Abstract
Prescribing of medicines during pregnancy is common, and for some groups of women is essential for maintaining maternal and therefore fetal health. Pregnant women and prescribers are rightly concerned, however, about the potential adverse fetal effects of medicines. These may include fetal death or stillbirth, congenital malformations, developmental impairment, neonatal effects or late carcinogenesis. It is therefore essential that the risks and benefits for mother and fetus are considered carefully before prescribing in pregnancy. This is often challenging because of the paucity of information available. To complicate the issue further, drug pharmacokinetics are commonly altered in pregnancy, potentially affecting optimal dosing as well as interpretation of plasma concentration measurements, with specific information on individual drugs seldom available. Most drugs cross the placenta, especially lipophilic drugs and those with low plasma protein binding. Active membrane transporters also have an important role in enhancing or preventing drug transfer, although this is not yet clearly understood. Animal studies have limited applicability to humans because of species-specific effects, and clinical trials in pregnancy are only undertaken in special circumstances. Prescribers therefore need to rely on observational studies of fetal outcomes following drug exposure in human pregnancy. These often involve limited numbers, and data are also subject to confounding and bias, making interpretation difficult. It therefore remains essential that appropriate mechanisms for systematic data collection, including congenital malformation registries, teratology information services, pregnancy registers and linked population registries, are maintained and enhanced to increase the amount and quality of information available.
Keywords: pregnancy, prescribing, teratogen
Introduction
Most women take a medicine at some point during pregnancy, and for more than 80% this includes at least one prescribed medication [1]. Use of medicines in pregnancy appears to be increasing, in part due to increasing rates of maternity in older women, who are more likely to have underlying medical conditions that require treatment. Drug treatment may also be required during pregnancy for acute illnesses and pregnancy-associated conditions.
Commonly used medicines include analgesics, antibiotics and antiemetics, and there is also evidence of increasing use of antidepressants during pregnancy in the UK [2] and the USA [3]. Many medicines involved are associated with substantial experience of safe use in pregnancy, but there is significant prescribing of drugs known to be associated with fetal risks, with 1–4% of women being prescribed medicines considered contraindicated [4] and a larger proportion prescribed medicines where information on safety in pregnancy is incomplete. These figures are in the context of over 650 000 maternities in England annually [5], up to 50% of which may be unplanned [6]. The delay between conception and recognition of pregnancy increases the risk of continuing exposure in early pregnancy, a critical period for fetal organ formation, to medicines prescribed for chronic conditions.
The objectives of this review are to discuss the complexities of assessing maternal and fetal risks and benefits of maternal drug therapy, taking into account the effects of pregnancy on drug pharmacology, and to consider the advantages and limitations of current methods of monitoring the safety of medicines use during human pregnancy.
Risks and benefits of medicines use in pregnancy
The decision to prescribe a drug should always involve consideration of the risks and benefits for recipients. Pregnancy is unique in that two individuals are exposed, with risks and benefits for each. A further consideration is that the maternal benefits and risks of a medication in pregnancy are not necessarily the same as for nonpregnant women, because altered physiology and pharmacology may affect the efficacy of treatment or the risk of adverse effects.
The great majority of medicines and/or their metabolites cross the placenta, and even those that do not may have fetal effects by altering maternal physiology. Since the thalidomide tragedy in the late 1950s and early 1960s, the potential adverse fetal affects of medicines have been in the forefront of the minds of pregnant women and prescribers, affecting willingness to prescribe and receive prescriptions, as well as adherence with any medicines that are prescribed.
Risks to the fetus, however, need to be considered in the context of the benefits of treatment to the mother and fetus from good maternal health. In the UK, underlying maternal conditions more commonly cause maternal deaths than direct pregnancy complications [6]. Many of these conditions are treatable, and adverse outcomes can often be avoided by appropriate therapy provided by clinicians with specific expertise and experience. For example, recent research suggests that poor asthma control during pregnancy increases the risk of pre-eclampsia, low birthweight and prematurity [7]. Likewise, it is well established that women with pre-pregnancy diabetes are at significantly increased risk of adverse maternal and fetal outcome, including a two- to threefold increased risk of congenital malformation in their offspring. These risks are reduced in diabetic women who achieve good glycaemic control before conception and throughout pregnancy [8, 9].
A major problem facing prescribers is a lack of information on risks and benefits of drug therapy in pregnancy. This particularly applies (but is not confined) to newer drugs. While there is some assessment of reproductive toxicology as part of drug development, studies performed in animals are of limited value, particularly because adverse effects may be species specific. Aspirin, for example, induces cardiac malformations in some animal species but not in humans [10]. Clinical trials in human pregnancy are rarely possible, so when drugs are licensed there is almost invariably inadequate evidence on which to base prescribing decisions. Subsequence experience may be obtained during clinical use, but this is observational and therefore subject to confounding and bias. As a result, the magnitude of any benefit or risk from drug treatment is poorly defined in pregnancy, especially in comparison to other treatment options, including nonpharmacological approaches.
An important historical example of inappropriate prescribing in pregnancy is that of diethylstilbestrol, which was advocated as reducing risks of pregnancy complications on the basis of a clinical trial with inadequate matching of controls. The drug was administered widely before the link with clear cell adenocarcinoma of the vagina and cervix and genital tract malformation in female offspring was recognized. Widespread prescribing continued even though an appropriately controlled clinical trial demonstrated no beneficial effect of diethylstilbestrol on rates of spontaneous abortion, prematurity or postmaturity [11].
Effects of pregnancy on pharmacology
Alterations in drug pharmacokinetics associated with pregnancy are described in detail elsewhere [12–16]. Briefly, as pregnancy progresses, gastric emptying is delayed, potentially delaying maximal drug concentrations after ingestion; gastric pH is increased, which may affect bioavailability of some drugs. Nausea and vomiting, a common problem in early pregnancy, will also have an important effect. The increase in total body water and fat stores and the reduction in plasma albumin associated with pregnancy increase the volume of distribution of many drugs. The increased cardiac output associated with pregnancy increases the speed at which distribution occurs.
Although blood volume and cardiac output are increased during pregnancy, hepatic artery flow appears unchanged. Portal venous blood flow and, as a result, total hepatic blood flow are, however, increased in the third trimester [17]. There are variable affects of pregnancy on hepatic metabolism. Processes catalysed by some cytochrome P450 isoenzymes (CYP2A6, CYP2C9, CYP2D6 and CYP3A4) and uridine 5′-diphospho-glucuronosyltransferases are increased, while others, such as the isoenzymes CYP1A2 and CYP2C19, are reduced (Table 1).
Table 1.
| Enzyme | Effect of pregnancy | Trimesters | Substrates (examples) |
|---|---|---|---|
| CYP1A2 | Decreased | I–III | Caffeine |
| Paracetamol | |||
| Theophylline | |||
| CYP2A6 | Increased | III | Nicotine |
| Sodium valproate | |||
| CYP2C9 | Increased | III | Angiotensin-converting enzyme inhibitors |
| Nonsteroidal anti-inflammatory drugs | |||
| Phenytoin | |||
| Warfarin | |||
| CYP2C19 | Decreased | II–III | Citalopram |
| Proguanil | |||
| CYP2D6 | Increased | I–III | Methadone |
| Metoprolol | |||
| Tricyclic antidepressants | |||
| Selective serotonin reuptake inhibitors | |||
| Venlafaxine | |||
| CYP3A4 | Increased | I–III | Carbamazepine |
| Nifedipine | |||
| Protease inhibitors | |||
| Uridine 5′-diphospho-glucuronosyltransferases | Increased | I–III | Lamotrigine |
| Morphine | |||
| N-Acetyltransferase 2 | Decreased | I | Isoniazid |
| Hydralazine |
Glomerular filtration rate increases during pregnancy, enhancing clearance of renally excreted drugs and metabolites, such as atenolol, digoxin, metformin, lithium and morphine glucuronides. Activity of P-glycoprotein is also enhanced, increasing renal tubular secretion of substrates such as digoxin.
These changes and their extent depend on the stage of pregnancy, so that for a woman prescribed the same drug dose, there may be clinically important changes in drug concentrations between the various trimesters of pregnancy and the early and late postpartum periods, as has recently been demonstrated for some antidepressants, for example [18].
Pharmacodynamic changes in pregnancy are less well studied, but increases in blood pressure and blood glucose associated with corticosteroids may be more common in pregnancy. The efficacy of vaccination may be affected by impairment of cell-mediated immunity, especially in late pregnancy. Sensitivity to the heart rate-lowering effects of β-blockade may also be increased in pregnancy [19]. These changes and their clinical implications are poorly understood.
Placental transfer
Most drugs have a molecular weight of <600 Da and, as such, are able to cross the placenta. The extent of placental transfer also depends on the plasma protein binding and lipid solubility of the drug [20]. The latter depends on pKa, and the lower fetal pH may result in some degree of fetal trapping of weak bases. Gestational age is also an important factor, owing to the reduction in the materno-fetal diffusion distance as pregnancy progresses.
There is increasing recognition that placental transfer also depends also on transporter activity, and accumulating evidence that blockade of P-glycoprotein can have important effects on the extent of fetal transfer of drugs and potentially the risk that these may produce [20, 21]. For example, transplacental movement of the antiviral drug lopinivir, a P-glycoprotein substrate, has been shown to be increased when P-glycoprotein is inhibited [22].
Identifying teratogenic drugs
In the past, teratogenesis has referred to the generation of structural fetal malformations; however, a wider definition is now in general use, encompassing fetal adverse effects arising from drug and chemical exposure, such as fetal death resulting in spontaneous abortion or stillbirth, developmental abnormalities, longer term developmental impairment and late carcinogenesis.
Congenital malformations may be induced when exposure to a teratogen happens above a threshold concentration during a time period critical for development of the relevant organ system [23]. As most organs and systems develop during the first trimester, it is exposure at that time which carries the highest risk, although central nervous system development continues into the second and third trimesters. For individual organ systems, the periods of risk are quite specific. For example, the critical period of palatal development is 6–9 weeks after conception, and exposure at other times is not expected to carry a high risk of palatal malformations. Effects on growth and development may occur as a result of exposure in the second and third trimesters of pregnancy, while exposure near term carries the greatest risk of producing adverse functional neonatal effects, such as neonatal toxicity following maternal prescription of opioid analgesics or maternal neonatal withdrawal effects following maternal use of selective serotonin reuptake inhibitors.
Most major congenital malformations occur rarely, with 2–3% overall risk and a much lower risk of specific malformations. As a result, information from large numbers of infants exposed in utero is needed to prove or disprove an association with drug therapy, unless risks are very high. As human research is observational, signals suggesting possible teratogenic effects may also arise (or be obscured) because of confounding or bias. Confounding by therapeutic indication, as a result of an adverse effect associated with the condition being treated, is particularly difficult to exclude. Other potential confounding factors that need consideration include maternal age, race, co-ingestion of other medicinal drugs, substance use such as smoking, alcohol, caffeine or recreational drugs, chronic illness, poor obstetric history, infections such as sexually transmitted diseases including HIV, maternal fever, poor nutritional status and quality of antenatal care. These need to be taken into account when interpreting observational data, but information on their presence is often incomplete.
Observational study methodology
A number of methods have been used to collect data on drug safety during human pregnancy after a drug is licensed. These include spontaneous reporting, congenital malformation registries, follow-up data from teratology information services, pregnancy registries and computerized population data, often linked with prescribing data sets. Problems common to many of these methods include lack of information about confounding factors, maternal adherence to prescribed medication, use of over-the-counter medicines, including herbal preparations and drugs of abuse, and difficulty in evaluating longer term effects. Some specific advantages and disadvantages of these methods are summarized in Table 2.
Table 2.
Observational methods for studying drug safety in human pregnancy
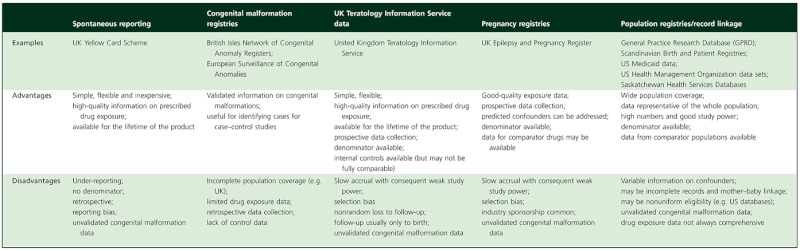 |
Spontaneous adverse drug reaction reporting
Spontaneous adverse drug reaction reporting, such as the UK Yellow Card Scheme, has been relatively ineffective for detecting teratogenic effects because of low reporting rates, the background rate of congenital malformations in the general population and lack of a denominator on which to base estimates of frequency. Nevertheless, reporting of suspected associations should be encouraged, because this method may be useful for detecting a signal suggesting a link between a drug and particular pattern of malformation, especially if the latter is rare in the absence of drug exposure.
Congenital malformation registries
Congenital malformation registries [24] provide high-quality data on the characteristics of malformations, but information on drug exposure or other maternal confounders is not necessarily part of core data collection. The European congenital malformation registries network (EUROCAT) has recently introduced collection of maternal medication exposure data for a subset of member registries. Combining data from these registries offers promise as a means of linking malformations with drug exposure, because better standardization of methodology for obtaining the drug history has been implemented [25].
Teratology Information Service data
Teratology information services, such as the UK Teratology Information Service, provide advice on safe use of medicines during pregnancy. When exposure has happened or is planned, attempts are made to follow up the pregnancy to obtain information on fetal outcome. It is a laborious process to collect sufficient outcomes on which reliable conclusions can be drawn, but these data remain useful, especially if information from several services is combined [26]. The information collected is also subject to bias; one important example is that more complex patients are likely to be referred. There may also be nonrandom loss to follow-up, with adverse pregnancy outcomes being more likely to be reported.
Pregnancy registries
Pregnancy drug registries involve an attempt to collect data on fetal outcome for as many pregnant women as possible prescribed a specific drug or drug group of interest [27, 28]. Such registries are used increasingly, and some have provided invaluable information, for example regarding antiepileptic drugs [29]. Registries can be designed to collect all essential data relevant to the drug under study, including important confounders. However, accrual of exposed pregnancies can be slow, and high-quality data collection is often costly. Some registries are industry sponsored or managed, and this may raise concerns about the independence of data and ensuing publications.
Population databases and record linkage
An attractive method for collecting large amounts of observational data is via health information databases, which have information on maternal medical history and drug treatment that can be linked to information on the health of the offspring, as well as useful information on potential confounders. Detailed reviews of available databases have been published [30]. This approach also allows comparison of outcomes between women with the same condition exposed to different drugs and with those who are untreated. More detailed information can sometimes be obtained by linking several databases together, including hospital discharge data, general practice records, pregnancy registries, congenital malformation registries and prescription databases. Such record linkage systems are available in Scandinavian countries and have been effective for identifying drug safety issues, for example involving selective serotonin reuptake inhibitor antidepressants [31] or antiepileptic drug therapy [32].
Knowledge gaps
More information is needed on basic mechanisms of teratogenesis, and experimental systems that better predict risk in humans are key to establishing safe use in pregnancy for new drugs in the future. Further research on maternal pharmacokinetics, pharmacodynamics and placental drug transfer is also required.
From a UK perspective, better quality observational data across large populations are required to allow more rapid and accurate investigation of drug safety issues. To identify a teratogen as soon as possible, it is necessary that information from as many exposed pregnancies as possible is collected; indeed, the prescribing of newer drugs during pregnancy without systematic collection of information on maternal and fetal outcome is a serious omission. Drug manufacturers have a responsibility to collect such data, and design of an effective method for data collection should form part of their pharmacovigilance planning. Responsibility, however, does not rest solely with manufacturers, and the inability to utilize appropriately linked anonymized pharmacoepidemiological information from data sets collected as a matter of routine across the National Health Service, including GP prescribing data, needs to be challenged. Population databases, such as the General Practice Research Database (GPRD) [33] and The Health Improvement Network primary care database (THIN) [2], are of great value but cover only a minority of the UK population and are limited by a lack of information on prescribing in secondary care [34].
For drugs where widespread use in pregnancy is likely, randomized controlled clinical trials would be of great value, but drug manufacturers are unlikely to sponsor these because of their inherent risks, unless the proposed indication is specific to pregnancy, so public funding would be needed. The limited available experience of such studies, however, suggests that not only are they challenging to design, but also difficult to recruit to.
Prescribing in pregnancy
General principles include obtaining and sharing accurate and balanced information with mothers. For many drugs, detailed evidence-based information is available from the UK Teratology Information Service (telephone +44 844 892 9090, website http://www.uktis.org/) and similar organizations in other countries. Drugs should be used where there is previous experience, avoiding newer drugs if possible. A prescription should be issued only if there is a clear indication, especially during the first trimester, when exposure to a culprit drug is most likely to be associated with an increased risk of congenital malformation, although other teratogenic effects may be associated with exposure in the second or third trimesters. Where use of a drug is clinically justified, the lowest effective dose should be prescribed for the minimal time required. For drugs subject to therapeutic drug monitoring, plasma concentrations should be measured regularly and results used to inform dose adjustments. Polypharmacy should be avoided as far as possible and appropriate folic acid supplementation provided. The use of high-dose folic acid is especially important for women prescribed drugs affecting folate metabolism, such as sodium valproate. Avoidance of alcohol, tobacco and other recreational substances is also recommended, as these have adverse effects on pregnancy outcomes and may also affect pharmacokinetics of prescribed medication. Implementation of these precautions offers the best prospect of a healthy outcome for mother and baby.
When prescribing drugs for which there are limited data on safety in pregnancy, prescribers are encouraged to provide outcome information to the appropriate pregnancy registries or teratology information services, because this will increase the information available on which future prescribing decisions can be made.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Irvine L, Flynn RW, Libby G, Crombie IK, Evans JM. Drugs dispensed in primary care during pregnancy: a record-linkage analysis in Tayside, Scotland. Drug Saf. 2010;33:593–604. doi: 10.2165/11532330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Petersen I, Gilbert RE, Evans SJ, Man SL, Nazareth I. Pregnancy as a major determinant for discontinuation of antidepressants: an analysis of data from The Health Improvement Network. Clin Psychiatry. 2011;72:979–85. doi: 10.4088/JCP.10m06090blu. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S, the National Birth Defects Prevention Study Medication use during pregnancy, with particular focus on prescription drugs: 1976–2008. Am J Obstet Gynecol. 2011;205:51.e1–8. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daw JR, Hanley GE, Greyson DL, Morgan SG. Prescription drug use during pregnancy in developed countries: a systematic review. Pharmacoepidemiol Drug Saf. 2011;20:895–902. doi: 10.1002/pds.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NHS Information Centre. NHS Maternity Statistics, England. 2009/10 Available at http://www.ic.nhs.uk/pubs/maternity0910 (last accessed 21 March 2012)
- 6.Centre for Maternal and Child Enquiries. Saving mothers' lives. Reviewing maternal deaths to make motherhood safer: 2006–2008. Br J Obstet Gynaecol. 2011;118(Suppl. 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 7.Murphy VE, Namazy JA, Powell H, Schatz M, Chambers C, Attia J, Gibson PG. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG. 2011;118:1314–23. doi: 10.1111/j.1471-0528.2011.03055.x. [DOI] [PubMed] [Google Scholar]
- 8.Steel JM, Johnstone FD, Hepburn DA, Smith AF. Can prepregnancy care of diabetic women reduce the risk of abnormal babies? BMJ. 1990;301:1070–4. doi: 10.1136/bmj.301.6760.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinsley B. Achieving better outcomes in pregnancies complicated by type 1 and type 2 diabetes mellitus. Clin Ther. 2007;29(Suppl. D):S153–60. doi: 10.1016/j.clinthera.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Werler MM, Mitchell AA, Shapiro S. The relation of aspirin use during the first trimester of pregnancy to congenital cardiac defects. N Engl J Med. 1989;321:1639–42. doi: 10.1056/NEJM198912143212404. [DOI] [PubMed] [Google Scholar]
- 11.Dieckmann WJ, Davis ME, Rynkiewicz LM, Pottinger RE. Does the administration of diethylstilbestrol during pregnancy have therapeutic value? Am J Obstet Gynecol. 1953;66:1062–81. doi: 10.1016/s0002-9378(16)38617-3. [DOI] [PubMed] [Google Scholar]
- 12.Wadelius M, Darj E, Frenne G, Rane A. Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther. 1997;62:400–7. doi: 10.1016/S0009-9236(97)90118-1. [DOI] [PubMed] [Google Scholar]
- 13.Tsutsumi K, Kotegawa T, Matsuki S, Tanaka Y, Ishii Y, Kodama Y, Kuranari M, Miyakawa I, Nakano S. The effect of pregnancy on cytochrome P4501A2, xanthine oxidae and N acetyltransferase activities in humans. Clin Pharmacol Ther. 2001;70:121–5. doi: 10.1067/mcp.2001.116495. [DOI] [PubMed] [Google Scholar]
- 14.Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 15.Koren G. Pharmacokinetics in pregnancy: clinical significance. J Popul Ther Clin Pharmacol. 2011;18:e523–e527. [PubMed] [Google Scholar]
- 16.Anger GJ, Piquette-Miller M. Pharmacokinetic studies in pregnant women. Clin Pharmacol Ther. 2008;83:184–7. doi: 10.1038/sj.clpt.6100377. [DOI] [PubMed] [Google Scholar]
- 17.Nakai A, Sekiya I, Oya A, Koshino T, Araki T. Assessment of the hepatic arterial and portal venous blood flows during pregnancy with Doppler ultrasonography. Arch Gynecol Obstet. 2002;266:25–9. doi: 10.1007/pl00007495. [DOI] [PubMed] [Google Scholar]
- 18.Sit DK, Perel JM, Helsel JC, Wisner KL. Changes in antidepressant metabolism and dosing across pregnancy and early postpartum. J Clin Psychiatry. 2008;6:652–8. doi: 10.4088/jcp.v69n0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin PC, Butters L, McCabe R, Kelman A. The influence of pregnancy on drug action; concentration-effect modelling with propranolol. Clin Sci. 1987;73:47–52. doi: 10.1042/cs0730047. [DOI] [PubMed] [Google Scholar]
- 20.Tomi M, Nishimura T, Nakashima E. Mother-to-fetus transfer of antiviral drugs and the importance of transporters at the fetal barrier. J Pharm Sci. 2011;100:3708–18. doi: 10.1002/jps.22642. [DOI] [PubMed] [Google Scholar]
- 21.Vähäkangas K, Pyllynen P. Drug transporters in the human blood-placental barrier. Br J Pharm. 2009;158:665–78. doi: 10.1111/j.1476-5381.2009.00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceccaldi P-F, Gavard L, Mandelbrot L, Rey E, Farinotti R, Jean-Marc Treluyer J-M, Gil S. Functional role of P-glycoprotein and binding protein effect on the placental transfer of lopinavir/ritonavir in the ex vivo human perfusion model. Obstet Gynecol Int. 2009 doi: 10.1155/2009/726593. doi: 10.1155/2009/726593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webster WS, Freeman JA. Prescription drugs and pregnancy. Expert Opin Pharmacother. 2003;4:949–61. doi: 10.1517/14656566.4.6.949. [DOI] [PubMed] [Google Scholar]
- 24.Dolk H. EUROCAT: 25 years of European surveillance of congenital anomalies. Arch Dis Child Fetal Neonatal Ed. 2005;90:F355–F358. doi: 10.1136/adc.2004.062810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijer WM, Cornel MC, Dolk H, de Walle HE, Armstrong NC, de Jong-van den Berg LT for the EUROCAT Working Group. The potential of the European network of congenital anomaly registers (EUROCAT) for drug safety surveillance: a descriptive study. Pharmacoepidemiol Drug Saf. 2006;15:675–82. doi: 10.1002/pds.1265. [DOI] [PubMed] [Google Scholar]
- 26.Garbis H, Elisabeth Elefant B, Diav-Citrin O, Mastroiacovo P, Schaefer C, Vial T, Clementi M, Valti E, McElhatton P, Smorlesi C, Rodriguez EP, Gnansia E, Merlob P, Peiker G, Pexieder T, Schueler L, Ritvanen A, Mathieu-Nolf M. Pregnancy outcome after exposure to ranitidine and other H2-blockers. A collaborative study of the European Network of Teratology Information Services. Reprod Toxicol. 2005;19:453–8. doi: 10.1016/j.reprotox.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy DL, Uhl K, Kweder SL. Pregnancy exposure registries. Drug Saf. 2004;27:215–28. doi: 10.2165/00002018-200427040-00001. [DOI] [PubMed] [Google Scholar]
- 28.Tomson T, Battino D, Craig J, Hernandez-Diaz S, Holmes LB, Lindhout D, Morrow J, French J for the ILAE Commission on Therapeutic Strategies. Pregnancy registries: differences, similarities, and possible harmonization. Epilepsia. 2010;51:909–15. doi: 10.1111/j.1528-1167.2010.02525.x. [DOI] [PubMed] [Google Scholar]
- 29.Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, Irwin B, McGivern RC, Morrison PJ, Craig J. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–8. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrenstein V, Sørensen HT, Bakketeig LV, Pedersen L. Medical databases in studies of drug teratogenicity: methodological issues. Clin Epidemiol. 2010;2:37–43. doi: 10.2147/clep.s9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Källén BAJ, Olausson PO. Maternal Use of Selective Serotonin Re-Uptake Inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79:301–8. doi: 10.1002/bdra.20327. [DOI] [PubMed] [Google Scholar]
- 32.Mølgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA. 2011;305:1996–2002. doi: 10.1001/jama.2011.624. [DOI] [PubMed] [Google Scholar]
- 33.Charlton RA, Cunnington MC, de Vries CS, Weil JG. Data resources for investigating drug exposure during pregnancy and associated outcomes. The General Practice Research Database (GPRD) as an alternative to pregnancy registries. Drug Saf. 2008;31:39–51. doi: 10.2165/00002018-200831010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Suissa S. Immeasurable time bias in observational studies of drug effects on mortality. Am J Epidemiol. 2008;168:329–35. doi: 10.1093/aje/kwn135. [DOI] [PubMed] [Google Scholar]


