Abstract
This study seeks to understand whether poverty very early in life is associated with early-onset adult conditions related to immune-mediated chronic diseases. It also tests the role that these immune-mediated chronic diseases may play in accounting for the associations between early poverty and adult productivity. Data (n = 1,070) come from the US Panel Study of Income Dynamics and include economic conditions in utero and throughout childhood and adolescence coupled with adult (age 30–41 y) self-reports of health and economic productivity. Results show that low income, particularly in very early childhood (between the prenatal and second year of life), is associated with increases in early-adult hypertension, arthritis, and limitations on activities of daily living. Moreover, these relationships and particularly arthritis partially account for the associations between early childhood poverty and adult productivity as measured by adult work hours and earnings. The results suggest that the associations between early childhood poverty and these adult disease states may be immune-mediated.
Keywords: health disparities, socioeconomic status
Existing research links early childhood income to adult productivity as measured by adult work hours and earnings (1), with much of the association related to variation in individuals’ ability to sustain full-time work in adulthood. Such sustained capacity for work may, in turn, be related to the presence or absence of chronic health conditions, which impair the physical and mental abilities required to achieve and maintain gainful employment. Commonalities among many of these prevalent, chronic conditions, such as hypertension and arthritis, include their propensities to disrupt activities of daily living (ADLs), their known linkages to socioeconomic status in both childhood and adult life (2, 3), and their plausible partial mediation by neuroimmunological processes (4–7). Whether immune-mediated chronic diseases play a role in associations between early socioeconomic conditions and adult productivity is, therefore, an important and understudied question (8). This study investigates both whether low income during very early childhood vs. other stages of childhood is associated with immune-mediated chronic health conditions in adulthood and the extent to which these adult health conditions might explain associations between very early childhood income and adult productivity. The reported analyses help to inform arguments not only for the role of early-life conditions and immune-mediated disease processes in adulthood but also implicating physical and mental health conditions in the intergenerational transmission of poverty (8).
Early childhood income and later disease processes, especially immune-related processes, may be plausibly connected through several potential pathways (9). First, the fetal origins hypothesis posits a biological programming process, where exposures and insults during the prenatal period have long-lasting implications for physiology and disease risk (10). Maternal diet and smoking, for example, have known effects on the neonatal development of the immune system, which could play a role in the pathogenesis of immune-mediated disease (11). Furthermore, low caloric intake during pregnancy is found to be associated with increases in chronic health conditions, such as coronary heart disease, hypertension, and obesity, among infants later in life (12). Low birth weight has also been associated with chronic low-grade inflammatory processes in later life (13).
Second, psychological stress has well-documented influences on cellular and humoral immune processes (14, 15), and chronic stress from growing up poor could also play a role in dysregulation across multiple physiological systems with effects that persist (or possibly compound) into adulthood (16). Childhood poverty may actually calibrate immune system responsivity, dysregulating inflammatory processes and resulting in a shift to proinflammatory states (17). Third, parental behaviors associated with low income could increase susceptibility to immune- or inflammation-mediated diseases (18, 19). Poverty-related adversity is known, for example, to impede parents’ abilities to engage in warm and sensitive interactions with their children (20), and parental warmth may moderate the effects of low socioeconomic status (SES) on inflammatory processes in children (21, 22).
Fourth, low-income children attain less education as adults, and education is an important determinant of health, in part through knowledge of healthy diet and health-inducing behaviors (23), which may help attenuate chronic inflammatory processes. Fifth, macrolevel conditions, such as the state of the business cycle during time of birth, are found to adversely impact health outcomes. Specifically, the risk of cardiovascular mortality increases among those individuals born during economic downturns (24, 25). Finally, low income in early childhood has been linked to poor mental health in adulthood (9). Disorders of mental health, especially depression, are associated with inflammation, and mental disorders are causally antecedent to inflammatory changes (21, 26).
Given the credibility of these immune mediation pathways, the present study sought to relate high-quality measures of very early childhood income to incident adulthood disease states with demonstrable ties to immune-mediated pathogenic processes. In contrast to many epidemiological studies that rely on retrospective data of childhood SES, our data allow us to follow individuals from birth to adulthood and use concurrent reports of family income gathered annually between the prenatal year and age 15 y. We examine the relationship between income in different stages of childhood and adult health (arthritis, hypertension, and conditions that limit daily activities) and labor market productivity (earnings and work hours). Our data also provide rich controls for conditions correlated with income at the time of the individuals’ birth, thus helping to rule out the influence of confounding factors.
Results
Data are drawn from the Panel Study of Income Dynamics (PSID), which has followed a nationally representative sample of about 5,000 families and their children since 1968. Our target study sample consisted of the 1,070 individuals born into the PSID households between 1968 and 1975, and these individuals constitute a representative national sample of children in these birth cohorts. Interviews taken between 1998 and 2009 provide self-reported information on adult health and labor market productivity.
Descriptive statistics for outcome and control variables are presented in Table S1. Key adult health outcome measures include the fractions of time that the individual reported arthritis, hypertension, or health conditions that limit daily activities in up to three interviews taken when the individual was between age 30 and 41 y (ADL). Key labor market outcomes include annual earnings, annual work hours, and hourly earnings, with each of these measures averaged over all available interview reports taken between age 30 and 41 y.
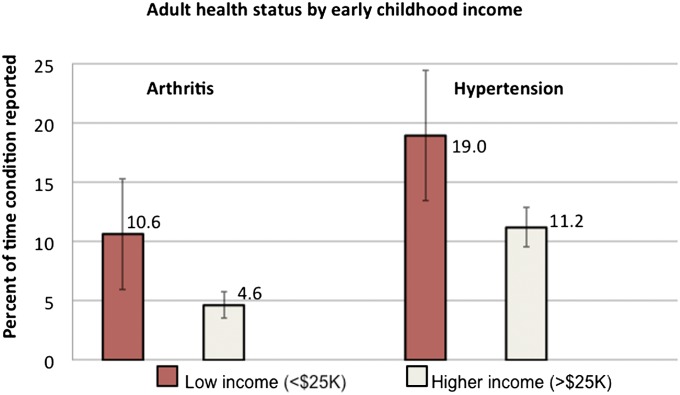
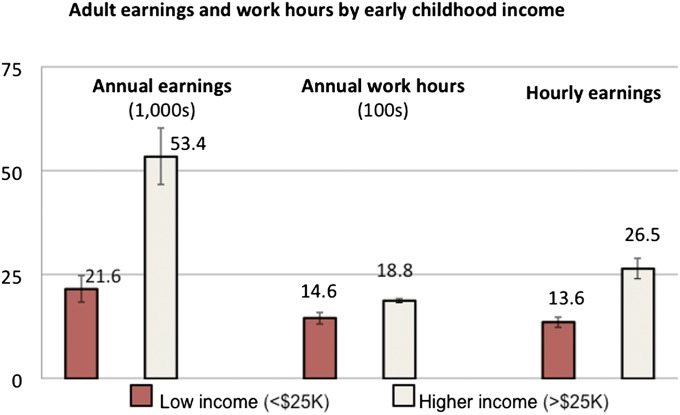
Fig. 1 shows that adult health differed markedly depending on a child’s family income during pregnancy and infancy, with poorer children more likely to report diagnoses of adult arthritis (10.6% vs. 4.6%, P < 0.01) and hypertension (19.0% vs. 11.2%, P < 0.01). Furthermore, as shown in Fig. 2, children in low-income families between the prenatal year and second year had lower annual earnings as adults ($21,600 vs. $53,400, P < 0.01), annual work hours (1,460 vs. 1,877 h, P < 0.001), and hourly earnings ($13.60 vs. $26.50/h, P < 0.01) in adulthood.
Fig. 1.
Percent of times that health conditions were reported between age 30 and 41 y by income status in early childhood. Low income includes children (n = 156) with average family income between their prenatal year and age 2 y below $25,000 (2010 dollars). Higher income includes children (n = 914) with average family income between their prenatal year and age 2 y greater than or equal to $25,000 (2010 dollars). Both differences were significant at P < 0.01. All data come from the PSID and are weighted.
Fig. 2.
Annual earnings, work hours, and hourly earnings between age 30 and 41 y by income status in early childhood. Low income includes children (n = 156) with average family income between their prenatal year and age 2 y below $25,000 (2010 dollars). Higher income includes children (n = 914) with average family income between their prenatal year and age 2 y greater than or equal to $25,000 (2010 dollars). Annual earnings are in $1,000 of 2010 dollars, annual work hours are in 100s of hours, and hourly earnings are in 2010 dollars. All differences significant at P < 0.01. All data come from the PSID and are weighted.
Although children born into low-income families are most likely to be low income throughout childhood and adolescence, there is still substantial mobility across income classes. Table S2 classifies children according to the average prenatal to age 2 y period (early childhood) and later incomes. Only about one-half of the children with early-life incomes that were below $15,000 had incomes that low between age 3 and 5 y (53%) or between age 6 and 15 y (59%). This income volatility enables us to estimate impacts of income very early in childhood while controlling for income in other childhood stages, which should be very helpful in controlling for the kinds of omitted variable biases present in most studies of poverty effects.
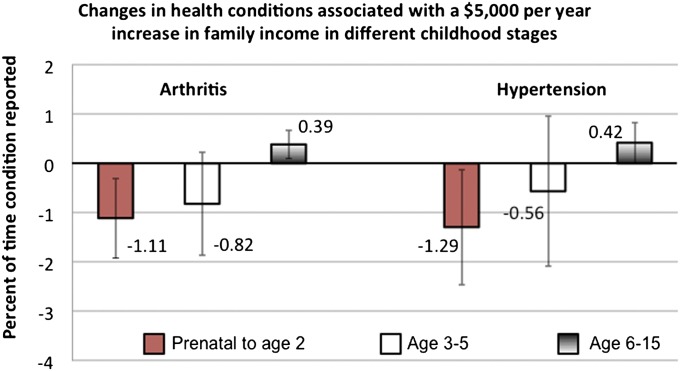
Fig. 3 (based on Table S3) summarizes regression results for three of the adult health outcomes. Key independent variables are family income (in 2010 dollars) averaged between the individual’s prenatal year and age 2 y, between age 2 and 5 y, and between age 6 and 15 y. Each of these income segments is treated as a piecewise linear (spline) function with a knot at $25,000. Figs. 3 and 4 show results for the first (low-income) segment only. Additional control variables included in the analysis are listed in Table S1.
Fig. 3.
Simulated change in the percentage of times that health conditions were reported between age 30 and 41 y in response to a $5,000 (in 2010 dollars) annual increase in income status in various childhood stages. Red boxes show results for the prenatal year through age 2 y, white boxes show results for age 3–5 y, and gradient boxes show results for age 6–15 y. Information is based on regression results presented in Table S3. All results are based on estimates for individuals with childhood incomes below $25,000. All data come from the PSID and are weighted.
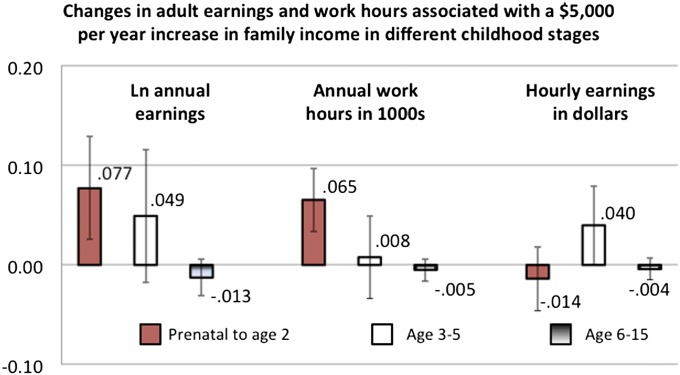
Fig. 4.
Simulated change in ln annual earnings, work hours, and hourly earnings between age 30 and 41 y in response to a $5,000 (in 2010 dollars) annual increase in income status in various childhood stages. Red boxes show results for the prenatal year through age 2 y, white boxes show results for age 3–5 y, and gradient boxes show results for age 6–15 y. Information is based on regression results presented in Tables S5 and S6. All results are based on estimates for individuals with childhood incomes below $25,000. All data come from the PSID and are weighted.
Increases in childhood income between prenatal and age 2 y are associated with reductions in hypertension, arthritis, and ADL limitations, but these associations are statistically significant only among low-income children. Specifically, a $5,000 increase in household income among low-income children in any of the years between the prenatal period and age 2 y (a total of 4 y) is associated with a 1.1 percentage point reduction [95% confidence interval (CI) = −2.46, −0.12] in the proportion of years that hypertension was reported between the age of 30 and 41 y, a 1.3 percentage point reduction (95% CI = −1.92, −0.30) in the proportion of years that arthritis was reported, and a 0.02 point reduction (95% CI = −0.037, −0.001) in the ADL index. Increments to low or higher income between age 3 and 5 y or 6 and 15 y did not have statistically significant associations with adult hypertension and ADLs. In the case of arthritis, the coefficients on the low-income spline segment on income averaged between age 6 and 15 y were unexpectedly positive and significant. The null hypothesis of equality of the low-income coefficients for all three childhood periods could be rejected at P < 0.06 or lower for all three health outcomes.
We also estimated to what extent hypertension and arthritis accounted for the association between early childhood income and adult ADLs by adding them to the ADL regression equation (Table S3, ADLs columns). We found that inclusion of these diagnoses reduced the coefficient on early childhood by one-half. Arthritis was a statistically significant predictor of ADL limitations. Although early income had significant associations with these three health outcomes, it was not uniquely predictive of all health outcomes that we examined. SI Text shows that there are no significant associations between early childhood income and mental health.
To estimate the extent to which immune-mediated chronic diseases accounted for associations between early income and adult labor market productivity, we first estimated models of three key productivity-related measures—annual earnings, work hours, and hourly earnings—all of which are drawn from reported answers when respondents were between ages 30 and 41 y. Independent variables were identical to those variables used in the health models. As shown in Fig. 4 and consistent with prior research (1), low income in very early childhood has highly significant associations with annual earnings and work hours but not hourly earnings. This finding suggests that variation in individuals’ ability to sustain full-time work careers rather than their hourly earnings for each hour worked is most consequential in explaining why children with low incomes early in life earn less per year as adults. Table S4 shows that the addition of hypertension and arthritis reduces the coefficient on early childhood income in the work hours model by 11% and that adding the ADL index reduces it by a total of 18.3%. Corresponding reductions for earnings are 23.8% and 30.2%, respectively.
Discussion
The outcomes examined here (hypertension and arthritis) are typically considered diseases of old age, but as the current study shows, adults of 30–41 y of age who were poor in early childhood reported hypertension about 19% of the time across three adult interviews and arthritis about 11% of the time. These rates are approximately two times the rates reported by adults who were not poor in early childhood. We, thus, hypothesized that the stressors associated with early childhood poverty may induce a proinflammatory state. In keeping with this hypothesis, we find that childhood income is associated with the risk of adult hypertension, arthritis, and limitations on daily activities. However, these associations seem to be highly specific. First, they are much stronger for income in the first years of life (between the prenatal year and age 2 y) than later in childhood. This finding is consistent with the hypotheses that these early years represent a sensitive period during which time social processes become embedded in biology and that epigenetic modifications could be responsible for these associations (27). Second, the associations are much stronger at lower, rather than higher, income levels. This finding corresponds to a number of recent studies showing that income plays a more potent role in later life outcomes for those children at the bottom end of the income distribution (1). These patterns of effects mirror the patterns found in prior studies of the association between poverty early in childhood and adult labor market productivity (1). Using data drawn from the same source, we replicate those prior results and extend them in showing that early-onset hypertension, arthritis, and limitation on daily activities account for a modest share of those associations.
One specific hypothesis that these findings support, which requires more investigation and direct measurement of immune parameters, is that inflammatory changes constitute a core biological process initiating and mediating associations between early childhood poverty and adult disease. Although other nonimmune-mediated processes may also be relevant (e.g., the role of diet and nutrition in the development of hypertension), emerging evidence suggests that inflammatory changes may be an additional common pathway to several chronic morbidities (28). Acute inflammation is a complex orchestration of changes in blood flow, capillary permeability, leukocyte migration, and expression of cytokines—regulatory glycoproteins that serve as chemical messengers among immune cells and between the immune system and the brain (13). Inflammatory processes are controlled by regulatory and counterregulatory events at the levels of the brain (e.g., the basal ganglia and the anterior cingulate cortex), neuroendocrine circuitry (i.e., the hypothalamic–pituitary–adrenocortical axis and the autonomic nervous system), and cellular function (e.g., helper T-lymphocyte differentiation into Th1 vs. Th2 cells) (17, 29, 30). These processes confer short-term protection by resisting and often eliminating infectious and other pathogenic agents to which the host has been exposed, but such benefits are also accompanied, when inflammatory conditions persist, by long-term tissue damages. Physical aging, for example, is thought to be related, in part, to the long-term accumulation of damage from repeated episodes of acute inflammation (31). Inflammatory changes are also an integral part of the human stress response and have been linked to the development of a broad range of adversity-related chronic diseases, such as coronary heart disease, asthma, and hypertension (6, 7, 13, 32), and conditions of aging (33, 34). Although such morbidities are likely to have multiple and interactive etiologic pathways [e.g., for hypertension (35)], immune-mediated processes have been increasingly implicated in their pathogenesis. Whether inflammatory processes represent an independent risk factor for disease or simply mark underlying processes is not yet known (36), and it remains unclear whether proinflammatory states are causally antecedent or consequent to chronic physical and mental morbidities (37–39).
Nonetheless, immune parameters that index proinflammatory states can be strong near-term predictors of the mortality and diminished job productivity associated with age-related chronic diseases (33). Animal models show that occupying subordinate social positions is associated with proinflammatory shifts in cytokine signaling pathways (40), and other recent evidence suggests that exposures to early low SES environments are linkable to similar immune changes in human children (36, 41, 42). Low childhood SES is associated with higher blood levels of the acute-phase reactant C-reactive protein, cytokine IL-6, and TNF-α (43), and low SES children are more likely to develop a proinflammatory phenotype, placing them at risk for inflammatory diseases such as atherosclerosis, autoimmune disorders, and cancer (44). Furthermore, immune-mediated chronic disease states, such as arthritis and other activity-limiting conditions, have established effects on job performance and market productivity, and even high blood pressure, plausibly mediated, in part, by proinflammatory changes, can hasten work-relevant declines in cognitive functioning (45–48).
Importantly, a major limitation of the current study in examining a proinflammatory hypothesis is the absence of direct measures of immune parameters. Nonetheless, our data offer many advantages relative to existing studies of the hypothesized nexus among childhood poverty, immune mediation, and adult disease processes. In particular, the present work gathers high-quality prospective information on family SES from a nationally representative sample. At the same time, our data track individuals only through age 41 y, which is too early to capture most of the eventual diagnoses of hypertension, arthritis, and activity limitations. Additionally, our data rely on self-reported diagnosis of conditions, which is subject to error. Studies suggest underreporting of chronic health conditions (49), although most validation studies are in older populations than our sample, with hypertension underreported across groups (50); self-reported diagnosis of arthritis has been judged valid for surveillance purposes (51). Moreover, our data lack other key mediating measures during childhood, such as childhood health conditions, physical activity, stress exposure, health risk behaviors, health status of the mother at the time of the child’s birth, and breastfeeding practices, all of which might help provide an understanding of the process by which early economic conditions matter for later life health.
All else equal, our results suggest that, for children living in low-income households, income increases in any of the years between the prenatal period and second year of life are associated with statistically significant reductions in adult hypertension and arthritis. Our point estimates imply that raising the average income of low-income children by $5,000/y over the entire 4-y interval is associated with about 5 percentage point reductions in the risks of adult arthritis and hypertension. Although $20,000 constitutes a sizeable income increase, the reductions in risk for both conditions (one-quarter for hypertension and close to one-half for arthritis) are also quite substantial, given the 19% and 11% rates, respectively, of hypertension and arthritis among those adults who were poor in early childhood.
These findings point to the potential importance of policies that affect the financial resources available to families with young children. Our findings indicate that the incomes of the most economically disadvantaged families should be of greatest concern, particularly during the years when these families have young children. Virtually all countries have a variety of tax and transfer programs that redistribute income. In the United States, the earned income tax credit pays up to $5,751 per year to low-income working families, the temporary assistance for needy families program provides cash grants to low-income families for limited periods, and the child tax credit grants a nonrefundable credit of up to $1,000/y per child (information from the 2011 tax year). Targeting these transfers or similar programs to families with very young children may offer the largest benefit for later life health and wellbeing, and this extra income may be especially important in an era of rapidly rising healthcare costs.
Materials and Methods
Data.
As detailed in SI Text, this study uses data from the PSID, which has followed a nationally representative sample of about 5,000 families and their children since 1968. Our target study sample consists of the 1,296 individuals who were born into the PSID households between 1968 and 1975. Parents of these individuals reported family income and other demographic information annually between their prenatal years and age 15 y. Some 1,070 of these individuals participated in the surveys between 1999 and 2009 as adults and had valid responses for at least one of the outcome health variables.
Dependent Variables: Health and Labor Market Productivity.
The PSID provides data on both the physical and mental health of survey respondents. We drew our data from health and labor market reports taken when the individuals were 30–41 y old. In these years, respondents are asked whether a doctor has ever told them that they have high blood pressure, hypertension, arthritis, or rheumatism. Respondents are asked whether they experience health or physical disability problems (ADLs) in each year between 1999 and 2009. Additional health measures include a subjective health rating and the Kessler Screening Scale for Psychological Distress (K6). Adult productivity is captured with annual earnings, work hours, and hourly earnings, which is again averaged over all available reports taken when the individuals were between age 30 and 41 y.
Childhood SES and Demographics.
Parent reports of annual total family income were averaged across three periods: the prenatal year through the calendar year in which the child turned 2 y, age 3–5 y, and age 6–15 y. Our analyses also control for the following demographic characteristics taken around the time of the child’s birth: birth year, race, child sex, whether the child’s mother was married at the time of his or her birth, region, age of the mother at the time of the birth, total number of siblings, and whether the child was the first birth of the mother. Completed schooling and a score on a sentence completion test taken from the head of the household (usually the father in two-parent households and the mother in single-parent households) are also included.
Analysis Plan.
To account for a possible differential impact of increments to low as opposed to higher family income, we allowed the coefficients on average income within each childhood period to have distinct linear effects for average incomes up to $25,000 and incomes $25,000 and higher.
Supplementary Material
Acknowledgments
Support for this work was provided by a small grant for research using the Panel Study of Income Dynamics data from the Survey Research Center, Institute for Social Research, University of Michigan (to K.M.Z.-G.).
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biological Embedding of Early Social Adversity: From Fruit Flies to Kindergartners,” held December 9–10, 2011, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/biological-embedding.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1203167109/-/DCSupplemental.
References
- 1.Duncan GJ, Ziol-Guest KM, Kalil A. Early-childhood poverty and adult attainment, behavior, and health. Child Dev. 2010;81:306–325. doi: 10.1111/j.1467-8624.2009.01396.x. [DOI] [PubMed] [Google Scholar]
- 2.Adler NE, Rehkopf DH. U.S. disparities in health: Descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 3.Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: Burden of infection, health, and socioeconomic status in U.S. children. Soc Sci Med. 2009;68:699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giraldo E, Garcia JJ, Hinchado MD, Ortega E. Exercise intensity-dependent changes in the inflammatory response in sedentary women: Role of neuroendocrine parameters in the neutrophil phagocytic process and the pro-/anti-inflammatory cytokine balance. Neuroimmunomodulation. 2009;16:237–244. doi: 10.1159/000212384. [DOI] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 6.Muller DN, Kvakan H, Luft FC. Immune-related effects in hypertension and target-organ damage. Curr Opin Nephrol Hypertens. 2011;20:113–117. doi: 10.1097/MNH.0b013e3283436f88. [DOI] [PubMed] [Google Scholar]
- 7.Zubcevic J, Waki H, Raizada MK, Paton JF. Autonomic-immune-vascular interaction: An emerging concept for neurogenic hypertension. Hypertension. 2011;57:1026–1033. doi: 10.1161/HYPERTENSIONAHA.111.169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currie J. Healthy, wealthy, and wise: Socioeconomic status, poor health in childhood, and human capital development. J Econ Lit. 2009;47:87–122. [Google Scholar]
- 9.Almond D, Currie JM. In: Handbook of Labor Economics. Ashenfelter O, Card D, editors. Cambridge, MA: National Bureau of Economic Research; 2010. [Google Scholar]
- 10.Strauss RS. Effects of the intrauterine environment on childhood growth. Br Med Bull. 1997;53:81–95. doi: 10.1093/oxfordjournals.bmb.a011608. [DOI] [PubMed] [Google Scholar]
- 11.Noakes PS, et al. Maternal smoking is associated with impaired neonatal toll-like-receptor-mediated immune responses. Eur Respir J. 2006;28:721–729. doi: 10.1183/09031936.06.00050206. [DOI] [PubMed] [Google Scholar]
- 12.Roseboom TJ, et al. Effects of prenatal exposure to the Dutch famine on adult disease in later life: An overview. Twin Res. 2001;4:293–298. doi: 10.1375/1369052012605. [DOI] [PubMed] [Google Scholar]
- 13.Labayen I, Ortega FB, Sjöström M, Ruiz JR. Early life origins of low-grade inflammation and atherosclerosis risk in children and adolescents. J Pediatr. 2009;155:673–677. doi: 10.1016/j.jpeds.2009.04.056. [DOI] [PubMed] [Google Scholar]
- 14.Mathews HL, Janusek LW. Epigenetics and psychoneuroimmunology: Mechanisms and models. Brain Behav Immun. 2011;25:25–39. doi: 10.1016/j.bbi.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol Bull. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc Natl Acad Sci USA. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hänsel A, Hong S, Cámara RJA, von Känel R. Inflammation as a psychophysiological biomarker in chronic psychosocial stress. Neurosci Biobehav Rev. 2010;35:115–121. doi: 10.1016/j.neubiorev.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Repetti RL, Taylor SE, Seeman TE. Risky families: Family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128:330–366. [PubMed] [Google Scholar]
- 19.Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Shonkoff JP, Phillips DA. From Neurons to Neighborhoods: The Science of Early Childhood Development. Washington DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 21.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shankardass K, et al. Parental stress increases the effect of traffic-related air pollution on childhood asthma incidence. Proc Natl Acad Sci USA. 2009;106:12406–12411. doi: 10.1073/pnas.0812910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan GJ, Yeung WJ, Brooks-Gunn J, Smith JR. How much does childhood poverty affect the life chances of children? Am Sociol Rev. 1998;63:406–423. [Google Scholar]
- 24.van den Berg GJ, Doblhammer G, Christensen K. Exogenous determinants of early-life conditions, and mortality later in life. Soc Sci Med. 2009;68:1591–1598. doi: 10.1016/j.socscimed.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 25.van den Berg GJ, Doblhammer-Reiter G, Christensen K. Being born under adverse economic conditions leads to a higher cardiovascular mortality rate later in life: Evidence based on individuals born at different stages of the business cycle. Demography. 2011;48:507–530. doi: 10.1007/s13524-011-0021-8. [DOI] [PubMed] [Google Scholar]
- 26.Kiecolt-Glaser JK, et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–9095. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31:329–347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]
- 28.Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nat Rev Immunol. 2012;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- 29.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci USA. 2010;107:8507–8512. doi: 10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung HY, et al. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen E, et al. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. J Allergy Clin Immunol. 2006;117:1014–1020. doi: 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- 33.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung HY, et al. Molecular inflammation as an underlying mechanism of the aging process and age-related diseases. J Dent Res. 2011;90:830–840. doi: 10.1177/0022034510387794. [DOI] [PubMed] [Google Scholar]
- 35.Sharma V, McNeill JH. The etiology of hypertension in the metabolic syndrome part four: The systemic perspective—the role of the neuroendocrine and immune systems, and the challenge of integration. Curr Vasc Pharmacol. 2006;4:349–381. doi: 10.2174/157016106778521599. [DOI] [PubMed] [Google Scholar]
- 36.Miller GE, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci USA. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun. 2009;23:936–944. doi: 10.1016/j.bbi.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duivis HE, et al. Depressive symptoms, health behaviors, and subsequent inflammation in patients with coronary heart disease: Prospective findings from the heart and soul study. Am J Psychiatry. 2011;168:913–920. doi: 10.1176/appi.ajp.2011.10081163. [DOI] [PubMed] [Google Scholar]
- 39.Raison CL, Miller AH. Is depression an inflammatory disorder? Curr Psychiatry Rep. 2011;13:467–475. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merlot E, Moze E, Dantzer R, Neveu PJ. Cytokine production by spleen cells after social defeat in mice: Activation of T cells and reduced inhibition by glucocorticoids. Stress. 2004;7:55–61. doi: 10.1080/1025389042000208150. [DOI] [PubMed] [Google Scholar]
- 41.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Danese A, et al. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong S, Nelesen RA, Krohn PL, Mills PJ, Dimsdale JE. The association of social status and blood pressure with markers of vascular inflammation. Psychosom Med. 2006;68:517–523. doi: 10.1097/01.psy.0000227684.81684.07. [DOI] [PubMed] [Google Scholar]
- 44.Miller G, Chen E. Unfavorable socioeconomic conditions in early life presage expression of proinflammatory phenotype in adolescence. Psychosom Med. 2007;69:402–409. doi: 10.1097/PSY.0b013e318068fcf9. [DOI] [PubMed] [Google Scholar]
- 45.Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning: A perspective in historical context. Hypertension. 2012;60:260–268. doi: 10.1161/HYPERTENSIONAHA.111.186429. [DOI] [PubMed] [Google Scholar]
- 46.Knecht S, Wersching H, Lohmann H, Berger K, Ringelstein EB. How much does hypertension affect cognition?: Explained variance in cross-sectional analysis of non-demented community-dwelling individuals in the SEARCH study. J Neurol Sci. 2009;283:149–152. doi: 10.1016/j.jns.2009.02.362. [DOI] [PubMed] [Google Scholar]
- 47.Kerola T, Kettunen R, Nieminen T. The complex interplay of cardiovascular system and cognition: How to predict dementia in the elderly? Int J Cardiol. 2011;150:123–129. doi: 10.1016/j.ijcard.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Scuteri A, Nilsson PM, Tzourio C, Redon J, Laurent S. Microvascular brain damage with aging and hypertension: Pathophysiological consideration and clinical implications. J Hypertens. 2011;29:1469–1477. doi: 10.1097/HJH.0b013e328347cc17. [DOI] [PubMed] [Google Scholar]
- 49.Gross R, Bentur N, Elhayany A, Sherf M, Epstein L. The validity of self-reports on chronic disease: Characteristics of underreporters and implications for the planning of services. Public Health Rev. 1996;24:167–182. [PubMed] [Google Scholar]
- 50.Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. J Clin Epidemiol. 2003;56:148–154. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- 51.Sacks JJ, et al. Validation of a surveillance case definition for arthritis. J Rheumatol. 2005;32:340–347. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.