Abstract
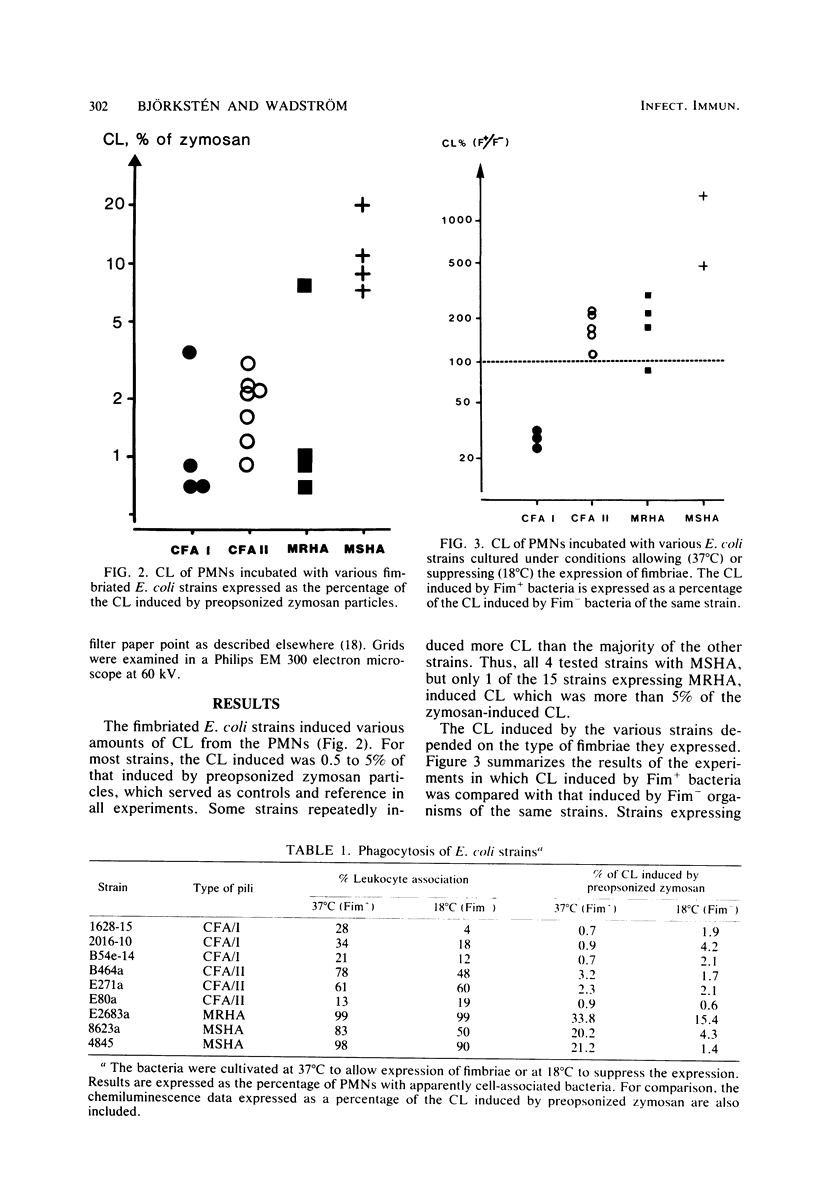
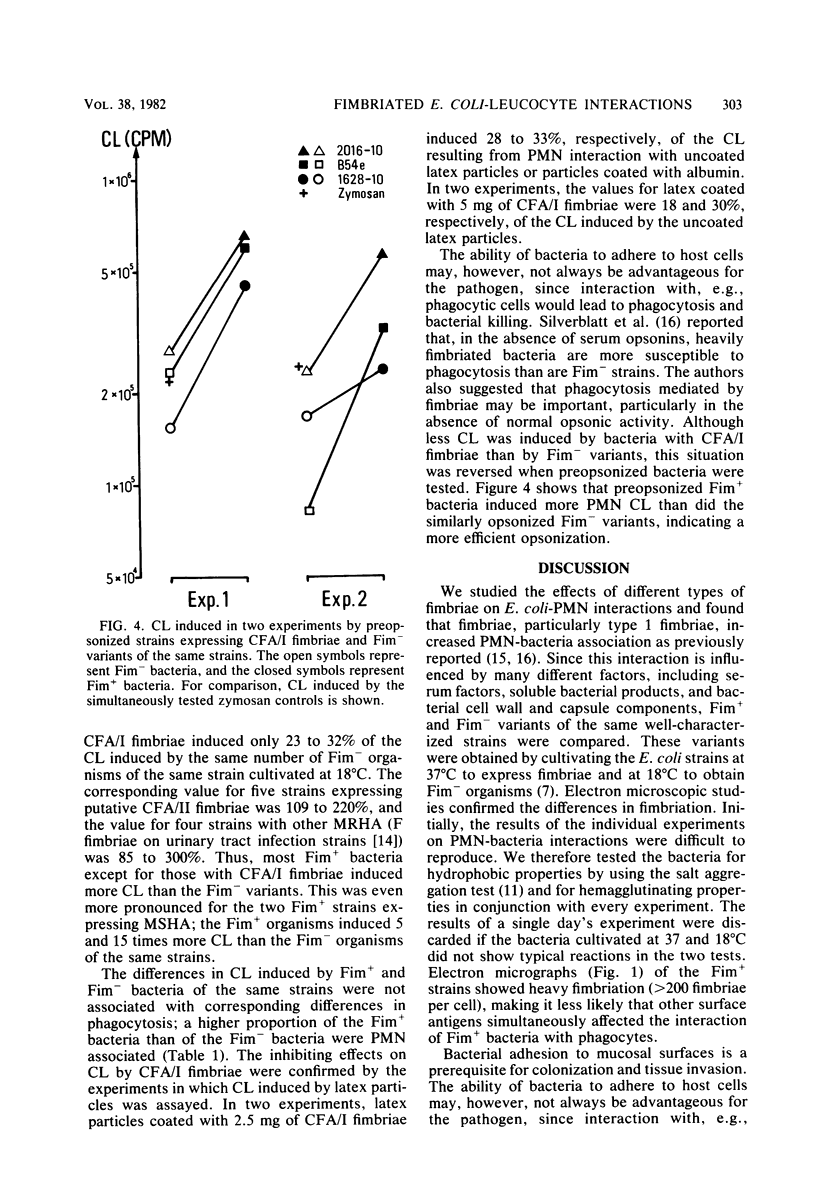
The effects of Escherichia coli strains with various fimbriae on bacteria-polymorphonuclear leukocyte (PMN) interactions were studied. Strains of E. coli were cultivated at 37°C to express and at 18°C to suppress the formation of fimbriae. The presence of fimbriae was confirmed by electron microscopic studies and hemagglutination and salt aggregation tests. Fimbriated E. coli strains were more readily PMN associated than the nonfimbriated strains in the absence of opsonins, confirming the results of previous studies. However, the PMN chemiluminescence (CL) induced by the various strains in the absence of serum opsonins depended on the type of fimbriae they expressed. Strains with type 1 fimbriae expressing mannose-sensitive hemagglutination induced 5 to 15 times more CL than the same strains grown at 18°C, i.e., not expressing this type of fimbriae. For strains showing mannose-resistant hemagglutination, the differences between fimbriated and nonfimbriated variants of the same strains grown at 37 and 18°C, respectively, were less pronounced. Analysis of enterotoxigenic strains expressing colonization factor antigen I (CFA/I) fimbriae showed that these induced only 25 to 33% of the CL induced by the same E. coli strains not expressing CFA/I, whereas enterotoxigenic strains expressing CFA/II fimbriae induced 100 to 200% of the CL induced by the nonfimbriated variants. Although less CL was induced by bacteria with CFA/I fimbriae than by nonfimbriated variants, this situation was reversed when the microorganisms were opsonized. Thus, CFA/I fimbriae, while enhancing adhesion to cells, induce less activation of PMN-killing mechanisms in a serum-free environment. These findings may be relevant for the virulence in certain body sites, since CFA/I fimbriae, while facilitating adhesiveness, may protect the bacteria from PMN killing. Our findings indicate that PMN interactions with fimbriated E. coli in the host defense may be complex. Certain fimbriae may indeed be advantageous to the bacteria, enabling them to interact with PMNs without activating the bactericidal oxidative metabolism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen B. R., Amirault H. J. Important variables in granulocyte chemiluminescence. Proc Soc Exp Biol Med. 1979 Oct;162(1):139–145. doi: 10.3181/00379727-162-40633. [DOI] [PubMed] [Google Scholar]
- Björkstén B., Kaijser B. Interaction of human serum and neutrophils with Escherichia coli strains: differences between strains isolated from urine of patients with pyelonephritis or asymptomatic bacteriuria. Infect Immun. 1978 Nov;22(2):308–311. doi: 10.1128/iai.22.2.308-311.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenstock E., Jann K. Adhesion of piliated Escherichia coli strains to phagocytes: differences between bacteria with mannose-sensitive pili and those with mannose-resistant pili. Infect Immun. 1982 Jan;35(1):264–269. doi: 10.1128/iai.35.1.264-269.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvath L., Amirault H. J., Andersen B. R. Chemiluminescence of human and canine polymorphonuclear leukocytes in the absence of phagocytosis. J Clin Invest. 1978 May;61(5):1145–1154. doi: 10.1172/JCI109029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G., James J. F., Swanson J. Studies on gonococcus infection. XI. Comparison of in vivo and vitro association of Neisseria gonorrhoeae with human neutrophils. J Infect Dis. 1978 Jan;137(1):38–43. doi: 10.1093/infdis/137.1.38. [DOI] [PubMed] [Google Scholar]
- Lambden P. R., Robertson J. N., Watt P. J. The preparation and properties of alpha and beta pili from variants of Neisseria gonorrhoeae P9. J Gen Microbiol. 1981 May;124(1):109–117. doi: 10.1099/00221287-124-1-109. [DOI] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Morse S. A. The biology of the gonococcus. CRC Crit Rev Microbiol. 1978;7(2):93–189. doi: 10.3109/10408417909083071. [DOI] [PubMed] [Google Scholar]
- Nelson R. D., Herron M. J., Schmidtke J. R., Simmons R. L. Chemiluminescence response of human leukocytes: influence of medium components on light production. Infect Immun. 1977 Sep;17(3):513–520. doi: 10.1128/iai.17.3.513-520.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A. Comparison of Escherichia coli fimbrial antigen F7 with type 1 fimbriae. Infect Immun. 1980 Feb;27(2):657–666. doi: 10.1128/iai.27.2.657-666.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]





