Abstract
AIMS
In clinical studies of glucagon-like peptide-1 (GLP-1) agonists used in the management of patients with type 2 diabetes, there is often a small accompanying fall in blood pressure. The mechanism underlying this effect is not known, although exenatide, a GLP-1 mimetic, has acute regional vasodilator properties in rats. We have therefore studied the haemodynamic effects of exenatide in healthy male volunteers.
METHODS
We compared the effects of a single 10 µg subcutaneous injection of exenatide with placebo in a double-blind, randomized, crossover study. For 2 h after dosing, haemodynamic measurements were made using a Finometer, venous occlusion plethysmography and Doppler ultrasound. The urine sodium : creatinine excretion ratio was determined.
RESULTS
At the end of the study when exenatide was compared with placebo, heart rate had risen by a mean of 8.2 (95% CI 4.2, 12.2, P < 0.01) beats min−1, cardiac output by a mean of 1.2 (95% CI 0.42, 20.3, P < 0.05) l min−1 and total peripheral resistance had fallen by 120 (95% CI −8, −233, P < 0.05) dyn s cm−5.There were no differences in blood pressure. The urinary sodium : creatinine ratio was increased by mean 12.4 (95% CI 4.6, 20.2, P < 0.05) mmol mmol−1 when exenatide was compared with placebo.
CONCLUSIONS
Exenatide has significant haemodynamic effects in healthy volunteers. The results of this study are consistent with exenatide having both vasodilator and natriuretic properties. The vascular changes may contribute to the hypotensive effect of exenatide when used chronically in patients with diabetes.
Keywords: blood pressure, exenatide, glucagon-like peptide-1, haemodynamic, natriuresis, urinary sodium : creatinine ratio
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Large clinical trials show that chronic use of GLP-1 agonists cause a fall in blood pressure independent of weight loss. Animal studies have shown acute haemodynamic and vasodilator effects of these drugs. The exact mechanism of the blood pressure drop and acute cardiovascular effects has not been studied in humans.
WHAT THIS STUDY ADDS
This study shows that exenatide also has acute haemodynamic effects in humans. This study demonstrates direct vascular effects and potentially direct cardiac effects. This will enable future studies to look at GLP-1 agonists as aids in the treatment of cardiovascular disease in type 2 diabetes.
Introduction
Glucagon-like peptide-1 (GLP-1) agonists are glucoregulatory agents which have the added benefit of inducing weight loss and are increasingly used in the management of patients with type 2 diabetes (T2DM).
There is increasing evidence that GLP-1 agonists may also have cardiovascular effects including lowering blood pressure (BP). During large phase 3 clinical trials in patients with diabetes, exenatide was associated with a reduction in systolic/diastolic BP of 5/2 mmHg [1]. In a 14 week study of liraglutide at various potentially therapeutic doses in subjects with T2DM there was a reduction in systolic BP of between 5 and 8 mmHg, with a small fall in diastolic BP [2]. The fall in BP in clinical studies is accompanied by a modest rise in resting heart rate [3] suggesting this might be due to vasodilatation or to intravascular volume depletion.
In most patients with T2DM a fall in BP is of potential benefit in cardiovascular risk factor management. However the mechanism for the BP lowering effect of GLP-1 agonists is not known. It is possible, although unlikely, that this relates to the associated weight loss in clinical studies, as the fall in BP occurs early in treatment, before significant weight change [4]. Of potential relevance is the observation that an infusion of GLP-1 induces a natriuresis, which may contribute to a reduction in BP [5]. Alternatively, GLP-1 agonists may have more direct haemodynamic effects.
Exenatide is a synthetic form of exendin-4 which was isolated from the Gila monster lizard. Exendin-4 displays similar properties to human GLP-1. In rats, exendin-4, at equivalent doses not much greater than used therapeutically in man, causes regionally selective and directionally opposite changes in blood flow, with a marked increase in the vascular conductance in the hindquarters but vasoconstriction in the mesenteric and, to a lesser extent, renal vascular beds [6]. Some of these responses appear to be due to indirect (but not hypoglycaemia-induced) sympatho-adrenal activation, although this does not explain all of the changes seen [7]. An infusion of GLP-1 in man has also been shown to have potentially beneficial effects on cardiac function. Some small studies have shown improvement in left ventricular ejection fraction (LVEF) in patients following angioplasty after myocardial infarction [8]. A further study of GLP-1 infusion over 5 weeks in patients with cardiac failure (NYHA II/IV) showed improvement in both LVEF and quality of life [9].
Evidence to date suggests that GLP-1 and associated agonists may therefore have potentially beneficial effects on cardiovascular function and on BP, although few mechanistic studies have been carried out in man. We therefore studied the acute haemodynamic effects of a single dose of exenatide vs. placebo in healthy male volunteers.
Methods
This was a single centre, randomized, placebo-controlled, double-blind, crossover study, approved by the Ethics Committee of the University of Nottingham and conducted in accordance with the Declaration of Helsinki, with participants giving written informed consent. Volunteers were recruited via poster advertisements around the Medical School. Each subject was studied on two occasions at least 1 week apart to compare the effect of exenatide 10 µg (the usual therapeutic dose) or an equivalent volume of saline (placebo) injected subcutaneously. Study randomization was performed using randomization.com.
Participants attended for an initial screening visit for health questionnaire, blood tests and ECG. Eight normal weight healthy males were recruited with mean age 24 (2.5) years and body mass index (BMI) 22.7 (1.4) kg m−2).
During studies, a Finometer™ was used to take continuous measurements of cardiovascular parameters including heart rate (HR), blood pressure (BP), cardiac output (CO) and total peripheral resistance (tPR) [10, 11] Finometry functions by using the volume clamp method. A finometer consists of a blood pressure cuff around the upper arm and a finger probe including a small cuff on the middle finger of the ipsilateral hand. Under normal circumstances blood exerts pressure on the arterial wall. If an equal counter pressure is applied the arterial wall is unloaded. In that situation the counter pressure is equal to intra-arterial pressure. The finger cuff has an infrared sensor connected to a pressure transducer and inflation system. This therefore can act as a clamp to prevent changes in diameter. If a change is detected, the pressure in the finger cuff is adjusted and this once again will lead to an equal pressure between finger cuff and intra- arterial pressure.
Studies took place in a thermally comfortable (25°C) room with subjects lying supine. Subjects attended after an overnight fast and voided prior to study. A retrograde intravenous cannula was inserted in the dorsum of the left hand which rested in a heated box and used to obtain arterial blood samples. Superior mesenteric artery (SMA) blood flow was measured using Doppler ultrasonography by an experienced scanner (LS) who undertook all the scans in the study. Leg blood flow (LBF) was calculated using venous occlusion plethysmography with the left leg slightly elevated.
After 1 h in which baseline measurements were taken, subjects then received a subcutaneous injection of either exenatide or placebo. Finometer readings were recorded continuously for 2 h and averaged over 10 min blocks for analysis. SMA and leg blood flow measurements were taken at half hourly intervals. Blood was sampled every 15 min for immediate determination of glucose and later analysis of insulin concentrations. After 120 min subjects were asked to void, urine volume was measured and samples analyzed for urinary sodium content (sodium : creatinine ratio).
Assays
Glucose concentrations were analyzed immediately using a HemoCue® device (Hemocue AB, Angelholm, Sweden). Serum insulin was measured using a labelled human insulin specific radioimmuno assay kit (Millipore, Massachusetts, USA) at the University of Nottingham. Urinary creatinine concentrations were measured by sarcosine oxidase enzymatic method and urinary sodium concentrations were measured by direct ion selective electrode technology (VITROS Chemistry Products, Ortho Clinical Diagnostics Inc, Rochester NY, USA) at Nottingham University Hospitals NHS Trust.
Statistical analysis
We did not undertake a priori power calculations as this was purely a mechanistic pilot study designed to see if we could detect appreciable haemodynamic effects of exenatide in healthy volunteers.
All results are expressed as means with standard deviations (SD) and absolute differences with 95% confidence intervals (CI). Figures show means and standard errors (SE). Statistical analysis was performed using SPSS version 16 (SPSS, Chicago, Illinois USA). In order to assess responses to exenatide compared with placebo, baseline subtracted data were compared using a two way analysis of variance with repeated measures (anova). Where there was a significant time–treatment interaction, results at individual time points were further compared using a paired t-test. Where there was no time−treatment interaction on anova, we looked for significant treatment or time effects. P values of <0.05 were taken as significant.
Results
All subjects completed both visits and none complained of significant nausea or developed symptomatic hypoglycaemia after exenatide.
Blood glucose and serum insulin (Figure 1)
Figure 1.
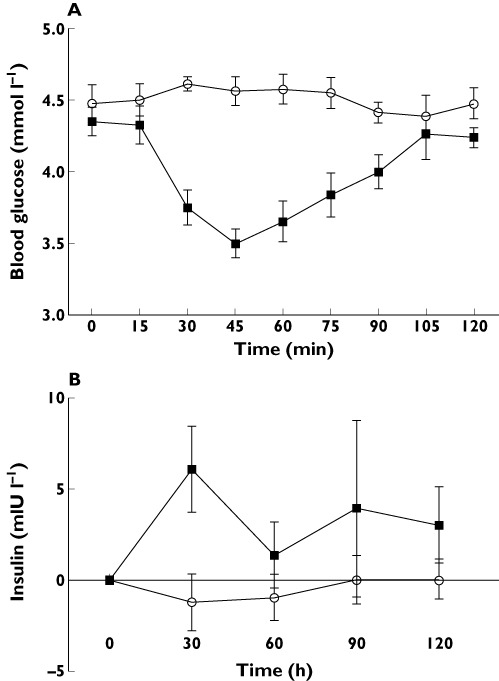
(A) Blood glucose (mmol l−1) and (B) serum insulin concentration (mIU l−1) after placebo (○) or exenatide ( ) injection
) injection
As expected with exenatide mean (SD) blood glucose concentrations fell from 4.5 (0.31) mmol l−1 to a nadir of 3.5 (0.3) mmol l−1 at 45 min and were at 4.2 (0.2) mmol l−1 at 120 min. The lowest individual blood glucose concentration seen was 3.1 mmol l−1 but with no reported symptoms of hypoglycaemia. The serum insulin concentration initially rose with exenatide but returned to near baseline levels at 120 min (P= 0.4, paired t-test at 120 min vs. placebo), Peak insulin increase was seen 30 min post injection (P= 0.03, paired t-test).
Cardiovascular measurements (Figures 2,3)
Figure 2.
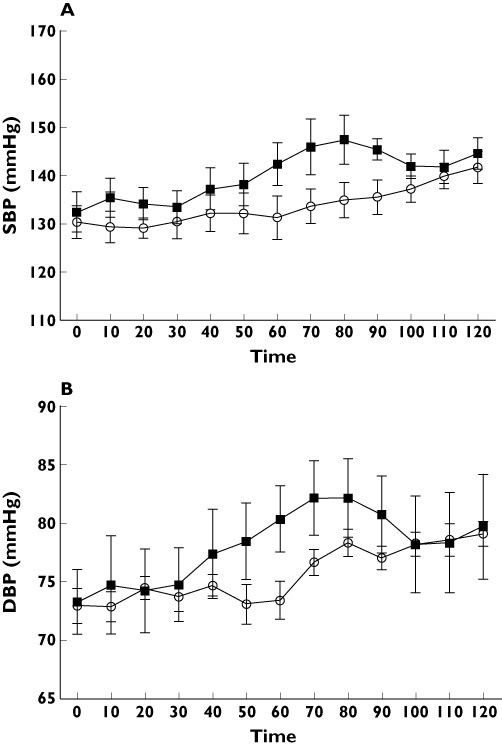
(A) systolic blood pressure (mmHg) and (B) diastolic blood pressure (mmHgl) after placebo (○) or exenatide ( ) injection
) injection
Figure 3.
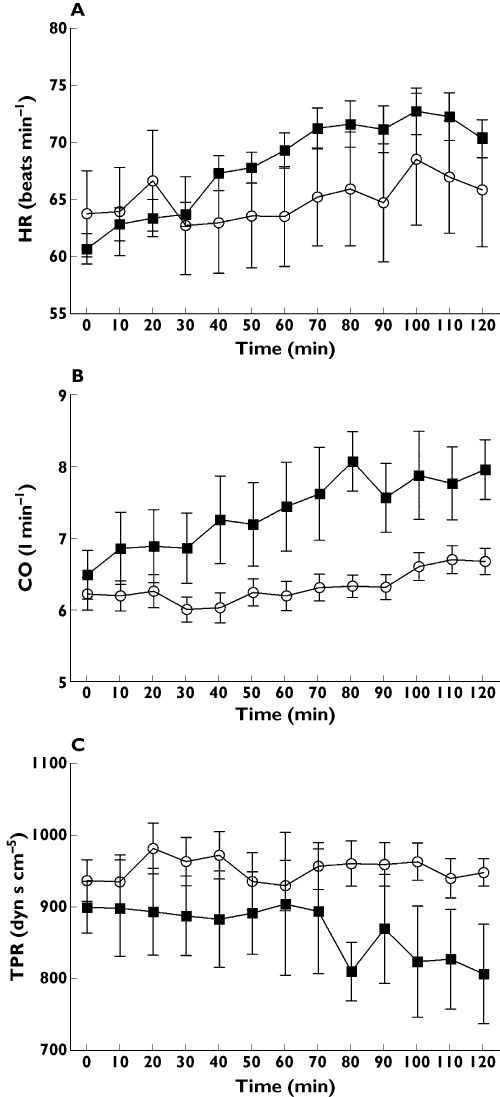
Change in (A) heart rate (beats min−1), (B) cardiac output (l min−1) and (C) total peripheral resistance (dyn s cm−5) after after placebo (○) or exenatide ( ) injection
) injection
There were no significant differences between visits in baseline HR, systolic BP, diastolic BP, CO, tPR, SMA or LBF.
BP (Figure 2)
Overall, both systolic and diastolic BP increased as the study progressed (both P < 0.01, time effects, anova) but there was no significant difference between treatments in the changes in systolic BP (P= 0.28, treatment effect, anova) or diastolic BP (P= 0.31).
HR, CO and tPR (Figure 3)
Over time, there was a greater rise in HR with exenatide compared with placebo (P < 0.01, treatment–time effect, anova). At the end of the study, the increases in HR above baseline were 10.4 (3.0) and 2.1(5.2) beats min−1 with exenatide and placebo respectively (P < 0.01, t-test) with a mean difference of 8.2 (95% CI 4.2, 12.2) beats min−1.
Over the course of the study, CO increased to a greater extent with exenatide than with placebo (P < 0.001, treatment–time effect, anova At the end of the study, CO increased by 1.23 (95% CI, 0.42, 2.03, P < 0.05) l min−1 more with exenatide compared with placebo. Over time, there was a significant fall in tPR with exenatide compared with placebo (P < 0.05, treatment–time effect, anova). At the end of the study, tPR decreased by 120 (95% CI, −8, −233, P < 0.05) dyn s cm−5 more with exenatide compared with placebo.
LBF (Figure 4)
Figure 4.
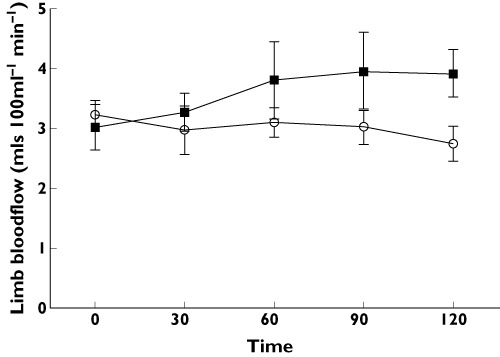
Limb blood flow (ml 100 ml−1 tissue min−1) vs. time after placebo (○) and exenatide ( )
)
There was an increase in LBF with exenatide (P < 0.05, treatment effect, anova) reaching 0.9 (1.3) ml 100 ml−1 tissue min−1 above baseline at 120 min compared with a fall of −0.5 (0.5) ml 100 ml−1 tissue min−1 with placebo.
SMA blood flow
Resting SMA blood flow was 7.8 (2.5) ml s−1 in the exenatide group and 8.8 (3.4) ml s−1 before placebo (NS). There was no significant difference between the changes in SMA blood flow with exenatide and placebo with final values of 9.1 (3.8) and 9.1 (2.4) ml s−1 respectively.
Urinary sodium : creatinine ratio
The urinary sodium : creatinine ratio was measured on the urine sample collected over the total 3 h of study. A urine sample was not collected for one subject so results are given for the remaining seven volunteers. The urinary sodium : creatinine ratio was higher in all subjects with exenatide vs. placebo, with mean values 25.4 (12.3) mmol mmol−1 and 13.0 (5.0) mmol mmol−1, respectively (P < 0.05, paired t-test) with a mean difference of 12.4 (95% CI 4.6, 20.2) mmol mmol−1
Discussion
This study demonstrates that exenatide, a GLP-1 mimetic, has acute haemodynamic effects in man. With exenatide, there were significant increases in HR, CO and LBF, and a decrease in tPR compared with placebo. There were no significant differences between exenatide and placebo in effects on systolic and diastolic BP or on SMA blood flow. The urinary sodium : creatinine ratio was also increased with exenatide. There was an expected increase in serum insulin concentration soon after the exenatide injection, and this was accompanied by a modest fall in blood glucose, but by the end of the study, insulin and glucose concentrations had returned to baseline levels.
The mechanism by which exenatide exerts haemodynamic effects cannot be determined with certainty from this study. However, the increases in HR and CO and the decrease in tPR are most consistent with the medication acting principally as a direct or indirect arterial vasodilator. There was also an increase in LBF with exenatide, consistent with the increase in hindquarter blood flow in studies using rats [6]. SMA blood flow was unaffected by exenatide but there may, of course, have been changes in blood flow or vascular resistance in other regions not measured in the current study. The increase in the urinary sodium : creatinine ratio is consistent with an increase in renal blood flow. One might have expected to observe a trend for BP to fall with vasodilation, which is what is seen in large clinical studies, whereas BP rose here, albeit to a similar extent following placebo and exenatide. In the present study, it is also possible that the increases in HR and CO with exenatide might also in part have been due to direct chronotropic and inotropic actions of the medication, although this does not explain all of the changes seen. An alternative explanation may be that the blood pressures are similar due to baroreflex activity.
A possible explanation for the observed cardiovascular effects of exenatide is that these result from a counter-regulatory sympatho-adrenal response to the modest fall in blood glucose. However in another study with a single subcutaneous injection of exenatide in healthy volunteers, we did not detect a change in arterial plasma epinephrine or norepinephrine concentrations [12]. In addition, the cardiovascular effects of exenatide appear to increase progressively over 120 min, whereas the fall in blood glucose here was maximal at 45 min and then returned to near normal levels. The peak exenatide concentration occurred between 1 and 2 h after subcutaneous injection [13].
There is some evidence for an effect of GLP-1 and agonists on cardiovascular parameters and in particular regional blood flow in animal studies. In rats, there are significant increases in HR and BP in both acute and more chronic settings [6, 14]. In an early study [14] there was a dose dependent increase in both BP and HR in normal rats. Others have shown a biphasic response to GLP-1 with an initial increase in BP followed by a hypotensive episode lasting 30 min [15]. In longer term human studies in patients with T2DM, with both exenatide and liraglutide there is a modest reduction in both systolic and diastolic BP [2, 16, 17]. Further evidence for effects on regional blood flow is seen in a number of studies which suggest peripheral vasodilatation occurs with GLP-1. GLP-1 has been noted to have a direct vasorelaxant action on the pulmonary artery [18] and femoral vessels [19] in rats. A chronic infusion of GLP-1 in hypertensive heart failure prone rats showed a significant increase in CO despite no changes in BP possibly due to peripheral vasodilatation [20]. In healthy human volunteers acute treatment with GLP-1 showed an increase in endothelium dependent blood flow in the forearm [21].
Exenatide has been shown to have beneficial effects on cardiovascular function in clinical studies. A small study of patients with diabetes and NYHA class II-III heart failure showed improvement in systolic and diastolic cardiac function after an infusion of GLP-1 [22]. A further study using a 5 week infusion of GLP-1 in a similar group showed improvements in left ventricular ejection fraction [9]. A longer term retrospective post-marketing surveillance study suggested a lower likelihood of cardiovascular disease in patients treated with exenatide compared with other anti-diabetic therapies [23]. In addition, in long term clinical studies in patients with T2DM, exenatide has been shown to induce significant reductions in BP at 12 weeks [24] and 3 years [25]. Liraglutide has similar effects [26].
We do not yet know whether the fall in BP with GLP-1 agonists in long term studies in patients with diabetes contrasts with the current study because of differences between acute and chronic effects of exenatide or because of variation in effect in a group of older patients with (generally) higher BP.
Our results and other mechanistic investigations, principally animal studies, are consistent with exenatide exerting direct vascular and/or cardiac effects. GLP-1 and its agonists have wide reaching actions beyond modulating pancreatic islet cell function. GLP-1 receptors are located not only on pancreatic islets but also at multiple other sites including the brain, heart, kidney and gastrointestinal tract [27, 28], endothelial and smooth muscle cells [29] and blood cells such as macrophages and monocytes [30]. The presence of GLP-1 receptors on vascular endothelium may be responsible for its effects on blood flow as GLP-1 has shown to increase flow mediated dilatation in patients with diabetes [19]. These effects on vascular relaxation may be induced via both nitric oxide dependent mechanisms and also independently of this by a direct effect on vascular smooth muscle cells via its receptors [31].
Other than causing vasodilatation and a decrease in tPR, an alternative explanation for the BP lowering effect of exenatide when used chronically is that it is due to increased sodium excretion. In the current study, there was a consistent increase in the urine sodium : creatinine ratio associated with exenatide. The true effect of exenatide may be greater than suggested by these data as the urine was collected over 3 h, one of which was prior to the exenatide injection. In Dahl salt sensitive rats, chronic treatment with GLP-1 lessened the development of hypertension, which may have been due to its natriuretic actions [32, 33].
A concern with the current study is that the absolute BP readings appeared to increase by about 10 mmHg in systolic BP and 6 mmHg in diastolic BP overall during both visits. One possibility is that the apparent rise in BP may be artefactual due to prolonged use of the Finometer device. However BP was similar between treatment arms both at the beginning and end of the study whereas CO and tPR were similar before but different after exenatide. In addition HR readings were independent of BP and LBF was measured using a different technique and both showed definite changes with exenatide.
The current study therefore clearly demonstrates significant acute haemodynamic effects resulting from a single dose of exenatide. This finding extends the previous literature concerning GLP-1 and agonists in animals and also in clinical studies in man, where there appear to be beneficial effects on cardiac function and, when used chronically, in BP lowering in patients with T2DM. It is possible that the apparent vasodilator properties of exenatide in man are the mechanism behind the chronic BP lowering effect in patients with diabetes. Considerable further work would be required to substantiate this hypothesis. It would, however, be potentially of great value to establish firmly the mechanism whereby GLP-1 agonists have a chronic hypotensive effect as this may facilitate optimization of BP lowering in combination treatment. It would also provide a rationale for GLP-1 agonists having potentially beneficial haemodynamic effects in the management of patients with T2DM.
Acknowledgments
Funding was provided by Nottingham University Hospitals, Department of Diabetes and Endocrinology research funds. Sally Cordon of the School of Biomedical Sciences, University of Nottingham, Nottingham, UK analyzed the insulin concentrations. Nicola Gilberthorpe of Clinical Pathology Department, Nottingham University Hospitals, Nottingham, UK analyzed the urine sodium and creatinine concentrations.
Professor Johannes J. van Lieshout, Acute Admissions Unit Internal Medicine & Laboratory of Clinical Cardiovascular Physiology, University of Amsterdam, the Netherlands advised regarding Finometer analysis.
Competing Interests
There are no competing interests to declare.
Contribution statement: BM, ES, IM and PM were all involved in the design of the study. BM and ES undertook the study procedures. BM, IM and PM have written and edited the manuscript.
REFERENCES
- 1.Nauck MA, Duran S, Kim D, Johns D, Northrup J, Festa A, Brodwos R, Trautmann M. A comparison of twice daily exenetide and biphasic insulin as part in patients with type 2 diabetes who were subopitmally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259–67. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- 2.Vilsbøll T, Zdravkovic M, Le-Thi T, Krarup T, Schmitz O, Courrèges JP, Verhoeven R, Bugánová I, Madsbad S. Liraglutide, a long acting human glucagon like peptide analogue, given as monotherapy significantly improves glycaemic control and lowers body weight in people with type 2 diabetes. Diabetes Care. 2007;30:1608–10. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- 3.Buse JB, Rosenstock J, Sesti GG, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 4.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simó R. Liraglutide versus insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes (LEAD 5-Met + SU) a randomised controlled trial. Diabetologia. 2009;52:2046–55. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutzwiller JP, Tscopp S, Bock A, Zehnder CE, Huber AR, Kreyenbuehl M, Gutmann H, Drewe J, Henzen C, Goeke B, Beglinger C. Glucagon like peptide induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89:3055–61. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner SM, March JE, Kemp PA, Bennett T. Mesenteric vasoconstriction and hindquarter vasodilatation accompany the pressor actions of exendin-4 in conscious rats. J Pharmacol Exp Ther. 2006;316:852–9. doi: 10.1124/jpet.105.093104. [DOI] [PubMed] [Google Scholar]
- 7.Gardiner S, March J, Kemp P, Bennett T. Autonomic nervous system-dependent and independent cardiovascular effects of exendin-4 infusions in conscious rats. Br J Pharmacol. 2008;154:60–71. doi: 10.1038/bjp.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nikolaidis L, Mankad S, Sokos G, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon like peptide in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 9.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–9. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 10.Jansen J, Screuder J, Wesseling K. A comparison of cardiac output derived from the arterial pressure wave against thermodilution in cardiac surgery patients. Br J Anaesth. 2001;87:212–22. doi: 10.1093/bja/87.2.212. [DOI] [PubMed] [Google Scholar]
- 11.Schutte A, Huisman H, van Rooyen J, Malan N, Schutte R. Validation of the Finometer device for measurement of blood pressure in Black women. J Hum Hypertens. 2004;18:79–84. doi: 10.1038/sj.jhh.1001639. [DOI] [PubMed] [Google Scholar]
- 12.Khoo E, Wallis J, Tsintzas K, Macdonald I, Mansell P. Effects of circulating glucose, insulin, glucagon, cortisol and catecholamines in healthy volunteers during exercise. Diabetologia. 2010;53:139–43. doi: 10.1007/s00125-009-1579-1. [DOI] [PubMed] [Google Scholar]
- 13.Kothare P, Linnebjerg H, Isaka Y, Uenaka K, Yamamura A, Yeo KP, de la Peña A, Teng CH, Mace K, Fineman M, Shigeta H, Sakata Y, Irie S. Pharmacokinetics, pharmacodynamics, tolerability, and safety of exenatide in Japanese patients with Type 2 diabetes mellitus. J Clin Pharmacol. 2008;48:1389–99. doi: 10.1177/0091270008323750. [DOI] [PubMed] [Google Scholar]
- 14.Barragan JM, Rodriguez RE, Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7-36) amide in rats. Am J Physiol. 1994;266:E459–66. doi: 10.1152/ajpendo.1994.266.3.E459. [DOI] [PubMed] [Google Scholar]
- 15.Bojanowska E, Stempniak B. Effects of glucagon-like peptide-1 (7-36)amide on neurohypophysial and cardiovascular functions under hypo- or normotensive hypovolaemia in the rat. J Endocr. 2002;172:303–10. doi: 10.1677/joe.0.1720303. [DOI] [PubMed] [Google Scholar]
- 16.Klonoff D, Buse J, Nielsen L. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275–86. doi: 10.1185/030079908x253870. [DOI] [PubMed] [Google Scholar]
- 17.Buse JB, Bergenstal RM, Glass LC. Use of twice daily exenatide in basal insulin treated patients with type 2 diabetes: a randomised controlled trial. Ann Intern Med. 2011;154:103–12. doi: 10.7326/0003-4819-154-2-201101180-00300. [DOI] [PubMed] [Google Scholar]
- 18.Golpon HA, Peuchner A, Welte T, Wichert PV, Federsen CO. Vasorelaxant effect of glucagon-like peptide – (7-36) amide and amylin on the pulmonary circulation of the rat. Regul Pept. 2001;102:81–6. doi: 10.1016/s0167-0115(01)00300-7. [DOI] [PubMed] [Google Scholar]
- 19.Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahrén B, Sjöholm A. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–15. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 20.Poornima I, Brown S, Bhashyam S, Parikh P, Bolukoglu H, Shannon R. Chronic glucagon-like peptide-1 infusion sustains left ventricular systolic function and prolongs survival in the spontaneously hypertensive, heart failure-prone rat. Circ Heart Fail. 2008;1:153–60. doi: 10.1161/CIRCHEARTFAILURE.108.766402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu A, Charkoudian N, Schrage W, Basu R, Joyner M. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide not glimepiride. Am J Physiol Endocrinol Metab. 2007;293:E1289–95. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- 22.Thraisndottir I, Malmberg K, Olsson A, Gutniak M, Ryde N. Initial experience with GLP-1 treatment on metabolic control and myocardial function in patients with type 2 diabetes mellitus and heart failure. Diab Vasc Dis Res. 2004;1:40–3. doi: 10.3132/dvdr.2004.005. [DOI] [PubMed] [Google Scholar]
- 23.Best J, Hoogwerf B, Herman W. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the GLP-1 receptor agonist exenatide twice daily or other glucose lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011;34:90–5. doi: 10.2337/dc10-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill A, Hoogwerf BJ, Burger J, Bruce S, Macconell L, Yan P, Braun D, Giaconia J, Malone J. Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus: a double-blind, placebo-controlled, randomized pilot study. Cardiovasc Diabetol. 2010;9:6. doi: 10.1186/1475-2840-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–47. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 26.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L. LEAD-4 Study Investigators. Efficacy and safety of the human glucagon-like peptide-1 analogue liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–30. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holst JJ. The physiology of glucagon like peptide. Physiol Rev. 2007;87:1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 28.Bullock BP, Heller RS, Habener JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon like 1 receptor. Endocrinology. 1996;137:298–2978. doi: 10.1210/endo.137.7.8770921. [DOI] [PubMed] [Google Scholar]
- 29.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117:2340–50. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 30.Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesions by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–7. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nystrom T, Gonon A, Sjoholm A, Pernow J. Glucagon like peptide-1 relaxes rat conduit arteries via an endothelium independent mechanism. Regul Pept. 2005;125:173–7. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–8. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 33.Hirata K, Kume S, Araki S, Sakaguchi M, Chin-Kanasaki M, Isshiki K, Sugimoto T, Nishiyama A, Koya D, Haneda M, Kashiwagi A, Uzu T. Exendin-4 has an anti-hypertensive effect in salt-sensitive mice model. Biochem Biophys Res Commun. 2009;380:44–9. doi: 10.1016/j.bbrc.2009.01.003. [DOI] [PubMed] [Google Scholar]


