Abstract
The variability of drug response in different patients can be caused by various factors including age, change in renal function, co-medication and genotype. Traditionally, these personal variables are considered by clinicians prior to issuing a prescription. This paper provides an overview of a process to individualize prescribing for a patient with an emphasis on how to train (learning) clinicians in skillful rational prescribing. For this purpose the 6STEP methodology, a concept-based learning strategy to achieve a structured therapeutic plan, has been introduced. In contrast to older educational approaches which focused primarily on the drugs or the process of prescribing, the 6STEP is a patient-centred method resulting in individualized therapy. The six interlinked steps provide the (training) prescriber with a structured framework that facilitates a rationalized therapeutic decision by focusing on the individual patient parameters that influence drug response. Educational tools for rational prescribing involve understanding of basic and clinical pharmacological principles, practicing to write 6STEP therapeutic plans, learning from feedback sessions on these plans and actively obtaining up to date information on drugs and therapeutic standards from online resources.
Keywords: individualized pharmacotherapy, medical education, structured therapeutic plan
Introduction
Getting the right drug, with the right dose via the right route of administration at the right time in the right patient is a complex quest for each prescriber. In daily practice clinicians consider age, gender, allergies, organ (dys)function, comorbidity and co-medication prior to issuing a prescription. Therefore one can state that, traditionally, prescribing is ‘personalized’ to the individual patient. However, the concept of personalized prescribing should be extended so that the selection of therapy for a patient is based on patient characteristics, scientific evidence and pharmacogenomic information (when available). But how can a training clinician tackle this enormous amount of information and formulate a rational prescription? In this short review we define the term personalized prescribing and show methodologies for individualizing the prescribing of medicine. In order to make future clinicians understand the importance and the need for personalized prescribing this paper focuses on how to teach and train for skillful rational prescribing.
The concept of personalized prescribing
Personalized prescribing is a term closely related to personalized medicine. The latter is described as ‘a comprehensive, prospective approach to preventing, diagnosing, treating and monitoring disease in ways that achieve optimal individual health care decisions’[1]. Personalized medicine is not actually new, it has its roots back to 500 BC to Hippocrates, the father of Western medicine [2, 3]. Almost one and a half decades ago ‘personalized prescription’ was defined as ‘tailoring drugs to a patient's genetic makeup’ in a Science Editorial [4]. Although pharmacogenomics are important in individualizing therapy as variability in drug response can be explained in part by genetic differences among patients, a more global definition is required. In the literature, many different terms are closely linked to the concept of personalized prescribing such as ‘balanced’, ‘rational’, ‘safely’, ‘appropriate’ or ‘optimum’ prescribing. All indicate the balancing act between therapeutic benefit and risk, between effectiveness and toxicity. Prescribing safely is the desire of every clinician and according to Aronson ‘safe prescribing is a process that recommends a medicine appropriate to the patient's condition and ideally optimizes the balance of benefit to harm’[5]. Driven by the current need for reducing health care costs and increasing patient adherence, economic and patient-centred values should be incorporated into the definition. Therefore, in a comprehensive view of personalized prescribing, clinicians need to consider scientific evidence for drug and disease therapies, patient characteristics and values, and economic factors (Table 1). This definition encompasses a lot of parameters but initially we have to ask why do we need personal prescribing?
Table 1.
Drug, prescriber and patient factors that influence rational drug and dosage selection that need to be considered during step 4 of the therapeutic plan (modified from [28])
| Drug factors |
|---|
| Pharmacokinetic |
| Route of administration |
| Bioavailability |
| Frequency of dosing |
| Routes of metabolism/excretion |
| Interactions |
| Pharmacodynamic |
| Target specificity |
| Safety |
| Therapeutic window/index |
| Frequency and severity of adverse effects |
| Ease with which adverse effects can be predicted, monitored and prevented |
| Cost |
| Availability of alternatives with similar efficacy |
| Evidence |
| Clinical impact on disease progression |
| Efficacy in relieving symptoms |
| Efficacy on morbidity/mortality/hospitalization |
| Prescriber factors |
|---|
| Familiarity and knowledge with prescribing choices |
| Ease of follow-up (may depend on resources) |
| Doctor–patient relationship |
| Communication |
| Ability to collect patient data |
| Patient factors |
|---|
| Age, weight, height |
| Ethnicity, gender |
| Concomitant disease states |
| Concomitant medication (interactions) |
| Renal function (creatinine clearance/proteinuria) |
| Liver function |
| Allergies |
| History of previous adverse effects |
| Diet |
| Consumption of alcohol, smoking |
| Health beliefs/literacy |
| Genotype and phenotype |
| Compliance |
Examples of drug response variability
The answer lies in the variability of drug response of the individual patient to the same drug. Variability can often be attributed to pharmacological mechanisms, i.e. pharmacodynamic and pharmacokinetic actions, which are altered due to genetic variability. It is estimated that genetic variability accounts for a wide range (from 20 to 95 %) of variability in drug disposition and effect [6] due to variance in gene sequences encoding drug metabolizing enzymes, drug transporters or drug targets [7–9]. Pharmacogenomics is the study of individual genetic variation and was defined as ‘individualization of drug therapy through medication selection or dose adjustment based upon direct (e.g. genotyping) or indirect (e.g. phenotyping) assessment of a person's genetic constitution for drug response’[10].
Pharmacodynamics describes the effect of a drug on the body. The varying response to a drug can be determined by the genetic variation of the drug target in the individual patient [11]. Genetic alterations in receptors or signal transduction pathways can affect the efficacy of a drug profoundly.
Pharmacokinetics can be described by the absorption, distribution, metabolism and excretion of a drug. Each of these parameters has an influence on drug exposure, and since all these processes are subject to inter-patient variation, plasma concentrations can be altered. For example, factors that affect drug absorption, such as gastric pH and emptying, intestinal motility, and blood flow, change with age and cause variation in drug response. Renal excretion is also known to change with age and has a significant impact on drug response variability. Certain drugs are characterized by non-linear kinetics, i.e. blood plasma concentrations that do not relate linearly to changes in the dose. This phenomenon can occur due to auto-inhibition or auto-induction of metabolizing enzymes (cytochrome P450 system), saturation kinetics and saturation absorption [12]. Moreover, drug–drug interactions of these metabolizing enzymes can significantly influence drug exposure and its effect. Clinically relevant examples for pharmacokinetic interactions are numerous and prescribers need to be aware that co-administering inhibiting or inducing drugs may lead to problems in efficacy or safety.
Relevant genetic pharmacokinetic variations mainly occur in the cytochrome P450 enzyme system and thus influence the metabolism of drugs [11]. Polymorphisms of these genes can result in phenotypes ranging from poor to ultrarapid metabolizers and require the clinician to make dose adjustments for some of the drugs prescribed. It is unfortunate that genotyping and pharmacogenomic techniques are in their early stages, expensive and limited in their availability [13]. Moreover, the clinical benefit of treatment guided by pharmacogenetic outcomes often has not yet been proven [14].
The one patient variable that has the potential to have the greatest impact on drug response is that of adherence. Adherence to long term therapy for chronic illnesses in developed countries averages as low as 50% [15] indicating the importance of training prescribers to improve it. An often underestimated effect of respectful communication between prescriber and patient is the enhancement of patient adherence to the drug regimen and success in getting the drug in the patient [16, 17].
Formats for personalized prescribing
It is evident that knowledge of the drug's pharmacology and patient-specific parameters is of importance for tailored treatment. While experienced clinicians manage to consider these aspects routinely it is a major challenge for inexperienced prescribers to process all parameters. Numerous reports have demonstrated the high occurrence of (preventable) errors in prescribing medication including fatal errors [18, 19]. The World Health Organization (WHO) has acknowledged that rational prescribing is of high importance for (training) physicians [20]. Traditional pharmacology education has focused more on theory than on practice. The education was ‘drug-centred’, and concentrated on mechanisms, indications and side effects of different drugs while the curricula were focusing on diseases. In continuation of this approach De Vries developed a normative problem-solving model to address the need for more emphasis on the process of how to make choices for a certain drug and prescribing drugs rationally and safely [21]. De Vries and colleagues combined medical problem solving and decision analysis, practical medical aspects, and pharmacological facts and basic principles and developed the Guide to Good Prescribing [22]. According to this WHO guideline the selection of the drug should be based on a logical, deductive process including comprehensive and objective information. The WHO guide suggests that physicians develop a formulary of personal drugs (P-drugs) [22]. P-drugs are effective, safe and inexpensive drugs that physicians regularly prescribe to treat common problems. However, the focus of determining your P-drug formulary is still on the drug. Further development of the rational prescribing process emphasizes the argumentation of the drug choice for an individual patient. The 6STEP therapeutic plan (Figure 1) provides six consecutive, interlinked steps in order to develop a rational therapeutic plan for an individual patient. The strength of the 6STEP is the patient-oriented format that provides a rationale for each therapeutic choice in step 4. By using the 6STEP approach, students learn to justify their choices and take responsibility for their therapeutic decisions. Moreover, a 6STEP therapeutic plan serves as a communication tool between health care providers with the aim of decreasing prescribing errors due to communication faults. The 6STEP should also include the feedback of the patient's preference for treatment. Communication with the patient is part of the assessment of treatment options. Improvement can be achieved if the training involves role plays including application of the 6STEP as basic information for the therapeutic consult [23]. It is important to note that the individual criteria for the 6STEP were validated in different groups of health care educators and students [24]. In medical curricula this concept-based learning method is recognized as it improves the education in pharmacotherapy [25–27]. For instance it was clearly demonstrated that the total rationality increased significantly from 55.2% to 87.1% for undergraduate students who had not undergone the (concept-based) pharmacotherapy programme vs. students who completed the entire preclinical pharmacotherapy programme [27]. In order to illustrate how the 6STEP can be incorporated into the medical curriculum an example from Leiden University is presented in Table 2.
Figure 1.
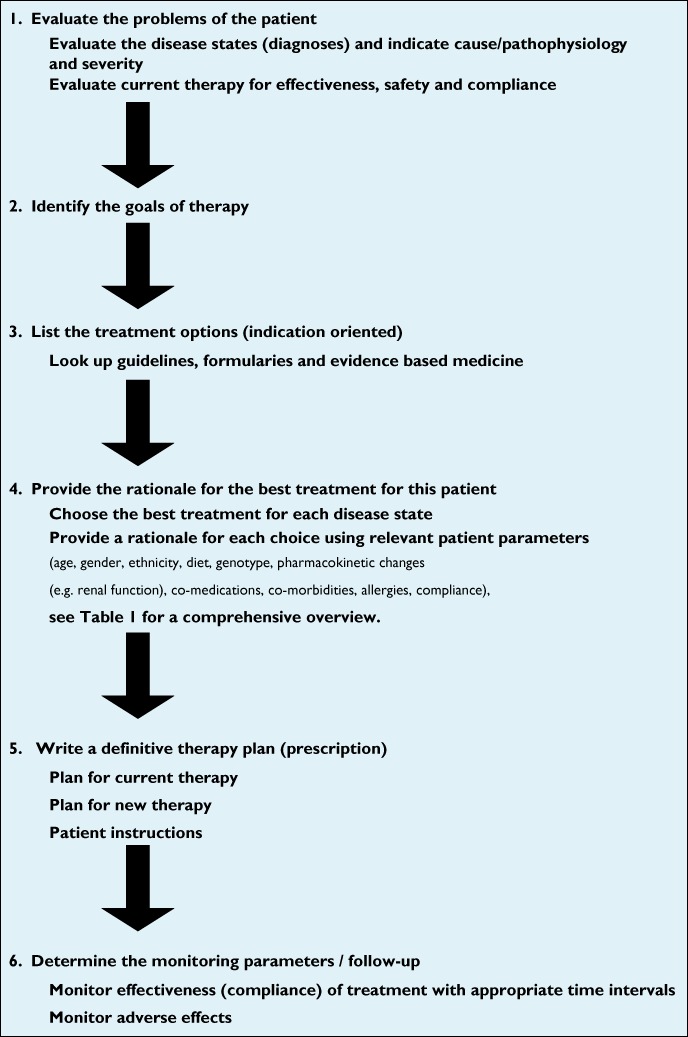
The 6STEP therapeutic plan format as applied in the Leiden University Medical curriculum gives a structured framework to achieve personalized prescribing, modified from [22] and [25]
Table 2.
Education in pharmacology and pharmacotherapy in Leiden [34]
| Teaching clinical pharmacology and rational prescribing to medicine students requires an integrated approach that is consistently provided across the Leiden curriculum. |
| Basic principles of pharmacology (pharmacodynamics and pharmacokinetics) and pharmacotherapy are taught early in the curriculum at the beginning of year 2. Thereafter, students apply these basic concepts and learn the clinical pharmacology in all (mostly organ specific) courses. All the gained knowledge needs then to be applied by writing 6STEP therapeutic plans. Practicing 6STEPs occurs in very different ways during the preclinical years. 6STEP teaching methods include interactive (plenary) lectures, self-study assignments, small group discussions, and tutorials and assignments on our pharmacology E-Learning program (Teaching Resource Centre). Students write a 6STEP for all commonly occuring disease states discussed in year 2 to 4. At the end of year 4, the students are able to write a 6STEP therapeutic plan for a simple (virtual, paper-based) patient case with a common medical problem. |
| Assessment of the prescribing skills takes place with open questions, multiple choice or extended matching questions in the final exam of every course. |
| This pharmacotherapy programme is continued during the clinical phase of the study (clerkship, year 5 and 6) when the student interns write 6STEPs for real patients. The complexity of the cases increases, because the real patient often has more than one medical problem and a longer medication list. The emphasis is now directed towards a practical, clinical 6STEP instead of the more tutorial-directed 6STEPs in the preclinical phase. The format for information gathering in a 6STEP is used to perform the therapeutic consult of a patient and that can be practiced in a role play fashion. |
| Assessment of 6STEP writing skills during the rotations is done by feedback sessions with the supervisor, creation of a 6STEP portfolio and a pharmacotherapeutic exam. |
Personalized prescribing with the 6STEP
The 6STEP anchors a medical problem solving process that includes the clinical presentation, differential diagnosis, diagnostic action plan and the therapeutic action plan or 6STEP. Individualizing therapy begins within the first step of the 6STEP therapeutic plan. When evaluating the patient's disease states, the prescriber needs to consider the cause/pathophysiology and the severity of the patient's problem, as well as evaluate current therapies for effectiveness, safety and compliance. In the second step, the therapeutic goal for each disease state is identified. This is patient-centred as it needs to be a realistic, achievable goal agreed upon by the patient. The third step is the only non-patient specific part within the 6STEP. Here the prescriber determines the (drug and non-drug) treatment options for each disease state of the patient using evidence-based medicine principles. In the fourth step, the best therapy for (all disease states of) the patient is selected from the treatment options identified in step 3. The therapy becomes personalized by rationalizing the choice based on patient-specific parameters and the information written in the first three steps. ‘The best drug’ for the patient should maximize the balance between benefit and harm [28] and fit with the patients individual characteristics. At this stage, the prescriber should scan all patient parameters with clinical pharmacological relevance (Table 1) and mention those which might affect the drug plasma concentration and drug response in the patient. The prescriber should evaluate clinical pharmacological characteristics such as the route of elimination of each drug as metabolism in the liver or excretion via the kidneys can be predictive for potential changes in drug response. When more than one medication is involved, the relevant drug–drug interactions should also be reviewed. All these findings together are the arguments for a rational drug choice and dosing. In step 5 the prescriber determines the definitive prescription(s) and plan for existing therapy and non-drug therapy, including instructions for the patient. The final step describes the monitoring plan. Each chosen therapy requires follow-up in order for the prescriber to determine its effectiveness, safety and compliance. In this step the prescriber explicitly describes the parameters (e.g. laboratory tests, physical examination) that need to be determined along with the appropriate time frame. An illustrative example of a 6STEP that is based on a virtual patient with diabetes mellitus type II is presented in Table 3.
Table 3.
6STEP example case from the third year in the Leiden Medical School
| CASE |
|---|
| A male Caucasian patient (age 56 years) was recently diagnosed with diabetes mellitus type 2 during a medical evaluation for his life insurance. He was told to visit his general practitioner for follow-up and treatment. |
| You are his GP and see the patient today. He has no specific complaints and does not use any medication. He smokes 15 cigarettes per day and works as accountant and sits behind his computer all day. |
| Weight 95 kg, height 1.80 m. |
| Blood pressure: 145/80 mmHg. |
| Laboratory results: HbA1c 8.6%; glucose (fasting)10 mmol l−1; lipids normal. |
| Creatinine 80 µmol l−1 |
| Albumin 40 g l−1 |
| Medication: none |
| Write a 6STEP therapeutic plan for this patient. |
| 6STEP answer model |
| STEP 1 – EVALUATION OF THE PROBLEM |
| Diabetes mellitus type 2: no complaints, but HbA1c and glucose are elevated. Probably due to increased insulin resistance. Attributing factors: overweight (BMI = 29 kg m−2), smoking and sedentary lifestyle. Treatment is required because of possible complications. No current therapy. |
| STEP 2 – GOAL |
| Prevention of macro- and microvascular damage. (cardiovascular disease, retinopathy, neuropathy and nephropathy) |
| STEP 3 – TREATMENT OPTIONS |
| Dutch GP guideline DMII: non-drug: weight loss (dietary restriction, less salt, less fat, less calories), stop smoking, physical exercise. |
| Drugs: oral antidiabetics: 1. biguanide (metformin), 2. sulphonylurea derivatives, 3. thiazolidinediones and 4. insulin. |
| STEP 4 – INDIVIDUALIZED THERAPY |
| The patient is overweight: a normal BMI could resolve the DMII. Losing weight is important. The patient should start a daily programme of physical exercise and restrict calories in his diet. Ask whether he wants to be coached in this. The patient smokes: stopping smoking significantly contributes to the goal of therapy. Non-drug treatment alone is not sufficient at this moment. Metformin is the first step in the treatment for diabetics. Metformin is renally cleared. Renal function is OK (eGFR (MDRD) is 92 ml min−1 1.73 m−2), so normal starting dose of metformin is adequate. |
| STEP 5 – PRESCRIPTION |
| Lifestyle: lose weight 3 kg month−1, stop smoking, daily exercise of 30 min. Start metformin 500 mg orally twice daily |
| Emphasize the importance of the lifestyle changes and drug compliance. Inform patient about ADRs of metformin (gastrointestinal complaints) |
| STEP 6 – FOLLOW-UP |
| After 6 weeks follow-up: check HbA1c (target value < 7%), blood pressure (120/80 mmHg), weight (BMI < 27 kg m−2) and lifestyle changes (did he stop smoking? Does he exercise regularly?). If NOT OK check compliance and adjust dose of metformin. If OK then yearly follow-up for retinopathy, renal function, nephropathy (microalbuminuria), lipids (hyperlipidaemia). Check for ADRs of metformin (nausea, diarrhoea) |
Tools for personal prescribing
It should be evident that the above process requires that the prescriber has a lot of knowledge at his/her disposal in order to make adequately the therapeutic choice with a solid rationale. Thus, the prescriber himself is also a determining factor for personalized prescription [28] (Table 1). How experienced is the prescriber with therapeutic decision making? Does the prescriber work with a structured format such as the 6STEP therapeutic plan? To individualize prescriptions, clinicians also have several conventional tools available. As evidence based medicine has found its way into the treatment guidelines, clinicians should appreciate the utility of these clinical treatment standards. In step 3 of the 6STEP therapeutic plan prescribers seek guidelines, formularies, and/or evidence based medicine to identify treatment options. When the lack of familiarity with certain drugs or their mechanisms of action arises, prescriber should seek information from text books, formularies or trusted, peer-reviewed online sites, such as the British National Formulary or UpToDate®.
An editorial by Begg advocated the use of equations to calculate an estimation of the renal clearance [29]. Formulae such as that of Cockroft & Gault [30] or the MDRD (obtained from the ‘Modification of diet in renal disease (MDRD) study [31]) provide important tools for clinicians, are easily comprehensible and give the user important information for dose individualization in a very short time frame. Adjusting the drug dosage due to renal insufficiency remains one of most important items in personal prescribing. Once more, the experienced prescriber will be aware of the importance of kidney function and by applying the 6STEP method the novice prescriber will be reminded to be cautious and consider pharmacokinetic differences in drug or dose selection.
Recently, decision support programmes and electronic medical records are also available to aid the prescriber to avoid serious drug–drug interactions [32]. Tamblyn and colleagues demonstrated that computer-based access to drug profiles and potential problematic prescriptions reduce the rate of potentially inappropriate prescribing [33]. Increasingly, decisions about patient therapy are not written, but recorded in electronic medical records. In these systems structured data recording is essential and the 6STEP provides a structure that can also be used to motivate treatment decisions in the electronic medical record.
Integration of the 6STEP into the curriculum
Let us return to two important questions that have previously been raised: How can a student learn to write a good and concise 6STEP using enormous amounts of information? And how do students know what is relevant and important to mention in the 6STEP? As for many complex tasks the answer is: practice makes the master. One important aspect of learning personalized prescribing is a strong foundation in basic pharmacology. Medical students need to obtain understanding of pharmacodynamic and pharmacokinetic concepts and on pharmacological mechanisms of action during the curriculum. Apart from these basic sciences, they should be motivated by the patient's problems to seek information from therapeutic guidelines, standards and drug formularies. Although our current students seem familiar with searching the Internet, they often get lost in the enormous amounts of available online information. Educators play an important role in guiding them towards trustworthy, scientific resources and interpreting the information found.
Educational research has shown that 6STEP writing and receiving feedback is the starting point for students to implement successfully a therapeutic plan [25]. During the medical curriculum (Table 2) it is recommended to practice writing therapeutic plans starting with simple one-problem patient cases and building up towards complex multi-disease with polypharmacy patients. The latter requires experience in therapeutic plan writing and well-developed analytical skills. Undergraduate students can practice personalized prescribing individually with online tutorial cases or in small group settings with peer review and tutor feedback. Discussions amongst students or with a tutor on written therapeutic plans are valuable educational strategies. This aids students to learn which patient specific information is relevant for the therapeutic decision.
Students on rotations and residents write personalized therapeutic plans on the patients they treat in the clinic. On these therapeutic plans they receive feedback from discussions with their supervisor and during patient presentations.
Broad utility of the 6STEP
It is important to realize that determining drug treatment is not the only intervention made by clinicians. The 6STEP is unique as it is not focused solely on drug treatment. As such, it is also suitable for a structured approach in the areas of diet, life-style modifications, surgery, radiotherapy and a combination of therapies.
In summary, personalized prescribing is already common practice as personal variables such as co-medication, gender, weight, pharmacogenomics and conditions of disease are considered when prescribing drugs. The prescriber has an important role of optimizing the risk : benefit ratio and considering the relevant patient specific parameters in order to make a rational therapeutic decision. Experience with and daily use in clinical practice of a structured therapeutic plan such as the 6STEP is recommended for personalized prescribing. Personalized prescribing requires access to knowledge of (clinical) pharmacology and evidence based medicine. The prescriber should use online available resources in order to obtain up to date information on drugs and their clinical application. Giving your patient the optimal therapy is still the ultimate goal of personal prescribing and the greatest responsibility of a clinician.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Lesko LJ. Personalized medicine: elusive dream or imminent reality? Clin Pharmacol Ther. 2007;81:807–16. doi: 10.1038/sj.clpt.6100204. [DOI] [PubMed] [Google Scholar]
- 2.Bulger RJ, Barbato AL. On the Hippocratic sources of Western medical practice. Hastings Cent Rep. 2000;30:S4–S7. [PubMed] [Google Scholar]
- 3.Sykiotis GP, Kalliolias GD, Papavassiliou AG. Pharmacogenetic principles in the Hippocratic writings. J Clin Pharmacol. 2005;45:1218–20. doi: 10.1177/0091270005281091. [DOI] [PubMed] [Google Scholar]
- 4.Science editorial. New research horizons. Science. 1997;278:2040. [Google Scholar]
- 5.Aronson JK. Balanced prescribing. Br J Clin Pharmacol. 2006;62:629–32. doi: 10.1111/j.1365-2125.2006.02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalow W, Tang BK, Endrenyi L. Hypothesis: comparisons of inter- and intra-individual variations can substitute for twin studies in drug research. Pharmacogenetics. 1998;8:283–9. doi: 10.1097/00008571-199808000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Evans WE, Johnson JA. Pharmacogenomics: the inherited basis for interindividual differences in drug response. Annu Rev Genomics Hum Genet. 2001;2:9–39. doi: 10.1146/annurev.genom.2.1.9. [DOI] [PubMed] [Google Scholar]
- 8.Evans WE, Relling MV. Pharmacogenomics: translating functional genomics into rational therapeutics. Science. 1999;286:487–91. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 9.McLeod HL, Evans WE. Pharmacogenomics: unlocking the human genome for better drug therapy. Annu Rev Pharmacol Toxicol. 2001;41:101–21. doi: 10.1146/annurev.pharmtox.41.1.101. [DOI] [PubMed] [Google Scholar]
- 10.Swen JJ, Huizinga TW, Gelderblom H, de Vries EG, Assendelft WJ, Kirchheiner J, Guchelaar HJ. Translating pharmacogenomics: challenges on the road to the clinic. PLoS Med. 2007;4:e209. doi: 10.1371/journal.pmed.0040209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein DB, Need AC, Singh R, Sisodiya SM. Potential genetic causes of heterogeneity of treatment effects. Am J Med. 2007;120:521–5. doi: 10.1016/j.amjmed.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 12.de Leon J. Future of personalized prescription in psychiatry. Adv Biol Psychiatry. 2010;25:118–34. [Google Scholar]
- 13.Swen JJ, Nijenhuis M, de BA, Grandia L, Maitland-van der Zee AH, Mulder H, Rongen GA, van Schaik RH, Schalekamp T, Touw DJ, van der Weide J, Wilffert B, Deneer VH, Guchelaar HJ. Pharmacogenetics: from bench to byte – an update of guidelines. Clin Pharmacol Ther. 2011;89:662–73. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- 14.Hunter DJ, Khoury MJ, Drazen JM. Letting the genome out of the bottle – will we get our wish? N Engl J Med. 2008;358:105–7. doi: 10.1056/NEJMp0708162. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. Switzerland: 2003. Adherence to long-term therapies – evidence for action. [Google Scholar]
- 16.Butrick M, Roter D, Kaphingst K, Erby LH, Haywood C, Jr, Beach MC, Levy HP. Patient reactions to personalized medicine vignettes: an experimental design. Genet Med. 2011;13:421–8. doi: 10.1097/GIM.0b013e3182056133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez-Sanroman A, Bermejo F. Review article: how to control and improve adherence to therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24(Suppl. 3):45–9. doi: 10.1111/j.1365-2036.2006.03060.x. [DOI] [PubMed] [Google Scholar]
- 18.Leendertse AJ, Egberts AC, Stoker LJ, van den Bemt PM. Frequency of and risk factors for preventable medication-related hospital admissions in the Netherlands. Arch Intern Med. 2008;168:1890–6. doi: 10.1001/archinternmed.2008.3. [DOI] [PubMed] [Google Scholar]
- 19.Lewis PJ, Dornan T, Taylor D, Tully MP, Wass V, Ashcroft DM. Prevalence, incidence and nature of prescribing errors in hospital inpatients: a systematic review. Drug Saf. 2009;32:379–89. doi: 10.2165/00002018-200932050-00002. [DOI] [PubMed] [Google Scholar]
- 20.Hogerzeil HV. Promoting rational prescribing: an international perspective. Br J Clin Pharmacol. 1995;39:1–6. doi: 10.1111/j.1365-2125.1995.tb04402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vries TP. Presenting clinical pharmacology and therapeutics: a problem based approach for choosing and prescribing drugs. Br J Clin Pharmacol. 1993;35:581–6. doi: 10.1111/j.1365-2125.1993.tb04185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries T, Henning RH, Hogerzeil HV, Fresle DA. World Health Organziation. Guide to good prescribing – practical manual, WHO/DAP/94 Edition, 1994.
- 23.Vollebregt JA, van OJ, Kox D, van Galen SR, Sturm B, Metz JC, Richir MC, de HM, Hugtenburg JG, de Vries TP. Evaluation of a pharmacotherapy context-learning programme for preclinical medical students. Br J Clin Pharmacol. 2006;62:666–72. doi: 10.1111/j.1365-2125.2006.02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Franson KL, Dubois EA, Meenhorst PL, Cohen AF. The 6-step: validation of the Dutch national standard for a therapeutic plan. Basic Clin Pharmacol Toxicol. 2007;101(Suppl. 1):33. [Google Scholar]
- 25.Franson KL, Dubois EA, de Kam ML, Burggraaf J, Cohen AF. Creating a culture of thoughtful prescribing. Med Teach. 2009;31:415–9. doi: 10.1080/01421590802520931. [DOI] [PubMed] [Google Scholar]
- 26.Richir MC, Tichelaar J, Geijteman EC, de Vries TP. Teaching clinical pharmacology and therapeutics with an emphasis on the therapeutic reasoning of undergraduate medical students. Eur J Clin Pharmacol. 2008;64:217–24. doi: 10.1007/s00228-007-0432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richir MC, Tichelaar J, Stanm F, Thijs A, Danner SA, Schneider AJ, de Vries TP. A context-learning pharmacotherapy program for preclinical medical students leads to more rational drug prescribing during their clinical clerkship in internal medicine. Clin Pharmacol Ther. 2008;84:513–6. doi: 10.1038/clpt.2008.82. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell S. Rational prescribing: the principles of drug selection. Clin Med. 2009;9:481–5. doi: 10.7861/clinmedicine.9-5-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begg EJ. Individualizing drug therapy, and ‘men behaving badly’. Br J Clin Pharmacol. 2004;58:449–51. doi: 10.1111/j.1365-2125.2004.02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 31.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 32.Kaushal R, Shojania KG, Bates DW. Effects of computerized physician order entry and clinical decision support systems on medication safety: a systematic review. Arch Intern Med. 2003;163:1409–16. doi: 10.1001/archinte.163.12.1409. [DOI] [PubMed] [Google Scholar]
- 33.Tamblyn R, Huang A, Perreault R, Jacques A, Roy D, Hanley J, McLeod P, Laprise R. The medical office of the 21st century (MOXXI): effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. CMAJ. 2003;169:549–56. [PMC free article] [PubMed] [Google Scholar]
- 34.Franson KL, Dubois EA, van Gerven JM, Cohen AF. Development of visual pharmacology education across an integrated medical school curriculum. J Vis Commun Med. 2007;30:156–61. doi: 10.1080/17453050701700909. [DOI] [PubMed] [Google Scholar]


