Abstract
Flies display transient social interactions in groups. However, whether fly–fly interactions are stochastic or structured remains unknown. We hypothesized that groups of flies exhibit patterns of social dynamics that would manifest as nonrandom social interaction networks. To test this, we applied a machine vision system to track the position and orientation of flies in an arena and designed a classifier to detect interactions between pairs of flies. We show that the vinegar fly, Drosophila melanogaster, forms nonrandom social interaction networks, distinct from virtual network controls (constructed from the intersections of individual locomotor trajectories). In addition, the formation of interaction networks depends on chemosensory cues. Gustatory mutants form networks that cannot be distinguished from their virtual network controls. Olfactory mutants form networks that are greatly disrupted compared with control flies. Different wild-type strains form social interaction networks with quantitatively different properties, suggesting that the genes that influence this network phenotype vary across and within wild-type populations. We have established a paradigm for studying social behaviors at a group level in Drosophila and expect that a genetic dissection of this phenomenon will identify conserved molecular mechanisms of social organization in other species.
Keywords: social network, emergent phenotypes, complex systems
Flies engage in a variety of social behaviors that include courtship, aggression, mating, and egg-laying (1–5). Recent studies also indicate that individuals have the ability to recognize others (6) and regulate their behavior according to group membership (7). The composition of such Drosophila groups forms a social environment that influences gene transcription, pheromone displays, and mating frequency among group members (7–9). However, the genetic and sensory determinants that underlie these observations are unknown.
To explore the possibility that Drosophila form organized social interaction networks (SINs), we quantified interactions formed by pairs of individuals within groups of 12 flies of the same sex placed in a circular arena (10). Using video sequences, three classes of behavior suggesting social interactions were identified: frontal approach, rear approach, and social preening (also known as “grooming,” in which a fly rubs its legs together, or uses the leg(s) to rub its own wings, head, or abdomen). Social preening has been previously associated with social interactions in Drosophila (11, 12). These behaviors were verified in detail using video sequences captured at high spatial and temporal resolution (Movie S1). Inspection of video sequences indicated that these classes of behaviors often involve physical contact between the fore- and middle legs of the interacting flies, and these were scored to evaluate their relative frequency of occurrence (Table S1). Drosophila legs harbor both touch- and taste-sensitive sensillae (13), suggesting that flies might exchange somatosensory and gustatory information during these interactions.
We used a computer vision system (14) to track the motion of the 12 flies in each arena, recording the position, orientation, and identity of each fly throughout a 30-min trial. An automated classifier was constructed that captured the interactions described above on the basis of the proximity of flies, their angular orientation during the interaction, and the duration of the interaction (details in Fig. 1A). This classifier captured interactions resulting from all three behaviors described above (Movies S2, S3, and S4). Because these interactions are intrinsically asymmetric (in that one fly actively approached another fly), we were also able to define one fly as the “principal interactor” and the other fly as the “interactee.”
Fig. 1.
Wild-type strains of Drosophila form SINs via fly–fly interactions. (A) Criteria for interaction between two flies are (i) the angle (α) subtended by the long axis of fly 1 (the interactor) and the line segment connecting fly 1’s center of area to that of fly 2 (the interactee) is less than or equal to 90°, (ii) the length of that line segment is less than or equal to two body lengths of the initiator fly (x), and (iii) these two conditions are maintained for at least 1.5 s. This classification scheme encodes the polarity of the interaction and defines an edge in the network. (B) Example network formed by flies (Movie S5). Each interaction that fulfills the criteria of A is represented by an arrow between individuals. The number of interactions (incoming and outgoing) that a fly has participated in (a fly’s degree) is indicated (the variance of the degrees in a network is used in G). (C–F) Effects of strain and sex on behavioral parameters during network formation between Canton-S males and females and Oregon-R males and females. (C) Mean locomotor rate (total distance traveled divided by trial length) was unaffected by sex or strain. (D) Canton-S and Oregon-R females formed interactions at a significantly higher rate than males (P < 0.001), and Canton-S flies made interactions more frequently than Oregon-R flies (P < 0.001). (E) Interaction duration exhibited a strain × sex effect, with Canton-S females interacting for longer durations than Canton-S males, whereas Oregon-R males and females displayed similar interaction durations (P < 0.001). (F) Proportion of interactions that were reciprocated by the receiver exhibited no strain, sex, or interaction effect. (G) Degree distribution variance of the networks formed by each strain/sex group was compared with both Erdős–Rényi (E-R) random networks as well as virtual network controls, which control for the encounter rates expected from the basic locomotor behavior of flies within our arena without social feedback (Fig. S2). Recombining Canton-S or Oregon-R trajectories for virtual networks always resulted in the formation of at least one network (at least 1,000 recombinations were done for each strain/sex). All wild-type groups displayed a significantly higher degree distribution variance than E-R networks (shaded line) and a significantly lower degree distribution variance from virtual network controls (hatched lines). (H) Each individual's degree in the first network iteration plotted against that same individual's degree in the last network iteration of the trial. The means of the last degree were connected and plotted along the first degree axis and show no correlation and no strain or sex difference. (I) The probability of being the receiver in an interaction increases with an individual's total degree. The mean probability of receiving an interaction is plotted, and this function of preferential attachment shows no sex or strain difference. (A–I) Colors indicate strain and sex: Canton-S males (n = 43, white) and females (n = 26, green) and Oregon-R males (n = 28, orange) and females (n = 23, purple). *P < 0.05, **P < 0.01, and ***P < 0.001, only when significance is maintained after multiple test corrections. B–F analyzed using two-way ANOVAs (Methods). Error bars indicate mean ± 1 SE. Boxplot whiskers indicate 1.5*(interquartile range). Measurements presented here represent networks at 25% density (33 unique directed interactions). Tables S2 and S3 show all relevant P values.
To evaluate a SIN, we then established a timeline of directed interactions and calculated iterative networks using a moving-window boxcar filter (15) (i.e., the first network represented the first 33 unique interactions [Fig. 1B and Movie S5], the second network represented the 33 unique interactions starting from the second interaction, and so on). Because no fly may interact with itself in our scheme, 33 such interactions represent a network density of 25% of the total number of interactions possible for 12 individuals. For each iteration, an interaction was considered unique if the principal interactor had not previously interacted with the other fly (Movie S5). This iterative process resulted in the calculation of several successive directed networks formed by the same wild-type flies over a 30-min trial. At first we selected a network density value of 25% a priori for statistical analysis of SINs, but later evaluated networks over a range of densities (12.5%, 20%, 50%, and 75%, representing 17, 27, 66, or 99 directed interactions, respectively) to assess the robustness of our results.
We compared SINs composed of only males and only females from each of two wild-type Drosophila strains (16). Analysis of the timeline of interactions revealed differences between strains and sexes in the rate by which interactions form (Fig. 1D) and a significant strain × sex interaction in the average duration of an interaction (Fig. 1E). Each same-sex group formed at least one SIN in >95% of the 30-min trials (Fig. S1). To test whether the occurrence of these SINs happened by chance, we compared the observed SINs with simulated random networks (Erdős–Rényi) (17). The observed SINs exhibited significantly different distribution of incoming and outgoing interactions per individual [i.e., “degree distribution” (18); see legend of Fig. 1B] from the Erdős–Rényi networks (Fig. 1G). As another control that corrected for the influence of our arena geometry on the spatial and temporal pattern of the flies’ locomotion, we randomly combined individual movement trajectories from different replicates within an experimental group and ran them through a modified classifier (keeps angle and distance but loses time as criteria; Methods, Fig. 1G, and Fig. S2). Again, the observed SINs had a significantly different degree distribution from the virtual network controls, suggesting that SINs do not arise simply from the physical constraints on the locomotor trajectories of individual flies within the arena (Fig. 1G).
To test whether wild-type flies maintained a consistent number of social interactions (their “degree”) over time, we compared each fly’s degree in the first iteration with its degree in the last iteration of a trial (Fig. 1H). We found no correlation, which indicates that individuals do not maintain a fixed number of contacts over time. This means that the stability of SIN dynamics over time (see below) cannot be explained by the constancy of each fly’s number of social connections, a finding consistent with other transient social networks (15). Stated another way, the SIN position of an individual fly varies over time, whereas the structure of the SIN persists within a trial.
Next we investigated whether an individual’s degree affects the probability of acquiring an incoming interaction [an attribute known as “preferential attachment” (19)]. Both strains and sexes displayed a high correlation between current degree and probability of acquiring an incoming interaction (Fig. 1I). This nonzero correlation is taken as evidence for preferential attachment. Because we see no correlation between the number of interactions received during the first and last iteration of a SIN (Fig. 1H), this cannot be explained by some persistent “attractiveness” of a fly. Rather, it suggests that an individual’s recent degree is somehow evident to others in the network. We see no strain differences in preferential attachment (Fig. 1I), despite the observations that strains differ in the rate of interaction and the interaction duration (Fig. 1 D and E).
The passage of information (e.g., the cues conveying an individual’s degree) could rely on the sending and receiving of signals between flies. To assess the sensory modalities involved, we investigated the role of visual, acoustic, olfactory, and gustatory pathways in the formation of SINs among male flies. For vision, we performed experiments using Canton-S flies in dim far-red light (850 nm), to which the flies are not sensitive (20). Similar to what has been reported for courtship behavior (21, 22), the absence of visual cues did not affect overall movement or the percentage of reciprocal interactions (Fig. 2 A–D). For hearing, we also performed experiments using hearing-impaired homozygous inactive mutants (iav1) (23). These mutants are hearing impaired and tend to display low levels of locomotor activity, but we observed no effect on movement (Fig. 2A). The rate of forming interactions in groups of homozygous mutant iav1 flies was not significantly different from that measured in Canton-S homozygous controls, although heterozygous iav1/Canton-S flies displayed significantly lower rates than either homozygous strain (Fig. 2B). We also observed a significant increase in the proportion of interactions reciprocated in mutant iav1 flies (Fig. 2D). Similar to the wild-type strains, the degree distribution within the networks formed by vision- and hearing-impaired flies was different from those predicted by simulated Erdős–Rényi networks and their respective virtual network controls (Fig. 2E).
Fig. 2.
Effects of sensory manipulations on male behavioral characteristics during network formation. (A) Movement during trials was unaffected by visual cues (Canton-S vs. Canton-S in the dark), acoustic cues (Canton-S vs. iav1/Canton-S vs. iav1/iav1; P = 0.0615), or gustatory cues (poxnSuper-A vs. poxnΔXBs6) but was affected by olfactory cues (Canton-S vs. Orco2/Canton-S vs. Orco2/Orco2; P = 0.23, not significant after multiple test correction). (B) Rate of interactions was significantly reduced by eliminating visual cues (P < 0.001) and was significantly reduced in heterozygotes for the iav1 mutation (P < 0.001). Olfactory cues significantly affect the rate of interaction, Orco2/Canton-S and Orco2/Orco2 exhibiting a slower rate than Canton-S (P ≤ 0.001). (C) Average duration of an interaction was affected only by visual cues, because Canton-S in the dark has significantly shorter interactions (P < 0.001). (D) Percentage of interactions reciprocated by the receiving fly was increased by affecting acoustic cues (P = 0.011) and olfactory cues (P < 0.001). (E) As in Fig. 1, variances of degree distribution were compared with Erdős–Rényi (E-R) random networks (shaded line) and virtual network controls (hatched lines) with a sign test. There was some variation in the success of generating these virtual networks (100% for Canton-S, Canton-S in the dark, iav1/Canton-S, and iav1/iav1, 99.9% for Orco2/Canton-S, 82% for Orco2, 99% for poxnSuper-A, and 71% for poxnΔXBs6 ; at least 500 virtual networks were established for each genotype). All genotypes had a significantly higher variance of degree distribution than E-R random networks. All groups besides poxnΔXBs6 had significantly lower variance of the observed degree distribution compared with their respective virtual networks. As poxnΔXBs6 was not significantly different from its virtual network controls (P = 0.061), this strain was excluded in further analysis. A–D analyzed with ANOVAs (Methods). Groups are color-coded: Canton-S (n = 43, white), Canton-S in the dark (n = 28, black), iav1/Canton-S (n = 26, light blue), iav1/iav1 (n = 26, blue), Orco2/Canton-S (n = 24, light red), Orco2/Orco2 (n = 14, red), poxnSuper-A (n = 21, light brown), and poxnΔXBs6 (n = 12, brown). *P < 0.05, **P < 0.01, ***P < 0.001, only when significance is maintained after multiple test corrections. Statistical group identities (lowercase letters) are color-coded by test. All significant effects of Orco2 were observed in both a Canton-S as well as a w1118 background (Fig. S8). Error bars indicate mean ± 1 SE. Boxplot whiskers indicate 1.5*(interquartile range). Measurements presented here represent networks at 25% density (33 unique directed interactions). Tables S2 and S3 show all relevant P values.
In contrast to flies with compromised vision and hearing, the chemosensory mutants ΔXBs6;poxnΔM22-B5 (poxnΔXBs6) and Orco2 disrupted the SIN phenotype. The gustatory mutant poxnΔXBs6 has a deletion of the poxn gene with a partial transgenic rescue that effectively transforms gustatory sensillae, including those on the forelegs, into mechanosensory sensillae (13). The control strain poxnΔM22-B5SuperA-158 (poxnSuperA) harbors this same deletion, but mutant effects are eliminated by complete transgenic rescue (13). The poxnΔXBs6 flies showed an extreme reduction in the ability to form SINs (40% of poxnΔXBs6 failed to accumulate enough interactions for at least one network; Fig. S1). When SINs did form, the degree distribution could not be distinguished from the virtual network controls (Fig. 2E), suggesting that the interactions formed in this genotype were not selective. For this reason, we did no analysis on the structure of SINs produced by this strain. We note that poxn strains were indistinguishable and normal for all other behavioral measures. One caveat is that the effects of this mutation could potentially be explained by an increased sensitivity to touch. Touch and taste are intertwined in the fly, and we are unaware of mutant strains that distinguish between these modalities. In any case, SINs strongly rely on contact, and the more conventional interpretation of this mutation is that it influences gustatory sensation.
The olfactory mutant Orco2 has a severe, although not complete, loss of smell (24, 25). Like poxnΔXBs6, these olfactory mutants show a drastic reduction in their ability to form a SIN (Fig. S1). However, unlike poxnΔXBs6, when Orco2 did form SINs they were not random: they differed from both the Erdős–Rényi networks and virtual network controls (Fig. 2E). The Orco2 SINs were characterized by a significantly lower rate of movement and interactions, as well as a significantly higher proportion of interactions that were reciprocated (Fig. 2 A, B, and D). We note that auditory mutants also display increased reciprocity. This suggests a tendency for both mutant strains to interact more with individuals with which they have had a previous interaction than do wild-type flies.
All individuals within a social network can play a role in forming contacts and transmitting information. In this context, we assessed the average role of individuals in our SINs with four parameters that have been used to indicate types of relationships within social networks (Fig. S3 provides graphical representation of these concepts). (i) Clustering coefficient: a measure of how interconnected neighbors are to one another (17). An individual interactor in a SIN with a relatively higher clustering coefficient is more likely to have interactees who interact with each other. (ii) Assortativity: the probability of an individual interacting with another individual of similar degree (17). Individuals in a SIN with lower assortativity would have a more heterogeneous (by degree) complement of interactors than in a SIN with higher assortativity values on average. (iii) Betweeness centrality: the number of shortest paths that traverse an individual, which indicates the relative importance of a given individual for communication relay (17). SINs with higher betweeness centrality are expected to have more members that are critically important for maintaining network cohesion and potentially transmitting information between subnetworks compared with networks with lower values. (iv) Global efficiency: a measure of redundant pathways (26). SINs with high global efficiency values suggest that the average distance between individuals is smaller in the network than when global efficiency is low.
Here, all four parameters are directed measures. We used z scores to normalize across groups, accounting for the differences in degree distribution (Methods and Figs. 1G and 2E). The fly SINs we measured were consistent with respect to these five metrics, both within a 30-min trial (Fig. S4 A and B) and across the interaction density levels used to define them (see Fig. 4 and Fig. S5). Because all five variables may be interrelated, we corrected for multiple tests (see legend to Fig. 3).
Fig. 4.
Network dynamics seem to be consistent at varying densities. Networks were evaluated at 12.5%, 20%, 25%, 50%, and 75% density to determine consistency of network dynamics (17, 27, 33, 66, and 99 unique directed interactions, respectively; see text). Z scores were based on 500 simulations, which preserved the in- and out-degree of the iterative networks. The difference between strains in betweeness centrality (A) displayed a strong trend from 20% to 50% (Canton-S males, n = 43, 43, 42, and 40; Canton-S females n = 26, 26, 26, 26, and 26; Oregon-R males n = 28, 28, 28, 25, and 19; Oregon-R females n = 23, 23, 23, 23, and 20, at 12.5%, 20%, 25%, 50%, and 75% density, respectively). The reduction of global network efficiency in Orco2 mutants (B) showed a consistent trend from 12.5% to 50% (Orco2/Canton-S n = 27, 25, 24, 21, and 11; Orco2 n = 23, 18, 14, 6, and 2 at 12.5%, 20%, 25%, 50%, and 75% density, respectively). (A–D) *P < 0.05, **P < 0.01, ***P < 0.001, if significance is maintained after multiple test corrections. Significant effects of Orco2 were observed in both a Canton-S as well as a w1118 background (Fig. S8). Error bars indicate mean ± 1 SE.
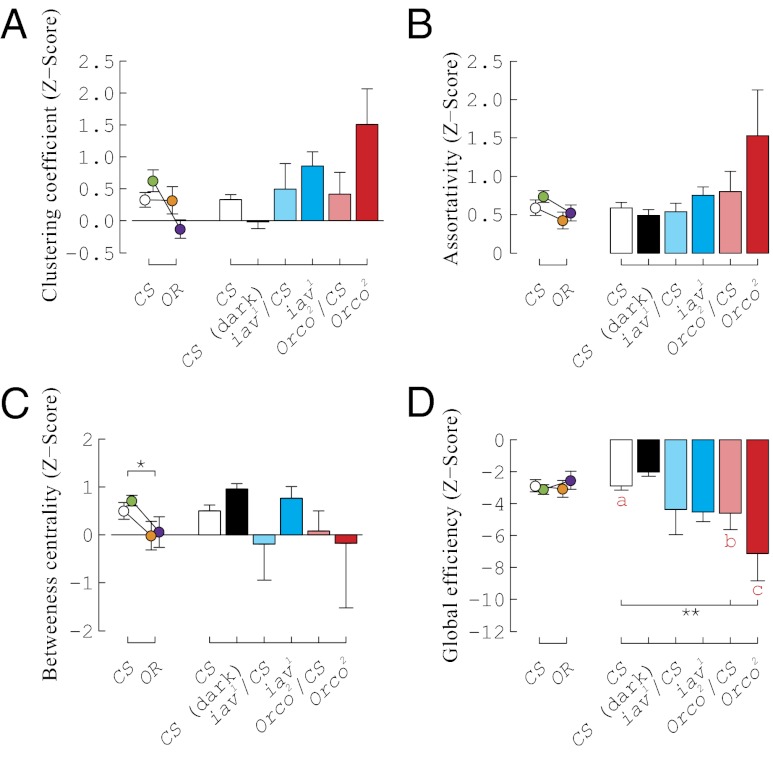
Fig. 3.
Strain and sensory mutation affect network organization. Measurements for each network were standardized to 10,000 random network simulations which preserved distribution of the in- and out-degrees of the iterative network, creating a z score. (A) Clustering of flies within the networks was not different between Oregon-R and Canton-S: neither strain (P = 0.019) nor sex × strain interaction (P = 0.023) were significant after multiple test correction. Canton-S in light vs. Canton-S in dark was also not different (P = 0.036) after multiple test correction. Mutations affecting acoustic and olfactory modalities did not affect the clustering coefficient (acoustic: Canton-S vs. iav1/Canton-S vs. iav1/iav1; P = 0.292, olfactory: Canton-S vs. Orco2/Canton-S vs. Orco2/Orco2, P = 0.019; not significant after multiple test correction). (B) Assortativity of the networks was not higher in Canton-S compared with Oregon-R (P = 0.0738) or in olfactory mutants compared with controls (P = 0.048; not significant after multiple test correction). (C) Betweeness centrality is significantly higher in Canton-S compared with Oregon-R (P = 0.012). Eliminating visual cues did not increase the betweeness centrality of Canton-S (P = 0.033; not significant after multiple-test correction). (D) Global efficiency did not increase by eliminating visual cues (P = 0.099) but significantly decreased in olfactory mutants (P = 0.005). A–D analyzed with ANOVAs (Methods). Groups are color-coded: Canton-S males (n = 43, white) and females (n = 26, green), Oregon-R males (n = 28, orange) and females (n = 23, purple), Canton-S in the dark (n = 28, black), iav1/Canton-S (n = 26, light blue), iav1/iav1 (n = 26, blue), Orco2/Canton-S (n = 24, light red), Orco2/Orco2 (n = 14, red), poxnSuper-A (n = 21, light brown), and poxnΔXBs6 (n = 12, brown). *P < 0.05, **P < 0.01, ***P < 0.001, if significance is maintained after multiple test corrections. Group identities (lowercase letters) are color-coded by test. All significant effects of Orco2 were observed in both a Canton-S as well as a w1118 background (Fig. S8). Error bars indicate mean ± 1 SE. Measurements presented here represent networks at 25% density (33 unique directed interactions).
We observed a significant effect of genetic strain on betweeness centrality (Fig. 3C). The difference in the measure of centrality indicates that, on average, each individual is more critically required for maintaining the overall pattern of network connectivity in Canton-S than in Oregon-R (Figs. 3C and 4A). Impairment of visual and auditory modalities did not affect any of the network properties after multiple test correction (see legend to Fig. 3). The Orco2 mutants produced a significantly lower score for global efficiency (Figs. 3D and 4B), indicating that the olfactory deficit leads to a set of social interactions resulting in a greater average network distance between individuals. We also note a tendency for olfactory mutants to have both a higher assortativity and higher clustering coefficient, although these are not significant after multiple test correction (Fig. 3 A and B).
We evaluated SINs at varying densities of unique interactions (see above and Fig. 4). Our a priori criterion (25%) yielded significant results in betweeness centrality (in strains) and global efficiency (for Orco2 mutants), and the trends were consistent across a wide range of different density criteria (Fig. 4). In addition, certain measurements (such as assortativity between Canton-S, Oregon-R, and Orco2, as well as clustering coefficient in Orco2) seem to be quite consistent at varying densities, despite a lack of statistical significance (Fig. 3 and Fig. S5). Our findings suggest that chemosensory function is required to support the emergent structural properties of SINs in Drosophila.
The dynamics we have presented here could be the result of behavioral interactions before the experiment because we evaluated groups that had been housed together from the time of adult eclosion. To test this hypothesis we compared the SINs of wild-type flies under three different rearing conditions: (i) housed together, (ii) drawn from distinct groups, and (iii) individually isolated from the time of eclosion. We found no statistical differences between these groups in any of the social network measurements (Fig. S6), suggesting that manipulation of prior adult social experience has little effect on the network dynamics. Along with strain-specific differences (Fig. 3), this result strengthens the suggestion of an innate feature of SIN formation in Drosophila.
Another structural analysis of networks focuses on patterns of triadic interactions known as motifs (27). Although the interpretation of individual motifs is unclear, patterns of triadic interactions that occur more or less frequently than expected may provide a signature for networks (28). We constructed discriminant classifiers based on triadic patterns of interaction. These classifiers were able to distinguish among genotypes with a high degree of efficiency (up to ∼98% accuracy; Fig. S7), promising an excellent method for future genetic screens of the SIN phenotype in Drosophila.
Our experiments indicate that Drosophila form nonrandom social networks that persist with a stable structure over time and density (Figs. S4 and S5). In addition, network structure varies in a strain-dependent manner and depends critically on chemosensory signals. We failed to find differences between networks as a consequence of prior adult social experience, and we observed that individuals display variability of their SIN position as the network evolves in time (Fig. 1H), suggesting that network dynamics form rapidly. The mechanisms by which a particular individual remains within the network but transitions continuously to a different network position remain unknown. The observation that individuals do not occupy a fixed position in a SIN while a constant structure is maintained suggests that there may be a temporal or numerical limitation on the influence of past interactions. On the other hand, it is noteworthy that the absence of such individual “memory” is associated with the long-lasting group structure revealed by our analysis. We think that this somewhat paradoxical relationship between persistent group structure and individual limitations may be resolved as we learn more about the molecular and physiological mechanisms that underlie the formation of SINs.
Observing and quantifying the structure of Drosophila SINs may lead to a greater understanding of an individual’s behavior within a group. The characterization of emergent group-level phenotypes such as betweeness centrality and global efficiency offers a first step toward identifying computational rules that guide individual behavior within the network. Such agent-based models would aid in the identification of candidate genes governing social rules of engagement.
Although not tested here, SINs may capture the spread of information throughout a group as it performs communal behaviors. Mated females have been shown to communicate a substrate preference both directly and through female intermediaries (29). We speculate that measurements of information relay, such as betweeness centrality, correlate to the ability of flies to transfer such preferences between individuals (Fig. 3C).
Here, the SIN phenotype has been measured under artificial conditions, and it remains to be seen what role such patterns of interaction play in the wild. The transient nature of the networks, however, is consistent with the natural history of Drosophila melanogaster (2). While active, flies frequently aggregate with conspecifics and closely related species on rotting fruit, although the composition of such aggregates changes over time. Drosophila modulates its behavior based on the group composition, reproductive status, and oviposition substrate preference of others in the group. A fly arriving on a rot would benefit from quickly determining these variables, and resident flies would benefit from acquiring similar information about new immigrants. Between individuals, features such as species and sex are represented by chemical tags (30). The ability of individuals to communicate their degree of interaction (Fig. 1I) hints that such tags also convey the number and quality of prior interactions. If a recent arrival to a rot were able to discriminate the chemical tags of others, it might evaluate its new social environment very quickly. We note that although Drosophila do exhibit social information transfer and may learn from conspecifics (29), it is unclear to what extent and in what direction information flows through the SINs we observed. Nevertheless, we were able to quantify differences in social interaction structure using metrics designed to characterize information flow.
Recent studies also show that strain-specific patterns of interaction may give rise to more complex social phenomena. For example, a strain-specific effect observed between Canton-S and Oregon-R demonstrates that in Drosophila, patterns of mixed mating have nonlinear effects on offspring genotypes (9). It is tempting to hypothesize that such mating patterns and assessment of social context emerges from the same strain-specific organizational properties found in the SINs. Our experimental method will permit us to approach such dynamic interactions quantitatively and further investigate the relationship between social behaviors and the structure of social interactions.
These results support classic ideas that social organization is best understood in terms of the natural history of the species (31, 32). Although it is possible that the genetic basis of social behavior has evolved independently, we favor the view that—like mechanisms underlying circadian clocks and learning (33)—the genetic dissection of Drosophila group behavior will identify molecular mechanisms of social structure conserved across a wide taxonomic base.
Methods
All collections were under light anesthesia (CO2) and grouped by genotype and sex into vials at time of eclosion. Each vial contained 12–16 flies (except for isolated; Fig. S5) and were kept at 12 h light/12 h dark for 3 d and at 25 °C before use in an experiment.
Thirty minutes of video was acquire with fview (34) and analyzed using Ctrax (14) to obtain fly orientation, position, and trajectories. Scripts were written in MATLAB to import tracked data and identify when an interaction occurred between specific flies, defined by rules outlined in Fig. 1A. Brain Connectivity Toolbox (35) MATLAB scripts were used for network measurements.
Iterative Network Analysis.
A connectivity matrix (CMi) was established, accumulating the critical number of interactions while ignoring the first i − 1 interactions. Although weighted connection matrices were produced, all subsequent analyses were carried out on unweighted (binary) connectivity matrices.
Randomness Tests.
We compared the mean variance for each trial against both the median of the variance of the degree distribution of 10,000 Erdős–Rényi graphs and the median of the mean variance of the degree distribution of their respective artificial networks. A sign test determined whether the variances were significantly different from the medians of the controls.
Preferential Attachment.
Each network within the trial was analyzed. The degree of a fly was determined when the network was five interactions short of completion. The subsequent five incoming interactions were then recorded against the receiving fly’s degree, and the normalized probability was calculated and plotted.
Fly Behavior Measurements.
Movement, average time spent per interaction, interaction rate, and percentage of interactions reciprocated were analyzed with ANOVAs. Each test’s F statistic (including factor and interaction) were compared with an empirical distribution (36). To correct for multiple tests, the false discovery rate was controlled between the behavioral measurements (37).
Structural Measurements.
All trials were standardized to control for the degree distribution. For each trial, random networks were generated with the same in- and out-degree distributions. Each measurement was calculated for the observed as well as every generated network to generate a z score (used for all statistical analyses of network properties):
Univariate analysis permutations and multiple test correction were carried out as above. To correct for multiple tests, the false discovery rate was controlled between the four network measurements. Fig. S7 shows analysis of triadic interactions [motifs (27)] and discriminant analysis classifiers.
Tables S2 and S3 show all behavioral and structural P values. More detailed methods are available in SI Methods.
Supplementary Material
Acknowledgments
We thank Jean-Christoph Billeter, Jayed Atallah, Rebecca Rooke, Altaf Ramji, Carl Bergstrom, Phillip Kim, Laurent Keller, Bruce Schneider, Andrew Straw, and Kristin Branson for their suggestions and discussion. This study was funded by grants from the Canada Research Chair, the Canadian Institutes of Health Research, and the National Sciences and Engineering Research Council of Canada (to J.D.L.) and by National Science Foundation Grant 0623527 (to M.H.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biological Embedding of Early Social Adversity: From Fruit Flies to Kindergartners,” held December 9–10, 2011, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/biological-embedding.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121252109/-/DCSupplemental.
References
- 1.Sarin S, Dukas R. Social learning about egg-laying substrates in fruitflies. Proc Biol Sci. 2009;276:4323–4328. doi: 10.1098/rspb.2009.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shorrocks B. Drosophila. London: Ginn; 1972. [Google Scholar]
- 3.Sokolowski MB. Social interactions in “simple” model systems. Neuron. 2010;65:780–794. doi: 10.1016/j.neuron.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 4.Wertheim B, van Baalen EJ, Dicke M, Vet LE. Pheromone-mediated aggregation in nonsocial arthropods: An evolutionary ecological perspective. Annu Rev Entomol. 2005;50:321–346. doi: 10.1146/annurev.ento.49.061802.123329. [DOI] [PubMed] [Google Scholar]
- 5.Mery F, et al. Public versus personal information for mate copying in an invertebrate. Curr Biol. 2009;19:730–734. doi: 10.1016/j.cub.2009.02.064. [DOI] [PubMed] [Google Scholar]
- 6.Yurkovic A, Wang O, Basu AC, Kravitz EA. Learning and memory associated with aggression in Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:17519–17524. doi: 10.1073/pnas.0608211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krupp JJ, et al. Social experience modifies pheromone expression and mating behavior in male Drosophila melanogaster. Curr Biol. 2008;18:1373–1383. doi: 10.1016/j.cub.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 8.Kent C, Azanchi R, Smith B, Formosa A, Levine JD. Social context influences chemical communication in D. melanogaster males. Curr Biol. 2008;18:1384–1389. doi: 10.1016/j.cub.2008.07.088. [DOI] [PubMed] [Google Scholar]
- 9.Billeter JC, Jagadeesh S, Stepek N, Azanchi R, Levine JD. Drosophila melanogaster females change mating behaviour and offspring production based on social context. Proc Biol Sci. 2012;279:2417–2425. doi: 10.1098/rspb.2011.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon JC, Dickinson MH. A new chamber for studying the behavior of Drosophila. PLoS ONE. 2010;5:e8793. doi: 10.1371/journal.pone.0008793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connolly K. The social facilitation of preening behaviour in Drosophila melanogaster. Anim Behav. 1968;16:385–391. doi: 10.1016/0003-3472(68)90023-7. [DOI] [PubMed] [Google Scholar]
- 12.Sexton OJ, Stalker HD. Spacing patterns of female Drosophila paramelanica. Anim Behav. 1961;9:77–81. [Google Scholar]
- 13.Krstic D, Boll W, Noll M. Sensory integration regulating male courtship behavior in Drosophila. PLoS ONE. 2009;4:e4457. doi: 10.1371/journal.pone.0004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Branson K, Robie AA, Bender J, Perona P, Dickinson MH. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kossinets G, Watts DJ. Empirical analysis of an evolving social network. Science. 2006;311:88–90. doi: 10.1126/science.1116869. [DOI] [PubMed] [Google Scholar]
- 16.Hoskins RA, et al. Single nucleotide polymorphism markers for genetic mapping in Drosophila melanogaster. Genome Res. 2001;11:1100–1113. doi: 10.1101/gr.178001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman M. Networks: An Introduction. Oxford: Oxford Univ Press; 2010. [Google Scholar]
- 18.Wasserman S, Faust K. Social Network Analysis. New York: Cambridge Univ Press; 1994. [Google Scholar]
- 19.Newman ME. Clustering and preferential attachment in growing networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;64:025102. doi: 10.1103/PhysRevE.64.025102. [DOI] [PubMed] [Google Scholar]
- 20.Stark WS. The effect of eye colour pigments on the action spectrum of Drosophila. J Insect Physiol. 1973;19:999–1006. doi: 10.1016/0022-1910(73)90026-7. [DOI] [PubMed] [Google Scholar]
- 21.Robie AA, Straw AD, Dickinson MH. Object preference by walking fruit flies, Drosophila melanogaster, is mediated by vision and graviperception. J Exp Biol. 2010;213:2494–2506. doi: 10.1242/jeb.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakai T, Isono K, Tomaru M, Fukatami A, Oguma Y. Light wavelength dependency of mating activity in the Drosophila melanogaster species subgroup. Genes Genet Syst. 2002;77:187–195. doi: 10.1266/ggs.77.187. [DOI] [PubMed] [Google Scholar]
- 23.Gong Z, et al. Two interdependent TRPV channel subunits, inactive and Nanchung, mediate hearing in Drosophila. J Neurosci. 2004;24:9059–9066. doi: 10.1523/JNEUROSCI.1645-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Vosshall LB, Hansson BS. A unified nomenclature system for the insect olfactory coreceptor. Chem Senses. 2011;36:497–498. doi: 10.1093/chemse/bjr022. [DOI] [PubMed] [Google Scholar]
- 26.Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- 27.Milo R, et al. Network motifs: Simple building blocks of complex networks. Science. 2002;298:824–827. doi: 10.1126/science.298.5594.824. [DOI] [PubMed] [Google Scholar]
- 28.Milo R, et al. Superfamilies of evolved and designed networks. Science. 2004;303:1538–1542. doi: 10.1126/science.1089167. [DOI] [PubMed] [Google Scholar]
- 29.Battesti M, Moreno C, Joly D, Mery F. Spread of social information and dynamics of social transmission within Drosophila groups. Curr Biol. 2012;22:309–313. doi: 10.1016/j.cub.2011.12.050. [DOI] [PubMed] [Google Scholar]
- 30.Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–991. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- 31.Allee WC. The Social Life of Animals. Boston: Beacon Press; 1958. [Google Scholar]
- 32.Tinbergen N. Social Behaviour in Animals; with Special Reference to Vertebrates. London: Chapman and Hall; 1971. [Google Scholar]
- 33.Bellen HJ, Tong C, Tsuda H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nat Rev Neurosci. 2010;11:514–522. doi: 10.1038/nrn2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Straw AD, Dickinson MH. Motmot, an open-source toolkit for realtime video acquisition and analysis. Source Code Biol Med. 2009;4:5. doi: 10.1186/1751-0473-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubinov M, Sporns O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Manly BFJ. Randomization, Bootstrap and Monte Carlo Methods in Biology. Boca Raton, FL: Chapman & Hall/CRC; 2007. [Google Scholar]
- 37.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.