Abstract
Introduction:
Fatigue is a major complain in breast cancer patients and survivors. Patterns and degree varies with schedule and type of the treatment. Different co-factors may aggravate fatigue. Multimodal approach is helpful in managing fatigue.
Aim:
To quantify prevalence, course and degree of fatigue in breast cancer patients on adjuvant treatment and effectiveness of different management approach.
Materials and Methods:
One Hundred and ten post-mastectomy breast cancer patients (Stage I to Stage III) were assessed. Patients on chemotherapy were assessed one week before, day after chemotherapy and two weeks later in every cycle. Patients on External Beam Radiation Therapy (EBRT) were assessed one week before and every week during radiation. Assessment was continued on second and fourth week of follow up. Functional Assessment of Chronic Illness Therapy - Fatigue subscale (FACIT-F) was used for assessment. Significant cofactors were also searched for.
Results:
Eighty four percent patients experienced fatigue. Fatigue was more prevalent during chemotherapy (91%) than EBRT (77%). Patients on Chemotherapy exhibit peak fatigue day after Chemotherapy and decreased level until the next cycle. Significant increase of fatigue was seen only in first cycle. Patient on EBRT had gradually increased fatigue during the course of treatment. Lower degree of fatigue was present in post treatment period. Anemia was a significant cofactor causing fatigue (P < 0.05). Blood Transfusion improved fatigue scores.
Conclusion:
Fatigue increases during chemotherapy and or EBRT. Different intervention strategies are needed to address the issue.
Keywords: Breast cancer, Chemotherapy, External beam radiation therapy, Fatigue
INTRODUCTION
Fatigue is the most common side effect of treatment with chemotherapy and radiation therapy in cancer patients.[1] Treatment related fatigue generally improves after therapy is completed, but some level of fatigue may persist for months or years. Fatigue is reported in 14–96% of patients undergoing cancer treatment and in 19-82% of patients during post-treatment. According to a survey of 1569 patients, fatigue is experienced by 80% of the patients receiving chemotherapy and/or radiotherapy.[2,3] Partly as a result of longer cancer survival, it is increasingly being realized that quality of life in cancer patients is affected by fatigue. Fatigue, like pain, is viewed as a self-perceived state. Patients may describe fatigue as feeling tired, weak, exhausted, lazy, weary, worn-out, heavy, slow, or like they do not have any energy or any get-up-and-go.[4]
During the last decade, breast cancer was one of the common cancers worldwide. Breast cancer in urban as well as in rural areas is increasing from last two decades. Adjuvant chemotherapy is part of the standard treatment in a large subset of patients. According to the literature, 58–94% of breast cancer patients experience fatigue during treatment with adjuvant chemotherapy.[5–7]
In a study by Sitzia and Huggins, fatigue incidence was 90% in breast cancer patients receiving six cycles of chemotherapy and severity remained stable throughout the treatment cycles.[6] Results from Berger et al. supported this finding.[8] Jacobsen et al. also reported similar results, though the prevalence and severity of fatigue significantly increased at the starting of chemotherapy, after which the prevalence of fatigue showed a stable pattern.[7]
Postoperative radiotherapy also increases fatigue in breast cancer patients. Rucinska and Langkje showed that the incidence of fatigue was nearly 75% in breast cancer patients undergoing postoperative radiotherapy and the intensity increased gradually during radiotherapy. It was more in younger women, smokers, and those who had a long traveling time to hospital.[9]
Age of presentation, menopausal status, stage of the disease, and anemia are the major contributing factors. Anemia is one of the common modifiable factors among all these.[10]
All these studies support the hypothesis that fatigue is highly prevalent during as well as post-treatment phase of chemotherapy and radiotherapy, but the exact nature of impact is quite unclear. It is also evident from the previous studies that anemia, one of the modifiable factors, directly influences the severity of fatigue.
In this context, our study aims to quantify
Prevalence, course, and degree of fatigue in breast cancer patients on adjuvant treatment;
Effect of treatment modalities (chemotherapy and/or radiotherapy) over it; and
Cofactors influencing the scenario.
MATERIALS AND METHODS
This prospective observational study was carried out by Palliative Care Unit at Department of Radiotherapy, Medical College and Hospitals, Kolkata, West Bengal, from December 2010 to August 2011. Patients were included with the following criteria: (i) stage I to stage III post-mastectomy breast cancer patient; (ii) no previous treatment with cytotoxic drugs or radiation therapy; (iii)Eastern Co-operative Oncology Group (ECOG) Performance Status 0–3; (iv) absence of chronic disease (such as hypertension, renal disease, cardiac disease, diabetes mellitus, etc.) or poor psychological state; (v) no use of morphine or narcoleptics; (vi) no deafness; (vii) more than 18 years of age; and (viii) Bengali or Hindi speaking. Patients having metastatic disease or any other malignancy and patients having ECOG Performance Status 4 were excluded from the study.
After getting informed consent from the eligible patients and institutional ethics committee clearance, the investigators (with adequate training for non-biased patient response) conducted the interviews.
The tumor–node–metastasis (TNM) clinical classification (AJCC, 7th ed., 2010) was used to describe the anatomic extent of breast cancer.
Patients were treated with six cycles of 5-Flurouracil, Doxorubicin, and Cyclophosphamide with standard dose schedule in 21–28 days interval according to current practice protocol of the study hospital. Patients having indication to radiotherapy were treated with 50Gy of external beam radiation in 25 fractions 5 days a week with Cobalt 60 machine (Theratron780C, Theratronics Inc, Kanata, Ontario, Canada). Pretreatment baseline hemoglobin levels were measured. For anemic patients, concentrated RBC was transfused to maintain the hemoglobin level >10g/dl throughout the treatment period.
Patients were interviewed with Functional Assessment of Illness Therapy – Fatigue subscale (FACIT-F)(version 4)multidimensional Quality of Life questionnaire in Bengali and/or Hindi language format (whichever was needed). Prior permission was taken from the owners of “FACIT system” to use this tool in this study. Patients were interviewed 2 weeks before starting of chemotherapy/radiotherapy (maintaining at least 1month gap between mastectomy and the first cycle of chemotherapy). During chemotherapy, patients were interviewed the day after therapy in each cycle, 2 weeks after chemotherapy in each cycle, and second and fourth week after completion of chemotherapy. During radiotherapy, patients were interviewed each week during radiotherapy, and second and fourth week after completion of therapy.
Fatigue scale and interpretations
FACIT-F scoring is a quality of life assessment tool used to assess cancer treatment related fatigue.[11] It has good test–retest reliability (r ranging from 0.82 to 0.92) and is sensitive to change over time. It has also been shown to have convergent and discriminate validity.[11–13] FACIT-F (version 4)is a 40-item self-report instrument. It includes core Functional Assessment of Cancer Therapy– General (FACT-G) scale with 27 items and one additional concern subscale (Fatigue) with 13 items. FACT-G items are divided into four subscale items:(a) Physical Well-being (PWB) (7 items), (b)Social/Family Well-being (SWB)(7 items), (c) Emotional Well-being (EWB) (6 items), and (d) Functional Well-being (FWB) (7 items).FACIT-F scores use a 5-point Likert-type score ranging from “0”(Not at all) to “4” (Very much).[11] Scores were obtained in each of the special domains and FACT-G score (includes summed score of PWB,SWB, EWB, and FWB).Total FACIT score was obtained by adding additional concern score(Fatigue) with FACT-G. Negatively stated items were reversed by subtracting the response from “4.” After reversing proper items, all subscale items were summed to a total, which was the subscale score. For all FACIT scales and symptom indices, the higher the score, the better was the Health-related Quality of Life (HRQoL). For missing and unanswered items, subscale scores were prorated as per administration guideline manual of FACIT-F score.[11] This was usually done by using the formula below:
Prorated subscale score = [Sum of item scores] × [N of items in subscale] ÷ [N of items answered].
When there were missing data, prorating subscale score in this way was acceptable as long as more than 50% of the items were answered (e.g. a minimum of 4 of 7 items, 4 of 6 items, etc.).The total score was then calculated as the sum of the unweighted subscale scores. The FACT scale was considered to be an acceptable indicator of patient quality of life as long as the overall item response rate was greater than 80% (e.g. at least 22 of 27 FACT-G items completed).[11]
The prevalence of fatigue at each measurement point was determined by choosing a cut-off score of <34 in the FACIT-F (additional concern item).[14]
Statistical analysis
In all analyses, the first measurement was taken as a reference baseline level and changes relative to the baseline measurement were analyzed. The effect of chemotherapy on the course of fatigue was represented by interaction terms between baseline variables and variables in each further interview. The course of fatigue, using FACIT-F scale, and the dependency on the type of adjuvant treatment were analyzed with a multivariate analysis using the SPSS program (version 19). Paired t test was done to find out significant difference of scores in any consecutive assessments. Mean values were calculated for FACT-G scores, additional concern (FACIT-F) scores, and FACIT Total scores separately with standard deviation values. Changes of mean values from baseline values (for cycle one in chemotherapy period and interview in the first week of radiation therapy period) or previous assessments were calculated separately for these three scales. This is the Minimally Important Difference (MID) value.[15] An MID is the “smallest difference in score in the domain of interest that patients perceive as important, either beneficial or harmful, and that would lead the clinician to consider a change in the patient's management.”[16] The MID estimates were developed based on distribution-and anchor-based methods, reflecting a growing consensus on the best approach for determining an MID. These MID values can be used to aid the interpretation of group differences and changes in HRQoL over time, and they can be useful in sample size calculations. MID values over 3–7 for Total FACT-G and over 3–4 for Fatigue subscale were considered significant. It is a valid and reliable (internal and test–retest reliability) interpretation for changes in quality of life. It is sensitive to changes over time and upon influencing factors.[16,17] MID values can enhance the interpretability of HRQoL scores by identifying differences likely to be meaningful to patients and clinicians.[18] To find out possible confounding factors in this analysis, several covariates were added: age, menopausal status, stage of breast cancer, baseline hemoglobin level (g/dl), mean interval between surgery and chemotherapy, and mean interval between chemotherapy and radiotherapy.
Multiple regression analysis was done for the cofactors to find out their relationship with fatigue score. Evaluation of the effect of blood transfusion over change of FACT-G and Fatigue subscale scores was done by paired t test.
RESULTS
A total of 79 eligible subjects were taken for this study. The principal investigator took 72% interviews. Rest was done by other investigators. Response rate for answers was 96%. The most frequently unanswered item was sexual activity in PWB subscale.19% patients preferred “not to answer it.”
Patients’characteristics
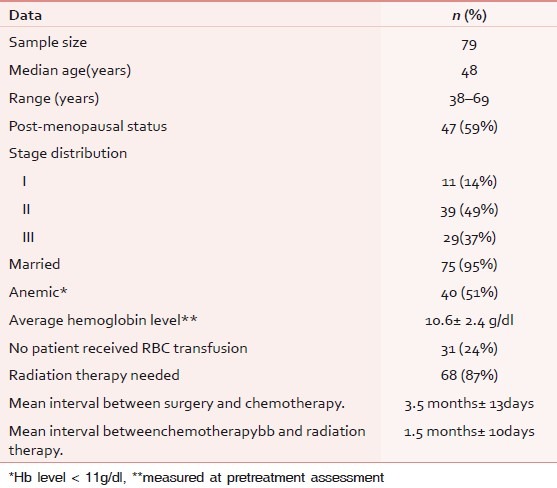
The patients’ characteristics are discussed in Table 1.
Table 1.
Patients' characteristics
Prevalence of fatigue
Sixty-four patients (83%) experienced fatigue (mean value considering all assessments).Distribution is discussed separately below and in Table 2.
Table 2.
Prevalence of fatigue during chemotherapy and radiotherapy
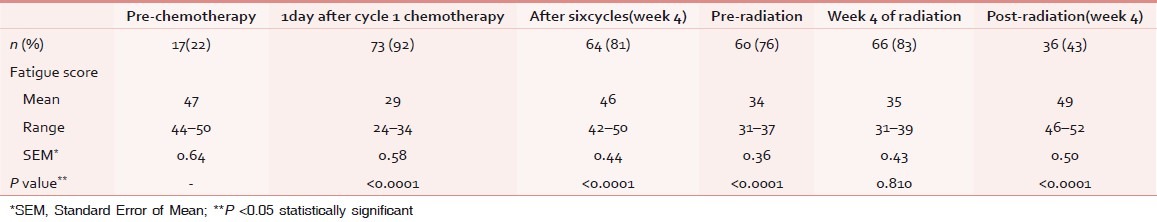
Chemotherapy period
Seventy-two patients (91%) had fatigue (considering mean values of scores of all assessments) during chemotherapy. Prevalence of fatigue increased significantly from second assessment onward [vide Table 2].
Changes of fatigue scores during chemotherapy period are given in Figures 1 and 2.
Figure 1.
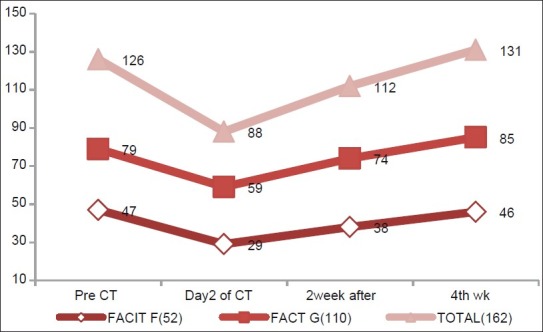
Changes in FACIT-F, FACT-G, FACIT Total score in different assessments during first cycle of chemotherapy, showing the mean value of these three parameters in different assessments
Figure 2.
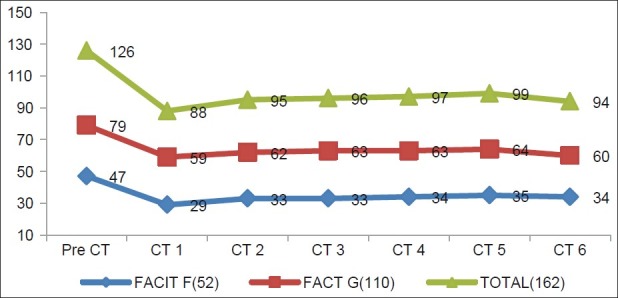
Changes in FACIT-F, FACT-G, FACIT Total score in different assessments during chemotherapy, showing the mean value of these three parameters on the day after chemotherapy
Multivariate analysis was done separately for assessment of scores of FACIT-F, FACT-G and FACIT Total for cycle one. We found that all the parameters were decreased during assessments on the day after chemotherapy and increased subsequently later on (during assessments on the second and fourth weeks). These changes were statistically significant for all three subscales (P<0.05). It is also interesting to note that all the changes occurred at the same point of time.
Figure 2 clearly shows that maximum decrease in scores occurred in cycle one, but in subsequent cycle, the scores were increased. From pre-chemotherapy assessment to cycle one assessment, all the three parameters were decreased significantly (P<0.0001 on paired t test). But from second cycle assessment onward, changes in FACIT-F subscale scores remained constant except in cycle six where it was increased. Changes of FACIT-F subscale scores from cycle one to cycle six assessments were statistically significant (P<0.05). Exactly similar trends occurred in FACT-G scores. But on analyzing the FACIT Total score, we found that scores were increased from cycle one to cycle two significantly, but from cycle two to cycle six, the scores were significantly decreased.
On analyzing the MID values of all parameters during cycle one, it has been found that there were significant changes of scores from pre-chemotherapy to the day after chemotherapy, second and fourth week assessment (18, 9, and 8, respectively) in FACIT-F scores (>3–4 is significant change).[13,15] The values for FACT-G were 20, 15, and 11, respectively (>3–7 is significant change).[13,15]
Radiation therapy period
Fifty-two patients (77%) had fatigue (considering mean values of scores of all assessments) during radiotherapy. Prevalence increased in the third week of radiotherapy and decreased subsequently [vide Table 2].
Changes of scores are shown in Figures 3 and 4.
Figure 3.
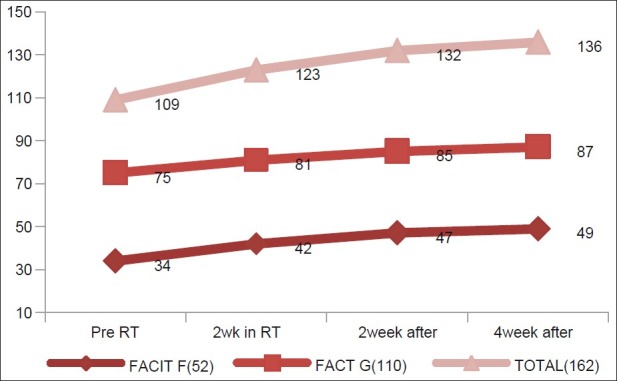
Changes in FACIT-F, FACT-G, FACIT Total score in different assessments during radiation therapy, showing the mean value of these three parameters in different assessments
Figure 4.
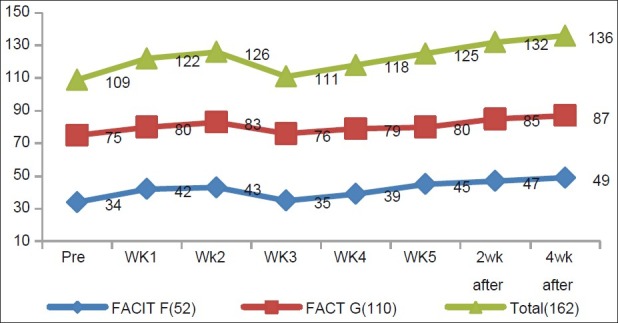
Changes in FACIT-F, FACT-G, FACIT Total score in different assessments during radiation therapy, showing the mean value of these three parameters in different assessments
From the figures, we observe that pre-radiotherapy assessment showed lower scores in all three parameters in contrast to pre-chemotherapy assessment, but the scores improved subsequently. All these changes were statistically significant on paired t test.
On analyzing all weekly assessments together, it was interesting to observe that the scores decreased in all parameters inthe fourth week. These changes were statistically significant when compared with the third week assessment data (P<0.05). In the subsequent weeks, scores of all three parameters were increased. In comparison to pre-radiotherapy assessment, the fatigue scores improved significantly in the fourth week post-radiotherapy assessment. MID values of FACIT-F and FACT-G scores from pre-radiotherapy to fourth week of radiotherapy were1 in both, which was insignificant (MID cut-off value for FACIT-F and FACT-G were 3–4 and 3–7, respectively).[13,15] These values were 12 and 9, respectively, on comparing scores from fourth week of radiotherapy and second week post-radiotherapy assessment, showing significant improvements in scores. But on comparing second week post-radiotherapy scores with fourth week post-radiotherapy scores, MID values were 2 in both, which was insignificant (MID cut-off value for FACIT-F and FACT-G were 3–4 and 3–7, respectively).[13,15]
Comparative analysis of chemotherapy and radiation therapy period
Figures 5–7 show the comparative analysis of all scores.
Figure 5.
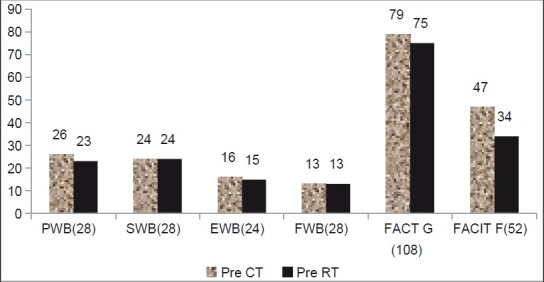
Changes in FACIT-F, FACT-G,PWB, SWB, EWB, and FWB in pretreatment assessment, showing the mean value of these three parameters
Figure 7.
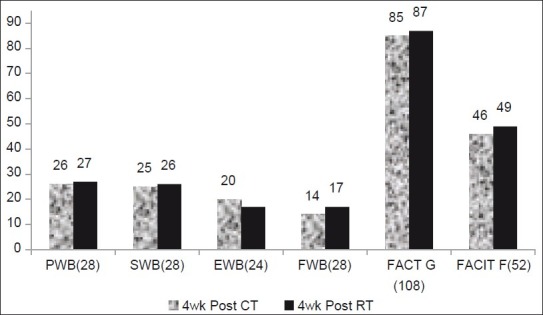
Changes in FACIT-F, FACT-G,PWB, SWB, EWB, and FWB during post-treatment week 4 assessments, showing the mean value of these three parameters
Figure 6.
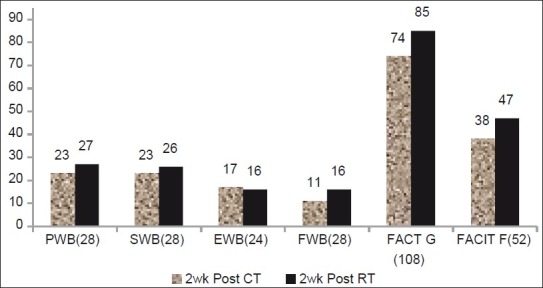
Changes in FACIT-F, FACT-G,PWB, SWB, EWB, and FWB during post-treatment week 2 assessment, showing the mean value of these three parameters
Analysis of cofactors
Prevalence of fatigue was assessed in anemic and non-anemic patients. Thirty-nine patients (96%) experienced fatigue (considering mean values of scores of all assessments) in anemic patients, whereas the value was 29(76%) in case of non-anemic patient group.
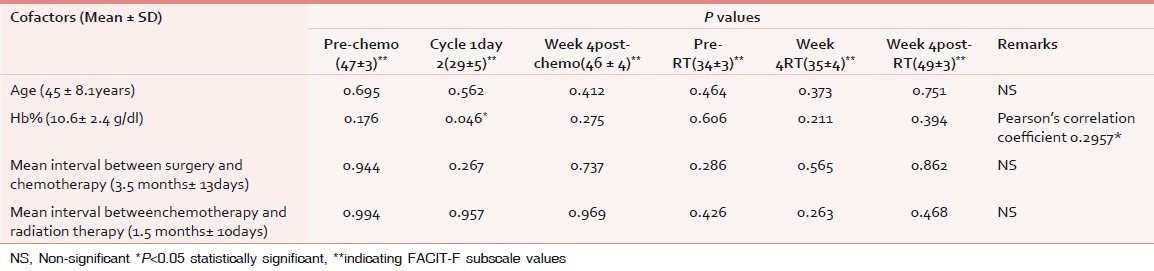
Multiple regression analysis was done for assessment of correlation of baseline characteristics on fatigue score [vide Table 3]. No significant correlation was evident in any of the cofactors except anemia (P 0.046). Anemia was positively correlated with degree of severity of fatigue, i.e. lesser the Hb level, lesser were the FACT-G and FACIT-F scores (Pearson's correlation coefficient 0.2957).Binary variables like marital status and menopausal status, and other variables like stage did not have statistically significant impact over the scores.
Table 3.
Cofactor analysis
Analysis of the effect of anemia correction over fatigue scores is given in Table 4.
Table 4.
Changes of scores on blood transfusion (paired t test)

DISCUSSION
In our study, the items were administered in a face-to-face interview, instead of presenting the items in a self-reported questionnaire for the practical reason that an unbiased response could be achieved as the patients were from diverse socioeconomic groups and were with different literacy status. In addition, filling in a self-report questionnaire was expected to be too time consuming for the patient.
In our study, majority of the patients were post-menopausal. Stage II was the most prevalent stage. Patients suffering from fatigue formed 83%. It was more prevalent in chemotherapy group than in radiotherapy group, and in anemic group in comparison to non-anemic group. In our study, we observed a significant increase in the prevalence of fatigue after the first cycle of chemotherapy. Then, it was stable in the subsequent cycles of chemotherapy. During post-chemotherapy, the prevalence of fatigue declined. Our findings corroborate the results of the study by de Jong et al.[19] During chemotherapy, fatigue scores were the lowest on the day after chemotherapy in the first cycle and increased in the subsequent cycles. Findings were comparable with earlier studies.[19] Apprehension and chemotherapy side effects, mainly nausea, may be a reason for the lowest score on day 2of the first cycle. Scores stabilized from cycle two onward. This finding supports the hypothesis that the intensity of fatigue stays stable throughout the treatment cycles. Sitzia and Huggins[6] found similar results. A reason for the stability could be habituation to the experience of fatigue.
Sixty-eight (87%) patients needed radiotherapy supplementary to chemotherapy. Interestingly, fatigue was more prevalent in pre-radiotherapy assessment than in pre-chemotherapy assessments.
Radiotherapy pretreatment scores were significantly low in comparison to chemotherapy period, corroborating previous results by de Jong et al.[19] It may be due to protracted effect of chemotherapy though the mean interval of chemotherapy and radiotherapy was long (1.5 months). Radiotherapy had a cumulative effect over fatigue scores. Scores were initially increased in the first cycle from pretreatment scores, but decreased in the third cycle to their lowest values. Scores were near pretreatment values at the fourth week follow-up. Janaki et al. found similar results.[20] Findings in radiotherapy period are explicable. It may be a limitation of our study that patients were not admitted during radiation therapy period unlike chemotherapy. Many patients had to travel a long distance every weekday for 5 weeks to have treatment. Besides, daily routine activities had to be adjusted to the treatments. Finally, it may be the effect of radiotherapy itself or a combination of therapies that makes these patients more fatigued. After completion of radiotherapy treatment, the scores became normalized to pretreatment values. The reasoning is partially supported by the study results of Irvine et al.[21]
MID values in chemotherapy period exactly showed similar changes as found using other statistical methodologies and affirmed by previous studies.[15,22] This was also true for fourth week assessment during radiotherapy and subsequent assessments, but not in early week assessments. Persistence of chemotherapy-induced changes during early weeks of radiotherapy may be a reason for this discordance.
Emotional and functional subscale scores were persistently low in the whole treatment duration, with minimal fluctuations, corroborating partly with the findings of Janaki et al.[20] This area should be addressed with importance during fatigue intervention.
Fatigue related to surgery and disease process itself did not influence our results because all the patients had Modified Radical Mastectomy and metastatic breast cancer patients were excluded from the study.
Anemia was the only factor that significantly influenced the scores. Anemic patients were more fatigued. More specifically, lower the hemoglobin level, lower was the score. It was true for all subscale measurements. Concentrated RBC was transfused for correction of anemia. Correction of anemia improved the scores significantly, contradicting the results by So-Osman et al.[23] But similar results were found by Prick et al.[24] Other baseline parameters were not found to influence the fatigue scores significantly unlike earlier studies.[19]
Our study had few limitations. Firstly, the mean intervals between surgery and chemotherapy (3.5 months ± 13days), and chemotherapy and radiotherapy (1.5 months ± 10days) were long. Reasons for this long delay were multiple. Twenty-eight patients (36%) had their surgery done outside. Majority of those patients were referred very late and not properly staged with standard work-up protocol. Four patients (6%) had postoperative complications and initiation of chemotherapy was delayed. But there was no loco-regional or distant failure reported in the study period. Patients are being followed up for chronic fatigue assessments. Impact of this delay over survival will be evident then. Delay in initiation of chemotherapy might not influence the fatigue scores; rather it was beneficial to overcome surgery-related fatigue. But long interval between chemotherapy and radiotherapy might be a confounding factor in radiotherapy assessments.
Secondly, in this study, we intended to find out the level of fatigue only up to fourth week of post-treatment period. Patients are under long-term follow-up to evaluate the nature of chronic fatigue in them. Findings will be reported later on.
Thirdly, except for anemia correction, we did not intervene to improve the fatigue scores specially addressing emotional and functional components of the patients.
CONCLUSION
Fatigue is a major, but often underestimated side effect in breast cancer patients during adjuvant therapy. Fatigue increases during chemotherapy and/or External Beam Radiation Therapy. But the frequency of fatigue appears to be higher with chemotherapy. Course is different in both groups. Anemia is an important contributor for fatigue. Blood transfusion improves the fatigue scores significantly. Different intervention strategies are needed to address the issue.
ACKNOWLEDGMENTS
No extra financial help was needed for this study. We are thankful to all of the patients who participated in the study. We are thankful to all the members of Palliative care team, Department of Radiotherapy, Medical College and Hospitals, Kolkata.
We express thanks to the following respected members of Department of Radiotherapy, Medical College and Hospitals, Kolkata: Dr. Anjali Dhar Majumdar MD, Prof., Dr. P.K.Sur MD, Prof., Dr. Debabrata Mitra MD, Associate Prof., Dr. Shantanu Pal, MD, Associate Prof., Dr. Swapan Kumar Mallick MD, Assistant Prof., Dr S.S.Adhiakry MD, R.M.O. cum Clinical Tutor Dr. ArnabAdhikary, 3rd year PG Trainee, Dr. Deban Banerjee, 3rd year PG Trainee, Dr. Pabitra Das, 3rd year PG Trainee, Dr. Dipnjan Majumder, 2nd year PGTrainee, Dr. Anindya Mukherjee, 1st year PGTrainee, Dr. Aramita Saha, 2nd year PGTrainee, Dr. Biswamit Bhattacharya, 2nd year PGTrainee, Dr. Md Azam, 1styear PGTrainee, Dr. Debasish Chatterjee, 1st year PGTrainee Dr Jaydeep Basu, 1st year PGTrainee, Dr Subhro Kanti Kundu, 1st year PGTrainee
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Prue G, Rankin J, Allen J, Gracy J, Cramp F. Cancer-related fatigue: A critical appraisal. Eur J Cancer. 2006;42:846–63. doi: 10.1016/j.ejca.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Fosså SD, Dahl AA, Loge JH. Fatigue, anxiety, and depression in long-term survivors of testicular cancer. J ClinOncol. 2003;21:1249–54. doi: 10.1200/JCO.2003.08.163. [DOI] [PubMed] [Google Scholar]
- 3.Clinical practice guidelines forcancer-related fatigue. NCCN guideline version 1.2012 updates. National Comprehensive Cancer Network. [Last accessed on 12/4/2012]. Available from: http://www.nccn.org .
- 4.Barsevick AM, Whitmer K, Walker L. In their own words: Using the common sense model toanalyze patient descriptions of cancer-related fatigue. OncolNurs Forum. 2001;28:1363–9. [PubMed] [Google Scholar]
- 5.Greene D, Nail LM, Fieler VK, Dudgeon D, Jones LS. A comparison of patient-reported side effects among three chemotherapy regimens for breast cancer. Cancer Pract. 1994;2:57–62. [PubMed] [Google Scholar]
- 6.Sitzia J, Huggins L. Side effects of cyclophosphamide, methotrexate, and5-fluorouracil (CMF) chemotherapy for breast cancer. Cancer Pract. 1998;6:13–21. doi: 10.1046/j.1523-5394.1998.1998006013.x. [DOI] [PubMed] [Google Scholar]
- 7.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: Characteristics, course, and correlate. J Pain Symptom Manage. 1999;18:233–42. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 8.Berger AM, Higginbotham P. Correlates of fatigue during and following adjuvant breastcancer chemotherapy: A pilot study. OncolNurs Forum. 2000;27:1443–8. [PubMed] [Google Scholar]
- 9.Rucinska M, Langkjer ST. Radiotherapy-related fatigue in breast cancer patients. 2005 ASCO Annual Meeting Proceedings. J ClinOncol. 2005;23(16S, Part I of II (June 1 Supplement)):8267. [Google Scholar]
- 10.Blair S, Bardwell WA, Podbelewicz-Schuller Y. Hemoglobin and fatigue in women undergoing adjuvant chemotherapy: Discussion. Clin Breast Cancer. 2008;8:522–6. doi: 10.3816/CBC.2008.n.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cella DF. Manual of the functional assessment of chronic illness therapy (FACIT) scales.Version 4.Evanston, Ill: Evanston Northwestern Healthcare. 1997 [Google Scholar]
- 12.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 13.Cella DF, Bonomi AE, Leslie WT, Von Roenn J, Tchekmeydian NS. Quality of life and nutritional wellbeing, Measurements and relationship. Oncology. 1993;79(suppl):105–11. [Google Scholar]
- 14.Minton O, Stone P. A systematic review of the scales used for the measurement ofcancer-relatedfatigue(CRF) Ann Oncol. 2009;20:17–25. doi: 10.1093/annonc/mdn537. [DOI] [PubMed] [Google Scholar]
- 15.Cella DF, Webster K, Yost K. MID:Minimally Important Difference value. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyatt GH, Osoba D, Wu AW, Wyrwich KW, Norman GR. Methods to explain the clinical significance of health status measures. Mayo ClinProc. 2002;77:371–83. doi: 10.4065/77.4.371. [DOI] [PubMed] [Google Scholar]
- 17.Webster K, Cella DF, Yost K. The functional assessment of chronicillnesstherapy (FACIT) measurement system: Properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yost K, Eton DT. Combining distribution- and anchor-based approaches to determine minimally important differences: The FACIT experience. Eval Health Prof. 2005;28:172–91. doi: 10.1177/0163278705275340. [DOI] [PubMed] [Google Scholar]
- 19.de Jong N, Candel MJ, Schouten HC, Abu-Saad HH, Courtens AM. Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol. 2004;15:896–905. doi: 10.1093/annonc/mdh229. [DOI] [PubMed] [Google Scholar]
- 20.Janaki MG, Kadam AR, Mukhesh S, Nirmala S, Ponni A, Ramesh BS, et al. Magnitude of fatigue in cancer patients receiving radiotherapy and its short term effect on quality of life. J Can Res Ther. 2010;6:22–6. doi: 10.4103/0973-1482.63566. [DOI] [PubMed] [Google Scholar]
- 21.Irvine DM, Vincent L, Graydon JE, Diane M, Bubela N. Fatigue in women with breast cancer receiving radiation therapy. Cancer Nurs. 1998;21:127–35. doi: 10.1097/00002820-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Robert L, Askew BA, Xing Y, Cella DF, Lemuel AM, Janice NC, et al. Evaluating minimal important differences for the FACT-Melanoma quality of life questionnaire. Value Health. 2009;12:1144–50. doi: 10.1111/j.1524-4733.2009.00570.x. [DOI] [PubMed] [Google Scholar]
- 23.So-Osman C, Nelissen R, Brand R, Brand A, Stiggelbout AM. Postoperative anemia after joint replacement surgery is not related to quality of life during the first two weeks postoperatively. Transfusion. 2011;51:71–81. doi: 10.1111/j.1537-2995.2010.02784.x. [DOI] [PubMed] [Google Scholar]
- 24.Prick BW, Gerard Jansen AJ, EricSteegers AP, Wim CJ, Essink-Bot ML, Uyl-de Groot CA. Dallas, Texas: Am J Obstet Gynecol; 2012. RBC transfusion leads to an improvement of physicalfatigue in women with acute postpartum anemia:The WOMB study: Abstract No 64.Annual Meeting, Society for Maternal and Fetal Medicine (Feb 06-11, 2012) (January supplement) [Google Scholar]


