Abstract
Context:
Palliative radiotherapy aims at symptom alleviation and improvement of quality of life. It may be effective in conferring a reasonable quantum of local control, as well as possibly prolonging survival on the short term. However, there can be rare instances where long-term survival, or even cure, results from palliative radiotherapy, which mostly uses sub-therapeutic doses.
Aim:
To categorize and characterize the patients with long-term survival and/or cure after palliative radiotherapy.
Materials and Methods:
This study is a retrospective analysis of hospital records of patients treated with palliative radiotherapy from 2001 to 2006 at the Regional Cancer Centre, Shimla.
Results:
Of the analyzed 963 patients who received palliative radiotherapy, 2.4% (n = 23) survived at least 5 years, with a large majority of these surviving patients (73.9%, n = 17) being free of disease.
Conclusions:
In addition to providing valuable symptom relief, palliative radiotherapy utilizing sub-therapeutic doses may, in a small proportion of patients, bestow long-term survival, and possibly cure. Rationally, such a favorable, but rare outcome cannot be expected with supportive care alone.
Keywords: Cure after palliative radiotherapy, Long-term survival after palliative radiotherapy, Palliative radiation therapy, Palliative radiotherapy, Therapeutic nihilism
INTRODUCTION
Radiotherapy (RT) in malignancies can be broadly classified into “radical” and “palliative” with regards to the intention of treatment. Radical RT primarily is delivered with an intention to cure, and associated toxicities can be anticipated and are acceptable as an expected side effect. However, when cure seems unlikely, the intention of RT becomes palliative, wherein the goal is to achieve durable symptom relief at the shortest expense of time and resources, and while inflicting the least possible toxicity.[1–3]
Given that “primum non nocere” (first, do no harm) is the principle behind palliative RT, the treatment program is designed to cause the least possible adverse impact upon the patient in all possible aspects.[4] While radical RT programs can often last for around half-a-dozen weeks, often with superimposed administration of concurrent chemotherapy, palliative RT most often consists of an overall lesser dose of radiation, delivered in large fraction sizes (hypofractionated) and alone without chemotherapy. Further, palliative RT is less expensive, since it involves simple planning and treatment without the need for elaborate conformal plans.[5,6]
Overall, palliative RT utilizes sub-therapeutic doses since the goal is not to achieve a “complete eradication of tumor.” As an example, while head and neck squamous cell carcinomas (HNSCC) often require >70 Gy, delivered concurrently with chemotherapy to achieve a reasonable tumor control probability (TCP), palliation can be achieved at much lesser doses in the order of 30–45 Gy delivered mostly in hypofractionated regimens (utilization of more than 2 Gy per fraction).[7–9]
In very rare instances, it is not impossible to come across patients who have achieved excellent survival after treatment with palliative intent, in spite of overall RT doses being well below the doses required for expectations of a good TCP. Thus, recognizing the existence of such outcomes in the clinic, we intended to analyze the frequency of such occurrences of excellent outcomes after palliative radiation doses.
MATERIALS AND METHODS
This study is a retrospective analysis of hospital records of the Regional Cancer Centre, Shimla, India. Patients treated with palliative RT between 1 January 2001 and 31 December 2006 were included, provided their hospital records fulfilled the inclusion criteria [Table 1].
Table 1.
Eligibility criteria for inclusion into analysis

We purposefully excluded malignancies of the breast and prostate, given that these malignancies often have a long natural history.[10–12] We also excluded malignancies of the central nervous system given the poor survival with or without RT. We also excluded salivary gland adenoid cystic malignancies, given that these malignancies too have a very long natural history wherein patients survive for years even after cannon-ball pulmonary metastases.[13,14] We excluded hematological and lympho-reticular malignancies since these patients often follow-up elsewhere at departments specializing in hematological oncology.
We excluded patients who, after initiation of palliative RT, later on received treatment with radical intent, either with the use of additional doses of radiation, chemotherapy, or surgery.
RESULTS
Overall, 963 patients were eligible for analysis [Table 2], of whom 23 patients (2.4%) were alive for at least 5 years since the initiation of palliative RT. Of these patients who had survived at least 5 years since palliative RT, 73.9% (17 of 23 patients) were free of disease without any clinical or radiological evidence of residual or recurrent malignancy.
Table 2.
Site-specific distribution of patients eligible for analysis
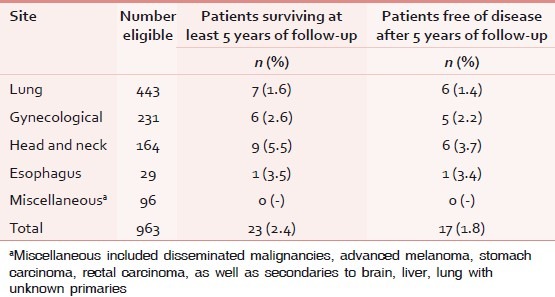
Interestingly, in spite of palliative RT being delivered after patients were considered unlikely to benefit from a full-course radical therapy, 1.6%, 2.6%, 5.5%, and 3.5% of patients with lung cancer, gynecological cancer, head/neck cancer, and esophageal cancer, respectively, were alive for more than 5 years of follow-up. Less than one-fourth of patients surviving for 5 years after treatment had any evidence of residual or recurrence.
The individual characteristics of patients who survived at least for 5 years since the initiation of palliative RT with regards to the diagnosis, age at diagnosis, sex, reason for choosing palliative approach, dose and fractionation of palliative RT, and current status have been presented in Tables 3–5.
Table 3.
Characteristics of patients of lung cancer surviving ≥5 years after palliative RT
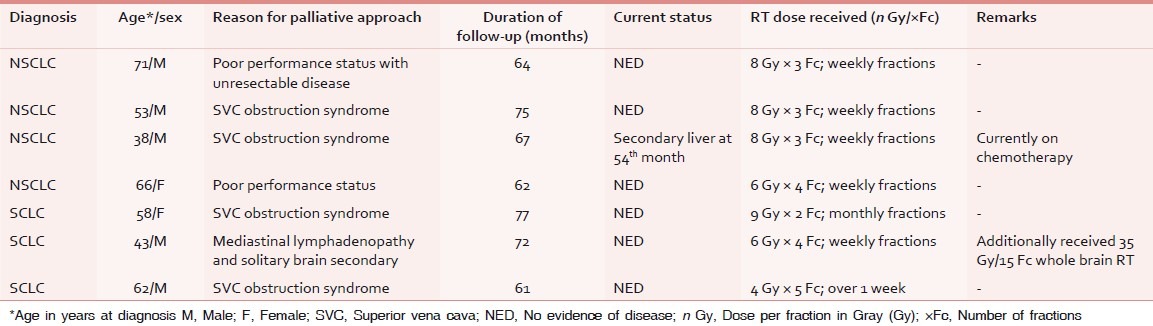
Table 5.
Characteristics of patients of head and neck, and esophageal cancer surviving ≥5 years after palliative RT
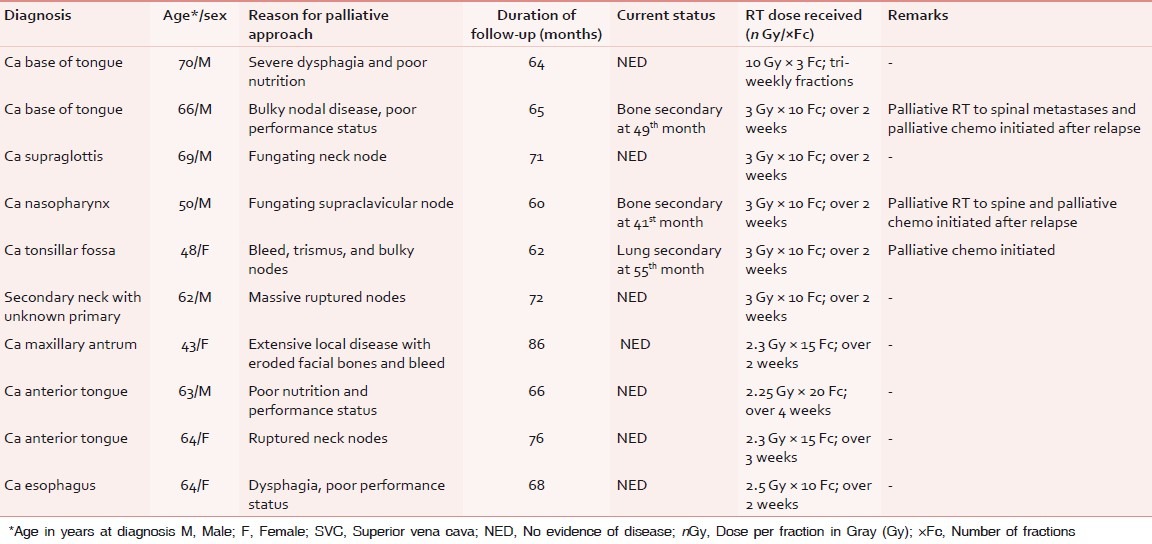
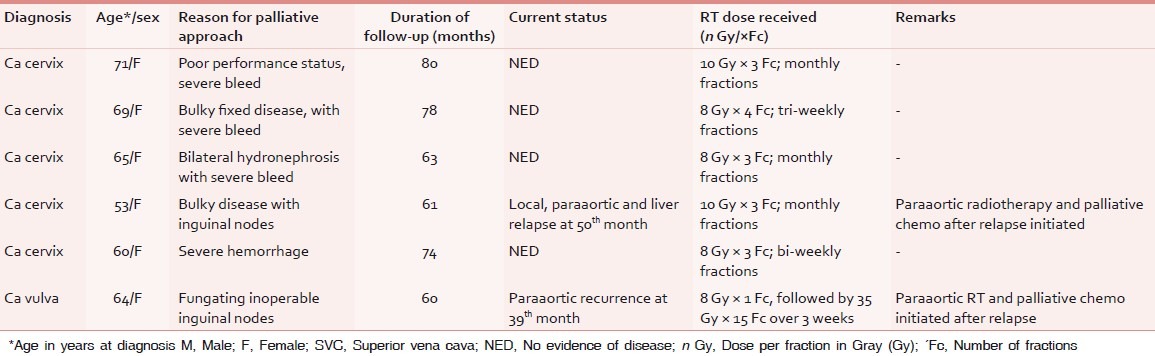
Table 4.
Characteristics of patients of gynecological cancer surviving ≥5 years after palliative RT
DISCUSSION
Palliative RT is based upon the principles of maximizing symptom relief with minimal consumption of time and resources, and causing the least possible concern to the patient with regards to span of treatment and toxicities afforded. Lower total time as well as lower total dose is the hallmark of palliative RT.[1–3] As an example, control of symptoms arising from superior vena caval obstruction syndrome with lung carcinoma is effective even with 20 Gy delivered over 5 days, with 5 fractions of 4 Gy each. However, a radical RT regime for a patient of lung cancer could take anywhere between 5 and 6 weeks.[15–19] Similarly, a patient of bulky neck nodal disease, which is threatening to rupture, may effectively be palliated with doses as less as 30 Gy delivered over 10 fractions of 3 Gy each. However, a radical regime consists of at least 70 Gy delivered over 35 fractions over 6–7 weeks, often with concurrent chemotherapy.[20,21]
The toxicities suffered from the higher total dose and the cost incurred by the protracted radical RT regimen can be justifiable, given the chance of cure. However, in the case of palliative RT, since there is an assumption of a low or absent probability of cure, the patient is offered a short course of radiation, which delivers higher dose per fraction, which can be expected to palliate the local symptoms, and at the same time not adding to agony by means of treatment-related toxicity.[20–24]
Often, a percentage of patients may not enjoy the expected survival outcomes even after a fully radical course of chemo-radiotherapy. This could be due to inter-individual variations in host and tumor biology. Conversely, the opposite, that is, expectations of cure or long-term survival can often be kindled (in the patient and in the clinician) when a patient initiated on palliative RT demonstrates very rapid tumor shrinkage. However, more often than not, patients succumb either due to disseminated disease, local recurrence with aggressive repopulation, or a combination of distant and local disease progression.
A detailed literature search done on literature indexes (PubMed, PubMed central, SCOPUS, Hinari, Index Copernicus, Google Scholar, EMBASE, CINAHL, and DOAJ) with the terms such as “long term survival after palliative radiotherapy,” “long term survival after palliative radiation therapy,” “cure after palliative therapy,” and “tumour control sub-therapeutic radiation dose” did not yield any study, either prospective or retrospective, regarding long-term survival after sub-therapeutic/palliative radiation doses.
We performed a retrospective analysis utilizing hospital records of patients who were treated with palliative RT from 1 January 2001 to 31 December 2006. We chose December 2006 as the cutoff so as to ensure that every patient included into the analysis had the opportunity to complete at least 5 years of follow-up.
Though our original intention was to perform a Kaplan–Meier analysis for survival of all eligible patients who had undergone palliative RT, we found it not feasible given the frequent migration of patients to other centers for treatment and a high attrition rate owing to the inherent logistic difficulties arising from the mountainous terrain of this region. Undeniably, many patients who died after having received symptom control could indeed have succumbed to intercurrent illnesses such as infectious diseases and other non-oncological causes concerning the cardiovascular and pulmonary systems. A prospective observational study design would be a reasonable approach.
Nevertheless, we observed that 2.4% of patients had indeed survived for at least 5 years in spite of the treatment intent not having focused on the goal of prolonged survival. Though the percentage value (2.4%) of patients surviving 5 years after palliative RT may seem trivial, the absolute numbers are significant given the large sample size. It is very likely that these very patients could not have survived if in case they were transitioned to supportive care instead of palliative RT.
This also is striking, given that often patients do not enjoy survival in spite of a full course of radical chemo-radiotherapy, whereas some patients (as illustrated in our analysis) very rarely may indeed benefit excellent survival outcomes even with sub-curative doses of palliative radiation.
This could be in part due to various biological factors, such as immunological clearance, induction of apoptotic signals by radiation, or possibly a lack of aggressive clonogens capable of accelerated repopulation.[25,26] While some cancers such as small cell lung cancer (SCLC) and poorly differentiated cervical carcinomas are more radiosensitive compared to other malignancies, it must also be acknowledged that tumors of the same diagnosis may have different biological behaviors in different patients.
Further, a prospective multivariate analysis of various potential factors contributing to exquisite radiosensitivity even with palliative doses can confirm or refute the role of parameters such as, but not limited to, histology, grade of differentiation, immunological indicators including circulating cytokines, anti-tumor antibodies, and growth factor receptors such as epidermal growth factor receptor 1 (EGFR1), her-2-neu, and vascular endothelial growth factor receptor (VEGFR).[27–29]
To conclude, we remind the reader that RT in a palliative role has excellent efficacy with regards to the control of pain and bleeding, and also with the relief of obstructive symptoms such as dyspnea and dysphagia. In addition, as our study suggests, there could be a benefit with regards to prolongation of survival. Thus, even seemingly unfit patients must be assessed for the potential utility of palliative RT before the decision is made to transition to terminal end-of-life supportive care.
The take-home message for oncologists and palliative care professionals would be that patients not given definitive therapy, but having responded exceptionally well to palliative treatment (e.g. a patient having achieved near-complete response after a course of palliative RT or a patient with markedly improved performance status after completion of palliative therapy, etc.) must be closely followed up and considered for further therapy, such as the use of additional chemotherapy for SCLC, or the use of additional RT and chemotherapy for patients of cervical and head/neck cancers. Summing it up, the noble principle of “primum non nocere,” which translates to “first, do not harm” should not be interpreted in a way as if it were encouraging “therapeutic nihilism,” wherein the clinician's pessimism with regards to the available therapeutic modalities could diminish a patient's chance of obtaining benefit from therapy.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ciezki JP, Komurcu S, Macklis RM. Palliative radiotherapy. Semin Oncol. 2000;27:90–3. [PubMed] [Google Scholar]
- 2.Lutz ST, Chow EL, Hartsell WF, Konski AA. A review of hypofractionated palliative radiotherapy. Cancer. 2007;109:1462–70. doi: 10.1002/cncr.22555. [DOI] [PubMed] [Google Scholar]
- 3.Konski A, Feigenberg S, Chow E. Palliative radiation therapy. Semin Oncol. 2005;32:156–64. doi: 10.1053/j.seminoncol.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Smith CM. Origin and uses of primum non nocere--above all, do no harm! J Clin Pharmacol. 2005;45:371–7. doi: 10.1177/0091270004273680. [DOI] [PubMed] [Google Scholar]
- 5.Donato V, Valeriani M, Zurlo A. Short course radiation therapy for elderly cancer patients. Evidences from the literature review. Crit Rev Oncol Hematol. 2003;45:305–11. doi: 10.1016/s1040-8428(02)00082-3. [DOI] [PubMed] [Google Scholar]
- 6.Lonardi F, Gioga G, Agus G, Coeli M, Campostrini F. Double-flash, large-fraction radiation therapy as palliative treatment of malignant superior vena cava syndrome in the elderly. Support Care Cancer. 2002;10:156–60. doi: 10.1007/s00520-001-0313-4. [DOI] [PubMed] [Google Scholar]
- 7.Al-Mamgani A, Tans L, Van Rooij PH, Noever I, Baatenburg de Jong RJ, Levendag PC. Hypofractionated radiotherapy denoted as the “Christie scheme”: An effective means of palliating patients with head and neck cancers not suitable for curative treatment. Acta Oncol. 2009;48:562–70. doi: 10.1080/02841860902740899. [DOI] [PubMed] [Google Scholar]
- 8.Porceddu SV, Rosser B, Burmeister BH, Jones M, Hickey B, Baumann K, et al. Hypofractionated radiotherapy for the palliation of advanced head and neck cancer in patients unsuitable for curative treatment--“Hypo Trial”. Radiother Oncol. 2007;85:456–62. doi: 10.1016/j.radonc.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Corry J, Peters LJ, Costa ID, Milner AD, Fawns H, Rischin D, et al. The ‘QUAD SHOT’--a phase II study of palliative radiotherapy for incurable head and neck cancer. Radiother Oncol. 2005;77:137–42. doi: 10.1016/j.radonc.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Jeong JH, Dignam J, Anderson S, Mamounas E, Wickerham DL, et al. Findings from recent National Surgical Adjuvant Breast and Bowel Project adjuvant studies in stage I breast cancer. J Natl Cancer Inst Monogr. 2001;30:62–6. doi: 10.1093/oxfordjournals.jncimonographs.a003463. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann M, Rody A. Long-term risk of breast cancer recurrence: The need for extended adjuvant therapy. J Cancer Res Clin Oncol. 2005;131:487–94. doi: 10.1007/s00432-005-0668-x. [DOI] [PubMed] [Google Scholar]
- 12.Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: Continued risk of biochemical failure after 5 years. J Urol. 2000;164:101–5. [PubMed] [Google Scholar]
- 13.Syed IM, Howard DJ. Should we treat lung metastases from adenoid cystic carcinoma of the head and neck in asymptomatic patients? Ear Nose Throat J. 2009;88:969–73. [PubMed] [Google Scholar]
- 14.Bradley PJ. Adenoid cystic carcinoma of the head and neck: A review. Curr Opin Otolaryngol Head Neck Surg. 2004;12:127–32. doi: 10.1097/00020840-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Beli I, Koukourakis G, Platoni K, Tolia M, Kelekis N, Kouvaris J, et al. Hypofractionated radiotherapy in non small cell lung cancer: A review of the current literature. Rev Recent Clin Trials. 2010;5:103–11. doi: 10.2174/157488710791233608. [DOI] [PubMed] [Google Scholar]
- 16.Cross CK, Berman S, Buswell L, Johnson B, Baldini EH. Prospective study of palliative hypofractionated radiotherapy (8.5 Gy × 2) for patients with symptomatic non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1098–105. doi: 10.1016/j.ijrobp.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Mohanti BK, Umapathy H, Bahadur S, Thakar A, Pathy S. Short course palliative radiotherapy of 20 Gy in 5 fractions for advanced and incurable head and neck cancer: AIIMS study. Radiother Oncol. 2004;71:275–80. doi: 10.1016/j.radonc.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Ghoshal S, Chakraborty S, Moudgil N, Kaur M, Patel FD. Quad shot: A short but effective schedule for palliative radiation for head and neck carcinoma. Indian J Palliat Care. 2009;15:137–40. doi: 10.4103/0973-1075.58460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baldini EH. Palliative radiation therapy for non-small cell lung cancer. Hematol Oncol Clin North Am. 1997;11:303–19. doi: 10.1016/s0889-8588(05)70432-9. [DOI] [PubMed] [Google Scholar]
- 20.Egelmeers A, Goor C, van Meerbeeck J, van den Weyngaert D, Scalliet P. Palliative effectiveness of radiation therapy in the treatment of superior vena cava syndrome. Bull Cancer Radiother. 1996;83:153–7. doi: 10.1016/0924-4212(96)81747-6. [DOI] [PubMed] [Google Scholar]
- 21.Chan RH, Dar AR, Yu E, Stitt LW, Whiston F, Truong P, et al. Superior vena cava obstruction in small-cell lung cancer. Int J Radiat Oncol Biol Phys. 1997;38:513–20. doi: 10.1016/s0360-3016(97)00094-1. [DOI] [PubMed] [Google Scholar]
- 22.Patrício MB, Tavares MA, Guimarães MF, Belo MC, Vilhena M. Haemostatic and antialgic effects of the 25 MV photon beam concentrated dose in the treatment of carcinoma of the cervix. J Surg Oncol. 1987;34:133–5. doi: 10.1002/jso.2930340213. [DOI] [PubMed] [Google Scholar]
- 23.Mishra SK, Laskar S, Muckaden MA, Mohindra P, Shrivastava SK, Dinshaw KA. Monthly palliative pelvic radiotherapy in advanced carcinoma of uterine cervix. J Cancer Res Ther. 2005;1:208–12. doi: 10.4103/0973-1482.19588. [DOI] [PubMed] [Google Scholar]
- 24.Onsrud M, Hagen B, Strickert T. 10-Gy single-fraction pelvic irradiation for palliation and life prolongation in patients with cancer of the cervix and corpus uteri. Gynecol Oncol. 2001;82:167–71. doi: 10.1006/gyno.2001.6233. [DOI] [PubMed] [Google Scholar]
- 25.Withers HR, Taylor JM, Maciejewski B. The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol. 1988;27:131–46. doi: 10.3109/02841868809090333. [DOI] [PubMed] [Google Scholar]
- 26.Dubben HH. Local control, TCD50 and dose-time prescription habits in radiotherapy of head and neck tumours. Radiother Oncol. 1994;32:197–200. doi: 10.1016/0167-8140(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Wang J, Liu F, You Z, Yang R, Zhao Y. EGFR inhibitor C225 increases the radiosensitivity of human lung squamous cancer cells. Cancer Cell Int. 2010;10:39. doi: 10.1186/1475-2867-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu F, Wang JJ, You ZY, Zhang YD, Zhao Y. Radiosensitivity of prostate cancer cells is enhanced by EGFR inhibitor C225. Urol Oncol. 2010;28:59–66. doi: 10.1016/j.urolonc.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Belkacémi Y. Innovation in adjuvant radiotherapy for breast cancer: New biologic parameters, a perspective for treatment tailoring. Bull Cancer. 2009;96:111–21. doi: 10.1684/bdc.2009.0800. [DOI] [PubMed] [Google Scholar]


