Abstract
Executive function (EF) abilities are increasingly recognized as an important protective factor for children experiencing adversity, promoting better stress and emotion regulation as well as social and academic adjustment. We provide evidence that early life adversity is associated with significant reductions in EF performance on a developmentally sensitive battery of laboratory EF tasks that measured cognitive flexibility, working memory, and inhibitory control. Animal models also suggest that early adversity has a negative impact on the development of prefrontal cortex-based cognitive functions. In this study, we report EF performance 1 y after adoption in 2.5- to 4-y-old children who had experienced institutional care in orphanages overseas compared with a group of age-matched nonadopted children. To our knowledge, this is the youngest age and the soonest after adoption that reduced EF performance has been shown using laboratory measures in this population. EF reductions in performance were significant above and beyond differences in intelligence quotient. Within the adopted sample, current EF was associated with measures of early deprivation after controlling for intelligence quotient, with less time spent in the birth family before placement in an institution and lower quality of physical/social care in institutions predicting poorer performance on the EF battery.
It has been argued that early experiences have an impact on neurobehavioral development, for good or ill, and thus influence lifelong health and disease (1). The search for protective processes in the individual that may reduce the impact of early adversity has begun to identify executive functions (EFs) as one candidate domain for intervention (2). EFs are a set of higher order cognitive processes that allow individuals to engage in planning and conscious, goal-directed problem solving (3). In children, EF is related to emotion regulation (4), conscience and moral development (5, 6), and math and literacy ability in kindergarten (7), and it is also predictive of later social and academic competence (8, 9). It is believed that EF may play an especially important role in adverse circumstances because of its role in balancing emotional arousal and cognitive processing (10). Thus, understanding the development of EF in children who experience early adversity may provide avenues for promoting their resilience.
Unfortunately, this factor that could help buffer children living in adverse rearing environments has also been shown to be impaired in these children. For instance, there is accumulating evidence that psychosocial deprivation in the form of being raised in an institution is associated with reduced performance in a variety of EF domains assessed years after adoption into an enriched family environment (11–15). Studies of children experiencing early adversity cannot definitively attribute reduced EF performance to their early experiences because of their frequent coexposure to multiple other prenatal and postnatal risk factors that stunt physical growth and cognitive development (16). However, animal models have shown causal links between social deprivation early in life and reduced performance on EF tasks along with abnormalities in brain regions supporting EF.
Animal Models of the Effects of Early Adversity on Cognition
Both rodent and primate studies have shown that disruptions in early life care result in abnormalities in brain development, including effects on the prefrontal cortex (PFC). Furthermore, these abnormalities in the PFC were associated with reduced EF performance during behavioral tasks. Rat pups that experience disruptions in maternal care exhibit decreasing levels of plasticity-supporting neurotrophins, which are most pronounced in the PFC (17), and show lower levels of performance on EF tasks like rule shifting (18). In rodents, chronic stress exposure more generally leads to dendritic alterations in the medial PFC and orbital frontal cortex that are associated with impaired attentional set-shifting performance (19). In nonhuman primates, social deprivation early in life has also been causally linked with reduced cognitive performance (20) and abnormalities in the development of the neural circuitry that supports EF, namely, the PFC and its associated areas (21). For instance, peer-reared primates exhibit enlarged dorsomedial PFC and dorsal anterior cingulate cortex (22). Additionally, there is evidence that disruptions in early caregiving affect not only the PFC circuits that restrain stress and threat responses but reward, motivation, and attention systems associated with dopaminergic activity. For instance, abnormal early postnatal rearing conditions have been shown to have an impact on the development of mesocorticolimbic dopamine systems, creating a sensitization to stress and vulnerability for compulsive/addictive behavior in rats (23). Alterations in both stress/emotion systems and cognitive functioning attributable to early adverse experiences have pointed to interconnections between these systems across development. For instance, the PFC is rich in glucocorticoid receptors, and evidence from rodent models shows that blocking these receptors during acute stress prevents transient cognitive impairments in performance that are usually observed during these episodes (24). Although translational models face challenges attributable to species differences in developmental timing of neural maturation and differences in how adverse circumstances are modeled (25), there is incontrovertible evidence based on animal models that adverse care early in life disrupts the normal development of PFC-based cognitive systems.
EF in Postinstitutionalized Children
The evidence drawn from animal models is consistent with correlational findings from children who experienced early adversity and were adopted from institutions overseas. For instance, one neuroimaging study of postinstitutionalized (PI) children [mean (M) age = 8.8 y] who had been adopted from Romanian orphanages used PET to find reduced glucose metabolism in the orbitofrontal gyrus and infralimbic PFC compared with nonadopted (NA) children and adults (26). Furthermore, a diffusion tensor imaging study on PI children found a diffuse pattern of frontostriatal connectivity that may explain their common symptoms of inattention/overactivity (27). In addition, electroencephalogram studies show reduced alpha power and increased relative theta power in frontal, temporal, and occipital regions of children adopted from institutions (28, 29), and these patterns of brain activity statistically mediated the relation between early deprivation and attention-deficit/hyperactivity symptoms at 54 mo of age (28). These neuroimaging and EEG results provide hints about the neural mechanisms that may underlie the behavioral findings. However, the latter results also indicated altered patterns of neural activation across the brain and not just in prefrontal regions. For this reason, it is important to provide evidence of impairments linked specifically to the PFC. Many behavioral studies of EF performance in PI children have not controlled for intelligence quotient (IQ); thus, we do not know whether reduced performance is specific to EF or reflects more general effects on brain and cognition. In this study, we controlled for IQ to examine this question.
Behavioral studies have shown that children who experienced early life deprivation but were adopted into nurturing homes exhibit reduced EF performance later in childhood, between the ages of 6 and 14 y (11–13, 15), usually measured at least 3–4 y after adoption. EF has been thought of as comprising three overlapping component skills: cognitive flexibility/rule shifting, updating/working memory, and inhibitory control (30). Previous laboratory studies with older PI children have shown reduced performance on each of these components: cognitive flexibility (11), working memory performance (11, 12, 15), and inhibitory control (13, 15, 31). Additionally, there are extensive reports that PI children show attention deficits and hyperactivity symptoms that persist into adolescence (32, 33), which could signal long-term difficulties with EF.
Despite these studies conducted with older children, little is known about the development of EF immediately after adoption, at a time of rapid improvement in functioning that presumably reflects high neural plasticity. The only studies of EF in preschool PI children used parent report to assess EF problems using the Behavior Rating Inventory of Executive Function Preschool Version (BRIEF-P) and reported mixed results. One study found no differences compared with a normative sample in adopted preschool children aged 2–5 y (14), whereas another found that 11% of children aged 4–5 y had EF problems in the clinical range according to parent ratings (34). The third study did not include a comparison group (35); thus, the authors were only able to conclude that parents of PI children reported more EF problems than teachers did. Observational laboratory studies are needed to clarify whether EF performance is lower in this group and can be detected in this age range. To address this gap in the literature, the present study examined EF 1 y postadoption in children adopted at the age of 16–36 mo using a developmentally sensitive battery of laboratory EF tasks (36).
Aims of Current Study
To summarize, the aims of the study were to investigate (i) whether children who experienced early life deprivation exhibit reduced EF performance soon (1 y) after adoption compared with a group of age-matched NA children, (ii) whether EF performance differences hold after controlling for differences in IQ, and (iii) whether EF performance is related to measures of preadoptive experience (age at placement in institution, duration and quality of institutional care) after controlling for IQ. We included these latter measures of early deprivation to strengthen the inference linking early deprivation with EF performance, because experimental studies of early adversity are not ethically possible in humans.
Results
Group Differences in EF.
A 2 × 2 (sex by group) ANOVA with EF composite scores as the dependent measure revealed a significant main effect of group on EF [F(1,79) = 25.4, P < .001, ηp2 = 0.24], such that PI children scored significantly lower (M = −0.23, SD = 0.6) than NA children (M = 0.51, SD = 0.75). There were no significant effects of sex [F(1,79) = 0.03, P = 0.87] or interactions of sex by group [F(1,79) = 0.92, P = 0.34].
Analysis of IQ as a Covariate.
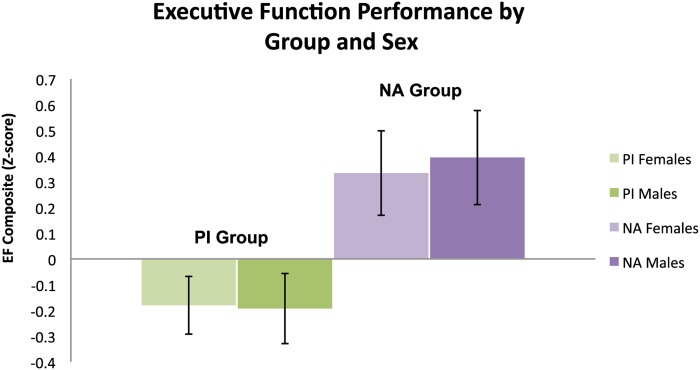
It was important to consider general cognitive ability as a covariate because measures of intelligence are consistently correlated with EF, especially in early childhood (e.g., 36–38), although less so in adulthood, consistent with increasing differentiation of cognitive function in development (e.g., 39). We first examined whether the groups differed on IQ. A 2 × 2 (sex by group) ANOVA with IQ scores as the dependent measure revealed a significant main effect of group [F(1,72) = 30.8, P < 0.001, ηp2 = 0.29], with no significant main effect or interaction effect with sex [F(1,74) = 0.64, P = 0.43 and F(1,74) = 1.15, P = 0.29, respectively]. As expected, PI children scored lower (M = 92.7, SD = 15.8) than NA children (M = 112.4, SD = 14.1). Given that IQ was significantly correlated with EF [r(78) = 0.54, P < 0.001], we reexamined group differences in EF to see whether they would hold above and beyond differences in IQ. Indeed, a 2 × 2 (sex by group) analysis of covariance with EF scores as the dependent measure and IQ as a covariate showed a significant main effect of group on EF, such that PI children scored lower on EF than NA children, even after controlling for IQ [F(1,73) = 10.45, P = 0.002, ηp2 = 0.13]. There were no main [F(1,73) = 0.03, P = 0.87] or interaction effects with sex [F(1,73) = 0.06, P = 0.80]. Estimated means of EF performance by group and sex after controlling for IQ are presented in Fig. 1.
Fig. 1.
Estimated means for EF performance by group and sex after controlling for IQ. Error bars represent SEMs.
Associations with Measures of Preadoptive Experience.
We examined relations between several measures of preadoption experiences (when available) and the EF composite in the PI group, while controlling for IQ. The EF composite was positively correlated with the amount of time children spent in their birth family before being placed for adoption, after controlling for IQ [partial r(43) = 0.29, P = 0.047], and it was also positively correlated with the mean rating of the physical and social quality of the orphanage environment according to parent reports and an adoption agency expert’s ratings, also controlling for IQ [partial r(37) = 0.37, P = 0.018]. The EF composite was not correlated with the total duration of institutional care after controlling for IQ [partial r(44) = 0.05, P = 0.75]. When examining links between IQ and measures of preadoptive experiences, IQ was negatively correlated with duration of institutional care [r(49) = −0.302, P = 0.035] but not correlated with age at placement in an institution [r(46) = −0.09, P = 0.56] or with the physical and social quality of institutional care [r(40) = 0.17, P = 0.29].
Discussion
The results reported here document the earliest objectively measured EF reductions in performance in children who were adopted from orphanages overseas and had experienced institutional care for various periods of their preadoptive lives. These reductions in performance were significant above and beyond the effects of early deprivation on global IQ, which have been noted in this study and previously reported in similar samples (40, 41). Although several studies have documented reduced EF performance in PI children (11–15), this is the youngest age (2.5–4 y old) and the soonest postadoption timing (1 y after adoption) to document lower EF performance using observational laboratory data. Given the protracted development of the PFC in humans (42, 43), it was important to investigate the question of how early hypothesized differences in EF would be noticeable as the basis for future longitudinal work examining change in EF in relation to postadoption experiences. Another strength of this study was that we assessed all three main components of EF that are typically subsumed by the construct (30): cognitive flexibility, working memory, and inhibitory control. PI children exhibited significantly reduced scores on our composite measure of EF and also within each of these three subtasks (SI Text). However, another strength is that our results were not undermined by floor effects in measurement. For instance, even though many PI children failed to pass any levels of the Dimensional Change Card Sort (DCCS) scale, we found evidence of reduced performance compared with nonadoptive children even when we excluded children from both groups who did not pass any level (SI Text).
We were also interested in associations between preadoptive environment characteristics and current EF. We found positive associations between present EF and the physical and social quality of the institutional care experienced early in life as rated by parents and an adoption expert. Additionally, children who spent more time in their birth family before placement in an institution fared better in terms of EF after controlling for IQ, suggesting a potentially protective role of early family care. The effects noted were small, accounting for less than 15% of the variance in EF. However, our information about preadoption conditions came from parent report rather than direct measures. Parents often have incomplete and sometimes inaccurate information about the actual living conditions before adoption, and their visits to the institutional setting are often brief. Thus, because of the likelihood of error in the preadoption measures, the correlations we noted may underestimate the true size of associations.
The main limitations of this study were its correlational nature and the scarcity of information on the birth families of these children (both common in studies of PI children). Because of these limitations, the objection could be raised that parents who give up their children for adoption may be more likely to have poor EF skills and that this risk factor is transmitted to their children. Even if there is some genetic or epigenetic transmission of this vulnerability, experimental studies in animals point to unique impacts of early parental deprivation on prefrontally mediated cognitive tasks (21, 22). Furthermore, our associations between EF and the quality of orphanage care, as well as the positive correlation between EF and more time spent in the birth family, support the argument that environmental input may play a role in the early development of EF.
A possible objection that could be raised is that the PI children might not have been versed in English well enough 1 y after adoption to understand the task instructions. There are several reasons why this is unlikely. First, average IQ in the PI group was in the normal range (M = 93), the measure of IQ that we used as a covariate both reflected and was influenced by verbal ability, and the EF measures were developed using simple language and requiring nonverbal responses (pointing, opening boxes, and waiting or ringing the bell). Second, the differences between groups held even when controlling for IQ. Finally, in the delay of gratification task, the majority of PI children who ended the task early did so by ringing the bell. Thus, even though they scored poorly on this task, they demonstrated their understanding of the verbal instruction.
The present findings raise the question of whether reduced performance in PI children is indicative of permanent deficits or temporary delays in development. Future studies should use a longitudinal design to examine this question. Even though previous literature documents lower levels of EF performance in older samples of children with similar backgrounds, longitudinal tracking of subjects is necessary for resolving the issue of deficits vs. delays. Such work will also help examine whether some children exhibit improvements in EF over time and may identify postadoption experiences associated with such improvements, which can then be targets for intervention trials. Indeed, we are following the PI children in this report and have begun assessing EF at the age of 5.5 y, along with measures of postadoption care. Thus, we will be able to investigate continuity of EF problems, as well as predictors of improvements or deteriorations over time. We are also measuring event-related potentials using electroencephalography at the age of 5.5 y to be able to reveal associations between behavioral measures of EF over time and patterns of underlying prefrontal neural activity. These physiological measures will allow us to make inferences regarding disruptions in developing neural circuitry associated with early life adversity.
Given that childhood EF is linked to future academic success (9) and positive social development (6), it is important to draw attention to these associations between early life adversity and EF performance. Furthermore, there is some indication that childhood EF may predict EF in adulthood. For instance, delay of gratification at the age of 4 y predicts cognitive control on a laboratory task 14 y later (44) and 40 y later (45), with evidence that individuals who had been less able to delay gratification as preschoolers showed less frontal activation and more activation of the ventral striatum when inhibiting motor responses to positive social cues 40 y later (45). Interventions aimed at promoting resilience in children living in adverse conditions by improving their EF would thus have the potential for long-term positive benefits, but they need to take note of these associations between early adversity and childhood EF. Preventing or reducing the impact of adversity on the developing brain may be an important avenue for improving child outcomes. Furthermore, adding adversity-reducing strategies to existing childhood interventions may be necessary to improve these programs that sometimes only focus on improvement through cognitive training. As some have argued (2), we need to protect brains rather than just stimulate minds.
Materials and Methods
Participants.
We recruited 60 internationally adopted PI and 30 NA community comparison children to participate in this study. PI children were recruited if they had been adopted between 16 and 36 mo of age (M = 24.1 mo, SD = 5.02) and had experienced periods of institutional care before adoption into the United States (range: 4–34 mo, M = 18.6 mo, SD = 7.7; 13.8–100% of preadoption life). We excluded from all analyses 4 PI children diagnosed with fetal alcohol syndrome, 2 PI children who were severely developmentally delayed, and 1 NA child who was diagnosed with epilepsy. The remaining 54 PI children (M = 3.08 y, SD = 0.43) and 29 NA children (M = 3.08 y, SD = 0.35) did not differ by age [t(81) = 0.04, P = 0.97] or sex (χ2 = 0.03, P = 0.86; 58.6 % female NA children and 57.4% female PI children). The groups also did not differ on parental socioeconomic status, which was higher than national norms (median household income in the PI group: $100,001–$125,000; median household income in the NA group: $75,001–$100,000). PI children were adopted from diverse countries of origin (13 countries) distributed across the following regions: 31.5% from Asia, 25.9% from Africa, 24% from Eastern Europe, 9.3% from Latin America, and 9.3% from Southeast Asia. Preliminary analysis of birth region revealed no significant associations with EF; thus, this was not considered further in analyses. Degrees of freedom for statistical tests vary in some instances based on some missing data on task performance measures or parent questionnaires.
Procedure.
PI and NA children completed a 2-h videotaped laboratory visit 12 mo after adoption, which included an EF battery (tasks described below in the order administered) and an IQ assessment. Parents of PI children also completed a telephone interview with an adoption expert during which they provided quantitative (e.g., duration) and qualitative ratings of institutional care based on their visit to the child’s birth country, when applicable.
Measures of EF.
DCCS scale.
The graded DCCS scale (46) measured cognitive flexibility and was adapted from the DCCS (47) for use with young preschoolers. We included four successive levels of difficulty appropriate for this sample: Categorization, Reverse Categorization, Separated Card Sorting, and Integrated Card Sorting. During Categorization, children were instructed to sort toy “baby” and “mommy” animals into the appropriate bucket, which were labeled with large pictures of a baby or a mother, respectively. After two demonstration trials and a verbal rule check, children completed six sorting trials (passing = minimum of 5 trials correct). During Reverse Categorization, children were instructed to sort the animals into the opposite bucket (i.e., “baby” animals were sorted into “mommy” buckets and vice versa; passing = minimum of 5 of 6 trials correct). For Separated Card Sorting, the child was seated in front of two boxes with rectangular slots cut in the top. A target card (e.g., black truck on a red background, black star on a blue background) was attached to the front of each box. The experimenter introduced the “shape game,” presenting a series of cards (black trucks on blue backgrounds and black stars on red backgrounds) and instructed the child to place star cards in the star box and truck cards in the truck box. If the child passed (minimum of 5 of 6 trials correct), the experimenter proceeded with the “color game,” where the same types of cards were to be sorted by background color instead of shape. To pass the overall Separated Card Sorting level, the child had to pass both the shape and color games (i.e., successfully switch rule sets). During the Integrated Card Sorting level, participants had to sort cards by color and then by shape. Integrated target and test cards were analogous to separated cards, with the exception that the color and shape were part of the same stimulus (e.g., a blue star on a white background). Passing criteria were the same as in the separated level. At every level, before each trial, the rule was repeated to diminish memory load (e.g., “Trucks go here, stars go here,” with the experimenter pointing to the correct boxes). Thus, perseverating on the old rule showed a lack of cognitive flexibility. Scores were computed as the highest level passed (range: 0–4).
Spin the pots.
The spin the pots task (48) measured working memory. A number of visually distinct boxes were arranged on a rotatable circular tray. In full view, the experimenter placed a sticker in all but two of the boxes. Children were shown the empty boxes, and the experimenter then covered the tray with a scarf and spun the tray 180°. The scarf was then removed, and children were instructed to choose one box; if a sticker was found, the child could keep it as a prize. Once children had chosen a box, the boxes were again covered with the scarf and the tray was rotated again. The number of boxes and trials depended on the age of the child: for 2.5-y-olds, 8 boxes and 12 spins; for 3-y-olds, 9 boxes and 14 spins; for 3.5-y-olds, 10 boxes and 16 spins; and for 4-y-olds, 11 boxes and 18 spins. The task continued until all stickers were found or the maximum number of spins was completed. Performance scores were calculated as number of stickers found of number available, as number of trials taken to complete the task, and as a dichotomous measure of task completion (1 = located all stickers, 0 = failed to locate all stickers).
Delay of gratification.
The delay of gratification task (9) measured inhibitory control. Children selected a favorite food reward (e.g., minimarshmallows); the experimenter placed 2 treats in a dish and 10 treats in a second identical dish and asked which dish the child preferred. Children nearly always chose the dish with more treats. The experimenter explained that she had to leave the room for awhile and that the child would have to wait until she returned if the child wanted the larger reward. Alternatively, the child could ring a bell to signal the experimenter to return but would then receive only the smaller pile of treats. After a verbal rule check, the experimenter left and returned when (i) the child rang the bell; (ii) the 8-min waiting period had passed; or (iii) the child began eating the treats, as observed by the experimenter watching the child from a different room. Latency to the first touch of the treats or bell, total time waited, and reasons for ending the task early (e.g., ringing bell, eating treat) were coded from video recordings of the session.
Composite measure of EF.
We used principal components analysis (PCA) to reduce the EF data. For tasks that had several highly intercorrelated performance indices, we selected only one measure to include in the PCA [e.g., latency to the first touch of the food reward was highly correlated with total time waited during the delay of gratification task (r = 0.88, n = 81, P < 0.001); thus, we only used one of the measures]. The PCA identified a single common factor with an eigenvalue larger than 1 that all the tasks were highly correlated with (loadings were 0.81, 0.69, and 0.69 for DCCS, spin the pots, and delay of gratification, respectively). Because the measures were highly intercorrelated, we converted the three performance measures to z-scores so that they would be measured on the same scale and averaged them to create an EF composite score reflecting the highest level achieved in DCCS, finding all the hidden stickers within the allotted trials during spin the pots and latency to first touch during delay of gratification. This EF composite was significantly correlated with IQ (r = 0.54, n = 78, P < 0.001) but did not completely overlap with it, showing 70.8% unique variance.
Measures of IQ.
The Abbreviated IQ Battery of the Stanford–Binet Intelligence Scales (fifth edition) (49) was used to assess general cognitive ability in NA children. This battery included both nonverbal performance and verbal (vocabulary) scales, and it is valid for measurement beginning at the age of 2 y. The Mullen Scales of Early Learning (50) were used to assess PI children, because this instrument can assess individuals from birth to the age of 5.5 y, and the design of the study anticipated that some PI children might be too impaired for the Stanford–Binet Intelligence Scales. Mullen Scales of Early Learning included visual reception, fine motor skills, receptive language, and expressive language. The Mullen Scales of Early Learning were a part of a comprehensive clinical assessment conducted by a trained clinician, which included feedback to parents and personalized referrals offered by this study as a cost-free service to PI children. This assessment was not available or necessary for NA children. For both the Stanford–Binet Intelligence Scales and the Mullen Scales of Early Learning, scale scores were added and the sum was converted to an age-adjusted estimated IQ score using the norms for each battery (based on M = 100 and SD = 15) to place them on the same scale. The Mullen Scales of Early Learning and the Stanford–Binet Intelligence Scales are both well validated on large national samples and produce estimates of IQ that are highly correlated (0.70–0.85) with other cognitive indices, such as the Bayley Mental Development Index and the IQ produced by the Wechsler Preschool and Primary Scales of Intelligence (51, 52).
Measures of Preadoptive Experiences.
Primary caregivers completed a telephone interview with an adoption expert within 4 mo of adoption. They described the timeline of adoption and the child’s life history based on the information available to them (age at placement into institutional care, total duration of institutionalization, number of transitions in care). They also described the care the child received and rated the physical and social characteristics of each institution the child lived in for each major time interval: 0–6 mo, 6–12 mo, 12–18 mo, and after 18 mo of life. Ratings were completed after the interviewer provided a description of the lowest, middle, and highest points on the scale. Ratings were five-point Likert scales (1 = very poor, 2 = poor, 3 = adequate, 4 = good, 5 = very good). When rating the physical quality of the institution, the parent was encouraged to consider factors like cleanliness, condition of paint/floors, and whether the child’s physical needs seemed to be met. Social conditions included ratings of the opportunities for engaging other caregivers and playing with other children. An important criterion was the ratio of caregivers to children (small groups of 3–4 children to 1 caregiver were considered very good, whereas groups of 10 or more with some 1:1 care for each child were considered adequate and groups larger than 10 without any 1:1 interaction with a caregiver were considered very poor). Based on parents’ qualitative descriptions of institutional conditions, the interviewer also produced her own ratings of the physical and social environment. Ratings were averaged across all time periods. To compute one index of Physical and Social Care Quality, both raters and both subscales were averaged (scale Cronbach’s α = 0.83).
Supplementary Material
Acknowledgments
We thank the families who participated in this research and the International Adoption Project at the University of Minnesota. We also thank Kristin A. Frenn, Shanna B. Mliner, Jamie M. Lawler, and Bao Moua for their assistance during data collection. This study was supported by Grants MH 080905 and MH 078105 (to M.R.G.); Grant R01HD051495 (to S.M.C); and, in part, by the Center for Neurobehavioral Development at the University of Minnesota.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biological Embedding of Early Social Adversity: From Fruit Flies to Kindergartners,” held December 9–10, 2011, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/biological-embedding.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1121246109/-/DCSupplemental.
References
- 1.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: Building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 2.Shonkoff JP. Protecting brains, not simply stimulating minds. Science. 2011;333:982–983. doi: 10.1126/science.1206014. [DOI] [PubMed] [Google Scholar]
- 3.Zelazo PD, Carlson SM, Kesek A. In: Handbook of Developmental Cognitive Neuroscience. Nelson C, Luciana M, editors. Cambridge, MA: MIT Press; 2008. pp. 553–574. [Google Scholar]
- 4.Carlson SM, Wang TS. Inhibitory control and emotion regulation in preschool children. Cogn Dev. 2007;22:489–510. [Google Scholar]
- 5.Kochanska G, Murray K, Coy KC. Inhibitory control as a contributor to conscience in childhood: From toddler to early school age. Child Dev. 1997;68:263–277. [PubMed] [Google Scholar]
- 6.Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Dev Psychol. 2000;36:220–232. [PubMed] [Google Scholar]
- 7.Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Dev. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 8.Mischel W, Ayduk O. Self-regulation in a cognitive-affective personality system: Attentional control in the service of the self. Self Ident. 2002;1(2):113–120. [Google Scholar]
- 9.Mischel W, Shoda Y, Rodriguez MI. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 10.Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Dev Psychopathol. 2008;20:899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biol Psychiatry. 2009;66:1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bos KJ, Fox N, Zeanah CH, Nelson CAIII. Effects of early psychosocial deprivation on the development of memory and executive function. Front Behav Neurosci. 2009;3:16. doi: 10.3389/neuro.08.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruce J, Tarullo AR, Gunnar MR. Disinhibited social behavior among internationally adopted children. Dev Psychopathol. 2009;21:157–171. doi: 10.1017/S0954579409000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Merz EC, McCall RB. Parent ratings of executive functioning in children adopted from psychosocially depriving institutions. J Child Psychol Psychiatry. 2011;52:537–546. doi: 10.1111/j.1469-7610.2010.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pollak SD, et al. Neurodevelopmental effects of early deprivation in postinstitutionalized children. Child Dev. 2010;81:224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutter M. English and Romanian Adoptees (ERA) Study Team Developmental catch-up, and deficit, following adoption after severe global early privation. J Child Psychol Psychiatry. 1998;39:465–476. [PubMed] [Google Scholar]
- 17.Roceri M, et al. Postnatal repeated maternal deprivation produces age-dependent changes of brain-derived neurotrophic factor expression in selected rat brain regions. Biol Psychiatry. 2004;55:708–714. doi: 10.1016/j.biopsych.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Lovic V, Fleming AS. Artificially-reared female rats show reduced prepulse inhibition and deficits in the attentional set shifting task—Reversal of effects with maternal-like licking stimulation. Behav Brain Res. 2004;148:209–219. doi: 10.1016/s0166-4328(03)00206-7. [DOI] [PubMed] [Google Scholar]
- 19.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812(1-2):38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- 21.Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: Evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- 22.Spinelli S, et al. Early-life stress induces long-term morphologic changes in primate brain. Arch Gen Psychiatry. 2009;66:658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19:1863–1874. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- 24.Butts KA, Weinberg J, Young AH, Phillips AG. Glucocorticoid receptors in the prefrontal cortex regulate stress-evoked dopamine efflux and aspects of executive function. Proc Natl Acad Sci USA. 2011;108:18459–18464. doi: 10.1073/pnas.1111746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunnar MR, Fisher PA. Early Experience, Stress, and Prevention Network Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Dev Psychopathol. 2006;18:651–677. [PubMed] [Google Scholar]
- 26.Chugani HT, et al. Local brain functional activity following early deprivation: A study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- 27.Behen ME, et al. Abnormal fronto-striatal connectivity in children with histories of early deprivation: A diffusion tensor imaging study. Brain Imaging Behav. 2009;3:292–297. doi: 10.1007/s11682-009-9071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McLaughlin KA, et al. Delayed maturation in brain electrical activity partially explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68:329–336. doi: 10.1016/j.biopsych.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarullo AR, Garvin MC, Gunnar MR. Atypical EEG power correlates with indiscriminately friendly behavior in internationally adopted children. Dev Psychol. 2011;47:417–431. doi: 10.1037/a0021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyake A, et al. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognit Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- 31.Colvert E, et al. Do theory of mind and executive function deficits underlie the adverse outcomes associated with profound early deprivation?: Findings from the English and Romanian adoptees study. J Abnorm Child Psychol. 2008;36:1057–1068. doi: 10.1007/s10802-008-9232-x. [DOI] [PubMed] [Google Scholar]
- 32.Kreppner JM, O’Connor TG, Rutter M. English and Romanian Adoptees Study Team Can inattention/overactivity be an institutional deprivation syndrome? J Abnorm Child Psychol. 2001;29:513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- 33.Stevens SE, et al. Inattention/overactivity following early severe institutional deprivation: Presentation and associations in early adolescence. J Abnorm Child Psychol. 2008;36:385–398. doi: 10.1007/s10802-007-9185-5. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs E, Miller LC, Tirella LG. Developmental and behavioral performance of internationally adopted preschoolers: A pilot study. Child Psychiatry Hum Dev. 2010;41(1):15–29. doi: 10.1007/s10578-009-0149-6. [DOI] [PubMed] [Google Scholar]
- 35.Groza V, Ryan SD, Thomas S. Institutionalization, Romanian adoptions and executive functioning. Child Adolesc Social Work J. 2008;25(3):185–204. [Google Scholar]
- 36.Carlson SM. Developmentally sensitive measures of executive function in preschool children. Dev Neuropsychol. 2005;28:595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- 37.Carlson SM, Zelazo PD, Faja S. In: Handbook of Developmental Psychology. Zelazo PD, editor. New York: Oxford Univ Press; [Google Scholar]
- 38.Hongwanishkul D, Happaney KR, Lee W, Zelazo PD. Hot and cool executive function: Age-related changes and individual differences. Dev Neuropsychol. 2005;28:617–644. doi: 10.1207/s15326942dn2802_4. [DOI] [PubMed] [Google Scholar]
- 39.Johnson MH, Munakata Y. Processes of change in brain and cognitive development. Trends Cogn Sci. 2005;9(3):152–158. doi: 10.1016/j.tics.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Beckett C, et al. Do the effects of early severe deprivation on cognition persist into early adolescence? Findings from the English and Romanian adoptees study. Child Dev. 2006;77:696–711. doi: 10.1111/j.1467-8624.2006.00898.x. [DOI] [PubMed] [Google Scholar]
- 41.O’Connor TG, Rutter M, Beckett C, Keaveney L, Kreppner JM. English and Romanian Adoptees Study Team The effects of global severe privation on cognitive competence: Extension and longitudinal follow-up. Child Dev. 2000;71:376–390. doi: 10.1111/1467-8624.00151. [DOI] [PubMed] [Google Scholar]
- 42.Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eigsti IM, et al. Predicting cognitive control from preschool to late adolescence and young adulthood. Psychol Sci. 2006;17:478–484. doi: 10.1111/j.1467-9280.2006.01732.x. [DOI] [PubMed] [Google Scholar]
- 45.Casey BJ, et al. Behavioral and neural correlates of delay of gratification 40 years later. Proc Natl Acad Sci USA. 2011;108:14998–15003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beck DM, Schaefer C, Pang K, Carlson SM. Executive function in preschool children: Test-retest reliability. J Cogn Dev. 2011;12(2):169–193. doi: 10.1080/15248372.2011.563485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zelazo PD. The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nat Protoc. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- 48.Hughes C, Ensor R. Executive function and theory of mind in 2 year olds: A family affair? Dev Neuropsychol. 2005;28:645–668. doi: 10.1207/s15326942dn2802_5. [DOI] [PubMed] [Google Scholar]
- 49.Roid GH. Stanford–Binet Intelligence Scales. 5th Ed. Rolling Meadows, IL: Riverside Publishing; 2003. [Google Scholar]
- 50.Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service, Inc.; 1995. [Google Scholar]
- 51.Bradley-Johnson S. Cognitive assessment for the youngest children: A critical review of tests. J Psychoed Assess. 2001;19(1):19–44. [Google Scholar]
- 52.Lichtenberger EO. General measures of cognition for the preschool child. Ment Retard Dev Disabil Res Rev. 2005;11(3):197–208. doi: 10.1002/mrdd.20076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.