Abstract
AIMS
Our aim was to identify and quantify the sources of variability in oxypurinol pharmacokinetics and explore relationships with plasma urate concentrations.
METHODS
Non-linear mixed effects modelling was applied to concentration–time data from 155 gouty patients with demographic, medical history and renal transporter genotype information.
RESULTS
A one compartment pharmacokinetic model with first order absorption best described the oxypurinol concentration–time data. Renal function and concomitant medicines (diuretics and probenecid), but not transporter genotype, significantly influenced oxypurinol pharmacokinetics and reduced the between subject variability in the apparent clearance of oxypurinol (CL/Fm) from 65% to 29%. CL/Fm for patients with normal, mild, moderate and severe renal impairment was 1.8, 0.6, 0.3 and 0.18 l h−1, respectively. Model predictions showed a relationship between plasma oxypurinol and urate concentrations and failure to reach target oxypurinol concentrations using suggested allopurinol dosing guidelines.
CONCLUSIONS
In conclusion, this first established pharmacokinetic model provides a tool to achieve target oxypurinol plasma concentrations, thereby optimizing the effectiveness and safety of allopurinol therapy in gouty patients with various degrees of renal impairment.
Keywords: allopurinol, gout, oxypurinol, pharmacogenetics, pharmacokinetics, urate
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Response to allopurinol in patients with gout is often suboptimal due to large variability in pharmacokinetics.
The sources of the variability in oxypurinol pharmacokinetics have not been systematically identified and quantified.
A therapeutic target 15–23 mg l−1 for oxypurinol concentrations has recently been established.
WHAT THIS STUDY ADDS
Renal function and concomitant diuretic/probenecid use significantly influence oxypurinol pharmacokinetics.
This model provides a tool to achieve target oxypurinol plasma concentrations, thereby optimizing the effectiveness and safety of allopurinol therapy in gouty patients with various degrees of renal impairment.
Model predictions show a failure to reach target oxypurinol concentrations using suggested allopurinol dosing guidelines.
Introduction
The cornerstone of the management of gout is the treatment of the proximate cause, namely hyperuricaemia. Allopurinol is the most frequently prescribed urate-lowering agent [1–3] due to its efficacy, tolerability and convenient, once daily dosing regimen [4]. Allopurinol is rapidly metabolized (half-life approximately 1 h) to its active metabolite oxypurinol. Oxypurinol is an inhibitor of xanthine oxidoreductase and has a considerably longer elimination half-life (approximately 23 h) [5, 6]. As a result, oxypurinol is responsible for the pharmacological activity of allopurinol [7].
The pharmacokinetics of both allopurinol and oxypurinol have been described primarily in healthy subjects [6]. There are limited data available regarding the pharmacokinetics of oxypurinol in patients with gout and even less information for gouty patients with renal impairment. The oral bioavailability of allopurinol is reasonably high, approximately 80% [6, 7] and is independent of age [8]. Of 100 mg allopurinol absorbed, 90 mg is metabolized to oxypurinol [6]. ‘Fm’ is herein used as the abbreviation to denote the fraction of the allopurinol dose systemically available as oxypurinol. The apparent volume of distribution of oxypurinol (V/Fm) is approximately 43 l and correlates with the body weight of healthy subjects [9], consistent with the low protein and tissue binding of oxypurinol [10]. Oxypurinol is primarily excreted by the kidneys and undergoes considerable active tubular reabsorption [6]. It is unclear which renal transporters mediate the reabsorption of oxypurinol. However, given the similar structure and molecular size to urate, organic anion transporters (SLC22A8, SLC22A11, SLC22A13) and other urate transporters (SLC2A9, ABCG2, SLC17A1) are the most likely candidates [11, 12].
Although there are relatively limited pharmacokinetic data in gouty patients [6] the total apparent clearance of oxypurinol (CL/Fm) has been shown to be reduced in older patients [8] and in patients with renal impairment [13–15]. During chronic dosing with allopurinol, steady-state plasma oxypurinol concentrations increase linearly with the dose of allopurinol, although there is considerable inter-patient variability, even in people with normal renal function [15]. The variability in oxypurinol pharmacokinetics during chronic administration in gouty patients with renal impairment and the effect of concomitant medications on pharmacokinetics are unknown. The exact causes of the inter-patient variability in oxypurinol pharmacokinetics remain unclear. However, variability in renal function, adherence, diet, drug–drug interactions and genetic variation in kidney transporters of oxypurinol are potential contributing factors.
Current allopurinol dosing guidelines are based on renal function alone [16]. Target plasma concentrations of oxypurinol have been suggested including, 5–15 mg l−1[13] and, more recently, 15–23 mg l−1[17] at 6–9 h post dose. Both ranges are consistent with the finding that 90% of xanthine oxidase activity is inhibited by oxypurinol concentrations of approximately 5 mg l−1[15]. Monitoring of plasma oxypurinol concentrations has been suggested in patients who do not achieve a satisfactory reduction in plasma urate concentration when dosed according to guidelines [17]. However, further work on the value of monitoring plasma oxypurinol concentrations is needed. A second approach to optimize allopurinol use, and for which there is increasing support [18–20], is to titrate the dose until target plasma urate concentrations are achieved. Nevertheless, further research is required to understand better the optimal dosing of allopurinol to reach target plasma urate concentrations, in particular, in patients with renal impairment and/or who are taking potentially interacting concomitant medications and where monitoring plasma oxypurinol concentrations may add value.
Therefore, the aims of the present study were (i) to assess the variability in the pharmacokinetics of oxypurinol in patients with gout and to evaluate and quantify the factors that affect oxypurinol pharmacokinetics and (ii) to correlate plasma oxypurinol concentrations with urate concentrations potentially to guide dosing of allopurinol.
Methods
Patients
An observational clinical study was conducted of 155 patients from the hospital and community setting prescribed allopurinol for the treatment of gout. The study protocol was approved (St Vincent's Hospital Human Research Ethics Committee approvals H06/107, H06/141; and Australian and New Zealand Clinical Trials register: ACTRN12611000743965, ACTRN012606000276550) and written informed consent was obtained from each participant.
Patients were included in the study if they were at least 18 years of age and had been taking allopurinol for at least 7 days. The indication for prescribing allopurinol, namely gout or hyperuricaemia, was established.
Study procedures
Medication history, including allopurinol dosing regimen and dose adjustments were recorded. Venous blood samples, up to 30 per person, were taken at a range of times over the dosage interval with accurate recording of sampling times. Plasma urate, creatinine and oxypurinol concentrations were measured. Where possible, at least two blood samples were collected within the dosing interval. All blood samples were centrifuged, and plasma stored at −20°C for analysis.
Drug assay
Oxypurinol concentrations in the plasma were measured using a validated high performance liquid chromatography method [21]. The limit of detection was 0.14 mg l−1 and the limit of quantification was 2.0 mg l−1. The assay was linear over the range 2 to 50 mg l−1 and the intra- and inter-day coefficients of variation were <2% for both high (15 mg l−1) and low (5 mg l−1) quality control samples.
Pharmacogenetic analysis
DNA was extracted from whole blood. Non-synonymous polymorphisms were determined using an oligo-ligation assay (SNPlex; Applied Biosystems, Foster City, CA) following the manufacturer's instructions. Polymerase chain reaction amplification of the genes of interest was performed using oligonucleotide primers (Supplementary Table S2) and using 10 ng DNA in a final volume of 5 µl. Amplified DNA was purified with exonuclease I and genotyped using the SequenomMassSpecIplex® system by the Australian Genome Research Facility Ltd (Garvan Institute of Medical Research, Sydney, Australia). SNP analysis was performed with the software Typer 4.0.3. (Applied Biosystems, Foster City, CA, USA).
Population pharmacokinetic modelling
The concentration–time data for oxypurinol in plasma were analyzed using the nonlinear mixed-effects modelling program, NONMEM® (Version 7.1.0, ICON Development Solutions, Ellicott City, MD, USA) using the first order conditional estimation (FOCE) method with interaction. PSN 3.4.2 and Xpose Version 4.3.0 were used for model diagnostic and validation. One and two compartmental pharmacokinetic models with linear elimination and a range of absorption models (zero or first order) were evaluated. Model derived values of CL/Fm and V/Fm for oxypurinol were estimated. In this analysis doses (mg day−1) of oxypurinol were assumed to be 90% of the allopurinol dose [6].
Between subject, between occasion and residual variability
The overall variability in the pharmacokinetic parameters was estimated as population parameter variability (PPV). The PPV incorporated the between subject variability (BSV) and the between-occasion variability (BOV). BOV includes both between occasion and within occasion variability. An occasion was defined as a change in concomitant medication(s) and/or readmission into hospital as assessed for each concentration–time data. The correlations between random variables were explored. Additive, proportional and combined error models were evaluated to describe the residual unexplained variability.
Covariate model development
The covariates analyzed were age, gender, total body weight (TBW), lean body weight (LBW) [22], creatinine clearance (CLCr) estimated using the Cockcroft–Gault equation [23] corrected for LBW or using TBW, concomitant medications (including probenecid, diuretics, low dose aspirin, colchicine, warfarin, angiotensin II receptor antagonists, β-adrenoceptor antagonists, angiotensin converting enzyme inhibitors, calcium channel blockers, HMG-CoA reductase inhibitors and antibiotics), adherence (as assessed by using the Beliefs about Medicine Questionnaire (BMQ) [24]) and genetic polymorphisms in renal transporters including SLC2A9, ABCG2, SLC22A13, SLC17A1, SLC22A11 and SLC22A8. Patients whose genotype was not assessable were assigned the reference (wild-type) genotype. The individual covariates were centered on the median value and all covariates were investigated. A stepwise approach was used to identify covariates that contributed to the CL/Fm and V/Fm of oxypurinol. The forward and backward thresholds were set at P < 0.05 and P < 0.01, respectively. Covariates were included in the final model (i) if they increased the likelihood of the base model, (ii) if they explained some of the proportional variability and (iii) if they were biologically plausible.
Model selection and validation
Model selection was based on a number of criteria including the χ2 test (likelihood ratio), goodness of fit plots, biologically plausible parameter estimates and numerical (NPC) and visual predictive checks (VPC) stratified for the significant covariates. Non-parametric bootstrap with re-sampling and replacement was performed [25] to assess the stability of the final model and to refine the uncertainty around the parameters.
Simulations
To understand the impact of important covariates on plasma concentrations of oxypurinol and their relationship with plasma concentrations of urate, model simulations were performed. Different dosing regimens and effects of dose adjustments and covariates on the pharmacokinetics of oxypurinol and plasma concentrations of urate were explored.
Results
Patients
Patients with gout (n = 155), comprising hospitalized inpatients (n = 129) and community patients (n = 26), were enrolled. Blood samples were obtained from 155 patients who were taking allopurinol for the treatment of gout (n = 146) or asymptomatic hyperuricaemia (n = 9). Patients with asymptomatic hyperuricaemia are referred to as gouty patients throughout the manuscript. The demographic and clinical characteristics of the gouty patients included in the analysis are shown in Table 1. All patients were being prescribed at least one other medication, with a median of 9 (range 1–18) medications other than allopurinol. This list of medications did not include vitamin and mineral supplements but did include over the counter medicines, such as aspirin. The frequency of concomitant medications largely reflected the frequency of comorbidities in the study population. Genotyping results for polymorphisms in renal transporter genes SLC2A9, ABCG2, SLC22A13, SLC17A1, SLC22A11 and SLC22A8 are presented in Supplementary Table S1. Due to genotyping failure and poor DNA quality, seven patients were assigned the reference (wild-type) genotype for the SLC2A9 variant, rs3733591, and two patients were assigned to the reference genotype for all polymorphisms. Genetic variation for the selected polymorphisms in SLC17A1, SLC2A9 and ABCG2 were common. By contrast, there were no patients carrying both variant alleles for the polymorphisms in SLC22A8, SLC22A11 or SLC22A13. All variation was within Hardy–Weinberg equilibrium.
Table 1.
The demographic and clinical profile of the 155 patients with gout
| Patient characteristic | Number (%) | Median | Range |
|---|---|---|---|
| Number of patients | 155 | ||
| Male/Female | 132/23 | ||
| Age (years) | 69.0 | 28.0–93.6 | |
| Height (cm) | 172 | 147–198 | |
| Weight (kg) | 83.0 | 42.5–139.0 | |
| BMI (kg m−2) | 27.6 | 17.4–46.4 | |
| Creatinine clearance (ml min−1)* | 37.6 | 6.0–130.4 | |
| Plasma urate concentration (mmol l−1) | 0.33 | 0.14–0.81 | |
| Allopurinol dose (mg day−1) | 300 | 50–400 | |
| Duration of gout (years)† | 11 | 0.2–>40 | |
| Tophi present | 20 (13) | ||
| Pharmacokinetic data | |||
| Number of blood samples | 1013 | ||
| Concentration (mg l−1) | 0.14–70.9 | ||
| Samples per patient | 1–30 | ||
| Number of occasions‡ | 1–5 | ||
| Concomitant medication | |||
| Diuretic§ | 72 (46) | ||
| Probenecid | 20 (13) |
SD, standard deviation; BMI, body mass index. *Estimated using the Cockcroft–Gault equation with total body weight; †Self-reported; ‡An occasion is classified as either (i) a new admission into hospital or (ii) commencement of a new concomitant medication and/or post a major event (e.g. surgery) §Including furosemide, thiazide diuretics and spironolactone.
Oxypurinol concentration–time observations (n= 1013, 1–30 per patient) ranging from 0.14–70.9 mg l−1, collected primarily during the dosing interval (0–24 h) were available for the population modelling. The number of occasions ranged from 1–5 per patient (Table 1) and individual allopurinol doses ranged from 50–400 mg day−1 (median of 300 mg day−1). The dose of allopurinol was altered in 21 patients. All data below the limit of quantification (<2 mg l−1, n= 23) were above the limit of detection and therefore measurable and included in the analysis. Seven patients were removed from the analysis because they were not at steady-state as demonstrated by large day to day fluctuations in plasma oxypurinol concentrations (n= 4), were undergoing dialysis (n= 2) or were in acute renal failure (n= 1).
Model building
A one compartment pharmacokinetic model with first order input and combined error model was found to best describe the observed concentration–time data. The BSV was estimated for CL/Fm and V/Fm, as well as the covariance between these parameters. The inclusion of BOV on CL/Fm resulted in a further reduction in the likelihood ratio and was found to be 21%. The diagnostic plots and VPC confirmed that the base model adequately described the data.
Covariate pharmacokinetic model
The final model included CLCr estimated using LBW, the concomitant use of diuretics (including thiazide or furosemide) and/or probenecid therapy as significant covariates for CL/Fm. V/Fm was allometrically scaled using LBW, despite not leading to a significant improvement in the likelihood or decrease in the BSV, since biologically V/Fm is likely to be dependent on body weight. The rs1165196 polymorphism in SLC17A1, which encodes the sodium-dependent phosphate transporter 1 NPT-1, increased the CL/Fm by 31%. However, after adjustment for CLCr, this effect was not observed (Figure 1, Table 2). Patients carrying the rs16890979 polymorphism in SLC2A9, which encodes the facilitated glucose transporter member 9 (GLUT9), had a 17% reduction in the CL/Fm, independent of CLCr and probenecid use. Interestingly, the reduction in CL/Fm was more pronounced (reduced by 41%) in patients taking a diuretic (Figure 1) and hence the effect of the polymorphism was not observed after accounting for diuretic use (Table 2). Overall, adherence to allopurinol therapy, as measured by the BMQ [24], was associated with a slight (20%), but not significant decrease in the ‘apparent’ oral availability of oxypurinol (Fm). Although age and gender improved the likelihood of the model, they did not explain any BSV in CL/Fm and were, therefore, not retained in the model (Table 2).
Figure 1.
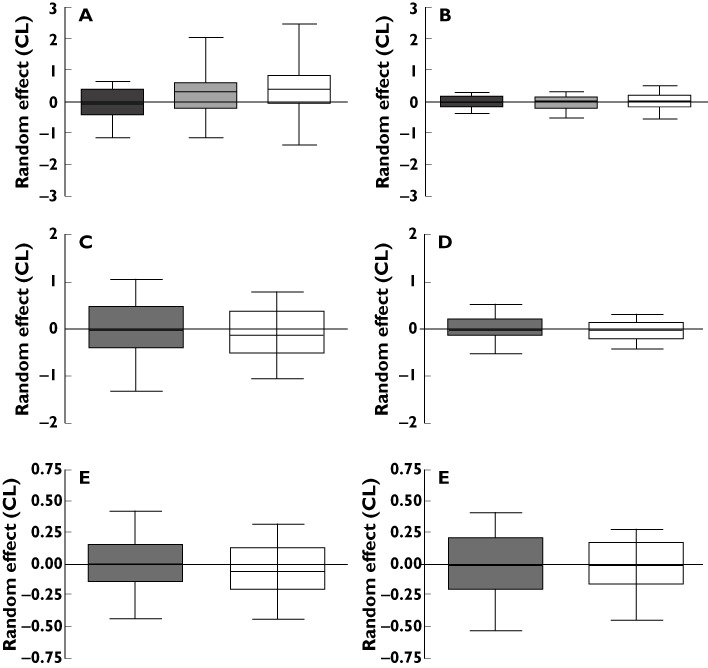
A box plot of the apparent renal clearance of oxypurinol (random effect) in all patients for the (A) NPT-1 rs1165196 polymorphism unadjusted for covariates (n= 31, 60, 64), (B) NPT-1 rs1165196 polymorphism adjusted for covariates (n= 31, 60, 64), (C) GLUT9 rs16890979 polymorphism unadjusted for covariates (n= 108, 47), (D) GLUT9 rs16890979 polymorphism adjusted for covariates (n= 108, 47), (E) GLUT9 rs16890979 polymorphism not taking a diuretic (n= 85) and (F) GLUT9 rs16890979 polymorphism in patients taking a diuretic (n= 70). Patients carrying both reference (wild-type) alleles (grey), one variant allele (light grey stripes) or both variant alleles (white)
Table 2.
The influence of significant covariates individually and in combination on the apparent clearance of oxypurinol
| Between subject variability (%) | Proportional error (%) | Likelihood ratio | |
|---|---|---|---|
| Base model | 65 | 21.7 | 3296 |
| rs1165196 polymorphism in SLC17A1 | 40 | 16.7 | 3291 |
| rs16890979 polymorphism in SLC2A9 | 36 | 16.7 | 3345 |
| CLCr | 39 | 13.7 | 3054 |
| CLCr+ rs16890979 polymorphism in SLC2A9 | 36 | 13.6 | 3162 |
| CLCr+ diuretic use | 31 | 13.4 | 3022 |
| CLCr+ diuretic use + probenecid use* | 28 | 13.3 | 3004 |
| CLCr+ diuretic use + probenecid use + gender | 27 | 13.2 | 2996 |
| CLCr+ diuretic use + probenecid use + gender + age | 27 | 13.1 | 2987 |
CLCr, estimated creatinine clearance using Cockcroft-Gault equation with lean body weight; *Final covariate model.
Inclusion of significant covariates showed substantial reduction in the BSV in CL/Fm from 65% to 28% and in V/Fm from 66% to 45%. The estimates of the final model were 0.62 l h−1 and 38.1 l kg−1 for CL/Fm and V/Fm, respectively. The results of the base and final (covariate) pharmacokinetic model are summarized in Table 3.
Table 3.
Parameter estimates of the base model, the covariate model and the 1000 bootstrap runs (median and 95th percentiles)
| Parameter | Base model | Covariate (final) model | 1000 bootstrap replicates median (95% CI) |
|---|---|---|---|
| Objective Function Value | 3296 | 3039 | 3015 |
| Fixed parameters | |||
| CL/Fm (l h−1)* | 1.587 | 0.595 | 0.594 (0.548, 0.646) |
| V/Fm (l)† | 32.6 | 38.1 | 38.3 (33.2, 44.4) |
| Kfm | 0.19 | 0.447 | 0.438 (0.301, 0.628) |
| Random parameters (CV%) | |||
| BSV CL/Fm | 65 | 28 | 28 (21, 33) |
| BSV V/Fm | 66 | 45 | 44 (33, 54) |
| COV (CL/V), R | −0.13 | 0.33 | 0.30 (−0.12, 0.48) |
| BOV CL/Fm | 26 | 21 | 20 (13, 28) |
| Effects of covariates on CL/Fm | |||
| Creatinine clearance (θ6) | 0.0250 | 0.025 (0.021, 0.028) | |
| Diuretics (θ7) | −0.294 | −0.298 (−0.386, −0.207) | |
| Probenecid (θ8) | 0.383 | 0.384 (0.264, 0.499) | |
| Residual Error | |||
| Additive (mg l−1) | 0.63 | 1.32 | 1.32 (0.77, 1.64) |
| Proportional (CV%) | 22.0 | 13.1 | 12.9 (9.4, 17.2) |
CL/Fm, apparent clearance of oxypurinol; V/Fm, apparent volume of distribution; Kfm, formation rate constant; Diuretics, the effect of diuretic use (thiazide, furosemide, spironolactone) on oxypurinol clearance; Probenecid, the effect of probenecid on oxypurinol clearance; BSV, between subject variability; COV, covariance; r, correlation coefficient; BOV, between occasion variability; *For a typical patient that has a estimated creatinine clearance of 37.6 ml min−1; †For a typical patient who has a lean body weight of 60 kg.
where CL/Fm, apparent clearance of oxypurinol, V/Fm, apparent volume of distribution of oxypurinol, CLCr, estimated creatinine clearance using the Cockcroft–Gault equation and based on LBW (ml min−1), DIUR, diuretic co-administration (DIUR = 1 if patient taking a diuretic), PROB, probenecid co-administration (PROB = 1 if patient taking probenecid), PPV, population parameter variability, ETA, individual random effect, BOVCL, the between occasion variability in the apparent clearance of oxypurinol and LBW, lean body weight (kg).
Model evaluation
The goodness of fit plots for the final (covariate) model showed no visual or statistical bias for the model predictions. The NPC (data not shown) and VPC (Figure 2) verified the appropriateness of the model. The VPC also demonstrated that the model was appropriate in patients with varying degrees of renal impairment including severe and moderate renal impairment (Figure 2B). The non-parametric bootstrap had a good convergence demonstrating that the model was stable (Table 3).
Figure 2.
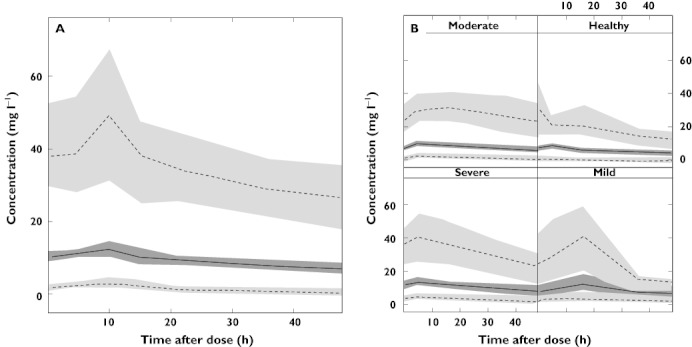
Visual predictive check showing the 95% percentiles (dashed line) and 50th percentile (solid line) and their respective confidence intervals for the model predicted data of the total cohort for (A) 48 h after allopurinol dosing in the total cohort and (B) 48 h after allopurinol dosing in patients with varying degrees of renal impairment. Healthy, CLCr >60 ml min−1; mild, CLCr 30–60 ml min−1; moderate, CLCr 15–30 ml min−1; severe, CLCr 0–15 ml min−1
Simulations
To investigate the impact of renal function, allopurinol dose changes and concomitant medicines on (i) the concentration–time profile of oxypurinol and (ii) the relationship between oxypurinol and urate concentrations, simulations and long-term model predictions, were derived. Representative patients are shown in Figure 3A–C. Simulations demonstrated that plasma concentrations of oxypurinol and urate were inversely related, hence plasma urate concentrations increased if plasma concentrations of oxypurinol decreased and vice versa (Figure 3A–C). The simulations also confirmed that renal function and allopurinol dose were important determinants of both plasma oxypurinol and urate concentrations (Figure 3A,B). Furthermore, commencement of probenecid therapy, which increases the CL/Fm, was associated with lower plasma concentrations of oxypurinol and urate (Figure 3C). Target plasma urate concentrations, less than 6 mg dl−1 (0.36 mmol l−1), were achieved with a range of plasma oxypurinol concentrations. Furthermore, model predictions highlighted the significance of renal function on the plasma oxypurinol concentrations achieved with a particular dose of allopurinol (Figure 4).
Figure 3.
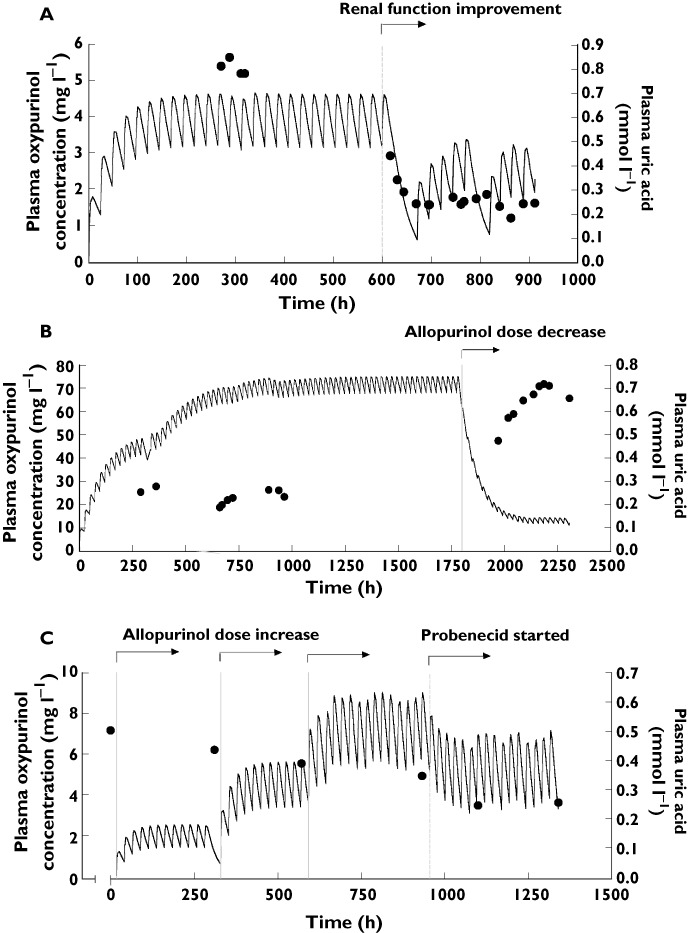
The dosing history and corresponding (simulated) plasma concentrations of oxypurinol (lines) and urate (black dots) of a patient with gout who (A) has significant changes to their renal function, (B) has a change in their allopurinol dose from 300 mg day−1 to 100 mg day−1 and (C) was commenced on allopurinol, had the doses of allopurinol increased from 100, 200 to 300 mg day−1 and was subsequently commenced on probenecid therapy during the period of observation. The dashed lines represent the time each change was implemented
Figure 4.
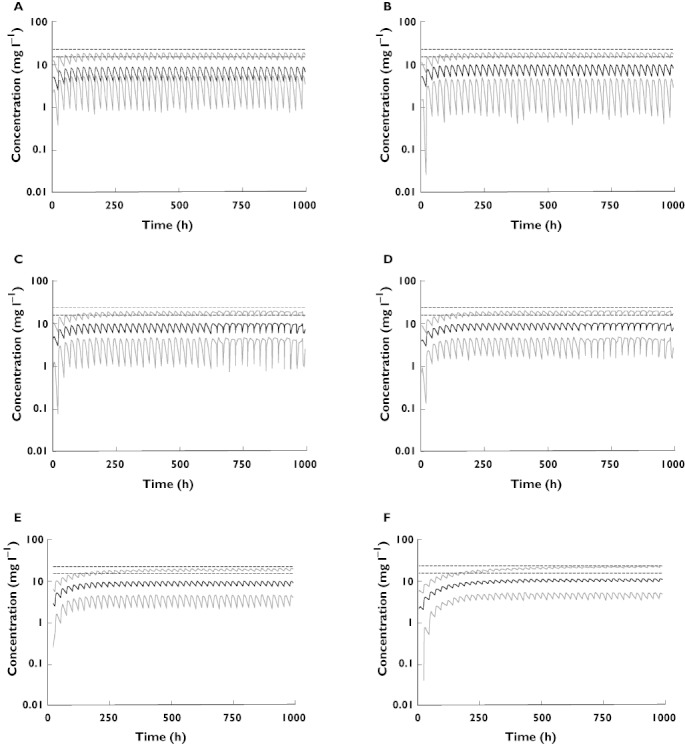
The model predicted median (black lines) and 95% confidence intervals (grey lines) plasma concentrations of oxypurinol over time for patients being dosed with allopurinol according to the current dosing guidelines. Each panel depicts a patient with particular renal function receiving an allopurinol dose of (A) 350 mg day−1 (CLCr= 120 ml min−1), (B) 300 mg day−1 (CLCr= 100 ml min−1), (C) 250 mg day−1 (CLCr= 80 ml min−1), (D) 200 mg day−1 (CLCr= 60 ml min−1), (E) 150 mg day−1 (CLCr= 40 ml min−1) and (F) 100 mg day−1 (CLCr= 20 ml min−1) according to current allopurinol dosing guidelines [16]. The dashed line represents the minimum recommended target plasma oxypurinol concentration 15.2 −22.8 mg l−1
Discussion
The population pharmacokinetic analysis has, for the first time, quantitatively explored the relative impact of a range of clinical characteristics, including genetic factors, on the pharmacokinetics of oxypurinol in people with hyperuricaemia and/or gout. This model has the potential to be used for dose adjustment based on target plasma oxypurinol concentrations and guide titration of allopurinol dose against plasma concentrations of urate to optimize clinical response.
A one compartment pharmacokinetic model with first order input described the concentration–time data well, which is consistent with previous work in a paediatric population [26]. The pharmacokinetic parameters found in this study of hospitalized and community patients are similar to those reported in previous non-compartmental analyses. The mean CL/Fm was similar to that in gouty patients [27]. However, it was almost half (approximately 47%) of that reported for healthy volunteers (1.3 l h−1) [6]. This is because healthy volunteers have a renal function of approximately 120 ml min−1 and the median CLCr of the present population was 37.6 ml min−1. The estimated population V/Fm of oxypurinol (38.1 l, 95% CI 33.2, 44.4) was similar to that reported for healthy volunteers (41.3 l) [6]. Given that the distribution of oxypurinol is similar to the fractional water content of the body, this finding is in agreement with the reduced fractional water content of the body and lean body mass in old age [28] and is also consistent with the pharmacokinetics of other low protein bound drugs, such as digoxin, when dosed in the elderly [29].
In the final model, CLCr and concomitant diuretic and probenecid therapy were identified as significant factors associated with inter-individual variability in the apparent clearance of oxypurinol. Approximately 26% of the variability in the apparent clearance of oxypurinol was explained by CLCr. This is consistent with the primarily renal excretion of oxypurinol [30] and with other clinical studies [16, 31]. From the present model, a reduction in CLCr of 10 ml min−1 causes a 4.23 ml min−1 decrease in CL/Fm. Between-occasion variability in CL/Fm was 21%. There are many factors which could influence the pharmacokinetics of oxypurinol within an individual between occasions. While adherence to prescribed dosing regimens of allopurinol is the most likely explanation, small changes in renal function between occasions may also contribute. Variation in diet, such as higher protein content, which increases the renal clearance of oxypurinol [32], and variable absorption of allopurinol from the gastrointestinal system or variable conversion of allopurinol to oxypurinol in the liver, are also possible contributors.
The estimation of renal function using the Cockcroft & Gault equation based upon LBW [22, 23], rather than TBW, more closely described the relationship between CL/Fm and renal function in this patient cohort. This can be explained on the basis that TBW often over estimates renal function in overweight or obese people whereas LBW normalizes the effect of obesity on renal function [33]. In the present cohort of gouty patients, 28% (n= 44) were obese (defined as BMI > 30 kg m−2).
The apparent clearance of oxypurinol was reduced by 29% in patients receiving concomitant diuretic therapy. This observation is consistent with previous studies demonstrating a decrease in the urinary excretion of both urate and oxypurinol in the presence of furosemide [34]. Both thiazide and loop diuretics increase plasma concentrations of urate [35, 36] through a combination of volume depletion and increased reabsorption of urate by transporters in the proximal tubule [37]. Given the similarity in the renal handling of urate and oxypurinol, the effects of diuretics on the renal transport of oxypurinol are thought to be similar to the effects of these drugs on urate transport. Hence, diuretics are thought to interact with allopurinol by increasing the reabsorption of oxypurinol. By contrast, the apparent clearance of oxypurinol was increased by 38% in patients receiving probenecid concomitantly. Studies have shown that probenecid increases the renal clearance of oxypurinol [21, 30] by decreasing the reabsorption of oxypurinol through inhibition of renal transporters [38]. Overall, the combined influence of diuretics and probenecid explained a further 4% of the variability in CL/Fm. These data suggest that individualizing allopurinol dosage should consider use of these medications either alone or in combination.
This present study is the first to investigate the influence of renal transporter polymorphisms on the pharmacokinetics of oxypurinol. The frequency of genetic variants in various renal transporters implicated in the bi-directional transport of urate was similar to previous studies in patients with gout [39–42]. Two polymorphisms, rs1165196 in NPT-1 and rs16890979 in GLUT9 were associated with CL/Fm (Figure 1). NPT-1 is expressed in the apical membrane of proximal tubule cells and is suggested to mediate the secretion of urate [43]. GLUT9 is expressed at both the apical and basolateral membrane of proximal tubule cells and is involved in the reabsorption urate [44]. Although oxypurinol transport via NPT-1 and GLUT9 has not been demonstrated, given the similar charge, structure and size to urate, it is a likely substrate. The rs1165196 polymorphism in NPT-1 was associated with a 32% increase in CL/Fm in patients carrying both variant alleles. The relationship between NPT-1 polymorphisms and the fractional clearance of urate has not been determined. However rs1165196 has been associated with reduced plasma concentrations of urate [39, 40] and a lower risk of developing gout [39]. Further molecular studies are required to elucidate the mechanism underlying these clinical associations. The rs16890979 polymorphism in GLUT9 was associated with a lower CL/Fm in patients carrying either one (by 17%) or both (by 21%) variant alleles, particularly in patients not receiving diuretic therapy (Figure 1). Similarly, other GLUT9 polymorphisms in linkage disequilibrium with rs16890979 have been associated with reduced fractional clearance of urate in gouty patients [45, 46]. Despite the initial influence of genetic variants in NPT-1 and GLUT9 on CL/Fm, none of the candidate polymorphisms could further explain the variability in oxypurinol pharmacokinetics after consideration of the estimated CLCr and use of diuretics. This may be because firstly, the study was not designed to investigate the influence of genetic variation in renal transporters on oxypurinol pharmacokinetics and, secondly, the number of patients carrying both variant alleles was small. Finally, the renal handling of both oxypurinol and urate is complex and the interplay of renal transporters with other renally eliminated medicines/compounds remains unclear. Overall, this study suggests that in addition to transporting urate, GLUT9 and NPT1 may also transport oxypurinol (or indirectly regulate oxypurinol transport) in the proximal tubule cell. However, confirmation from in vitro studies is required.
The current study also investigated the effect of adherence on the variability in CL/Fm. Non-adherence tended, though not significantly, to be associated with a lower Fm. Adherence to allopurinol is known to be poor even when compared with other chronic conditions such as type II diabetes [47, 48]. The reason this effect did not reach statistical significance may be because most patients reported a high level of adherence to allopurinol. This is not unexpected for the present cohort given the older age, higher frequency of comorbidities and hospitalization, which are all reported to increase adherence to gout therapy [47, 48]. Although, the BMQ accounts for approximately 20% of the variance in adherence [24, 49], implementation of an electronic monitoring system, the current gold standard in adherence measures, may provide a more sensitive measure of adherence to allopurinol in future studies. Although some effects of non-adherence between hospital admissions may be accounted for in the between occasion variability, clearly further investigation into the relative contribution of adherence to the overall influence on the pharmacokinetics of oxypurinol in community patients is warranted.
There have been few systematic pharmacokinetic–pharmacodynamic studies of oxypurinol, making it difficult to estimate the appropriate dose of allopurinol required to achieve a target oxypurinol concentration to optimize efficacy, viz. the urate lowering effect. A recent study in gouty patients suggested that plasma oxypurinol concentrations between 15.2–22.8 mg l−1 (6–9 h post dose) are required to achieve plasma urate concentrations below 0.6 mg dl−1 (0.36 mmol l−1) [17]. These target concentrations are higher than previous recommendations [13]. The simulations demonstrate that target plasma urate concentrations can be achieved with a range of allopurinol doses (Figure 3A–C). For example, some patients achieve target plasma concentrations of urate with plasma oxypurinol concentrations below 5.1 mg l−1 while others need concentrations greater than 23 mg l−1. There is an inverse relationship between plasma concentrations of oxypurinol and urate consistent with previous studies [13]. However, patient characteristics and concomitant medications can influence plasma concentrations of both analytes (Figure 3A–C). Indeed drugs which increase the CL/Fm, such as probenecid, lower both plasma oxypurinol and urate concentrations. Together, these data indicate that a more detailed assessment of the efficacy of oxypurinol in patients with gout and varying comorbidities is required.
Allopurinol dosing guidelines based on renal function have been widely accepted. Predictions from the current model suggest that following suggested allopurinol dosing guidelines [16] would result in failure to achieve target plasma oxypurinol concentrations in the majority of patients (Figure 4). For example, a gouty patient with normal renal function receiving the recommended 300 mg day−1 would have average steady-state oxypurinol concentrations of 7 mg l−1 (Figure 4A). This is significantly less than the suggested target concentration of 15.2 mg l−1. Indeed, clinical studies demonstrate an inadequate reduction in plasma urate concentrations using suggested dosing guidelines [20]. Clearly, revision of the current dosing guidelines is required to avoid under treatment of this potentially curable condition.
In this study of patients with gout, we have developed and validated, for the first time, a robust population pharmacokinetic model for oxypurinol. This study highlights the significance of renal function as well as certain concomitant medications, such as diuretics and probenecid in determining the pharmacokinetics of oxypurinol, and hence the dose selection of allopurinol, in patients with gout. Findings from this study may enable the establishment of allopurinol dosing guidelines to produce the most favourable reduction in plasma urate concentrations for an individual and reduce the incidence of tophaceous gout and acute attacks [42] of gout, a goal we believe can be achieved in the majority of patients.
Acknowledgments
We thank Arthritis Australia National and NH&MRC (Program Grant 568612) and Professor Kathy Giacomini (GM61390) for supporting this work. We would like to thank Mr Jim Shima for his advice with the genetic analysis and Dr Pavel Bitter for help with the genotyping of the samples.
Competing Interests
There are no competing interests to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1
Frequencies of SLC2A9, ABCG2, SLC22A13, SLC17A1, SLC22A11and SLC22A8 genotypes in patients with gout
Table S2
The primer sequences of the SNPlex™ assays for the coding of non-synonymous single nucleotide polymorphisms (n = 14) in candidate genes
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Annemans L, Spaepen E, Gaskin M, Bonnemaire M, Malier V, Gilbert T, Nuki G. Gout in the UK and Germany: prevalence, comorbidities and management in general practice 2000–2005. Ann Rheum Dis. 2008;67:960–6. doi: 10.1136/ard.2007.076232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riedel AA, Nelson M, Wallace K, Joseph-Ridge N, Cleary M, Fam AG. Prevalence of comorbid conditions and prescription medication use among patients with gout and hyperuricemia in a managed care setting. J Clin Rheumatol. 2004;10:308–14. doi: 10.1097/01.rhu.0000147049.12220.32. [DOI] [PubMed] [Google Scholar]
- 3.Bellamy N, Brooks PM, Emmerson BT, Gilbert JR, Campbell J, McCredie M. A survey of current prescribing practices of anti-inflammatory and urate-lowering drugs in gouty arthritis in New South Wales and Queensland. Med J Aust. 1989;151:531–2. doi: 10.5694/j.1326-5377.1989.tb128510.x. [DOI] [PubMed] [Google Scholar]
- 4.Terkeltaub RA. Clinical practice. Gout. N Engl J Med. 2003;349:1647–55. doi: 10.1056/NEJMcp030733. [DOI] [PubMed] [Google Scholar]
- 5.Walter-Sack I, de Vries JX, Kutschker C, Ittensohn A, Voss A. Disposition and uric acid lowering effect of oxipurinol: comparison of different oxipurinol formulations and allopurinol in healthy individuals. Eur J Clin Pharmacol. 1995;49:215–20. doi: 10.1007/BF00192382. [DOI] [PubMed] [Google Scholar]
- 6.Day RO, Graham GG, Hicks M, McLachlan AJ, Stocker SL, Williams KM. Clinical pharmacokinetics and pharmacodynamics of allopurinol and oxypurinol. Clin Pharmacokinet. 2007;46:623–44. doi: 10.2165/00003088-200746080-00001. [DOI] [PubMed] [Google Scholar]
- 7.Walter-Sack I, de Vries JX, Kreiner C, Ittensohn A, Stenzhorn G, Voss A, Weber E. Bioequivalence of allopurinol preparations: to be assessed by the parent drug or the active metabolite? Clin Investig. 1993;71:240–6. doi: 10.1007/BF00180109. [DOI] [PubMed] [Google Scholar]
- 8.Turnheim K, Krivanek P, Oberbauer R. Pharmacokinetics and pharmacodynamics of allopurinol in elderly and young subjects. Br J Clin Pharmacol. 1999;48:501–9. doi: 10.1046/j.1365-2125.1999.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzner JE, Buchberger D, Laeuter J, Pech R. Untersuchungen zur Bioaequivalenz einer neuen Allopurinol-tablettenformulierung im Verglueich zu einer Referenzformulierung. Arzneim-Forsch/Drug Res. 1997;47:1236–41. [PubMed] [Google Scholar]
- 10.Elion GB, Yü TS-F, Gutman AB, Hitchings GH. Renal clearance of oxipurinol, the chief metabolite of allopurinol. Am J Med. 1968;45:69–77. doi: 10.1016/0002-9343(68)90008-9. [DOI] [PubMed] [Google Scholar]
- 11.Iwanaga T, Kobayashi D, Hirayama M, Maeda T, Tamai I. Involvement of uric acid transporter in increased renal clearance of the xanthine oxidase inhibitor oxypurinol induced by a uricosuric agent, benzbromarone. Drug Metab Dispos. 2005;33:1791–5. doi: 10.1124/dmd.105.006056. [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Ohshiro N, Tsuchiya A, Kohyama N, Ohbayashi M, Yamamoto T. Renal transport of organic compounds mediated by mouse organic anion transporter 3 (mOat3): further substrate specificity of mOat3. Drug Metab Dispos. 2004;32:479–83. doi: 10.1124/dmd.32.5.479. [DOI] [PubMed] [Google Scholar]
- 13.Emmerson B, Gordon R, Cross M, Thomson D. Plasma oxipurinol concentrations during allopurinol therapy. Br J Rheumatol. 1987;26:445–9. doi: 10.1093/rheumatology/26.6.445. [DOI] [PubMed] [Google Scholar]
- 14.Peterson GM, Boyle RR, Francis HW, Oliver NW, Paterson J, von Witt RJ, Taylor GR. Dosage prescribing and plasma oxipurinol levels in patients receiving allopurinol therapy. Eur J Clin Pharmacol. 1990;39:419–21. doi: 10.1007/BF00315424. [DOI] [PubMed] [Google Scholar]
- 15.Graham S, Day R, Wong H, McLachlan A, Bergendal L, Miners J, Birkett D. Pharmacodynamics of oxypurinol after administration of allopurinol to healthy subjects. Br J Clin Pharmacol. 1996;41:299–304. doi: 10.1046/j.1365-2125.1996.03116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity. Description and guidelines for prevention in patients with renal insufficiency. Am J Med. 1984;76:47–56. doi: 10.1016/0002-9343(84)90743-5. [DOI] [PubMed] [Google Scholar]
- 17.Stamp LK, Barclay ML, O'Donnell JL, Zhang M, Drake J, Frampton C, Chapman PT. Relationship between serum urate and plasma oxypurinol in the management of gout: determination of minimum plasma oxypurinol concentration to achieve a target serum urate level. Clin Pharmacol Ther. 2011;63:412–21. doi: 10.1038/clpt.2011.113. [DOI] [PubMed] [Google Scholar]
- 18.Stamp LK, O'Donnell JL, Zhang M, James J, Frampton C, Barclay ML, Chapman PT. Using allopurinol above the dose based on creatinine clearance is effective and safe in patients with chronic gout, including those with renal impairment. Arthritis Rheum. 2011;63:412–21. doi: 10.1002/art.30119. [DOI] [PubMed] [Google Scholar]
- 19.Panomvana D, Sripradit S, Angthararak S. Higher therapeutic plasma oxypurinol concentrations might be required for gouty patients with chronic kidney disease. J Clin Rheumatol. 2008;14:6–11. doi: 10.1097/RHU.0b013e318164dceb. [DOI] [PubMed] [Google Scholar]
- 20.Dalbeth N, Kumar S, Stamp L, Gow P. Dose adjustment of allopurinol according to creatinine clearance does not provide adequate control of hyperuricemia in patients with gout. J Rheumatol. 2006;33:1646–50. [PubMed] [Google Scholar]
- 21.Stocker SL, Williams KM, McLachlan AJ, Graham GG, Day RO. Pharmacokinetic and pharmacodynamic interaction between allopurinol and probenecid in healthy subjects. Clin Pharmacokinet. 2008;47:111–8. doi: 10.2165/00003088-200847020-00004. [DOI] [PubMed] [Google Scholar]
- 22.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. Quantification of lean bodyweight. Clin Pharmacokinet. 2005;44:1051–65. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 23.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 24.Horne R, Weinman J. Patients' beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–67. doi: 10.1016/s0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 25.Ette EI, Williams PJ, Kim YH, Lane JR, Liu MJ, Capparelli EV. Model appropriateness and population pharmacokinetic modeling. J Clin Pharmacol. 2003;43:610–23. [PubMed] [Google Scholar]
- 26.van Kesteren C, Benders MJ, Groenendaal F, van Bel F, Ververs FF, Rademaker CM. Population pharmacokinetics of allopurinol in full-term neonates with perinatal asphyxia. Ther Drug Monit. 2006;28:339–44. doi: 10.1097/01.ftd.0000211808.74192.86. [DOI] [PubMed] [Google Scholar]
- 27.Stocker SL, Graham GG, McLachlan AJ, Williams KM, Day RO. Pharmacokinetic and pharmacodynamic interaction between allopurinol and probenecid in patients with gout. J Rheumatol. 2011;38:904–10. doi: 10.3899/jrheum.101160. [DOI] [PubMed] [Google Scholar]
- 28.Tumer N, Scarpace PJ, Lowenthal DT. Geriatric pharmacology: basic and clinical considerations. Annu Rev Pharmacol Toxicol. 1992;32:271–302. doi: 10.1146/annurev.pa.32.040192.001415. [DOI] [PubMed] [Google Scholar]
- 29.Cusack B, Kelly J, O'Malley K, Noel J, Lavan J, Horgan J. Digoxin in the elderly: pharmacokinetic consequences of old age. Clin Pharmacol Ther. 1979;25:772–6. doi: 10.1002/cpt1979256772. [DOI] [PubMed] [Google Scholar]
- 30.Elion GB, Yu TF, Gutman AB, Hitchings GH. Renal clearance of oxipurinol, the chief metabolite of allopurinol. Am J Med. 1968;45:69–77. doi: 10.1016/0002-9343(68)90008-9. [DOI] [PubMed] [Google Scholar]
- 31.Saji M. A study of serum oxipurinol concentration and renal function in patients administered allopurinol. Nippon Jinzo Gakkai Shi. 1996;38:640–50. [PubMed] [Google Scholar]
- 32.Berlinger WG, Park GD, Spector R. The effect of dietary protein on the clearance of allopurinol and oxypurinol. N Engl J Med. 1985;313:771–6. doi: 10.1056/NEJM198509263131302. [DOI] [PubMed] [Google Scholar]
- 33.Janmahasatian S, Duffull SB, Chagnac A, Kirkpatrick CM, Green B. Lean body mass normalizes the effect of obesity on renal function. Br J Clin Pharmacol. 2008;65:964–5. doi: 10.1111/j.1365-2125.2008.03112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Toshikazu H. Effect of furosemide on renal excretion of oxypurinol and purine bases. Metabolism. 2001;50:241–5. doi: 10.1053/meta.2001.19489. [DOI] [PubMed] [Google Scholar]
- 35.Laragh JH, Cannon PJ, Stason WB, Heinemann HO. Physiologic and clinical observations on furosemide and ethacrynic acid. Ann N Y Acad Sci. 1966;139:453–65. doi: 10.1111/j.1749-6632.1966.tb41219.x. [DOI] [PubMed] [Google Scholar]
- 36.Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med. 2005;165:742–8. doi: 10.1001/archinte.165.7.742. [DOI] [PubMed] [Google Scholar]
- 37.Hasannejad H, Takeda M, Taki K, Shin HJ, Babu E, Jutabha P, Khamdang S, Aleboyeh M, Onozato ML, Tojo A, Enomoto A, Anzai N, Narikawa S, Huang XL, Niwa T, Endou H. Interactions of human organic anion transporters with diuretics. J Pharmacol Exp Ther. 2004;308:1021–9. doi: 10.1124/jpet.103.059139. [DOI] [PubMed] [Google Scholar]
- 38.Enomoto A, Kimura H, Chairoungdua A, Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T, Igarashi T, Matsuo H, Kikuchi Y, Oda T, Ichida K, Hosoya T, Shimokata K, Niwa T, Kanai Y, Endou H. Molecular identification of a renal urate anion exchanger that regulates blood urate levels. Nature. 2002;417:447–52. doi: 10.1038/nature742. [DOI] [PubMed] [Google Scholar]
- 39.Urano W, Taniguchi A, Anzai N, Inoue E, Kanai Y, Yamanaka M, Endou H, Kamatani N, Yamanaka H. Sodium-dependent phosphate cotransporter type 1 sequence polymorphisms in male patients with gout. Ann Rheum Dis. 2010;69:1232–4. doi: 10.1136/ard.2008.106856. [DOI] [PubMed] [Google Scholar]
- 40.Yang Q, Kottgen A, Dehghan A, Smith AV, Glazer NL, Chen MH, Chasman DI, Aspelund T, Eiriksdottir G, Harris TB, Launer L, Nalls M, Hernandez D, Arking DE, Boerwinkle E, Grove ML, Li M, Linda Kao WH, Chonchol M, Haritunians T, Li G, Lumley T, Psaty BM, Shlipak M, Hwang SJ, Larson MG, O'Donnell CJ, Upadhyay A, van Duijn CM, Hofman A, Rivadeneira F, Stricker B, Uitterlinden AG, Pare G, Parker AN, Ridker PM, Siscovick DS, Gudnason V, Witteman JC, Fox CS, Coresh J. Multiple genetic loci influence serum urate levels and their relationship with gout and cardiovascular disease risk factors. Circ Cardiovasc Genet. 2010;3:523–30. doi: 10.1161/CIRCGENETICS.109.934455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hollis-Moffatt JE, Gow PJ, Harrison AA, Highton J, Jones PB, Stamp LK, Dalbeth N, Merriman TR. The SLC2A9 non-synonymous Arg265His variant and gout; evidence for a population-specific effect on severity. Arthritis Res Ther. 2011;13:R85. doi: 10.1186/ar3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phipps-Green AJ, Hollis-Moffatt JE, Dalbeth N, Merriman ME, Topless R, Gow PJ, Harrison AA, Highton J, Jones PB, Stamp LK, Merriman TR. A strong role for the ABCG2 gene in susceptibility to gout in New Zealand Pacific Island and Caucasian, but not Maori, case and control sample sets. Hum Mol Genet. 2010;19:4813–9. doi: 10.1093/hmg/ddq412. [DOI] [PubMed] [Google Scholar]
- 43.Iharada M, Miyaji T, Fujimoto T, Hiasa M, Anzai N, Omote H, Moriyama Y. Type 1 sodium-dependent phosphate transporter (SLC17A1 Protein) is a Cl(-)-dependent urate exporter. J Biol Chem. 2010;285:26107–13. doi: 10.1074/jbc.M110.122721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anzai N, Ichida K, Jutabha P, Kimura T, Babu E, Jin CJ, Srivastava S, Kitamura K, Hisatome I, Endou H, Sakurai H. Plasma urate level is directly regulated by a voltage-driven urate efflux transporter URATv1 (SLC2A9) in humans. J Biol Chem. 2008;283:26834–8. doi: 10.1074/jbc.C800156200. [DOI] [PubMed] [Google Scholar]
- 45.Kolz M, Johnson T, Sanna S, Teumer A, Vitart V, Perola M, Mangino M, Albrecht E, Wallace C, Farrall M, Johansson A, Nyholt DR, Aulchenko Y, Beckmann JS, Bergmann S, Bochud M, Brown M, Campbell H, Connell J, Dominiczak A, Homuth G, Lamina C, McCarthy MI, Meitinger T, Mooser V, Munroe P, Nauck M, Peden J, Prokisch H, Salo P, Salomaa V, Samani NJ, Schlessinger D, Uda M, Volker U, Waeber G, Waterworth D, Wang-Sattler R, Wright AF, Adamski J, Whitfield JB, Gyllensten U, Wilson JF, Rudan I, Pramstaller P, Watkins H, Doering A, Wichmann HE, Spector TD, Peltonen L, Volzke H, Nagaraja R, Vollenweider P, Caulfield M, Illig T, Gieger C. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vitart V, Rudan I, Hayward C, Gray NK, Floyd J, Palmer CN, Knott SA, Kolcic I, Polasek O, Graessler J, Wilson JF, Marinaki A, Riches PL, Shu X, Janicijevic B, Smolej-Narancic N, Gorgoni B, Morgan J, Campbell S, Biloglav Z, Barac-Lauc L, Pericic M, Klaric IM, Zgaga L, Skaric-Juric T, Wild SH, Richardson WA, Hohenstein P, Kimber CH, Tenesa A, Donnelly LA, Fairbanks LD, Aringer M, McKeigue PM, Ralston SH, Morris AD, Rudan P, Hastie ND, Campbell H, Wright AF. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat Genet. 2008;40:437–42. doi: 10.1038/ng.106. [DOI] [PubMed] [Google Scholar]
- 47.Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008;28:437–43. doi: 10.1592/phco.28.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harrold LR, Andrade SE, Briesacher BA, Raebel MA, Fouayzi H, Yood RA, Ockene IS. Adherence with urate-lowering therapies for the treatment of gout. Arthritis Res Ther. 2009;11:R46. doi: 10.1186/ar2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phatak HM, Thomas J., 3rd Relationships between beliefs about medications and nonadherence to prescribed chronic medications. Ann Pharmacother. 2006;40:1737–42. doi: 10.1345/aph.1H153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


