Abstract
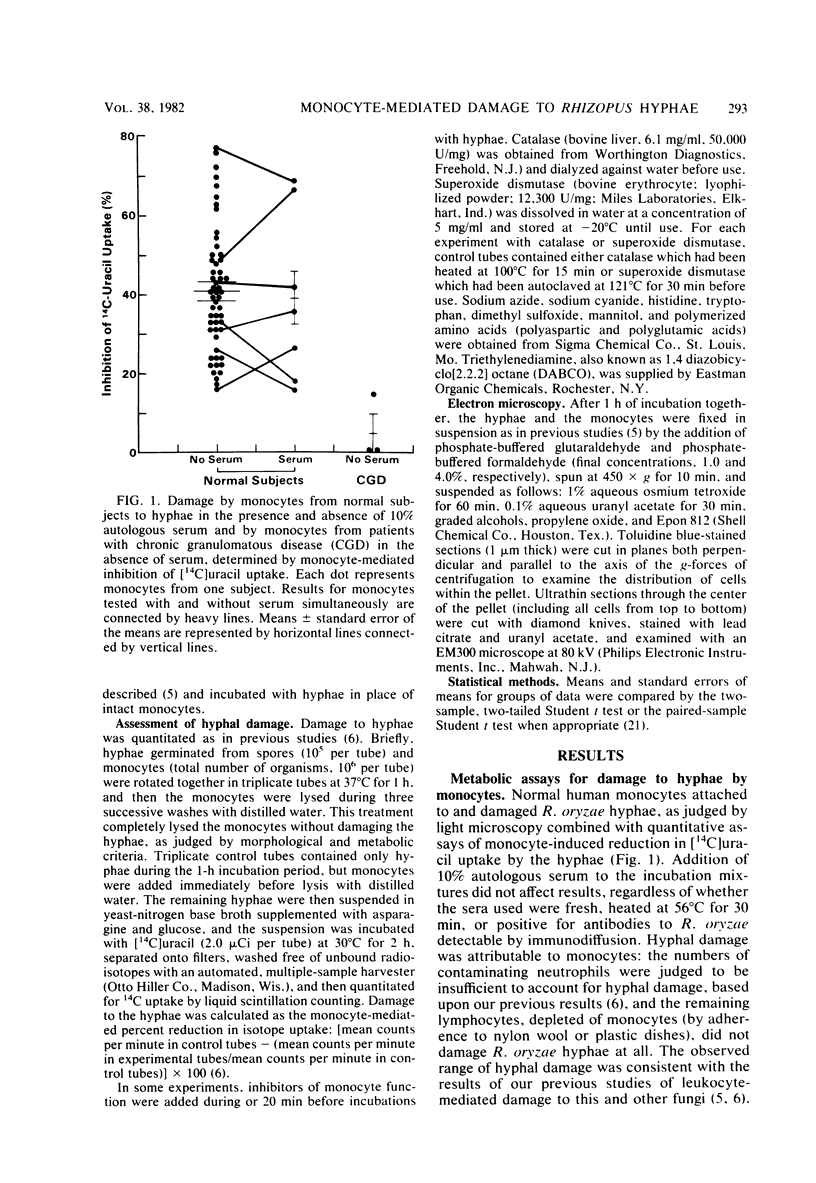
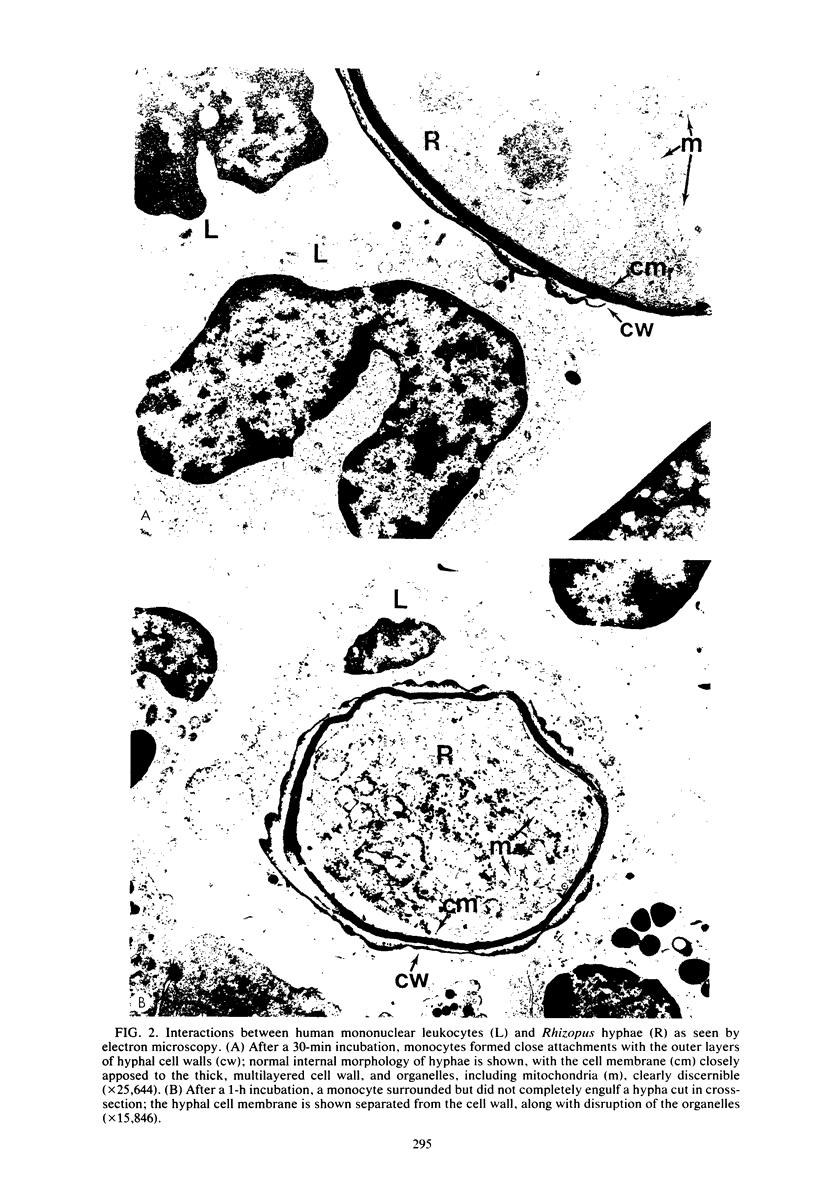
Clinicopathological correlations from human cases and experimental animal studies suggest that neutrophils are critical components of the host response to mucormycosis but that other cellular defense mechanisms appear to be important as well. Since our previous studies demonstrated that Rhizopus oryzae hyphae which are too large to be ingested completely can be damaged and probably killed by human neutrophils, we studied the antihyphal activity of human monocytes. As with neutrophils, light and electron microscopic studies indicated that monocytes attached to hyphae and appeared to destroy them in the absence of serum. As judged by our previously described assay for the leukocyte-induced inhibition of [14C]uracil uptake by hyphae, quantitative damage to hyphae by monocytes was 40.8 +/- 2.2% in 54 experiments. Neither attachment to nor damage of hyphae by monocytes was augmented by the presence of 10% human serum. As with neutrophils, monocyte-mediated damage of R. oryzae was significantly decreased by some inhibitors of oxidative metabolism and scavengers of the potentially microbicidal oxidative leukocyte products, which included 10(-4)M sodium azide, 10 (-3) M sodium cyanide, catalase, 10(-3) M histidine, 10(-3) M tryptophan, and 10(-4) M 1,4-diazobicyclo[2.2.2]octane but not superoxide dismutase, 1.4 X 10(-2) M dimethyl sulfoxide, and 4.0 X 10(-1) M mannitol. Moreover, monocytes from three patients with chronic granulomatous disease failed to damage hyphae at all. In contrast to our previous data for neutrophils, polyanions (10(-5) M polyaspartic or polyglutamic acid) did not inhibit monocyte-mediated hyphal damage. Thus, monocytes can damage and probably kill R. oryzae hyphae by oxidative mechanisms and so may be involved in host defense mechanisms against mucormycosis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUER H., FLANAGAN J. F., SHELDON W. H. Experimental cerebral mucormycosis in rabbits with alloxan diabetes. Yale J Biol Med. 1955 Sep;28(1):29–36. [PMC free article] [PubMed] [Google Scholar]
- BAUER H., FLANAGAN J. F., SHELDON W. H. The effects of metabolic alterations on experimental Rhizopus oryzae (mucormycosis) infection. Yale J Biol Med. 1956 Sep;29(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- BAUER H., SHELDON W. H. Leukopenia with granulocytopenia in experimental mucormycosis (Rhizopus oryzae infection). J Exp Med. 1957 Oct 1;106(4):501–508. doi: 10.1084/jem.106.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Lehrer R. I., Territo M. C., Golde D. W. UCLA Conference. Monocytes and macrophages: functions and diseases. Ann Intern Med. 1978 Jan;88(1):78–88. doi: 10.7326/0003-4819-88-1-78. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Haudenschild C. C. Monocyte-mediated serum-independent damage to hyphal and pseudohyphal forms of Candida albicans in vitro. J Clin Invest. 1981 Jan;67(1):173–182. doi: 10.1172/JCI110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- Drath D. B., Karnovsky M. L. Superoxide production by phagocytic leukocytes. J Exp Med. 1975 Jan 1;141(1):257–262. doi: 10.1084/jem.141.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. E., Watson B. D., Schultz J. Myeloperoxidase and singlet oxygen: a reappraisal. FEBS Lett. 1978 Aug 15;92(2):327–332. doi: 10.1016/0014-5793(78)80780-7. [DOI] [PubMed] [Google Scholar]
- Held A. M., Hurst J. K. Ambiguity associated with use of singlet oxygen trapping agents in myeloperoxidase-catalyzed oxidations. Biochem Biophys Res Commun. 1978 Apr 14;81(3):878–885. doi: 10.1016/0006-291x(78)91433-x. [DOI] [PubMed] [Google Scholar]
- Hodgson E. K., Fridovich I. The production of superoxide radical during the decomposition of potassium peroxochromate(V). Biochemistry. 1974 Aug 27;13(18):3811–3815. doi: 10.1021/bi00715a030. [DOI] [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase: contribution to the microbicidal activity of intact leukocytes. Science. 1970 Sep 11;169(3950):1095–1097. doi: 10.1126/science.169.3950.1095. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I. The fungicidal mechanisms of human monocytes. I. Evidence for myeloperoxidase-linked and myeloperoxidase-independent candidacidal mechanisms. J Clin Invest. 1975 Feb;55(2):338–346. doi: 10.1172/JCI107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson V. L., Hansing R. L., McClary D. O. The role of metabolic energy in the lethal action of basic proteins on Candida albicans. Can J Microbiol. 1977 Feb;23(2):166–174. doi: 10.1139/m77-024. [DOI] [PubMed] [Google Scholar]
- Peterson E. M., Calderone R. A. Inhibition of specific amino acid uptake in Candida albicans by lysosomal extracts from rabbit alveolar macrophages. Infect Immun. 1978 Aug;21(2):506–513. doi: 10.1128/iai.21.2.506-513.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repine J. E., Eaton J. W., Anders M. W., Hoidal J. R., Fox R. B. Generation of hydroxyl radical by enzymes, chemicals, and human phagocytes in vitro. Detection with the anti-inflammatory agent, dimethyl sulfoxide. J Clin Invest. 1979 Dec;64(6):1642–1651. doi: 10.1172/JCI109626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELDON W. H., BAUER H. Activation of quiescent mucormycotic granulomata in rabbits by induction of acute alloxan diabetes. J Exp Med. 1958 Jul 1;108(1):171–178. doi: 10.1084/jem.108.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh H., Vadasz J. A. Singlet oxygen: a major reactive species in the furocoumarin photosensitized inactivation of E. coli ribosomes. Photochem Photobiol. 1978 Oct-Nov;28(4-5):539–545. doi: 10.1111/j.1751-1097.1978.tb06966.x. [DOI] [PubMed] [Google Scholar]
- Slivka A., LoBuglio A. F., Weiss S. J. A potential role for hypochlorous acid in granulocyte-mediated tumor cell cytotoxicity. Blood. 1980 Feb;55(2):347–350. [PubMed] [Google Scholar]
- Weiss S. J., King G. W., LoBuglio A. F. Evidence for hydroxyl radical generation by human Monocytes. J Clin Invest. 1977 Aug;60(2):370–373. doi: 10.1172/JCI108785. [DOI] [PMC free article] [PubMed] [Google Scholar]