Abstract
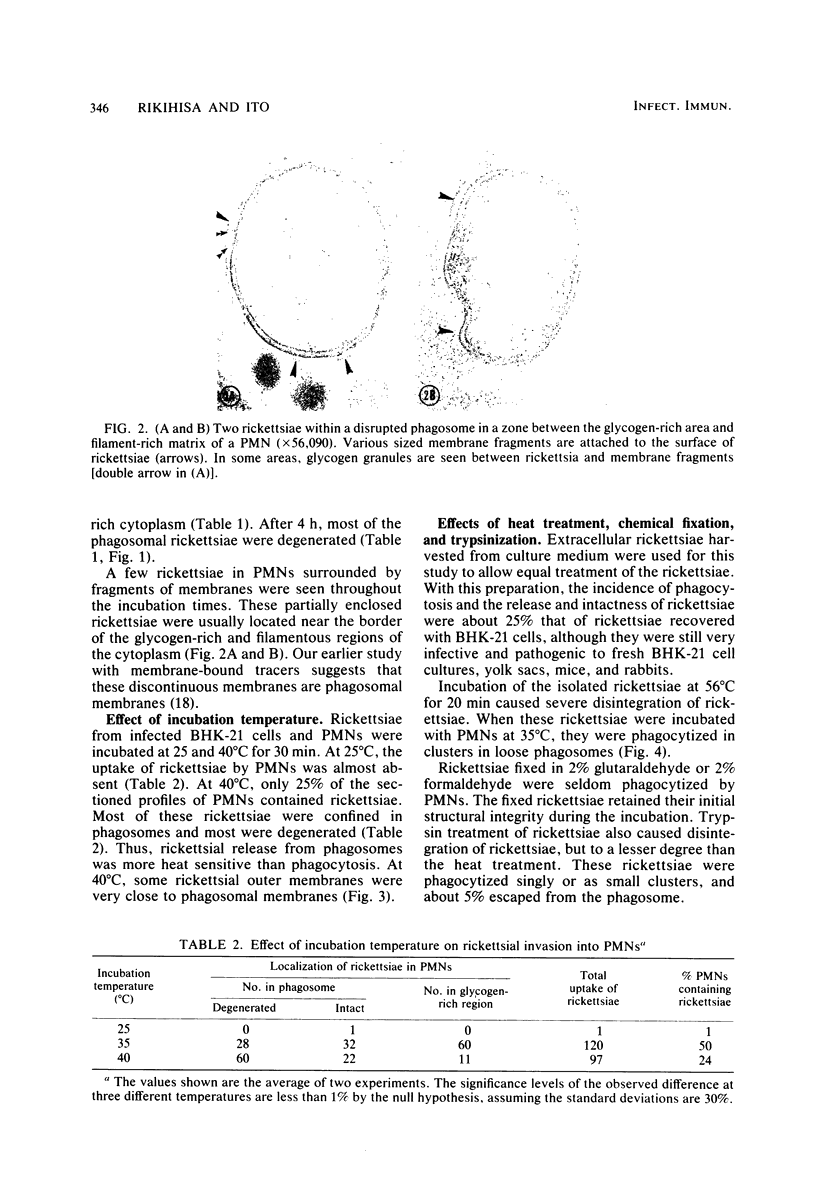
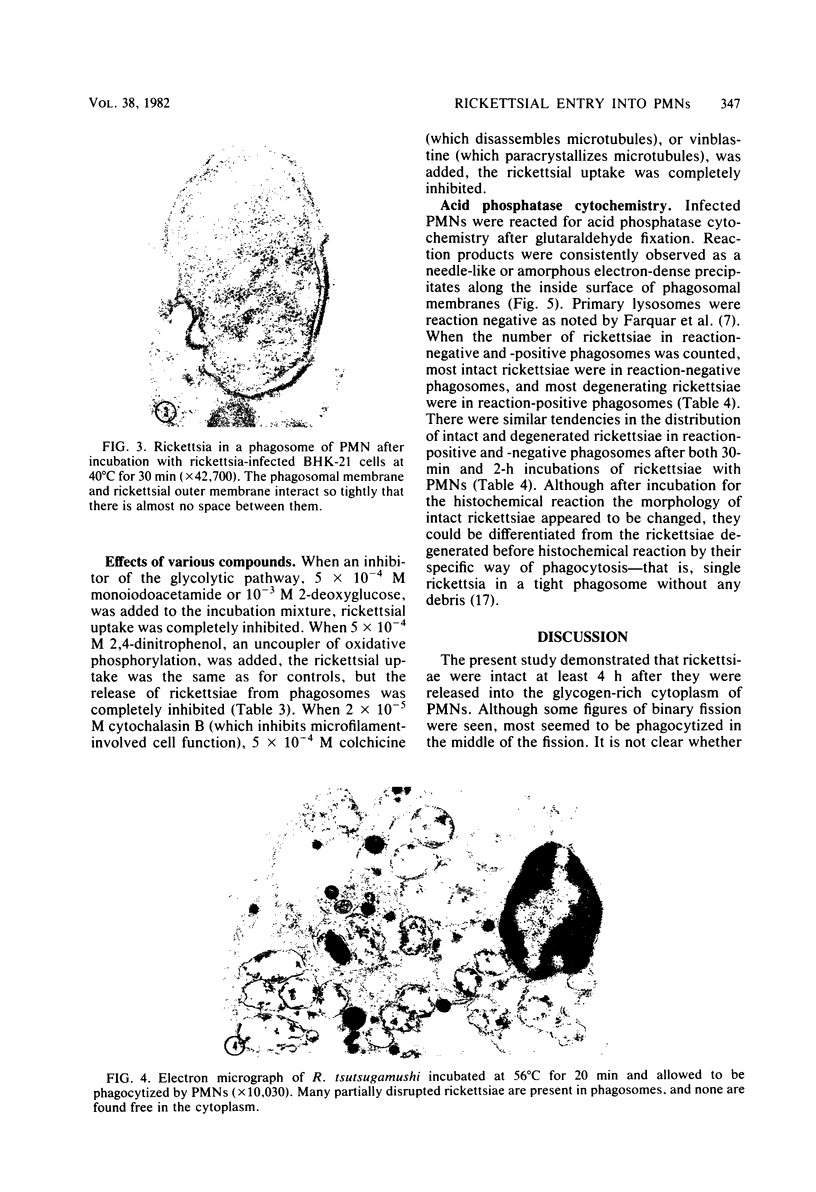
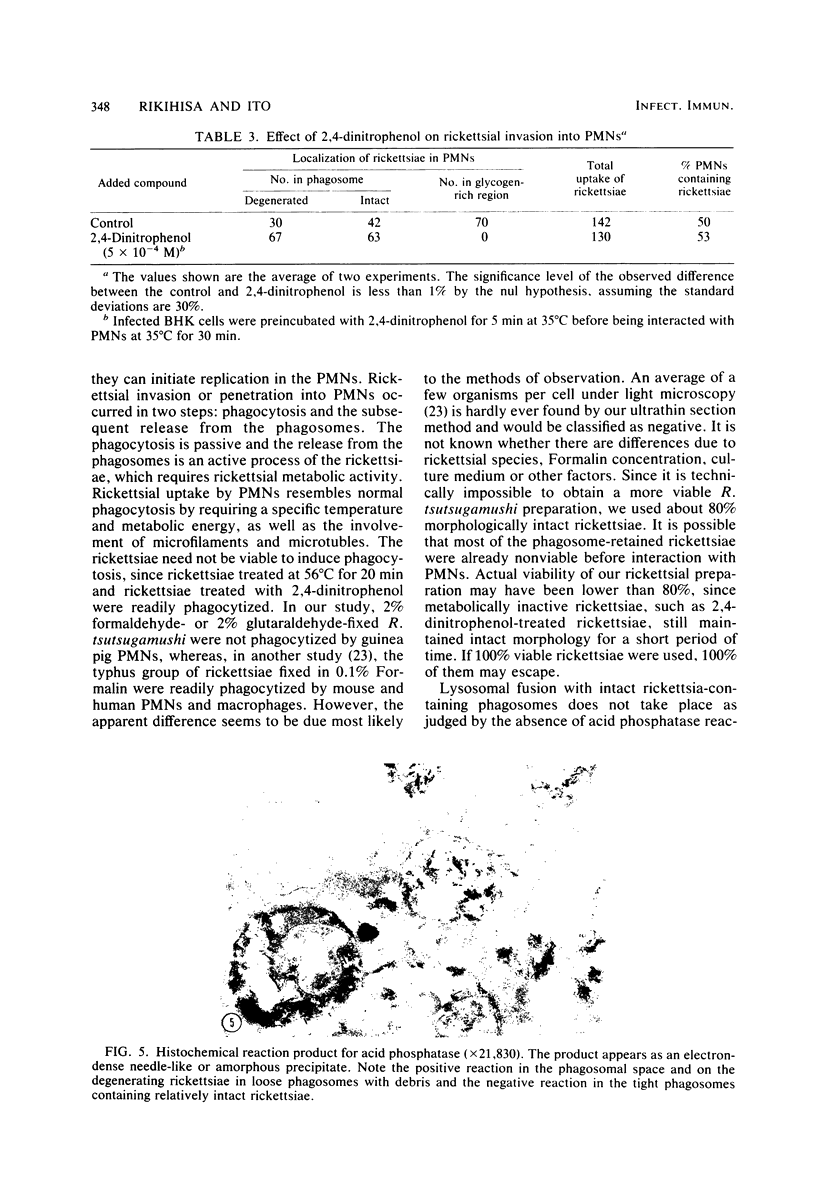
Factors involved in the phagocytosis and entry into polymorphonuclear leukocytes (PMNs) of Rickettsia tsutsugamushi were studied by electron microscopy. R. tsutsugamushi propagated in baby hamster kidney cell cultures was incubated with guinea pig peritoneal PMNs in vitro at 35 degrees C. Structurally intact and degenerating rickettsiae were found in phagosomes, but only intact rickettsiae escaped phagosomes and specifically entered the glycogen-rich cytoplasm. The extraphagosomal cytoplasmic rickettsiae were found within 30 min after incubation; continued incubation for 4 h increased the rickettsial entry about fourfold as seen in ultrathin sections. Most rickettsiae in phagosomes were degenerating after 4 h of incubation. When incubated at 25 degrees C, no entry and very few phagocytized rickettsiae were observed. At 40 degrees C, rickettsial entry was greatly reduced, but more rickettsiae were found in phagosomes than at 35 degrees C. Preincubation of rickettsiae at 56 degrees C for 20 min with trypsin or with 2,4-dinitrophenol inhibited entry, but many rickettsiae were in phagosomes. Glutaraldehyde or formaldehyde fixation of rickettsiae and addition of 2-deoxyglucose, iodoacetamide, cytochalasin B, colchicine, or vinblastine inhibited all rickettsial uptake by PMNs. Acid phosphatase cytochemistry of infected PMNs revealed the enzyme activity only in phagosomes with degenerated rickettsiae and not in those with intact rickettsiae. These observations indicated that rickettsiae are passively phagocytized by PMNs, and only those that are intact actively escape from phagosomes, which selectively inhibits lysosomal fusion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., BOZEMAN F. M., CAMPBELL J. M., HUMPHRIES J. W., SAWYER T. K. Study on growth of Rickettsia. V. Penetration of Rickettsia tsutsugamushi into mammalian cells in vitro. J Exp Med. 1959 Mar 1;109(3):271–292. doi: 10.1084/jem.109.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong A. S., Hak T. J., Van Duijn P., Daems W. T. A new dynamic model system for the study of capture reactions for diffusable compounds in cytochemistry. II. Effect of the composition of the incubation medium on the trapping of phosphate ions in acid phosphatase cytochemistry. Histochem J. 1979 Mar;11(2):145–161. doi: 10.1007/BF01002992. [DOI] [PubMed] [Google Scholar]
- Ewing E. P., Jr, Takeuchi A., Shirai A., Osterman J. V. Experimental infection of mouse peritoneal mesothelium with scrub typhus rickettsiae: an ultrastructural study. Infect Immun. 1978 Mar;19(3):1068–1075. doi: 10.1128/iai.19.3.1068-1075.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G., Bainton D. F., Baggiolini M., de Duve C. Cytochemical localization of acid phosphatase activity in granule fractions from rabbit polymorphonuclear leukocytes. J Cell Biol. 1972 Jul;54(1):141–156. doi: 10.1083/jcb.54.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambrill M. R., Wisseman C. L., Jr Mechanisms of immunity in typhus infections. I. Multiplication of typhus rickettsiae in human macrophage cell cultures in the nonimmune system: influence of virulence of rickettsial strains and of chloramphenicol. Infect Immun. 1973 Oct;8(4):519–527. doi: 10.1128/iai.8.4.519-527.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981 May;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Osterman J. V. Host defenses in experimental scrub typhus: inflammatory response of congenic C3H mice differing at the Ric gene. Infect Immun. 1981 Mar;31(3):1014–1022. doi: 10.1128/iai.31.3.1014-1022.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Ito S. Intracellular localization of Rickettsia tsutsugamushi in polymorphonuclear leukocytes. J Exp Med. 1979 Sep 19;150(3):703–708. doi: 10.1084/jem.150.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Ito S. Localization of electron-dense tracers during entry of Rickettsia tsutsugamushi into polymorphonuclear leukocytes. Infect Immun. 1980 Oct;30(1):231–243. doi: 10.1128/iai.30.1.231-243.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Rota T., Lee T. H., MacDonald A. B., Ito S. Changes in immunoferritin labeling of Rickettsia tsutsugamushi after serial cultivation in 60Co-irradiated BHK cells. Infect Immun. 1979 Nov;26(2):638–650. doi: 10.1128/iai.26.2.638-650.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr Interaction of Rickettsiae and phagocytic host cells. II. Chemotactic action of typhus Rickettsiae on human polymorphonuclear leukocytes in vitro. J Immunol. 1961 Oct;87:468–471. [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, TABOR H. INTERACTION OF RICKETTSIAE AND PHAGOCYTIC HOST CELLS. IV. EARLY CELLULAR RESPONSE OF MAN TO TYPHUS RICKETTSIAE AS REVEALED BY THE SKIN WINDOW TECHNIQUE, WITH OBSERVATIONS ON IN VIVO PHAGOCYTOSIS. J Immunol. 1964 Nov;93:816–825. [PubMed] [Google Scholar]
- Walker T. S., Winkler H. H. Penetration of cultured mouse fibroblasts (L cells) by Rickettsia prowazeki. Infect Immun. 1978 Oct;22(1):200–208. doi: 10.1128/iai.22.1.200-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner E. Interactions between Encephalitozoon cuniculi and macrophages. Parasitophorous vacuole growth and the absence of lysosomal fusion. Z Parasitenkd. 1975 Aug 21;47(1):1–9. doi: 10.1007/BF00418060. [DOI] [PubMed] [Google Scholar]