholar]
- Roberto M, Siggins GR. Nociceptin/orphanin FQ presynaptically decreases GABAergic transmission and blocks the ethanol-induced increase of GABA release in central amygdala. PNAS. 2006;103:9715–9720. doi: 10.1073/pnas.0601899103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology. 2008;196:523–531. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Sahuque LL, Kullberg EF, McGeehan AJ, Kinder JR, Hicks MP, Blanton MG, Janak PH, Olive MF. Anxiogenic and aversive effects of corticotropin-releasing factor (CRF) in the bed nucleus of the stria terminalis in the rat: role of CRF receptor subtypes. Psychopharmacology. 2006;186:122–132. doi: 10.1007/s00213-006-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin J, Ogren SO, Terenius L. Nociceptin/orphanin FQ modulates spatial learning via ORL-1 receptors in the dorsal hippocampus of the rat. Brain Res. 2004;997:222–233. doi: 10.1016/j.brainres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biological Psychiatry. 2010;67:804–811. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotrophin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, De Lecea L. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 2004;24:11439–11448. doi: 10.1523/JNEUROSCI.3459-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuei X, Flury-Wetherill L, Almasy L, Bierut L, Tischfield J, Schuckit M, et al. Association analysis of genes encoding the nociceptin receptor (OPRL1) and its endogenous ligand (PNOC) with alcohol or illicit drug dependence. Addict Biol. 2008;13:80–87. doi: 10.1111/j.1369-1600.2007.00082.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Valdez GR, Fekete EM, Rivier JE, Vale WW, Rice KC, et al. Subtype-Selective Corticotropin-Releasing Factor Receptor Agonists Exert Contrasting , but Not Opposite , Effects on Anxiety-Related Behavior in Rats. JPET. 2007a;323:846–854. doi: 10.1124/jpet.107.123208. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcoholism, clinical and experimental research. 2007b;31:1505–1515. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology. 2001;158:374–381. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Mean (±SEM) of motor output during the elevated plus maze and shock probe defensive burying tests 1 week following the termination of chronic ethanol (ethanol-dependent) or vehicle (nondependent) administration.
Table S2. Mean (±SEM) of motor output during the elevated plus maze and shock probe defensive burying tests 3 weeks following the termination of chronic ethanol (ethanol-dependent) or vehicle (nondependent) administration. N/OFQ (2 µg) increased freezing in dependent but not in nondependent controls. * p < 0.05 vs. vehicle (0 dose).