Summary
Lymphocytes provide optimal responses against pathogens with minimal inflammatory pathology. However, the intrinsic mechanisms regulating these responses are unknown. Here, we report that deletion of both transcription factors Egr2 and Egr3 in lymphocytes resulted in a lethal autoimmune syndrome with excessive serum proinflammatory cytokines but also impaired antigen receptor-induced proliferation of B and T cells. Egr2- and Egr3-defective B and T cells had hyperactive signal transducer and activator of transcription-1 (STAT1) and STAT3 while antigen receptor-induced activation of transcription factor AP-1 was severely impaired. We discovered that Egr2 and/or Egr3 directly induced expression of suppressor of cytokine signaling-1 (SOCS1) and SOCS3, inhibitors of STAT1 and STAT3, and also blocked the function of Batf, an AP-1 inhibitor, in B and T cells. Thus, Egr2 and Egr3 regulate B and T cell function in adaptive immune responses and homeostasis by promoting antigen receptor signaling and controlling inflammation.
Graphical Abstract
Highlights
► Deletion of Egr2 and Egr3 in lymphocytes results in a lethal autoimmune syndrome ► Deficiency in both Egr2 and Egr3 impairs antigen receptor-induced proliferation ► Egr2 and Egr3 are required for AP-1 activity by blocking Batf ► Egr2 and Egr3 induce expression of SOCS1 and SOCS3
Introduction
The immune system is finely balanced between providing immune responses against infectious pathogens while remaining tolerant to self-antigen (Goodnow et al., 2010; Kitaura et al., 2007; Schwartz, 2003; Sprent and Surh, 2011; von Boehmer and Melchers, 2010). In optimal immune responses, antigen and costimulatory molecules from activated antigen presenting cells (APCs) or helper T cells induce strong mitogenic signals in naive T or B cells, leading to proliferation and differentiation of effector cells; yet these same stimuli also induce suppressors, such as induced regulatory T (iTreg) cells, to prevent excessive immune pathology. Under homeostatic conditions, such as interaction with self-antigens and/or cytokines, T and B cells either do not respond or undergo homeostatic proliferation (Sprent and Surh, 2011; von Boehmer and Melchers, 2010). Recent studies suggest that the control of responses to self-antigen under homeostatic conditions is an active process involving induction of anergic molecules, such as E3 ubiquitin ligases and negative regulators of T cell receptor signaling (Bandyopadhyay et al., 2007; King et al., 2008; Macián et al., 2002; MacDonald et al., 2011; Puga et al., 2008; Thomas et al., 2007). These molecules can effectively reduce activation of MAP kinase and AP-1 transcription factor, the hallmarks of tolerant T cells (Schwartz, 2003). In addition to the control of antigen receptor signaling, the regulation of the concerted action of pro- and anti-inflammatory cytokines is also important for the maintenance of self-tolerance (Davey et al., 2005; MacDonald et al., 2011; Tamiya et al., 2011). Deficiency in suppressor of cytokine signaling-1 (SOCS1) or SOCS3, suppressors of signal transducer and activator of transcription 1 (STAT1)- and STAT3-mediated proinflammatory cytokine signaling, results in the development of severe inflammatory autoimmune syndromes and/or renders the mice susceptible to the induction of autoimmune diseases (Chong et al., 2005; Croker et al., 2004; Davey et al., 2005; Marine et al., 1999; Tamiya et al., 2011).
Egr2 and Egr3 are zinc-finger transcription factors of the early growth response gene (Egr) family (O’Donovan et al., 1999) that have critical functions in hindbrain development and myelination of the peripheral nervous system (Topilko et al., 1994; Tourtellotte and Milbrandt, 1998) and are also involved in the development of T and/or B cells (Lazarevic et al., 2009; Li et al., 2011; Xi et al., 2006). The involvement of Egr2 and Egr3 in the regulation of T cell tolerance was first suggested by the induction of their expression in tolerant B and T cells (Anderson et al., 2006; Harris et al., 2004; Safford et al., 2005). T cell lines overexpressing Egr2 or Egr3 show an upregulation of E3-ligase Cbl-b and reduced production of interleukin-2 (IL-2), suggesting that Egr2 and Egr3 are important for the maintenance of T cell tolerance by negatively regulating T cell activation (Harris et al., 2004; Safford et al., 2005). Previously, we found that Egr2 is expressed in CD44hi effector phenotype T cells under homeostatic conditions and a defect in Egr2 in T cells results in accumulation of interferon-γ (IFN-γ)- and IL-17-producing CD44hiCD4 T cells, leading to the development of a lupus-like syndrome in later life (Zhu et al., 2008). However, Egr2-deficient T cells are not hyperproliferative in response to primary T cell receptor (TCR) stimulation (Zhu et al., 2008). This normal response to TCR engagement could be due to functional compensation by Egr3.
Here, we report that mice with deficiency of both Egr2 and Egr3 in B and T cells developed a lethal and early-onset systemic inflammatory autoimmune syndrome. However, IL-2 production and proliferation of B and T cells in response to mitogenic antigen receptor stimulation in vitro were severely impaired as a result of a defect in AP-1 activity. Our results demonstrate that Egr2 and Egr3 reciprocally control the inflammatory responses and antigen receptor signaling of B and T cells in both homeostasis and antigen receptor-mediated immune responses.
Results
Deficiency in Egr2 and Egr3 in B and T cells Results in Severe Autoimmune Diseases
Previously, we found the development of systemic autoimmunity in CD2-specific Egr2-deficient (CD2-Egr2−/−) mice in later life, but B and T cell responses to antigen receptor stimulation in vitro were unchanged (Zhu et al., 2008), although there were no autoimmune symptoms found in Egr3−/− mice (data not shown). To explore the functions of Egr2 and Egr3 in lymphocytes, we interbred Egr3−/− and CD2-Egr2−/− mice to establish mice (CD2-Egr2−/−Egr3−/−) with defects in both Egr2 and 3 in T and B cells (Figures S1A and S1B available online). In our previous study, we observed incomplete excision of the floxed Egr2 gene in B cells from CD2-specificEgr2−/− mice due to some of these mice being heterozygous for the cre-transgene (Zhu et al., 2008). However, in CD2-Egr2−/−Egr3−/− mice homozygous for the cre-transgene, the Egr2 gene was completely deleted (Figures S1A and S1B). Total numbers of thymocytes and bone marrow B cells in CD2-Egr2−/−Egr3−/−mice were reduced (Figures S2A–S2D). However, the numbers of the major subpopulations of mature T and B lymphocytes in the lymph nodes and spleen of 5-week-old CD2-Egr2−/−Egr3−/− mice were similar to age-matched wild-type mice and the development of Treg cells was unchanged (Figures S3A and S2A). Furthermore, the function of peripheral Treg cells from 5-week-old CD2-Egr2−/−Egr3−/− mice in suppressing the activation of naive CD4+ T cells from wild-type mice was also normal (Figure S3B). However, at 2 months of age, both male and female CD2-Egr2−/−Egr3−/− mice developed a severe systemic autoimmune syndrome with lymphocytic infiltration in multiple organs, high levels of anti-self antibodies and glomerulonephritis (Figures 1A–1D; Figure S3C). Splenomegaly and super-enlarged lymph nodes were among the most prominent features of sick CD2-Egr2−/−Egr3−/− mice with increased numbers of highly activated B and T cells (Figures 1E and 1F; Figure S4A). The CD2-Egr2−/−Egr3−/− mice became moribund at ∼8 months of age because of multiorgan inflammation, which was associated with high levels of serum inflammatory cytokines, including IL-3, IL-6, IL-10, IL-17A, and IL-17F, granulocyte macrophage colony stimulating factor (GM-CSF), and IFN-γ, and large numbers of IL-17- and IFN-γ-producing CD4+ T cells (Figures 2A and 2B; Figures S3D and S4B). Interestingly, although B and T cells from sick mice displayed hyperactivated phenotypes, their proliferation and production of IL-2 were severely impaired in response to antigen receptor stimulation in vitro (Figures 2C–2E), suggesting that the hyperactivated phenotypes were not due to reduced antigen receptor activation thresholds.
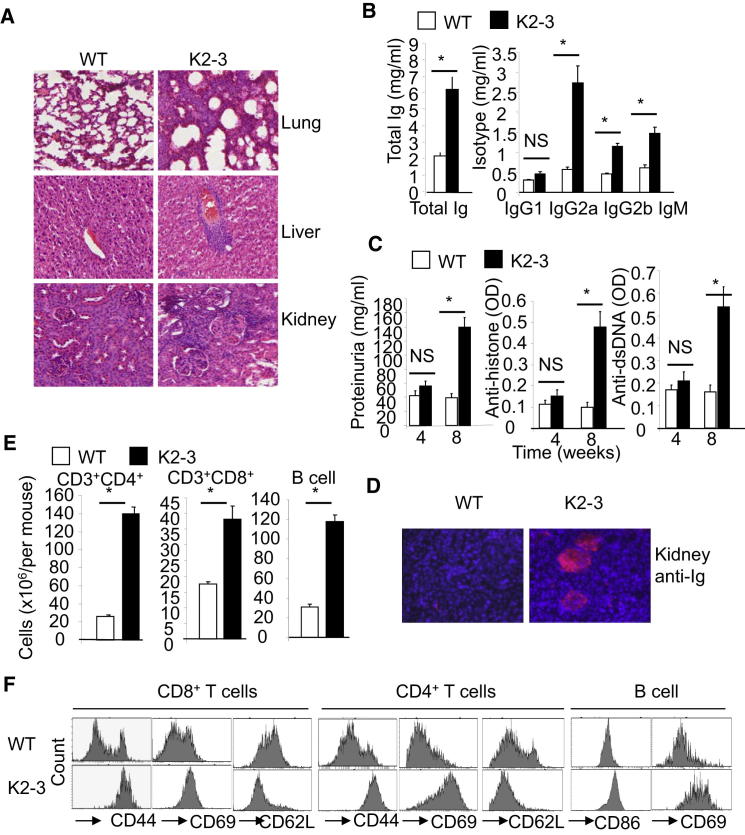
Figure 1.
Mice Lacking Egr2 and Egr3 in B and T cells Develop a Severe Systemic Autoimmune Syndrome
(A) Pathological analysis of lung, liver, and kidney sections from 3-month-old mice showing lymphocytic infiltration in CD2-Egr2−/−Egr3−/− (K2-3) tissues (hematoxylin and eosin [HE]).
(B) Serum Ig in 3-month-old K2-3 and wild-type (WT) mice.
(C) Self-reactive antibodies and urine protein levels in K2-3 and WT mice at the indicated ages.
(D) Glomerular Ig deposits in 3-month-old K2-3 mice (Texas red-labeled anti-mouse Ig).
(E) Absolute numbers of splenic B and T cells in 8-week-old mice.
(F) Surface marker expression on splenic B and T cells from 8-week-old WT and K2-3 mice. Data in (B), (C), and (E) are the mean ± standard deviation from four mice; the remaining data are representative of four mice. NS, not significant; ∗p < 0.05 (unpaired Student’s t test).
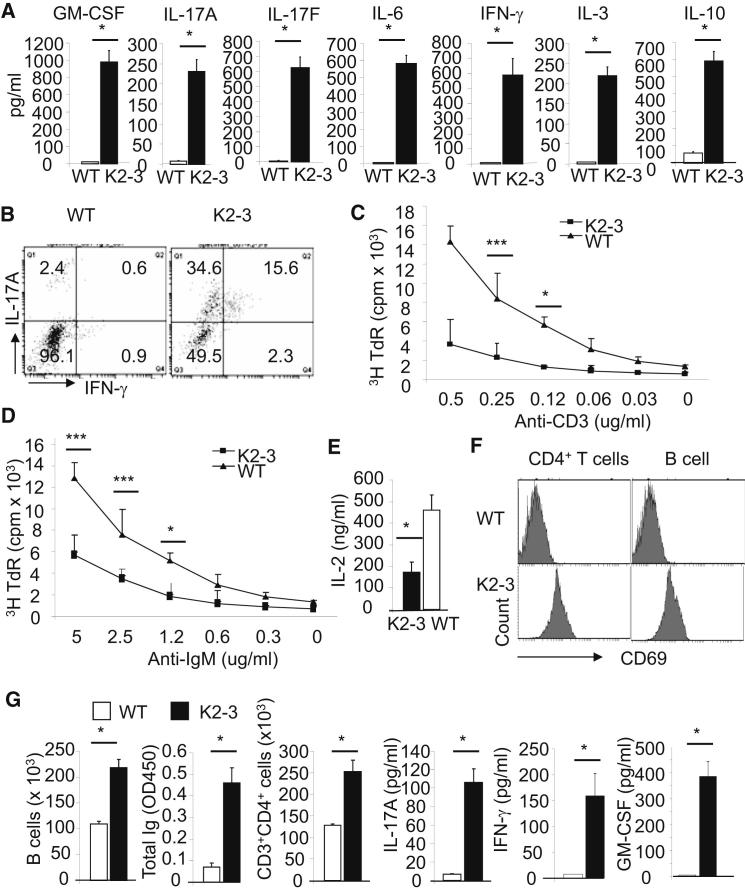
Figure 2.
Egr2 and Egr3 Control the Homeostasis of, and Production of Inflammatory Cytokines by, B and T Cells
(A) Serum cytokine levels in 3-month-old wild-type (WT) and CD2-Egr2−/−Egr3−/− (K2-3) mice.
(B) IL-17A- and IFN-γ-producing CD4+ T cells from spleens of 8-week-old mice.
(C and D) Proliferation of CD4+ T (C) and B cells (D) from 3-month-old mice, as measured by 3H-TdR incorporation, after in vitro stimulation with anti-CD3 and anti-CD28, for CD4+ T cells, or anti-IgM, for B cells, for 3 days.
(E) Production of IL-2 by CD4+ T cells after in vitro stimulation with anti-CD3 and anti-CD28 for 24 hr.
(F and G) Naive CD4 or B cells from spleens of 4-week-old WT and K2-3 mice were adoptively transferred into Rag2−/− mice at 1 × 106 cells/per mouse. Six weeks after transfer, the spleen and lymph nodes were harvested and surface marker expression (F), cell numbers and serum immunoglobulin and cytokines (G) were analyzed. Data in (A), (C)–(E), and G are the mean ± standard deviation from four mice; the remaining data are representative of two independent experiments. NS, not significant; ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.005 (unpaired Student’s t test).
To determine whether these functions of Egr2 and Egr3 were cell intrinsic, we transferred either resting B or naive CD4+ T cells from 4-week-old wild-type or CD2-Egr2−/−Egr3−/− mice, before the onset of lymphadenopathy and the signs of autoimmunity, into Rag2−/− mice (Figure 1C; Figures S3C and S3D). Six weeks after transfer, the B and T cells from wild-type mice largely displayed a resting phenotype in Rag2−/− recipient mice, whereas Egr2- and Egr3-deficient B and CD4+ T cells in Rag2−/− recipient mice were activated and produced high levels of serum Ig and cytokines, respectively (Figures 2F and 2G). A similar activated phenotype on Egr2- and Egr3-deficient, but not wild-type, CD4+ T and B cells was observed in Rag2−/− recipient mice, which received a 50:50 mixture of lymphocytes from 4-week-old CD45.1+ wild-type and CD45.2+ CD2-Egr2−/−Egr3−/− mice (Figure S4D). Furthermore, the percentages of Egr2- and Egr3-deficient B and T cells were increased 6 weeks after transfer with 62% CD2-Egr2−/−Egr3−/− versus 38% wild-type CD4+ T cells, and 71% CD2-Egr2−/−Egr3−/− versus 29% wild-type B cells. Thus, Egr2 and Egr3 regulate the homeostasis of both B and T cells in a cell-intrinsic manner.
Egr2 and Egr3 Are Required for Antigen Receptor-Induced B and T cell Proliferation and the Control of Inflammatory Cytokine Production
Egr2 and Egr3 have been found to negatively regulate T cell activation in vitro (Harris et al., 2004; Safford et al., 2005). To confirm this, we isolated naive B and T cells from spleens of CD2-Egr2−/−Egr3−/− mice before the onset of autoimmune symptoms were stimulated with anti-IgM, or anti-CD3 and anti-CD28, respectively. To our surprise, defects in Egr2 and Egr3 resulted in severely impaired B and T cell activation as demonstrated by defects in proliferation and production of IL-2 (Figures 3A–3C), whereas the proliferation of B and T cells with a single defect in either Egr2 or Egr3 was normal (Figure S5). Conversely, B and CD4+ T cells constitutively expressing Egr2, from CD2-specific Egr2 transgenic (Egr2 cTg) mice (Li et al., 2011), showed enhanced proliferative responses and increased IL-2 production in response to antigen receptor stimulation (Figures 3A–3C; Figure S5). The impaired proliferative responses of Egr2- and Egr3-deficient B and T cells were not due to increased cell death given that apoptosis was normal (Figure S5). Our results demonstrate that Egr2 and Egr3 are required for efficient proliferation of naive B and T cells in response to mitogenic antigen receptor stimulation.
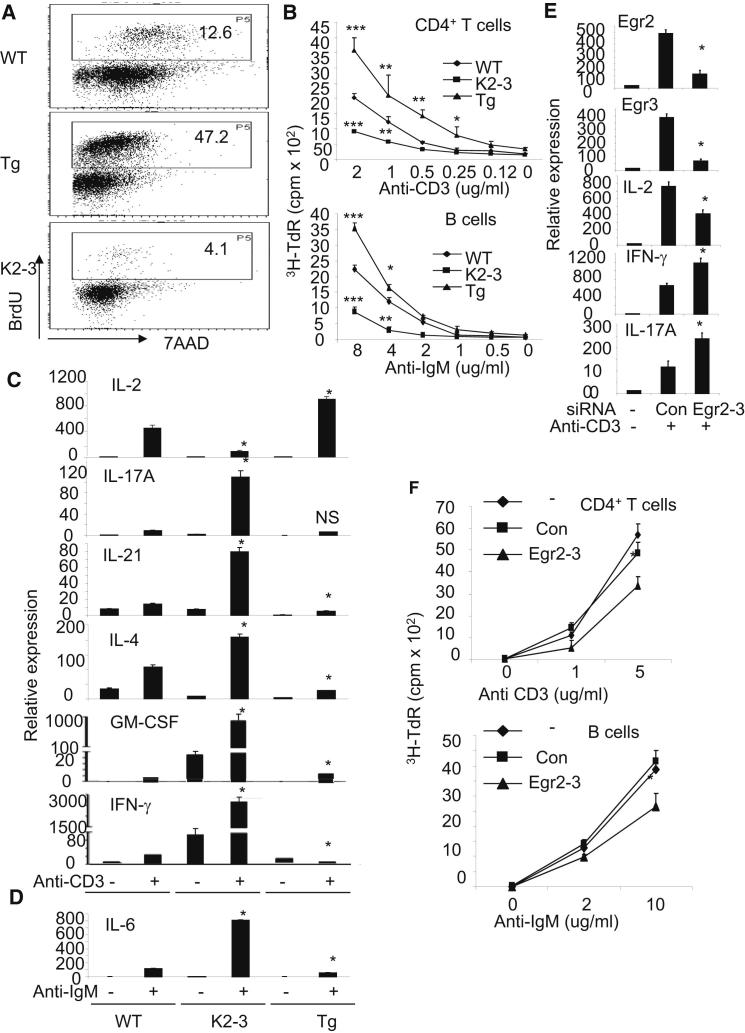
Figure 3.
Lack of Egr2 and Egr3 Results in Impaired Responses to Antigen Receptor Stimulation, and Excessive Production of Inflammatory Cytokines In Vitro
(A) BrdU incorporation by naive splenic CD4+ T cells from 4-week-old wild-type (WT), CD2-Egr2−/−Egr3−/− (K2-3) and Egr2 cTg (Tg) mice after stimulation in vitro for 3 days with anti-CD3 and anti-CD28.
(B) Proliferation of naive CD4+ T and B cells, as measured by 3H-TdR incorporation, after in vitro stimulation with anti-CD3 and anti-CD28, for CD4+ T cells or anti-IgM, for B cells, for 3 days.
(C) mRNA expression levels of cytokines in naive CD4+ T cells after stimulation with anti-CD3 and anti-CD28 for 16 hr as measured by RT-PCR. Note that the y axes in the IFN-γ and GM-CSF plots have been broken.
(D) mRNA expression of IL-6 in resting B cells after stimulation with anti-IgM for 16 hr as measured by RT-PCR.
(E) Expression of Egr2, Egr3, and cytokines in CD4+ T cells from WT mice after transfection with siRNAs against Egr2 and Egr3.
(F) Proliferation of CD4+ T and B cells from WT mice after transfection with siRNAs against Egr2 and Egr3. Results in (C)–(E) are presented relative to the expression of β-actin mRNA. Data are representative of three (A–D) or two (E and F) independent experiments. ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.005 (unpaired Student’s t test).
In contrast to the defects in proliferation and IL-2 expression, mitogenic antigen receptor stimulation induced high amounts of expression of inflammatory cytokines, including IL-1β (Figure S4C), IL-4, IL-17A, IL-21, GM-CSF, and IFN-γ in CD4+ T cells and IL-6 in B cells (Figures 3C and 3D), which is consistent with the inflammatory cytokines detected in the serum of CD2-Egr2−/−Egr3−/− mice (Figure 2). Conversely, B and CD4+ T cells overexpressing Egr2, from Egr2 transgenic (Egr2 cTg) mice, showed reduced inflammatory cytokine production (Figures 3C and 3D). To determine whether the reduced antigen receptor-induced proliferation of lymphocytes from CD2-Egr2−/−Egr3−/− mice is due to the inflammatory environment, we isolated CD45.1+ wild-type and CD45.2+Egr2−/−Egr3−/− B and CD4+ T cells from the same Rag2−/− recipient mouse 6 weeks after transfer for proliferative responses in vitro. Egr2- and Egr3 -deficient, but not wild-type, B and CD4+ T cells displayed defective antigen receptor-driven proliferation (Figure S4E), again suggesting again an intrinsic role for Egr2 and Egr3 in the regulation of antigen receptor-induced proliferation. To exclude the possibility that the impaired responses to antigen receptor stimulation were due to alterations in lymphocyte development, we silenced Egr2 and Egr3 in wild-type CD4+ and B cells (Figure S1C, Figure 3E). The reduction of Egr2 and Egr3 expression resulted in defective proliferation and IL-2 production, but increased production of inflammatory cytokines, in response to antigen receptor stimulation (Figures 3E and 3F), consistent with the alterations seen in Egr2- and Egr3-deficient CD4+ T and B cells. Our results demonstrate that Egr2 and Egr3 are required for both optimal B and T cell proliferation and the control of inflammatory cytokine expression.
Egr2 and/or Egr3 Control STAT1 and STAT3 Activation and Regulate the Expression of SOCS1 and SOCS3
The impaired proliferation of, and IL-2 production by, CD4+ T cells after antigen receptor stimulation indicated that the excessive production of inflammatory cytokines by Egr2- and Egr3-deficient B and T cells was not due to hyperactivation of antigen receptor signaling pathways. We therefore analyzed the major cytokine signaling pathways in B and T cells. We found hyperactivation of STAT3 in B and T cells in the lymph nodes of 3-month-old CD2-Egr2−/−Egr3−/− mice (Figure 4A). Next, we examined the activation of STAT1, STAT3, and STAT5 in naive B and T cells isolated from 4-week-old CD2-Egr2−/−Egr3−/− mice. We found that B and CD4+ T cells from CD2-Egr2−/−Egr3−/− mice showed increased activation of STAT1 and STAT3, but not STAT5 (data not shown), in response to antigen receptor stimulation in vitro, compared with B and T cells from wild-type mice (Figure 4B). Previously, we and others found that SOCS1 and SOCS3, suppressors of STAT1 and STAT3 activation, can be induced in T cells by TCR stimulation (Anderson et al., 2003; Diehl et al., 2000). To investigate whether Egr2 and/or Egr3 can regulate expression of SOCS molecules, we analyzed the expression of SOCS1, SOCS2, and SOCS3 in naive CD4+ T and B cells from 4-week-old mice after antigen receptor stimulation. The expression of SOCS1 and SOCS3, but not SOCS2, was highly induced in B and T cells from Egr2 cTg mice whereas, conversely, deficiency in Egr2 and Egr3 resulted in defective expression of SOCS1 and SOCS3 (Figure 4C). To determine the importance of impaired expression of SOCS1 and SOCS3 to the phenotypes observed in Egr2- and Egr3-deficient CD4+ T cells, we transduced SOCS1 or SOCS3 into naive Egr2- and Egr3-deficient CD4+ T cells by using SOCS-encoding lentiviruses. Previously, we demonstrated that the SOCS3 lentivirus can efficiently suppress activation of STAT3 (Miao et al., 2006). Indeed, expression of SOCS1 reduced IFN-γ production in Egr2- and Egr3-deficient CD4+ T cells, whereas SOCS3 suppressed STAT3 activation and production of IL-17A (Figures 4D and 4E). Next, we analyzed the proximal promoters of SOCS1 and SOCS3 for potential Egr binding sites. We discovered a number of potential Egr2 binding sites in the conserved intergenic elements of the SOCS3 locus and the promoters of the SOCS1 and SOCS3, but not SOCS2, genes (Figure S6). Chromatin immunoprecipitation assays (ChIPs) demonstrated that Egr2 directly interacted with the SOCS1 and SOCS3 promoters and a conserved intergenic element in the SOCS3 locus (Figure 4F). To determine whether Egr2 can trans-activate the SOCS1 and SOCS3 promoters, we cloned the proximal promoter regions of SOCS1 and SOCS3 into basic luciferase reporter constructs and measured the promoter activity in the presence of Egr2. The results showed that Egr2 induced increased SOCS1 and SOCS3 promoter activity (Figure 4G). Thus, we have discovered a function of Egr2 and/or Egr3: direct regulation of SOCS1 and SOCS3 expression that may be important to control the activation of STAT1- and STAT3-mediated cytokine signaling in B and T cells in both homeostatic conditions and mitogenic antigen receptor-mediated responses.
Figure 4.
Dysregulation of STAT1 and STAT3 Pathways in Egr2- and Egr3-deficient B and T Cells
(A) Phosphorylation of STAT3 in B and T cells in lymph nodes from wild-type (WT) and CD2-Egr2−/−Egr3−/− (K2-3) mice at 3 months of age.
(B and C) Phosphorylation of STAT1 and STAT3 (B) and expression of SOCS1, SOCS2, and SOCS3 (C) in naive CD4+ T or B cells from 4-week-old WT and K2-3 mice after stimulation with anti-CD3 and anti-CD28 antibodies, for CD4, or anti-IgM, for B cells for 6 hr.
(D) Expression of cytokines and SOCS3 or SOCS1 in CD4+ T cells from K2-3 mice after transduction with SOCS1- or SOCS3-lentivirus-IRES-GFP. After lentivirus infection, CD4 T cells were stimulated with anti-CD3 and anti-CD28 for 16 hr. The transduced cells were isolated by cell sorting for GFP-positive cells. Lentivirus-IRES-GFP served as a control.
(E) Phosphorylation of STAT3 in Egr2- and Egr3-deficient CD4+ T cells transduced with SOCS3-lentivirus.
(F) Binding of Egr2 to the proximal regions of the SOCS1 and SOCS3 promoters. CD4 T cells from Egr2 transgenic mice (Egr2cTg) were stimulated with anti-CD3 and anti-CD28 for 3 hr and used in a chromatin immunoprecipitation assay (CHIP) with primers flanking Egr binding sites in the promoters of SOCS1, SOCS3, and Nab. Total input DNA and anti-H3 precipitates served as positive controls and anti-Ig precipitates as a negative control.
(G) Activity of the SOCS1 and SOCS3 promoters. Control pGL3 (p) or SOCS1 (S1) or SOCS3 (S3) reporter gene constructs were transfected into HEK293 cells in the presence of Egr2 (E) or GFP control (G). Luciferase activity was measured 24 hr after transfection. Results in (C) and (D) are presented relative to the expression of β-actin mRNA. Data are representative of three (A–C and G) or two (D–F) independent experiments. ∗p < 0.05 (unpaired Student’s t test).
Egr2 and Egr3 Are Required for the Activation of the AP-1 Transcription Factor in Naive B and T cells after Antigen Receptor Signaling
In contrast to the increased activation of STAT1 and STAT3 and excessive production of proinflammatory cytokines, the proliferation of, and production of IL-2 by, naive B and/or T cells from CD2-Egr2−/−Egr3−/− mice was severely impaired in response to mitogenic antigen receptor stimulation (Figure 3). Therefore, we next examined the antigen receptor signaling pathways in naive B and CD4+ T cells isolated from CD2-Egr2−/−Egr3−/− mice before the onset of autoimmune disease. The activation of most of the intracellular signaling molecules, including MAP kinases, demonstrated by phosphorylation of Erk, and the transcription factors NF-κB and NFAT in naive B and CD4+ T cells from CD2-Egr2−/−Egr3−/− and Egr2 cTg mice in response to antigen receptor ligation in vitro was not altered compared with B and T cells from wild-type controls (Figures 5A and 5B). However, the activation of AP-1 was severely impaired in Egr2- and Egr3-deficient CD4+ T and B cells (Figures 5A and 5B). Conversely, overexpression of Egr2 increased AP-1 DNA binding activity in CD4+ T and B cells (Figures 5A and 5B). To further confirm the importance of Egr2 and Egr3 for efficient AP-1 activation, we carried out an AP-1 reporter gene assay. Consistent with the DNA binding results, anti-CD3 and anti-CD28 induced AP-1 reporter gene activity was reduced in CD4+ T cells from CD2-Egr2−/−Egr3−/− mice, as well as in wild-type CD4+ T cells after silencing Egr2 and Egr3 (Figures 5C and 5D). These results demonstrate that Egr2 and Egr3 are required for antigen receptor-induced AP-1 activation, which is essential for the production of IL-2 and cell cycle progression in T and B cells (Foletta et al., 1998; Rincón and Flavell, 1994).
Figure 5.
Lack of Egr2 and Egr3 Selectively Impairs AP-1 Activation in B and T Cells
(A) Naive CD4+ T and resting B cells were stimulated with anti-CD3 and anti-CD28 or anti-IgM. Then, the cytoplasmic extracts were analyzed for Erk phosphorylation, using anti-phospho-Erk antibody, while the nuclear extracts were analyzed for the activation of AP-1, NF-κB and NFAT by EMSA using their respective consensus DNA probes. The arrows indicate the alternative complex in CD2-Egr2−/−Egr3−/− (K2-3) samples that bound to the AP-1 DNA probe. B. Quantitation of AP-1 band intensities on a phosphorimager after normalization relative to the intensity of an internal loading control, SP1. C. AP-1 reporter gene assay in CD4 cells from wild-type and K2-3 mice. After transfection with an AP-1 reporter gene (AP-1-Luc), CD4+ T cells were stimulated with anti-CD3 and anti-CD28 before AP-1 activity was assessed by luciferase assay. D. AP-1 reporter gene assay in wild-type CD4 cells after co-transfection with siRNAs against Egr2 and 3, and AP-1-Luc. The transfected cells were stimulated with anti-CD3 and anti-CD28 before AP-1 activity was assessed by luciferase assay. E. EMSA supershift analysis, using the indicated antibodies, of nuclear extracts from CD4+ T cells stimulated with anti-CD3 and anti-CD28 using the consensus AP-1 DNA probe. Data are representative of three (A), (B), (D) or two (C), and (E) independent experiments. ∗p < 0.05, ∗∗p < 0.01 (unpaired student’s t test).
Interestingly, although electrophoretic mobility shift assay (EMSA) analysis showed a defect in the authentic AP-1 complex bound to the AP-1 DNA probe, an alternative molecular complex, which migrated more quickly than the traditional AP-1 complex, was detected in the nuclear extracts from Egr2- and Egr3-deficient, but not wild-type or Egr2 overexpressing, B and CD4+ T cells (Figures 5A and 5B). The conventional AP-1 complex is a dimer of cFos and Jun. However, the complex in Egr2- and Egr3-deficient cells was not affected by anti-cFos, but was effectively supershifted by anti-JunB (Figure 5E). It has been found recently that Batf, a member of the AP-1 family of transcription factors that is induced in CD4+ T cells after stimulation, forms a heterodimer with the transcription factors c-Jun and/or JunB, which binds to the consensus AP-1 DNA probe and migrates more quickly than the conventional AP-1 complex in EMSA (Macián et al., 2001; Schraml et al., 2009). Batf has multiple functions in the regulation of B and T cells, including inhibition of AP-1 activation (Quigley et al., 2010; Williams et al., 2001), inducing Th2 and Th17 cell derived cytokines (Betz et al., 2010; Schraml et al., 2009), and regulating T follicular helper (Tfh) cells and B cell function (Betz et al., 2010; Ise et al., 2011). Therefore, we considered the possibility that Batf may be part of this alternative AP-1 DNA binding complex in Egr2- and Egr3-deficient B and T cells.
Egr2 Blocks Batf DNA Binding and Batf Function in Suppression of AP-1 Activation
To test whether Egr2 is involved in the regulation of Batf function, we first examined the expression of Batf in B and T cells. We found that Batf, Egr2, and Egr3 were expressed in CD44hi T cells (Figure S7B). However, antigen receptor stimulation induced transient expression of Egr2 and Egr3, but a sustained expression of Batf in naive CD4+ T and B cells (Figures 6A–6C). The Batf expression was not influenced by the constitutive expression or absence of Egr2 or Egr3 (Figure S7A), leading us to conclude that Egr2 and Egr3 do not regulate Batf transcription.
Figure 6.
Egr2 Interacts with Batf and Blocks Its Binding to the AP-1 DNA Consensus Probe
(A–C) mRNA expression levels of Batf (A) and Egr2 and Egr3 (C) as measured by RT-PCR, and protein levels of Batf in nuclear extracts (B) from naive CD4+ T or B cells from wild-type mice after stimulation with anti-CD3 and anti-CD28 or anti-IgM. Results in (A) and (C) (mean and SEM) are presented relative to the expression of β-actin mRNA.
(D) HEK293 cells transfected with Flag-tagged Egr2 or Flag-tagged Egr2 and Myc-tagged Batf were lysed. The lysates were used for immunoprecipitation with anti-Flag-tag or anti-Myc-tag reagent. The precipitates were immunoblotted with anti-Myc and anti-Egr2, for anti-Flag precipitates, or with anti-Flag and anti-Batf, for anti-Myc precipitates. Immunoblotting with anti-Egr2 on total lysates served as loading controls.
(E) EMSA supershift analysis, using the indicated antibodies, of nuclear extracts from HEK293 cells transfected with the indicated genes using the consensus AP-1 DNA probe.
(F) Coimmunoprecipitation of Egr2 and Batf in CD4 T cells from Egr2 cTg mice after stimulation with anti-CD3 and anti-CD28 for 16 hr. Data are representative of two (B, D, and F) or three (A, C, and E) independent experiments.
We next examined the interplay between Batf and Egr2 proteins by cotransfection of these molecules into HEK293 cells (Figure S7C). We discovered a physical interaction between Batf and Egr2 (Figure 6D), which blocked the binding of Batf to the AP-1 DNA probe (Figure 6E). This interaction between Batf and Egr2 was also detected in CD4+ T cells from Egr2 cTg mice (Figure 6F). To investigate whether Batf suppresses AP-1 activation in Egr2- and Egr3-deficient B and T cells, we silenced Batf in wild-type and Egr2- and Egr3-deficient B and T cells in vitro (Figures 7A and 7B). We found that silencing of Batf expression in activated CD4+ T and B cells from CD2-Egr2−/−Egr3−/− mice restored the activation of AP-1, proliferation, and production of IL-2 (Figures 7C and 7D). In addition, reduction of Batf expression reduced the production of the inflammatory cytokine IL-17A by Egr2- and Egr3-deficient CD4+ T cells (Figure 7E). Taken together, these findings demonstrate that suppression of Batf function by Egr2 and/or Egr3 in activated B and T cells is important for AP-1 activation and the control of IL-17 expression.
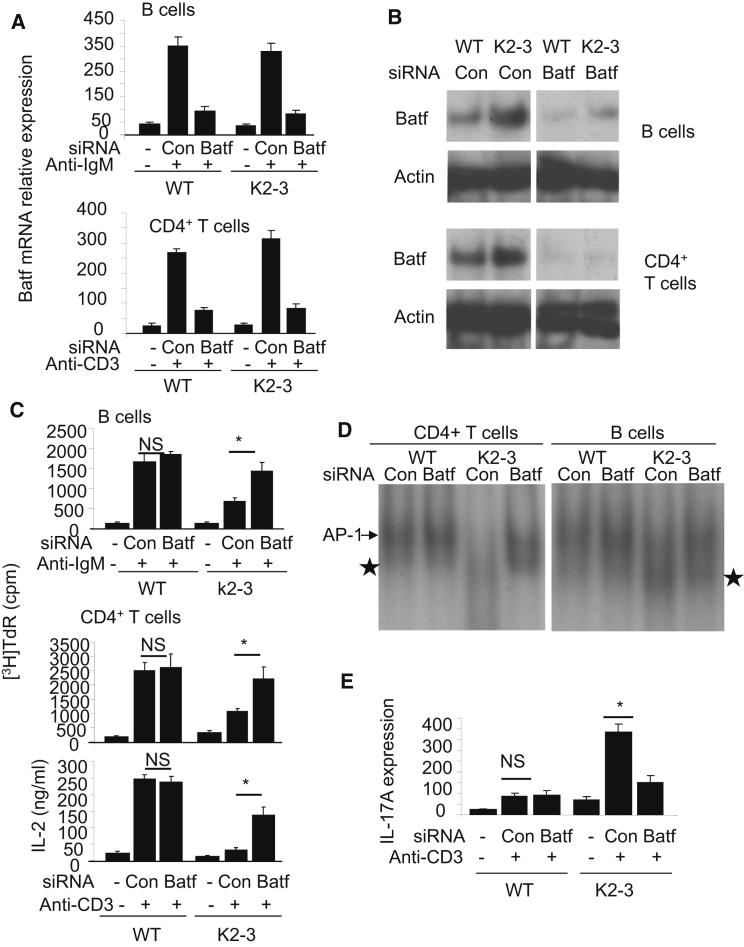
Figure 7.
Silencing of Batf in Egr2- and Egr3-Deficient B and T Cells Restores AP-1 Activation and Proliferative Responses and Reduces IL-17 Expression
(A and B) CD4+ T or CD19+ B cells from wild-type (WT) or CD2-Egr2−/−Egr3−/− (K2-3) mice were transfected with control siRNA (con) or Batf-specific siRNA (Batf). The transfected cells were stimulated with anti-CD3 and anti-CD28, for CD4, or anti-IgM, for CD19 cells, for 16 hr before analysis of Batf mRNA (A) or protein (B).
(C) Proliferation of, and IL-2 expression by, CD4+ T or CD19+ B cells from WT or K2-3 mice transfected with control or Batf siRNA after stimulation with anti-CD3 and anti-CD28 or anti-IgM for 48 hr.
(D) EMSA analysis of nuclear extracts from control or Batf siRNA-transfected CD4+ T or CD19+ B cells from WT or K2-3 mice with the consensus AP-1 DNA probe after stimulation with anti-CD3 and anti-CD28 for CD4+ T or anti-IgM for CD19+ B cells.
(E) IL-17A mRNA expression by CD4+ T cells from WT or K2-3 mice transfected with control or Batf siRNA after stimulation with anti-CD3 and anti-CD28 for 16 hr. Results in (A) and (E) are presented relative to the expression of β-actin mRNA. ∗p < 0.05 (unpaired Student’s t test). Data are representative of three (A–C) or two (D–F) independent experiments.
Discussion
In this study, we demonstrated that Egr2 and Egr3 have major regulatory functions in B and T cells for both the control of inflammation and antigen receptor-induced proliferation in a cell-intrinsic manner. Deficiency in both Egr2 and Egr3 in B and T cells resulted in lethal inflammatory autoimmune disease. However, in contrast to other molecules required for the maintenance of immune self-tolerance such as E3 ligases (Bandyopadhyay et al., 2007; King et al., 2008; Kitaura et al., 2007; Macián et al., 2002; Puga et al., 2008; Thomas et al., 2007; Zhu and Paul, 2010), which suppress B and T cell activation by downregulation of antigen receptor signaling, Egr2 and Egr3 promoted antigen receptor signaling and proliferation of B and T cells in response to mitogenic antigen receptor stimulation. Despite the impaired proliferation and IL-2 production of Egr2- and Egr3-deficient B and T cells, antigen receptor stimulation induced excessive production of inflammatory cytokines. These results demonstrate that Egr2 and Egr3 uncouple the proliferation of naive B and T cells from production of inflammatory cytokines during mitogenic antigen stimulation, which may be important for providing optimal adaptive immune responses with minimum immunopathology. The transfer of either naive B or CD4+ T cells from CD2-Egr2−/−Egr3−/− mice into Rag2−/− mice resulted in the development of inflammation in recipient mice demonstrating that Egr2 and Egr3 function via cell-intrinsic mechanisms to control inflammatory autoimmune responses of both B and T cells.
Egr2 and Egr3 have been found to be involved in the development of natural killer T (NKT) cells and thymocytes (Lazarevic et al., 2009; Xi et al., 2006). We found a reduction of thymocytes and bone marrow B cell precursors in CD2-Egr2−/−Egr3−/− mice. However, the subpopulations of mature B and T cells in the periphery were unchanged. The reduced thymocytes may be due to defects at the early stages of thymocyte development as observed for Egr3-deficient thymocytes (Xi et al., 2006). Nevertheless, the defective proliferation and enhanced production of inflammatory cytokines in wild-type B and T cells after silencing of Egr2 and Egr3 demonstrate that the developmental defects in B and T cell precursors are not responsible for the observed phenotype of lymphocytes from CD2-Egr2−/−Egr3−/− mice.
Egr2 and Egr3 are induced in both naive and tolerant T cells (Anderson et al., 2006; Harris et al., 2004; Safford et al., 2005). Silencing of Egr2 in T cells in vitro results in resistance to anergy induction (Harris et al., 2004) suggesting that Egr2 and Egr3, induced in response to TCR stimulation in the absence of costimulatory signals, play important roles in the maintenance of immune self-tolerance (Harris et al., 2004; Safford et al., 2005). However, although Egr2- and Egr3-deficient B and T cells in CD2-Egr2−/−Egr3−/− mice were increased in number, displayed hyperactivated phenotypes, and produced excessive levels of proinflammatory cytokines, the B and T cells from diseased mice showed defective responses to antigen receptor stimulation in vitro, with impaired proliferation and defective IL-2 production, suggesting that the induction of autoimmunity in CD2-Egr2−/−Egr3−/− mice is not due to reduced antigen receptor activation thresholds, but rather is related to the uncontrolled production of inflammatory cytokines and dysregulated responses to proinflammatory cytokines.
The overproduction of inflammatory cytokines by B and T cells in CD2-Egr2−/−Egr3−/− mice was associated with the hyperactivation of STAT1 and STAT3, the major inflammatory cytokine pathways controlling the development of Th1 and Th17 cells and expression of inflammatory cytokines, indicating that Egr2 and Egr3 play important roles in the control of inflammatory cytokine signaling under homeostatic conditions. We have demonstrated that Egr2 and Egr3 directly regulated SOCS1 and SOCS3 expression and deficiency in Egr2 and Egr3 resulted in reduced expression of SOCS1 and SOCS3. SOCS1 and SOCS3 are essential regulators for the control of STAT1- and STAT3-mediated cytokine expression and differentiation of Th1 and Th17 cells (Tamiya et al., 2011).
Deficiency in SOCS1 results in severe lymphopenia, activation of peripheral T cells, and multiorgan inflammation resulting in death at an early age (Marine et al., 1999). SOCS1 deficient T cells are hypersensitive to multiple cytokines resulting in IFN-γ production in the absence of TCR ligation (Chong et al., 2005). SOCS3 is essential for the control of Th17 cell differentiation by desensitizing STAT3 to activating cytokines, such as IL-23, thereby controlling the expression of Th17 cell-derived cytokines (Chen et al., 2006; Tanaka et al., 2008). Deficiency of SOCS1 or SOCS3 specifically in lymphoid and myeloid cells results in the development of inflammatory diseases (Chong et al., 2005; Croker et al., 2004). However, although deficiency of SOCS1 specifically in T cells results in increased IFN-γ production and enhanced development of Th1 cells, the mice do not develop spontaneous inflammatory pathologies and SOCS1 deficient T cells are resistant to Th17 cell differentiation (Tanaka et al., 2008). A T cell specific SOCS3 defect renders the cells more susceptible to Th17 cell differentiation, but also increases IL-10 production and reduces Th1 cell polarization (Taleb et al., 2009). These findings suggest that inflammatory pathology, which normally results from pathological functions of both Th1 and Th17 cell-derived cytokines, is controlled by both SOCS1 and SOCS3. We have now demonstrated that both SOCS1 and SOCS3 are induced in B and T cells in response to antigen receptor stimulation. Although we cannot rule out additional mechanisms contributing to the lethal inflammatory autoimmune responses in CD2-Egr2−/−Egr3−/− mice, the excessive production of Th1 and Th17 cell-derived cytokines and activation of STAT1 and STAT3 in Egr2- and Egr3-deficient B and T cells suggest that regulation of SOCS1 and SOCS3 expression by Egr2 and Egr3 is vital, not only for preventing the development of autoimmune diseases, but also for limiting immunopathology during productive adaptive immune responses. Previously, we found that Egr2 regulates expression of p21CIP1 (Zhu et al., 2008), which was supported by a recent study (Pospisil et al., 2011). Although the expression of p21CIP1 is also reduced in Egr2- and Egr3-deficient CD4+ T cells after T cell receptor stimulation, the level of reduction is similar to that in CD4+ T cells deficient in Egr2 alone, suggesting that the severe early-onset inflammatory autoimmunity in CD2-Egr2−/−Egr3−/− mice is not due to decreased expression of p21CIP1.
An important, but unexpected, function of Egr2 and Egr3 is the positive regulation of antigen receptor-induced proliferation. Egr2 and Egr3 have previously been considered to be negative regulators of T cell receptor signaling and T cell activation that function to induce expression of E3 ligases, leading to degradation of T cell receptor signaling molecules (Harris et al., 2004; Safford et al., 2005). However, naive T cells lacking either Egr2 or Egr3 did not show increased activation in response to antigen receptor stimulation (Zhu et al., 2008. In contrast, deficiency in both Egr2 and Egr3 resulted in impaired proliferation of naive B and T cells in response to antigen receptor stimulation. Collectively, these data suggest that Egr2 and Egr3 may play different roles in naive and anergic T cells. In anergic T cells, MAP kinase, AP-1, and NF-κB are suppressed while NFAT is activated because of partial antigen receptor stimulation. Therefore, suppression of Batf by Egr2 and Egr3 in anergic T cells does not enhance AP-1 activity; instead, Egr2 and Egr3 function to maintain the tolerance of anergic T cells by induction of E3 ligases (Harris et al., 2004; Safford et al., 2005).
We have now demonstrated that Egr2 and Egr3 have an overlapping function that is required for the induction of AP-1 activity in B and T cells in response to antigen receptor stimulation, which is mediated by directly blocking the function of Batf, a suppressor of AP-1 (Quigley et al., 2010; Williams et al., 2001). In addition to restoring AP-1 activity and proliferative responses, silencing of Batf in Egr2- and Egr3-deficient CD4+ T cells also reduced expression of IL-17, consistent with previous findings on the role of Batf in IL-17 expression (Betz et al., 2010; Schraml et al., 2009), suggesting that suppression of Batf function by Egr2 and/or Egr3 also contributes to the control of inflammatory pathology.
The expression of Egr2 and Egr3 in resting B and T cells induced by antigen receptor stimulation was transient, whereas Batf expression was sustained for much longer. The differential expression kinetics of Egr2 and Egr3 and Batf implies a model in which the transient repression of Batf function by Egr2 and Egr3 only occurs at the early stages of antigen-mediated responses, thus allowing the expansion of activated B and T cells, whereas the subsequent rapid cessation of Egr2 and Egr3 expression leads to the restoration of Batf function, thereby facilitating the production of antibodies and effector cytokines.
Recently, it has been reported that deletion of JunB and/or c-Jun specifically in epithelial cells results in skin inflammation leading to a systemic lupus erythematosus (SLE)-like syndrome with increased expression of serum proinflammatory cytokines and a myeloproliferation disorder (Guinea-Viniegra et al., 2009; Meixner et al., 2008; Pflegerl et al., 2009; Zenz et al., 2005), suggesting that normal and controlled AP-1 activity under homeostatic conditions is important for preventing inflammation and maintaining skin homeostasis. In addition to our previous finding of Egr2 expression in CD44hi effector phenotype T cells (Zhu et al., 2008), Egr-3 is also expressed in CD44hi T cells under homeostatic conditions, Whether the function of c-Jun and/or JunB is regulated by Egr2 and/or Egr3 in effector phenotype lymphocytes in response to homeostatic stimulation and, if so, the importance of this for the control of inflammatory cytokine expression in B and T cells has yet to be investigated.
The impaired antigen receptor-induced proliferation in vitro but inflammatory activity in vivo discovered in Egr2- and Egr3-deficient B and T cells resemble the findings from B and T cells in both lupus patients and lupus models, which show hyperinflammatory activity in vivo and yet defective AP-1 activation and IL-2 expression in vitro (Crispín et al., 2011; Jenks and Sanz, 2009; Rauen et al., 2011), supporting the notion that dysregulation of Egr2 and Egr3, reported recently in both lupus models and lupus patients (Myouzen et al., 2010; Sela et al., 2008), may play a part in the development of lupus-like systemic autoimmune diseases. Our results uncover an intrinsic regulatory mechanism mediated by Egr2 and Egr3 in both B and T cells for the regulation of immune homeostasis and antigen-specific immune responses.
Experimental Procedures
Mice
Egr3−/− and CD2-specific Egr2−/− (CD2-Egr2−/−Egr3−/−) mice were generated by interbreeding Egr3−/− (Tourtellotte and Milbrandt, 1998) and CD2-specific Egr2−/− mice (Zhu et al., 2008) on the C57BL/6 background. All mice were maintained in the Animal Unit, Brunel University, and used in accordance with established guidelines of institutional ethical committee under the authority of a UK Home Office project license (Guidance on the Operation of Animals, Scientific Procedures Act 1986).
Proliferation
For measuring proliferation, purified CD4+ T or B cells (5 × 104 cells/200 μl) in 96-well plates were stimulated in triplicate. A total of 1 μCi of [3H]TdR was added for the last 8 hr of culture, and the cells were then harvested and subjected to scintillation counting to measure [3H]TdR incorporation. Alternatively, a total of 10 uM BrdU was added for the last hour of culture, and cells were then harvested and stained with PE-conjugated anti-BrdU antibody and 7AAD with the BrdU flow kit (BD Biosciences). The percentage of T cells that had incorporated BrdU was analyzed by flow cytometry.
EMSA
The consensus probes for AP-1 (5′-AGCTTCGCTTGATGAGTCAGCCG-3′), NFκB (5′-CAGAGGGGACTTTCCGAGA-3′), SP1 (5′-ATTCGATCGGGGCGGGGCCAG-3′), and NFAT (5′- CTGTATCAAACAAATTTTCCTCTTTGG-3′) were labeled with [α-32P]dCTP using Ready-to-Go DNA labeling beads (Amersham Biosciences UK Ltd., Pollards Wood, Bucks) and used in binding reactions with nuclear extracts from lymphocytes stimulated with anti-CD3 and anti-CD28, for CD4+ T cells, or anti-IgM, for B cells, for 24 hr, and then restimulated for 30 min (Zhu et al., 2008). For supershift reactions anti-Myc, anti-cFos and anti-JunB were added after 10 min of incubation. The samples were electrophoresed on 5% polyacrylamide gels in 0.5× TBE. The gels were processed and then exposed to X-ray film or analyzed on a Storm 860 PhosphorImager (Molecular Dynamics). The intensity of the AP-1 band was normalized relative to the activity of SP1.
ChIP Assays
ChIP assays were performed in accordance with the protocol supplied with the Kit (cat 9003) from Cell Signaling Technology. In brief, 5 × 107 CD4+ T cells from Egr2 cTg mice were stimulated with anti-CD3 and anti-CD28 for 3 hr. The cells were then crosslinked with 1% formaldehyde for 10 min at room temp. After quenching of formaldehyde with 125 mM glycine, the cell nuclei were collected for nuclease digestion. The fragmented chromatin was ∼300–1000 bp as analyzed on agarose gels. After preclearing, chromatin (500 μg) was subjected to each immunoprecipitation with specific anti-Egr2 Ab (Covance) or anti-histone 3 Ab as positive control or anti-Ig as negative control at 4°C overnight. Immunocomplexes were recovered by incubation with blocked protein A beads. DNA was purified in accordance with the kit and used as template for PCR with specific primers SOCS1 (Chr 16, 7786214 –7786320) sense (5′-CTTCAAAGGAAGCCTAAGGCG-3′) and antisense (5′-CCACGTAGTAAGAGTGCAGAG-3′), SOCS3-1 (Chr 11, 29364759 –29364951) sense (5′-CAAGGATTTCACAAACGCCTG-3′) and antisense (5′-GAGAGGCCTGTAGTACACCA-3′), SOCS3-2 (Chr 11, 29371868 –29372072) sense (5′-CCAACTTCTCATTCACACTTTCC-3′) and antisense (5′-TACATGAGGACCTCGGAGTG-3′), and Nab2 sense (5′-ATAGCTCGGCCTCGGTCAC-3′) and antisense (5′-GGACTCAAGAATCGGGCTC-3′).
Messenger RNA Silencing
Specific short interfering oligonucleotides (siRNA) against the mRNA sequence of BATF, Egr2 and Egr3: siBatf 2, 5′-GAACGCAGCUCUCCGCAAA-3′; siBatf 4, 5′-GGACUCAUCUGAUGAUGUG-3′, siEgr2-1, GCUGCUAUCCAGAAGGUAU-3′; siEgr2-2, 5′-CGACCUCGAAAGUACCCUA-3′, and siEgr3-1, 5′-GCGACUCGGUAGCCCAUUA-3′; and siEgr3-2, 5′-GCAGUUUGCUAAAUCAAUU-3′, were used. Irrelevant scrambled siRNAs obtained from QIAGEN, CAT: 1027281, were used as negative controls. Primary naive CD4+ T and resting B cells isolated from 4-week-old mice were transfected with an Amaxa Nucleofector in accordance with the manufacturer’s instructions with 1 μM siRNA and assayed no more than 72 hr later. The Batf siRNAs sustained specific reductions in Batf expression for at least 72 hr, as monitored by RT-PCR, with BATF siRNA2 more effective than BATF siRNA4. Therefore, BATF siRNA2 was used throughout this study.
Adoptive Transfer
A total of 106 naive CD4+ T or resting B cells from 4-week-old mice were suspended in 100 μl of physiological saline and injected i.v. into the dorsal tail vein of female Rag2−/− mice at 12 weeks of age. Two or six weeks after transfer, CD4+ T and B cells were isolated from the spleen, and serum antibodies and cytokines were measured.
Acknowledgments
We thank J. Milbrandt for providing Egr3−/− mice, G. Warnes for cell sorting, and J. Walker for animal experiments. This work was supported by Arthritis Research UK.
Published online: September 27, 2012
Footnotes
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and one table can be found with this article online at http://dx.doi.org/10.1016/j.immuni.2012.08.001.
Supplemental Information
References
- Anderson P., Sundstedt A., Li L., O’Neill E.J., Li S., Wraith D.C., Wang P. Differential activation of signal transducer and activator of transcription (STAT)3 and STAT5 and induction of suppressors of cytokine signalling in T(h)1 and T(h)2 cells. Int. Immunol. 2003;15:1309–1317. doi: 10.1093/intimm/dxg130. [DOI] [PubMed] [Google Scholar]
- Anderson P.O., Manzo B.A., Sundstedt A., Minaee S., Symonds A., Khalid S., Rodriguez-Cabezas M.E., Nicolson K., Li S., Wraith D.C., Wang P. Persistent antigenic stimulation alters the transcription program in T cells, resulting in antigen-specific tolerance. Eur. J. Immunol. 2006;36:1374–1385. doi: 10.1002/eji.200635883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S., Duré M., Paroder M., Soto-Nieves N., Puga I., Macián F. Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood. 2007;109:2878–2886. doi: 10.1182/blood-2006-07-037754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz B.C., Jordan-Williams K.L., Wang C., Kang S.G., Liao J., Logan M.R., Kim C.H., Taparowsky E.J. Batf coordinates multiple aspects of B and T cell function required for normal antibody responses. J. Exp. Med. 2010;207:933–942. doi: 10.1084/jem.20091548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Laurence A., Kanno Y., Pacher-Zavisin M., Zhu B.M., Tato C., Yoshimura A., Hennighausen L., O’Shea J.J. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong M.M., Metcalf D., Jamieson E., Alexander W.S., Kay T.W. Suppressor of cytokine signaling-1 in T cells and macrophages is critical for preventing lethal inflammation. Blood. 2005;106:1668–1675. doi: 10.1182/blood-2004-08-3049. [DOI] [PubMed] [Google Scholar]
- Crispín J.C., Apostolidis S.A., Finnell M.I., Tsokos G.C. Induction of PP2A Bβ, a regulator of IL-2 deprivation-induced T-cell apoptosis, is deficient in systemic lupus erythematosus. Proc. Natl. Acad. Sci. USA. 2011;108:12443–12448. doi: 10.1073/pnas.1103915108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croker B.A., Metcalf D., Robb L., Wei W., Mifsud S., DiRago L., Cluse L.A., Sutherland K.D., Hartley L., Williams E. SOCS3 is a critical physiological negative regulator of G-CSF signaling and emergency granulopoiesis. Immunity. 2004;20:153–165. doi: 10.1016/s1074-7613(04)00022-6. [DOI] [PubMed] [Google Scholar]
- Davey G.M., Starr R., Cornish A.L., Burghardt J.T., Alexander W.S., Carbone F.R., Surh C.D., Heath W.R. SOCS-1 regulates IL-15-driven homeostatic proliferation of antigen-naive CD8 T cells, limiting their autoimmune potential. J. Exp. Med. 2005;202:1099–1108. doi: 10.1084/jem.20050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl S., Anguita J., Hoffmeyer A., Zapton T., Ihle J.N., Fikrig E., Rincón M. Inhibition of Th1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- Foletta V.C., Segal D.H., Cohen D.R. Transcriptional regulation in the immune system: all roads lead to AP-1. J. Leukoc. Biol. 1998;63:139–152. doi: 10.1002/jlb.63.2.139. [DOI] [PubMed] [Google Scholar]
- Goodnow C.C., Vinuesa C.G., Randall K.L., Mackay F., Brink R. Control systems and decision making for antibody production. Nat. Immunol. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- Guinea-Viniegra J., Zenz R., Scheuch H., Hnisz D., Holcmann M., Bakiri L., Schonthaler H.B., Sibilia M., Wagner E.F. TNFalpha shedding and epidermal inflammation are controlled by Jun proteins. Genes Dev. 2009;23:2663–2674. doi: 10.1101/gad.543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J.E., Bishop K.D., Phillips N.E., Mordes J.P., Greiner D.L., Rossini A.A., Czech M.P. Early growth response gene-2, a zinc-finger transcription factor, is required for full induction of clonal anergy in CD4+ T cells. J. Immunol. 2004;173:7331–7338. doi: 10.4049/jimmunol.173.12.7331. [DOI] [PubMed] [Google Scholar]
- Ise W., Kohyama M., Schraml B.U., Zhang T., Schwer B., Basu U., Alt F.W., Tang J., Oltz E.M., Murphy T.L., Murphy K.M. The transcription factor BATF controls the global regulators of class-switch recombination in both B cells and T cells. Nat. Immunol. 2011;12:536–543. doi: 10.1038/ni.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks S.A., Sanz I. Altered B cell receptor signaling in human systemic lupus erythematosus. Autoimmun. Rev. 2009;8:209–213. doi: 10.1016/j.autrev.2008.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C.G., Buckler J.L., Kobayashi T., Hannah J.R., Bassett G., Kim T., Pearce E.L., Kim G.G., Turka L.A., Choi Y. Cutting edge: requirement for TRAF6 in the induction of T cell anergy. J. Immunol. 2008;180:34–38. doi: 10.4049/jimmunol.180.1.34. [DOI] [PubMed] [Google Scholar]
- Kitaura Y., Jang I.K., Wang Y., Han Y.C., Inazu T., Cadera E.J., Schlissel M., Hardy R.R., Gu H. Control of the B cell-intrinsic tolerance programs by ubiquitin ligases Cbl and Cbl-b. Immunity. 2007;26:567–578. doi: 10.1016/j.immuni.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarevic V., Zullo A.J., Schweitzer M.N., Staton T.L., Gallo E.M., Crabtree G.R., Glimcher L.H. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat. Immunol. 2009;10:306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Symonds A.L., Zhu B., Liu M., Raymond M.V., Miao T., Wang P. Early growth response gene-2 (Egr2) regulates the development of B and T cells. PLoS ONE. 2011;14:e18498. doi: 10.1371/journal.pone.0018498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T.T., Monteleone I., Fantini M.C., Monteleone G. Regulation of homeostasis and inflammation in the intestine. Gastroenterology. 2011;140:1768–1775. doi: 10.1053/j.gastro.2011.02.047. [DOI] [PubMed] [Google Scholar]
- Macián F., López-Rodríguez C., Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20:2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- Macián F., García-Cózar F., Im S.H., Horton H.F., Byrne M.C., Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- Marine J.C., Topham D.J., McKay C., Wang D., Parganas E., Stravopodis D., Yoshimura A., Ihle J.N. SOCS1 deficiency causes a lymphocyte-dependent perinatal lethality. Cell. 1999;98:609–616. doi: 10.1016/s0092-8674(00)80048-3. [DOI] [PubMed] [Google Scholar]
- Meixner A., Zenz R., Schonthaler H.B., Kenner L., Scheuch H., Penninger J.M., Wagner E.F. Epidermal JunB represses G-CSF transcription and affects haematopoiesis and bone formation. Nat. Cell Biol. 2008;10:1003–1011. doi: 10.1038/ncb1761. [DOI] [PubMed] [Google Scholar]
- Miao T., Wu D., Zhang Y., Bo X., Subang M.C., Wang P., Richardson P.M. Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J. Neurosci. 2006;26:9512–9519. doi: 10.1523/JNEUROSCI.2160-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myouzen K., Kochi Y., Shimane K., Fujio K., Okamura T., Okada Y., Suzuki A., Atsumi T., Ito S., Takada K. Regulatory polymorphisms in EGR2 are associated with susceptibility to systemic lupus erythematosus. Hum. Mol. Genet. 2010;19:2313–2320. doi: 10.1093/hmg/ddq092. [DOI] [PubMed] [Google Scholar]
- O’Donovan K.J., Tourtellotte W.G., Millbrandt J., Baraban J.M. The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience. Trends Neurosci. 1999;22:167–173. doi: 10.1016/s0166-2236(98)01343-5. [DOI] [PubMed] [Google Scholar]
- Pflegerl P., Vesely P., Hantusch B., Schlederer M., Zenz R., Janig E., Steiner G., Meixner A., Petzelbauer P., Wolf P. Epidermal loss of JunB leads to a SLE phenotype due to hyper IL-6 signaling. Proc. Natl. Acad. Sci. USA. 2009;106:20423–20428. doi: 10.1073/pnas.0910371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisil V., Vargova K., Kokavec J., Rybarova J., Savvulidi F., Jonasova A., Necas E., Zavadil J., Laslo P., Stopka T. Epigenetic silencing of the oncogenic miR-17-92 cluster during PU.1-directed macrophage differentiation. EMBO J. 2011;30:4450–4464. doi: 10.1038/emboj.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga I., Rao A., Macian F. Targeted cleavage of signaling proteins by caspase 3 inhibits T cell receptor signaling in anergic T cells. Immunity. 2008;29:193–204. doi: 10.1016/j.immuni.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley M., Pereyra F., Nilsson B., Porichis F., Fonseca C., Eichbaum Q., Julg B., Jesneck J.L., Brosnahan K., Imam S. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat. Med. 2010;16:1147–1151. doi: 10.1038/nm.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen T., Benedyk K., Juang Y.T., Kerkhoff C., Kyttaris V.C., Roth J., Tsokos G.C., Tenbrock K. A novel intronic CREM promoter is regulated by AP-1 and accounts for altered activation-induced CREM expression in T cells from patients with systemic lupus erythematosus. J. Biol. Chem. 2011;286:32366–32372. doi: 10.1074/jbc.M111.245811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincón M., Flavell R.A. AP-1 transcriptional activity requires both T-cell receptor-mediated and co-stimulatory signals in primary T lymphocytes. EMBO J. 1994;13:4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safford M., Collins S., Lutz M.A., Allen A., Huang C.T., Kowalski J., Blackford A., Horton M.R., Drake C., Schwartz R.H., Powell J.D. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat. Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- Schraml B.U., Hildner K., Ise W., Lee W.L., Smith W.A., Solomon B., Sahota G., Sim J., Mukasa R., Cemerski S. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R.H. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Sela U., Dayan M., Hershkoviz R., Lider O., Mozes E. A peptide that ameliorates lupus up-regulates the diminished expression of early growth response factors 2 and 3. J. Immunol. 2008;180:1584–1591. doi: 10.4049/jimmunol.180.3.1584. [DOI] [PubMed] [Google Scholar]
- Sprent J., Surh C.D. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat. Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleb S., Romain M., Ramkhelawon B., Uyttenhove C., Pasterkamp G., Herbin O., Esposito B., Perez N., Yasukawa H., Van Snick J. Loss of SOCS3 expression in T cells reveals a regulatory role for interleukin-17 in atherosclerosis. J. Exp. Med. 2009;206:2067–2077. doi: 10.1084/jem.20090545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamiya T., Kashiwagi I., Takahashi R., Yasukawa H., Yoshimura A. Suppressors of cytokine signaling (SOCS) proteins and JAK/STAT pathways: regulation of T-cell inflammation by SOCS1 and SOCS3. Arterioscler. Thromb. Vasc. Biol. 2011;31:980–985. doi: 10.1161/ATVBAHA.110.207464. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ichiyama K., Hashimoto M., Yoshida H., Takimoto T., Takaesu G., Torisu T., Hanada T., Yasukawa H., Fukuyama S. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J. Immunol. 2008;180:3746–3756. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]
- Thomas R.M., Chunder N., Chen C., Umetsu S.E., Winandy S., Wells A.D. Ikaros enforces the costimulatory requirement for IL2 gene expression and is required for anergy induction in CD4+ T lymphocytes. J. Immunol. 2007;179:7305–7315. doi: 10.4049/jimmunol.179.11.7305. [DOI] [PubMed] [Google Scholar]
- Topilko P., Schneider-Maunoury S., Levi G., Baron-Van Evercooren A., Chennoufi A.B., Seitanidou T., Babinet C., Charnay P. Krox-20 controls myelination in the peripheral nervous system. Nature. 1994;371:796–799. doi: 10.1038/371796a0. [DOI] [PubMed] [Google Scholar]
- Tourtellotte W.G., Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat. Genet. 1998;20:87–91. doi: 10.1038/1757. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Melchers F. Checkpoints in lymphocyte development and autoimmune disease. Nat. Immunol. 2010;11:14–20. doi: 10.1038/ni.1794. [DOI] [PubMed] [Google Scholar]
- Williams K.L., Nanda I., Lyons G.E., Kuo C.T., Schmid M., Leiden J.M., Kaplan M.H., Taparowsky E.J. Characterization of murine BATF: a negative regulator of activator protein-1 activity in the thymus. Eur. J. Immunol. 2001;31:1620–1627. doi: 10.1002/1521-4141(200105)31:5<1620::aid-immu1620>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Xi H., Schwartz R., Engel I., Murre C., Kersh G.J. Interplay between RORgammat, Egr3, and E proteins controls proliferation in response to pre-TCR signals. Immunity. 2006;24:813–826. doi: 10.1016/j.immuni.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Zenz R., Eferl R., Kenner L., Florin L., Hummerich L., Mehic D., Scheuch H., Angel P., Tschachler E., Wagner E.F. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–375. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- Zhu J., Paul W.E. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B., Symonds A.L., Martin J.E., Kioussis D., Wraith D.C., Li S., Wang P. Early growth response gene 2 (Egr-2) controls the self-tolerance of T cells and prevents the development of lupuslike autoimmune disease. J. Exp. Med. 2008;205:2295–2307. doi: 10.1084/jem.20080187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.