Abstract
The prefrontal cortex (PFC) receives input from all other cortical regions and functions to plan and direct motor, cognitive, affective, and social behavior across time. It has a prolonged development, which allows the acquisition of complex cognitive abilities through experience but makes it susceptible to factors that can lead to abnormal functioning, which is often manifested in neuropsychiatric disorders. When the PFC is exposed to different environmental events during development, such as sensory stimuli, stress, drugs, hormones, and social experiences (including both parental and peer interactions), the developing PFC may develop in different ways. The goal of the current review is to illustrate how the circuitry of the developing PFC can be sculpted by a wide range of pre- and postnatal factors. We begin with an overview of prefrontal functioning and development, and we conclude with a consideration of how early experiences influence prefrontal development and behavior.
Keywords: neural plasticity, dendritic spines, prenatal stress, psychoactive drugs, metaplasticity
The development of the cerebral cortex reflects more than a simple unfolding of a genetic blueprint; rather, it represents a complex dance of experiential and genetic factors that mold the emerging cerebrum. Pre- and postnatal environmental events, such as sensory stimuli, hormones, parent–child relationships, stress, and psychoactive drugs, modify cerebral development and, ultimately, adult behavior. Although all cerebral regions are influenced by early experience, the effects of experience are significantly different in specific cortical regions. The goal of this article is to review the ways in which one specific region, the prefrontal cortex (PFC), is sculpted by a wide range of pre- and postnatal factors. We begin with an overview of the nature and function of the PFC, followed by a review of experience-dependent modification of prefrontal organization and function.
What Is the PFC?
Kaas (1) proposed that a few basic areas of cerebral cortex are present in all mammals. These include primary and secondary visual and somatosensory areas (i.e., V1, V2, S1, S2), at least one auditory area and one taste area, a motor area, a transitional strip of cortex that relates the amygdala and hippocampus to other cortical areas (i.e., perirhinal cortex, entorhinal cortex), and a region referred to as the PFC. The definition of the sensory regions is relatively straightforward insofar as they receive unimodal input from the sensory receptor systems (e.g., eye, ear, tongue), and the outputs of the motor cortex are ultimately directed via polysynaptic pathways to effector organs. The outputs of all cortical regions are also components of feedback loops through which the cortex and subcortical regions of the brain mutually influence each other. Although there is no universally acceptable definition of the PFC, it can be regarded to be the region of the cortex that receives its principal thalamic inputs from the mediodorsal nucleus of the thalamus (e.g., 2). This cortical region is located somewhere at the anterior end of the cerebral hemispheres and refers not to a single region but to a group of related regions.
The PFC expanded greatly in primate evolution. Pandya and Yeterian (3) proposed that the expansion of PFC subareas is correlated directly to the expansion of the sensory areas. The implication from this conclusion is that the PFC must have some function in the integration of sensory information from different modalities, and as more information is processed, the PFC must have enlarged.
Why Is There a PFC?
A key concept in answering this question is to remember that it is behavior rather than the cortex that is selected. Thus, the question becomes “What behaviors were selected that led to the development of the PFC?” Warren and Kolb (4) argued that although the details of behavior vary, in general, mammals must solve many of the same problems in their daily lives. For example, all mammals detect and interpret sensory stimuli, relate this information to past experience, and generate behavioral strategies. Although the details of what the animals do vary with species and likely have been selected to promote survival in a specific niche (i.e., species-typical behaviors), there are general behavioral capacities demonstrable in all mammalian species that could be designated as class-common behaviors.
However, what is the class-common function(s) of the PFC? This is a difficult question, but theories of frontal lobe function converge on a common theme: The PFC is involved in the temporal organization of behavior (e.g., 5–9). The general idea is that the PFC supports cognitive functions that are necessary to organize behavior in time and in context, with a good example being social behavior. The complexity of PFC functions is greatest in humans and much simpler in mammals, such as rodents; however, in each mammalian order, there are class-common functions that require the PFC. It is pretty safe to suggest that if early experiences dramatically alter PFC circuitry and behavior in laboratory animals, such as rats, the experiences are likely to have even larger effects in a more complexly organized PFC, such as in humans.
Development of the PFC
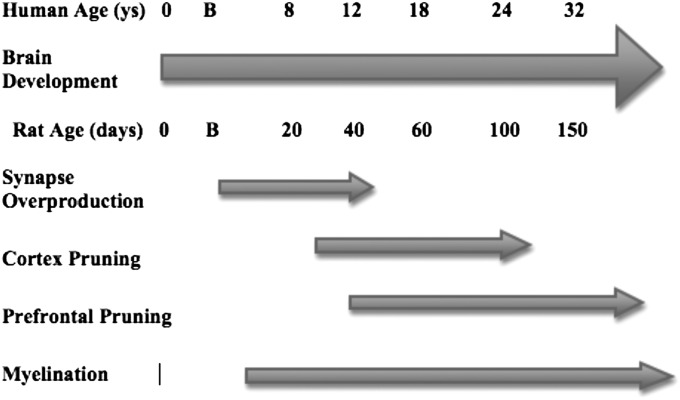
All cerebral areas go through several stages of development (Fig. 1). Cells destined to produce the nervous system begin to form about 3 wk after fertilization in humans, and cerebral maturation is not complete until an individual reaches his or her fourth decade of life. In short, neurons are born, migrate to their appropriate cerebral region, mature, form synapses, and develop glia that will form both myelin and other support cells. Owing to the complexity of building brains with a minimum of sensory information, there is an early life overproduction of neurons and their connections, which are later sculpted by neural activity. Thus, it is possible to use a minimum of genetic instructions to build brains that are appropriate for the specific ecological niche of an animal. In the human brain, the peak of synaptic density is reached between 1 and 5 y, depending on the region of cortex. Sensory regions have peak synapse density around 1 vs. 5 y (or later) for some PFC regions (10). There appears to be a caudal-to-rostral gradient, with posterior (sensory) regions peaking sooner than more anterior (PFC) ones.
Fig. 1.
Time line of brain development in humans and rodents. B, birth.
Two features of cerebral development are very important for understanding how experiences can modify cortical organization. First, dendritic spine density, which is an approximate surrogate for the number of excitatory synapses on a neuron, is two- to threefold greater in childhood than in adulthood. This overproduction of synapses is reversed beginning in late childhood, and in the PFC, it continues well into the third decade of life (10). Second, the overproduction of spines is greatest in the PFC, which then shows the slowest rate of synapse elimination (11). This extraordinarily long period of synapse elimination in the PFC has implications for understanding the environmental influences in puberty on adult cognitive capacities. Nonetheless, the period of synapse production may be as important in understanding adult cognitive and emotional capacities as the later adolescent pruning. It has been argued that it is in the period from early childhood to kindergarten in which the PFC forms the basic neural circuitry that will later underlie higher cognitive functions (12). Experiences early in life can therefore lay down the basic circuitry that is modified in adolescence. Early experiences (aversive or other) set up the PFC trajectories and have lifelong consequences on behavioral regulation. It is the effect of experiences during this early period of synaptic production that is the topic of this review.
Although we have specifically been describing developmental events in the human cerebrum, the basic principles remain the same for other mammals, including common laboratory animals like rats. The time line is slightly different from that of humans because rats are born developmentally younger than humans (roughly equivalent to the end of the human second trimester) and they mature more quickly. The peak in synapse density appears to be somewhere around 30 d in sensorimotor regions (13, 14), but there do not appear to be published data for the PFC. Van Eden et al. (15) reported that the cytoarchitectonic characteristics of the sensorimotor cortex stabilize around 24 d, whereas those of the rat PFC do not stabilize until about day 30. Given that the synaptic changes appear to follow the cytoarchitectonic features, it seems that the synaptic changes occur later in the PFC. Adolescence runs from about 35–60 d, and is therefore likely characterized by significant synaptic pruning in PFC regions, much as it is in humans.
Measuring Prefrontal Plasticity
Persistent changes in behavior and psychological function that occur as a consequence of experience are likely mediated by reorganization or strengthening of synaptic connections in specific neural circuits, a property referred to as neural plasticity. This idea has been a fundamental assumption underlying research on the neural basis of learning and memory for nearly 100 y (e.g., 16). Although neural plasticity can be inferred from behavioral, electrophysiological, and molecular measures, much of the research on plasticity has been on the morphology of dendrites and dendritic spines. The vast majority of synaptic inputs onto neurons are on dendrites and spines, and the amount of synaptic input a cell receives varies with the amount of dendritic surface available (14). It is estimated that over 90% of excitatory synapses are on dendritic spines (17); thus, it is common for researchers to focus on the density of spines on neurons, which can be quantified from tissue that is stained with one of many Golgi-related techniques. Our focus, therefore, is on spine density unless other measures give different results. More recently, studies have begun to use epigenetic measures that include both global methylation and gene expression. This type of analysis is likely to expand dramatically in the coming decade; thus, we have tried to include epigenetic studies whenever possible.
One important theoretical point is that measurements of either spine density or gene expression may show increases or decreases in response to specific experiences. However, it is not obvious how this relates to changes in neural networks. Historically, the literature implies that more synapses are better; however, given that pruning is also a key element of development, increased synapse numbers could reflect a failure to prune, which could be functionally detrimental. In Down syndrome, spine density is increased, although spine morphology (and probably function) are altered (18). At our current level of understanding, we really cannot do much more than identify the changes and perhaps correlate them with functional changes. We shall see that the same experiences can produce opposite changes in the spine density of PFC neurons depending on the age and sex of the animals studied.
Factors Influencing Prefrontal Development
When researchers began to study experience-dependent changes in the developing brain, there was a natural assumption that changes in brain development would be obvious only in response to rather large changes in experience, such as being raised in darkness. It is now clear that even fairly innocuous-looking experiences can profoundly affect brain development and that the range of experiences that can alter brain development is much larger than had once been believed. In addition, although it has been known for some time that sensory cortical regions are very responsive to early experiences, it has only recently been shown that the PFC is at least as sensitive to a wide range of stimuli (Table 1). We consider each in turn. We have summarized studies looking at different regions of the medial PFC (mPFC), which we collapse together as the mPFC, or the orbital and agranular insular region, which we collapse together as the orbital frontal cortex (OFC) (7).
Table 1.
Summary of effects of developmental experiences on PFC in rodents
| Experience | mPFC spines | OFC spines | FC methylation |
| Sensory/motor | |||
| Infant tactile stimulation (A) | ↑ | ↑ | |
| Prenatal paternal complex housing (W) | ↓ | ||
| Prenatal maternal complex housing (W) | ↓ | ||
| Adult complex housing (A) | ↑* | ↓* | |
| Stress | |||
| Prenatal mild stress (W) | ↑ | ↑ | ↑ |
| Prenatal mild stress (A) | ↓* | -* | ↓ |
| Prenatal bystander stress (W) | ↑ | ↑ | |
| Prenatal moderate stress (W) | ↑ | ||
| Prenatal moderate stress (A) | ↓ | ↓ | |
| Adult moderate stress (A) | ↓* | ↑* | ↓ |
| Parent–infant relationship | |||
| Paternal deprivation (A) | —* | ↓* | |
| Maternal separation (A) | ↑ | ↑ | |
| Peer relationships | |||
| Increased play (A) | ↓ | ↓ | |
| Psychoactive drugs | |||
| Infant antipsychotics (A) | ↓ | ↓ | |
| Juvenile antipsychotics (A) | ↑ | ↑ | |
| Prenatal stimulants (A) | ↑ | ↑ | |
| Juvenile stimulants (A) | ↑* | ↓* | |
| Adult stimulants | ↑* | ↓* | |
| Prenatal valproic acid (W) | ↑ | ↑ | |
| Prenatal valproic acid (A) | ↓ | ↓ | |
| Prenatal ethanol (A) | ↓ | ↓ |
A, tissue collected in adulthood; FC, frontal cortex; W, tissue collected at weaning; —, no change.
*Indicates conditions in which mPFC and OFC responded differently.
Sensory and Motor Experience.
There is a history of investigators raising young animals in conditions of restricted sensory experience (19, 20), but it is only recently that investigators considered the opposite phenomenon, namely, enriching animals’ sensory experiences to see if sensory processing could be enhanced (21, 22). We are unaware of any studies examining either the effects of restricted or enriched sensory experiences on PFC development, but because of the importance of tactile stimulation in the development of hypothalamic-pituitary-adrenal axis reactivity in rats (23), we elected to study the effects of enhanced tactile stimulation on the PFC. In these studies, infant rats were given tactile stimulation with a small brush for 15 min three times per day for 10–15 d beginning at birth (24). When the infants were studied in adulthood, they showed both enhanced skilled motor performance and spatial learning, as well as changes in synaptic organization across the cerebral cortex (25). Although the precise mechanism of action of the tactile stimulation is not known, we hypothesize that tactile stimulation leads to an increase in the production of a neurotrophic factor, FGF-2, in both skin and brain. FGF-2 has previously been found to play an important role in both neurogenesis and neuronal maturation (26, 27).
To determine the effects of tactile stimulation on synaptic development, we compared the pyramidal neurons in the mPFC and OFC of adult rats with and without the enhanced tactile stimulation as infants. There was a 15% increase in spine density in both prefrontal regions in the treated animals (23). This increase is at least partly responsible for the enhanced behavioral capacities of these animals on PFC-related tasks.
Muhammad and Kolb (28) stimulated pregnant dams using the same procedure as in the earlier tactile stimulation studies and examined the brains of the adult offspring. Although the authors did not report spine density, they showed that OFC of the stimulated rats was nearly 20% thinner than normal, suggesting more extensive pruning of OFC neurons. There were also a variety of behavioral effects, including a reduction in play behavior and an attenuated response to psychomotor stimulants, both of which are related to PFC functioning.
Another way to enhance sensory and motor functions is to place animals in complex environments in which they can interact with a changing sensory and social environment, and increase their motor activity. Studies in the 1960s by Rosenzweig et al. (29) and in the 1980s by Greenough and Chang (30) and Sirevaag and Greenough (31) showed that this type of experience led to increased dendritic length in pyramidal cells in various cortical regions. We now know that there are many neural changes associated with this form of “enrichment,” including increases in brain size, cortical thickness, neuron size, dendritic branching, spine density, synapses per neuron, glial numbers and complexity, and vascular arborization (e.g., 32, 33). Curiously, although virtually all cerebral structures show this result, the PFC of adult rats does not show changes in dendritic length or branching if the animals are placed in enriched environments as adults (34). There is a change in spine density in mPFC and OFC, but this is sexually dimorphic: Males show a decrease (35) and females show an increase (34). The decrease in the males is puzzling, and we predict that if animals are placed in the complex environments sooner (at weaning and then for 4 mo), we might see a different effect. When we placed rats in complex environments for 4 mo at weaning vs. adulthood, we found decreased spine density in parietal and visual cortex in the younger group compared with increased spine density in the older group (32). PFC neurons were not analyzed.
One final enrichment study is provocative. Mychasiuk et al. (36) placed adult male rats in complex housing for 28 d before pairing them in standard cages with females that had been housed in standard laboratory cages. A different group of female rats was also housed in complex environments for 28 d (7 d before conception and for the duration of the pregnancy). Brain tissue from the frontal cortex (mPFC and OFC) and hippocampus was harvested for global DNA methylation analysis when the pups reached weaning age (day 21). Both paternally and maternally enriching animals led to a significant decrease in gene methylation levels in frontal cortex and hippocampus. It is surprising that the changes induced by both paternal and maternal experiences were essentially identical. Although a measure of global methylation reflects the sum of all changes and the actual gene changes in the paternal and maternal treatments may turn out to be different, the result is provocative because it is generally assumed that maternal experiences will have a bigger influence on offspring than paternal experiences.
We can draw three conclusions from the complex housing studies. First, the PFC is more sensitive to this experience during development than in adulthood. Second, the precise age at which the animal experiences enrichment makes a large difference. Third, the PFC shows a different pattern of changes than other cerebral regions.
Early Stress.
There is an enormous literature showing the effects of stress on brain and behavior in adults, but it is only more recently that the role of perinatal stress in infants has been appreciated. It is now known that both gestational and infant stress predisposes individuals to a variety of maladaptive behaviors and psychopathologies. For example, prenatal stress is a risk factor in the development of schizophrenia, attention-deficit hyperactivity disorder (ADHD), depression, and drug addiction (33, 37). Experimental studies with laboratory animals have confirmed these findings, with the overall result being that perinatal stress, in rodents as well as nonhuman primates, produces behavioral abnormalities, such as an elevated and prolonged stress response, impaired learning and memory, deficits in attention, altered exploratory behavior, altered social and play behavior, and an increased preference for alcohol (38).
The synaptic changes in the PFC of perinatally stressed animals are different from those related to adult stress (39). Furthermore, the developmental effects appear to be related to the details of the stressful experience. For example, Liston et al. (40) showed that adult stress led to a decrease in spine density in mPFC but an increase in OFC. In contrast, Mychasiuk et al. (41) found an increase in spine density in both mPFC and OFC when animals were exposed to mild stress from embryonic days 12–16 (E12–E16) and the brains were examined at weaning. Using the same stress paradigm, Muhammad and Kolb (42) found a decrease in spine density in mPFC and no effect in OFC when the brains were examined in adulthood. Finally, Murmu et al. (43) reported that moderate prenatal stress from E16–E21 resulted in decreased spine density and dendritic length in both the mPFC and OFC of adult degus. Taken together, these studies show that differences in the timing of prenatal stress and the age at which the brain is examined result in differing plastic changes in neuronal circuits and that these alterations evolve over protracted intervals.
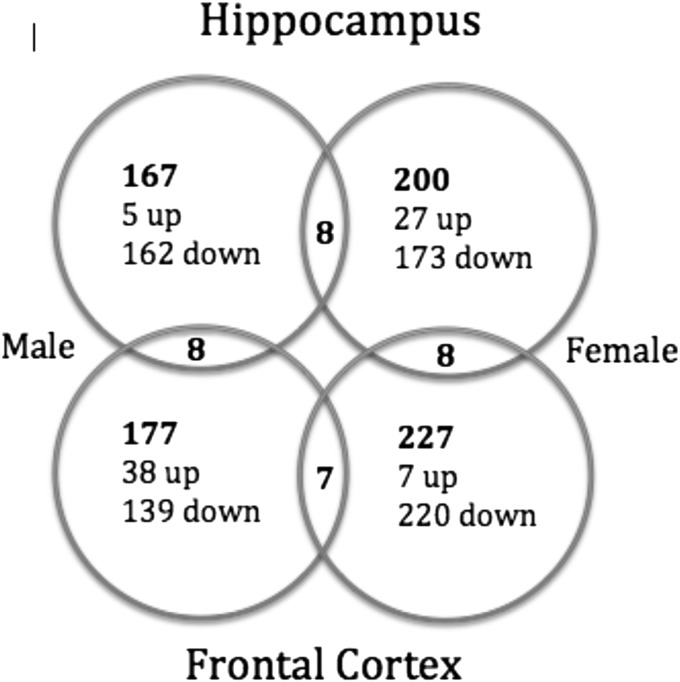
Mychasiuk et al. (44) provide one explanation for these differences. These authors varied the intensity of stress (again using E12–E16) and examined the brains for changes in global methylation. Mild prenatal stress increased global methylation in both frontal cortex (using a combined sample of mPFC and OFC) and hippocampus, whereas high prenatal stress had the opposite effect. A subsequent study (45) used a whole-genome microarray analysis to show that over 700 genes in the frontal cortex and hippocampus were differentially expressed following prenatal stress, with most genes being down-regulated. One surprising result was that the epigenetic changes demonstrated sexually dimorphic and region-specific profiles, with little overlap between sexes and brain area (Fig. 2). The qualitative difference between the effects of prenatal stress in the frontal cortex and hippocampus suggests that measures of epigenetic change in peripheral measures, such as blood or saliva, used in human studies are unlikely to reflect changes in specific brain regions.
Fig. 2.
Venn diagrams summarizing the changes in gene expression in the offspring of rats exposed to prenatal stress (45).
The effects of prenatal stress are clearly large and important for understanding the effects of early experience on PFC structure and function. It appears, however, that early stress can be quite subtle. Mychasiuk et al. (46) housed two pregnant dams together throughout their pregnancies. One dam was given mild prenatal stress as in the earlier studies, whereas the other dam was not. The authors compared the methylation and gene expression patterns of the offspring of the “bystander stressed” dam with those of unstressed dam. The results were reminiscent of the findings for the effects of direct prenatal stress in their earlier study: Global DNA methylation increased in the frontal cortex and hippocampus, and microarray analysis revealed significant gene expression level changes in 589 different genes, of which only 10 exhibited overlap between males and females or brain regions. Furthermore, there were marked changes in dendritic length and branching in mPFC and OFC that were also sex and areal-dependent (47). Both regions and sexes showed an increase in spine density, which contrasts with decreases seen in the offspring of the directly stressed dams. Presumably, both of the pregnant dams were stressed but in very different ways. Furthermore, they responded to the stress in different ways. The authors measured ultrasonic vocalizations of the two females when the stressed rat was returned to the communal cage. The stressed rat sang a 22-KHz distress call for several hours, whereas the bystander rat replied with a 55-KHz happy song. It is tempting to anthropomorphize from this finding.
Parent–Infant Relations.
Mammalian infants face a significant challenge in early life. They are dependent on their parents and must learn to identify, remember, and prefer their caregivers. Parent–infant relationships therefore can initiate long-term developmental effects that persist into adulthood (48). Rodent studies have shown that the time spent in contact and the amount of maternal licking and grooming correlate with behavioral and somatic differences. Meaney and colleagues (49, 50) have shown that these maternal–infant interactions modify the development of the hypothalamic-adrenal stress axis and emotional and cognitive behaviors in adulthood. These changes are correlated with changes in hippocampal structure and gene expression.
Although the effects of parent–infant interactions are less well studied in the other brain regions, there are effects in the hypothalamus and amygdala (51) and PFC (52–54). In one study (52), the authors looked at the effect of paternal deprivation on the development of spine density in the OFC of the offspring of a biparental rodent, the degu. Degu fathers participate in infant care, and single mothers do not increase their maternal care to compensate for the lack of paternal care. Degus raised without a father exhibit a marked suppression in dendritic length and spine density in the OFC. Thus, the paternal deprivation changes the development of PFC circuits.
It is obviously impractical to remove maternal care for extended periods because the young are dependent on their mothers for nourishment. It is possible to consider the effects of maternal separation for brief periods. Muhammad and Kolb (55) separated rat pups from their dams for 3 h per day from day 3 after birth until weaning (day 21). Later play behavior when the pups were adolescents was disrupted by this experience, and in adulthood, there was an increase in spine density in their mPFC and OFC. It is difficult to compare the paternal and maternal studies directly because the separation is permanent in the paternal case, whereas it is intermittent in the maternal study. Nonetheless, both studies show that a negative manipulation of parent–infant relations changes the development of PFC circuits (53).
Peer Relationships.
Peer relationships have been known to influence adult behavior since the studies of Harlow and Harlow (56). One of the most powerful peer relationships is play, which has been demonstrated to be important for the development of adult social competence (57). The PFC plays an essential role in play behavior, and infant injury to either the mPFC or OFC compromises play behavior, although in different ways (58). We therefore hypothesized that development of the two PFC regions would be differentially altered if play behavior was manipulated in development. Juvenile rats were given the opportunity to live with one or three adult rats or to live and play with one or three other juvenile animals. There was virtually no play with the adults, but play behavior increased as more juveniles were present. Analysis of the PFC cells showed that neurons of the OFC responded to the number of peers present (spine density decreased with number of playmates) but did not respond to whether or not play occurred. In contrast, the neurons of mPFC showed a decrease in spine density proportional to the amount of play but not the number of conspecifics (59). Thus, more play increases pruning in mPFC, whereas more social interaction increases pruning in OFC. Our working hypothesis is that play may lead to greater neural plasticity later in life.
We have subsequently shown that a variety of early experiences alter rat play behavior, including prenatal stress and pre- or postnatal tactile stimulation (42, 60); in each case, there are changes in prefrontal development. There may be an important lesson here when we consider conditions in which human childhood play is not normal, such as in autism or ADHD. The abnormalities in play behavior may influence PFC development and later adult behavior.
Psychoactive Drugs.
Early exposure to alcohol is known to be deleterious to brain development, but it has only recently been shown that other psychoactive drugs, including therapeutic drugs, can dramatically alter PFC both in adulthood and development. Using adult rats, Robinson and Kolb (61) administered repeated doses of psychomotor stimulants or opiates. When animals receive daily doses of amphetamine, cocaine, or nicotine, there is an incremental increase in locomotor activity in response to the drug, an effect referred to as drug-induced behavioral sensitization. In each case, the sensitization was correlated with a chronic increase in spine density in mPFC and nucleus accumbens (NAcc). In contrast, there was either a decrease or no change in these measures in OFC. It has now been shown that adult exposure to virtually every class of psychoactive drugs also produces changes in spine density in PFC and that the effects are consistently different (usually opposite) in the two PFC regions.
Less is known about the effects of psychoactive drugs on the developing brain, however. Prenatal exposure to nicotine increases dendritic length in both PFC regions when the brains are examined at weaning and, in addition, increases spine density when brains are studied in adulthood (62). Frost et al. (63) studied adult mice treated with a paradigmatic typical (haloperidol) or atypical (olanzapine) antipsychotic drug in infants (postnatal days 3–10 or 3–20). Both drugs reduced spine density in the mPFC and OFC in adulthood. In a later study using rats, the authors gave olanzapine on postnatal days 28–49 and found the opposite result, namely, an increase in spine density in both mPFC and OFC (64). When the authors looked at dopamine-receptor binding, they found D1 binding was reduced in the olanzapine-treated animals in both mPFC and OFC, whereas D2 binding was increased in mPFC but did not change in OFC. These animals showed impairments in PFC-related neuropsychological tasks, such as working memory. The simplest explanation for the mouse/rat difference is that there are different critical periods of drug-exposure effects on PFC development. This needs more investigation and has implications for the drug treatment not only of women with psychosis but potentially with other classes of drugs, such as selective serotonin reuptake inhibitors and anxiolytics.
Other studies have looked at the effects of stimulants given during the juvenile period. Diaz Heijtz et al. (65) found that doses of amphetamine producing blood plasma levels equivalent to what is found in children medicated for ADHD produced an increase in spine density in mPFC. The higher spine density in mPFC could be an indirect result of reduced play, although this was not examined directly.
Although there have been many studies of the effect of prenatal alcohol on brain development, there are surprisingly few studies on PFC. Two studies showed decreased spine density in OFC and either no change (66) or a decrease (67) in mPFC in adult brains.
Finally, prenatal exposure to valproic acid changes spine density and behavior in the offspring (68–70). Specifically, when tissue was collected for Golgi analysis at weaning, both mPFC and OFC showed increased spine density, but when the tissue was collected in adulthood, there was a decrease. Valproic acid has been used as a treatment for depression, bipolar disease, and epilepsy, and prenatal exposure has been implicated in autism (68, 71, 72).
On the basis of earlier studies in adult rats, we had anticipated that the effects of early experience on the two prefrontal regions would be different. For example, it has been shown that psychoactive drugs (61), stress (40), and complex housing (34) differentially change PFC regions (Table 1). No mechanism for such differential effects is known, although there are differences in connectivity (2, 3). There could be differential gene expression in the two regions, but we know of no evidence. When we look at the effects of experience in development, in general, the effects were similar in both prefrontal regions. The similarities could reflect similar effects of experience on the major afferents of both regions (e.g., dorsomedial thalamus, amygdala, ventral tegmental area). Alternatively, they could reflect a similar pattern of epigenetic changes in both regions. The differing predictions of epigenetic changes in adults and young animals point to the need for parallel epigenetic studies in animals with similar experiences at different ages. However, what is still not known is if there are common factors across different experiences that drive the synaptic changes or whether there are multiple mechanisms of change. Untangling this is going to be long and complex process.
Metaplasticity in the PFC
In the preceding section, our focus was on how singular experiences change PFC development. However, life is made up of more than singular experiences. Thus, as the brain develops, it is influenced by multiple experiences that may occur concurrently or sequentially. A key question is how one early experience might alter the effect of other experiences, either in development or later in life. Abraham and Bear (73) coined the term “metaplasticity” to refer to the idea that plastic changes in the brain will modulate later plasticity: One early life experience can change cerebral organization, and thus alter the brain’s responses to subsequent experiences. A corollary is that if the brain is changed by an early experience, a later experience could act to reverse, or at least modify, the early changes.
Metaplasticity is developmentally significant because it provides a mechanism for the development of individual differences in behavior, and in the extreme, primes the brain to take an abnormal developmental trajectory in response to later experiences that might otherwise be benign. Although few studies have examined metaplasticity in PFC development, several recent ones are intriguing.
In adult rats, exposing animals to sensitizing doses of amphetamine, cocaine, or nicotine completely blocked the effect of complex housing (74, 75). Not only did the complex housing have no effect on the PFC or NAcc, both regions that are consistently changed by the drugs in normal rats, but other regions, such as the parietal cortex, which show no synaptic changes in response to the drugs, also failed to change in response to enrichment. The powerful effects of psychoactive drugs in blocking later cerebral plasticity have important implications for the potential effects of perinatal drug exposure on later experience-dependent changes as juveniles or adults. Few laboratory studies have addressed this question, although one study showed that prenatal treatment with FGF-2 enhanced postnatal plasticity in response to prefrontal injury (76). Reviews of the effects of prenatal exposure to legal, illicit, and prescription drugs on brain and behavioral development in children conclude that the effects are complex and modulated by such factors as timing and dose of drug (e.g., 77). Similarly, in the treatment of childhood behavioral disorders, drug exposure could have beneficial, or harmful, long-term effects (78). Although little is known about PFC changes in such therapies, given the prominent role of PFC dysfunction in psychiatric disorders, this is an important topic of future study.
Another manipulation that influences later plasticity is play. We noted earlier that increased play is associated with decreased spine density in mPFC. It is likely that these changes will interact with later experiences, such as complex housing, learning new tasks, or even the effects of psychoactive drugs. If so, one conclusion is that the effect of play on the mPFC may be to enhance plasticity of the neurons later in life.
Other studies have looked at the effect of pre- or postnatal tactile stimulation or lifelong housing (beginning prenatally) in complex environments on drug-induced behavioral sensitization to amphetamine in adulthood. All treatments significantly attenuated the effect of the drug on both behavior and prefrontal anatomy (28, 60). Thus, early positive experiences can reduce the brain’s later response to psychoactive drugs. It would be interesting to know if negative experiences, such as prenatal stress, might increase synaptic changes in response to drugs.
Adolescence is a behavioral transitional period that is characterized by metaplasticity (79). The onset of gonadal changes, which are linked to the production of gonadal hormones, is correlated with changes in the brain, and especially PFC. Experiences early in life may modify the later effects of gonadal hormones in adolescence. For example, prenatal stress blocks the masculinizing effects of testosterone in adolescent males (80). Few studies have examined hormone-related changes in spine density in PFC, although adult males have longer dendrites and higher spine density in mPFC than females (32, 81). Given that experiences like complex housing in adulthood have large, sexually dimorphic effects in the cortex and hippocampus (82), one would expect additional metaplastic effects of manipulating early gonadal hormone levels. This is especially probable, given that there is a growing literature on the epigenetics of sex differences in the brain (83) and the correlation with sex differences in the incidence of many mental disorders (84). In addition, we have already shown that prenatal stress produces sexually dimorphic differences in gene expression in both hippocampus and PFC.
One implication of the metaplastic changes in the cortex is that they might permit the reversal or moderation of the effects of early experiences with additional developmental experiences. For example, it might be possible to reduce the effects of prenatal stress by subsequent postnatal tactile stimulation. We are unaware of any such studies, but there is evidence that the effects of perinatal brain injury, which is an extreme early experience, can be significantly reduced by treatments like postinjury tactile stimulation or even by prenatal maternal stimulation (85).
In sum, examinations of metaplastic effects are just beginning but, clearly, will have important implications for understanding PFC development and later adult behavior.
Concluding Comments
Variations in the effect of experience on the synaptic organization and function of the PFC occur at preconception, prenatal, infant, and juvenile periods of infant development. Whereas the mPFC and OFC nearly always respond in opposite directions to experiences in adulthood (e.g., increased vs. decreased spine density), experiences in development generally influence the two PFC regions similarly.
We have focused on the changes in spine density in the current review, but many other measures of cerebral structure related to neural plasticity can be used, including dendritic length, dendritic complexity, neuron number, glial number, transmitter expression, and receptor number. For example, prenatal bystander stress not only increases spine density but changes the number of both neurons and glial cells in a sexually dimorphic manner (41). This is important because if spine density per neuron drops but the number of neurons increases, the implications for total synapse number in a given region change. Furthermore, as might be expected, there are changes in such regions as the hippocampus. In addition, there are transient changes in receptor levels, such as dopamine receptors, in PFC (86).
A further complicating factor relates to the age at which the brain is examined. Table 1 illustrates examples in which there are opposite changes in spine density when the brain tissue is collected at weaning or in adulthood. In general, there are increases in spine density at weaning, which are reversed in adulthood. There are two issues here. First, experience or experimental manipulations at any stage of development take time to play out. As a result, the effects of early experiences may only be seen well down the developmental trajectory, especially given how much the adolescent brain changes (78). Second, the substrate on which experience acts (the brain) changes across the life span. Thus, the behavioral and neurobiological changes caused by a given experience early in life interact with the particular developmental state that the brain is in. One possible effect of an early experience is to accelerate or slow cortical maturation. Thus, adolescent experiences are acting on different cerebral phenotypes.
One important question that has not yet been addressed is whether there are cross-generational effects of early experiences on PFC development. We have shown that both prenatal paternal and maternal experiences alter gene methylation in frontal cortex (36) of offspring. It is reasonable to propose that the gene expression changes will alter both PFC development and behavior. Similarly, we can hypothesize that the offspring of mothers that were prenatally stressed will show changes in PFC development. Unpredictable maternal separation in mice has led to transgenerational behavioral effects that are correlated with an altered profile of DNA methylation and gene expression as well as impaired serotonergic pathway function (87). These studies did not focus on PFC, but the epigenetic effects suggest that PFC effects are likely, given that maternal separation does change dendritic organization in PFC in the first generation.
Another cross-generational effect is suggested by a study of the effects of paternal behavior on the brains of adult male marmosets (88). Although the study did not examine the offspring, the study did show that paternal experience increased spine density on pyramidal cells in PFC in the fathers. The question is whether this change might affect the brains of subsequent offspring, leading to changes in the PFC, and possibly in the paternal behavior, of the male offspring. Recall that preconceptual experiences of male rats produce changes in global methylation patterns in subsequent offspring.
One shortcoming of this review is that its focus has been on one species, the laboratory rat, which has been the species of choice for most studies. We have noted studies in other rodents when available, including degus and mice; however, although there are studies of cerebral plasticity in other species, there are few looking at effects of early experience on PFC. Nonetheless, the available studies show results consistent with those in rats. Thus, although it is important to have additional work on other species, there does not appear to be any compelling reason not to generalize to other rodent species and to mammals in general.
In sum, one of the most intriguing questions in behavioral neuroscience concerns the manner in which the brain, and especially the cerebral cortex, modifies its structure and function in response to experience in development. As this review has suggested, the PFC can be changed dramatically by events beginning prenatally and throughout the life span. The plasticity and prolonged development of the PFC provide an opportunity for continual modification of cognitive function but, in addition, create a potential susceptibility to the formation of abnormal circuitry, leading to compromised behavioral function (89).
Acknowledgments
This research was supported by grants from the Natural Sciences and Engineering Research Council of Canada (to R.G. and B.K.) and by a grant from the National Institutes of Health (to D.O.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biological Embedding of Early Social Adversity: From Fruit Flies to Kindergartners,” held December 9–10, 2011, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/biological-embedding.
References
- 1.Kaas JH. The organization of neocortex in mammals: Implications for theories of brain function. Annu Rev Psychol. 1987;38:129–151. doi: 10.1146/annurev.ps.38.020187.001021. [DOI] [PubMed] [Google Scholar]
- 2.Kolb B. Do all mammals have a prefrontal cortex? In: Kaas J, editor. Evolution of Nervous Systems: A Comprehensive Reference. Vol 3. New York: Elsevier; 2006. pp. 443–450. [Google Scholar]
- 3.Pandya DN, Yeterian EH. Architecture and connections of cortical association areas. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol 4. New York: Plenum; 1985. pp. 3–61. [Google Scholar]
- 4.Warren JM, Kolb B. Generalizations in neuropsychology. In: Finger S, editor. Brain Damage, Behavior and the Concept of Recovery of function. New York: Plenum; 1978. [Google Scholar]
- 5.Goldman-Rakic PS. 1987. Circuitry of the primate prefrontal cortex and regulation of behavior by representational memory. Handbook of Physiology: The Nervous System. Part 1: Higher Functions of the Brain, ed Plum F (American Physiological Society, Bethesda), Vol 5.
- 6.Fuster J. The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. 3rd Ed. New York: Raven; 1998. [Google Scholar]
- 7.Kolb B. Functions of the frontal cortex of the rat: A comparative review. Brain Res. 1984;320:65–98. doi: 10.1016/0165-0173(84)90018-3. [DOI] [PubMed] [Google Scholar]
- 8.Passingham RE. The Frontal Lobes and Voluntary Action. Oxford: Oxford Univ Press; 1993. [Google Scholar]
- 9.Wilson CR, Gaffan D, Browning PG, Baxter MG. Functional localization within the prefrontal cortex: Missing the forest for the trees? Trends Neurosci. 2010;33:533–540. doi: 10.1016/j.tins.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elston GN, Oga T, Fujita I. Spinogenesis and pruning scales across functional hierarchies. J Neurosci. 2009;29:3271–3275. doi: 10.1523/JNEUROSCI.5216-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsujimoto S. The prefrontal cortex: Functional neural development during early childhood. Neuroscientist. 2008;14:345–358. doi: 10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- 13.Miller MW. Development of projection and local circuit neurons in neocortex. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol 7. New York: Plenum; 1988. pp. 133–176. [Google Scholar]
- 14.Harris KM, Kater SB. Dendritic spines: Cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 15.van Eden CG, Kros JM, Uylings HBM. The development of the rat prefrontal cortex. Its size and development of connections with thalamus, spinal cord and other cortical areas. Prog Brain Res. 1990;85:169–183. doi: 10.1016/s0079-6123(08)62680-1. [DOI] [PubMed] [Google Scholar]
- 16.Hebb DO. 1949. The Organization of Behavior (Wiley, New York) [DOI] [PubMed]
- 17.Greenough WT, Withers GS, Wallace CS. 1990. Morphological changes in the nervous system arising from behavioral experience: What is the evidence that they are involved in learning and memory? The Biology of Memory, Symposia Medica Hoechst, eds Squire LR, Lindenlaub E (Springer, New York), Vol 23, pp 159–185.
- 18.Kaufmann WE, Moser HW. Dendritic anomalies in disorders associated with mental retardation. Cereb Cortex. 2000;10:981–991. doi: 10.1093/cercor/10.10.981. [DOI] [PubMed] [Google Scholar]
- 19.Riesen AH. Studying perceptual development using the technique of sensory deprivation. J Nerv Ment Dis. 1961;132:21–25. [PubMed] [Google Scholar]
- 20.Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- 21.Prusky GT, Silver BD, Tschetter WW, Alam NM, Douglas RM. Experience-dependent plasticity from eye opening enables lasting, visual cortex-dependent enhancement of motion vision. J Neurosci. 2008;28:9817–9827. doi: 10.1523/JNEUROSCI.1940-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Champagne FA, Curley JP. Maternal care as a modulating influence on infant development. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford Handbook of Developmental Behavioral Neuroscience. Oxford: Oxford Univ Press; 2010. pp. 323–341. [Google Scholar]
- 23.Richards S, Mychasiuk R, Kolb B, Gibb R. Tactile stimulation during development alters behaviour and neuroanatomical organization of normal rats. Behav Brain Res. 2012;231:86–91. doi: 10.1016/j.bbr.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 24.Schanberg SM, Field TM. Sensory deprivation stress and supplemental stimulation in the rat pup and preterm human neonate. Child Dev. 1987;58:1431–1447. [PubMed] [Google Scholar]
- 25.Kolb B, Gibb R. Tactile stimulation facilitates functional recovery and dendritic change after neonatal medial frontal or posterior parietal lesions in rats. Behav Brain Res. 2010;214:115–120. [Google Scholar]
- 26.Gómez-Pinilla F, Lee JWK, Cotman CW. Distribution of basic fibroblast growth factor in the developing rat brain. Neuroscience. 1994;61:911–923. doi: 10.1016/0306-4522(94)90412-x. [DOI] [PubMed] [Google Scholar]
- 27.Riva MA, Mocchetti I. Developmental expression of the basic fibroblast growth factor gene in rat brain. Brain Res Dev Brain Res. 1991;62:45–50. doi: 10.1016/0165-3806(91)90188-o. [DOI] [PubMed] [Google Scholar]
- 28.Muhammad A, Kolb B. Prenatal tactile stimulation attenuates drug-induced behavioral sensitization, modifies behavior, and alters brain architecture. Brain Res. 2011;1400:53–65. doi: 10.1016/j.brainres.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 29.Rosenzweig MR, Krech D, Bennett EL, Diamond MC. Effects of environmental complexity and training on brain chemistry and anatomy: A replication and extension. J Comp Physiol Psychol. 1962;55:429–437. doi: 10.1037/h0041137. [DOI] [PubMed] [Google Scholar]
- 30.Greenough WT, Chang FF. Plasticity of synapse structure and pattern in the cerebral cortex. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol 7. New York: Plenum; 1989. pp. 391–440. [Google Scholar]
- 31.Sirevaag AM, Greenough WT. A multivariate statistical summary of synaptic plasticity measures in rats exposed to complex, social and individual environments. Brain Res. 1988;441:386–392. doi: 10.1016/0006-8993(88)91420-5. [DOI] [PubMed] [Google Scholar]
- 32.Kolb B, Gibb R, Gorny G. Experience-dependent changes in dendritic arbor and spine density in neocortex vary qualitatively with age and sex. Neurobiol Learn Mem. 2003;79(1):1–10. doi: 10.1016/s1074-7427(02)00021-7. [DOI] [PubMed] [Google Scholar]
- 33.Anda RF, et al. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolb B, Gorny G, Söderpalm AH, Robinson TE. Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse. 2003;48:149–153. doi: 10.1002/syn.10196. [DOI] [PubMed] [Google Scholar]
- 35.Comeau WL, McDonald RJ, Kolb BE. Learning-induced alterations in prefrontal cortical dendritic morphology. Behav Brain Res. 2010;214:91–101. doi: 10.1016/j.bbr.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 36.Mychasiuk R, et al. Parental enrichment and offspring development: Modifications to brain, behavior and the epigenome. Behav Brain Res. 2012;228:294–298. doi: 10.1016/j.bbr.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 37.Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75:1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 38.Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Liston C, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mychasiuk R, Gibb R, Kolb B. Prenatal stress alters dendritic morphology and synaptic connectivity in the prefrontal cortex and hippocampus of developing offspring. Synapse. 2012;66:308–314. doi: 10.1002/syn.21512. [DOI] [PubMed] [Google Scholar]
- 42.Muhammad A, Kolb B. Mild prenatal stress-modulated behavior and neuronal spine density without affecting amphetamine sensitization. Dev Neurosci. 2011;33:85–98. doi: 10.1159/000324744. [DOI] [PubMed] [Google Scholar]
- 43.Murmu MS, et al. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- 44.Mychasiuk R, Ilnytskyy S, Kovalchuk O, Kolb B, Gibb R. Intensity matters: Brain, behaviour and the epigenome of prenatally stressed rats. Neuroscience. 2011;180:105–110. doi: 10.1016/j.neuroscience.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 45.Mychasiuk R, Gibb R, Kolb B. Prenatal stress produces sexually dimorphic and regionally specific changes in gene expression in hippocampus and frontal cortex of developing rat offspring. Dev Neurosci. 2011;33:531–538. doi: 10.1159/000335524. [DOI] [PubMed] [Google Scholar]
- 46.Mychasiuk R, et al. Prenatal bystander stress alters brain, behavior, and the epigenome of developing rat offspring. Dev Neurosci. 2011;33:159–169. doi: 10.1159/000330034. [DOI] [PubMed] [Google Scholar]
- 47.Mychasiuk R, Gibb R, Kolb B. Prenatal bystander stress induces neuroanatomical changes in the prefrontal cortex and hippocampus of developing rat offspring. Brain Res. 2011;1412:55–62. doi: 10.1016/j.brainres.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 48.Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, Hofer MA. Maternal behavior of SHR rats and its relationship to offspring blood pressures. Dev Psychobiol. 1989;22(1):29–53. doi: 10.1002/dev.420220104. [DOI] [PubMed] [Google Scholar]
- 49.Weaver ICG, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 50.Weaver ICG, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci USA. 2006;103:3480–3485. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Front Neuroendocrinol. 2006;27(2):180–192. doi: 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helmeke C, et al. Paternal deprivation during infancy results in dendrite- and time-specific changes of dendritic development and spine formation in the orbitofrontal cortex of the biparental rodent Octodon degus. Neuroscience. 2009;163:790–798. doi: 10.1016/j.neuroscience.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Seidel K, Poeggel G, Holetschka R, Helmeke C, Braun K. Paternal deprivation affects the development of corticotrophin-releasing factor-expressing neurones in prefrontal cortex, amygdala and hippocampus of the biparental Octodon degus. J Neuroendocrinol. 2011;23:1166–1176. doi: 10.1111/j.1365-2826.2011.02208.x. [DOI] [PubMed] [Google Scholar]
- 54.Helmeke C, Ovtscharoff W, Jr, Poeggel G, Braun K. Juvenile emotional experience alters synaptic inputs on pyramidal neurons in the anterior cingulate cortex. Cereb Cortex. 2001;11:717–727. doi: 10.1093/cercor/11.8.717. [DOI] [PubMed] [Google Scholar]
- 55.Muhammad A, Kolb B. Maternal separation altered behavior and neuronal spine density without influencing amphetamine sensitization. Behav Brain Res. 2011;223:7–16. doi: 10.1016/j.bbr.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Harlow HF, Harlow MK. The affectional systems. In: Schier A, Harlow HF, Stollnitz F, editors. Behaviour of Nonhuman Primates. Vol 2. New York: Academic; 1965. [Google Scholar]
- 57.Pellis S, Pellis V. The Playful Brain. New York: Oneworld Publications; 2010. [Google Scholar]
- 58.Pellis SM, et al. The effects of orbital frontal cortex damage on the modulation of defensive responses by rats in playful and nonplayful social contexts. Behav Neurosci. 2006;120:72–84. doi: 10.1037/0735-7044.120.1.72. [DOI] [PubMed] [Google Scholar]
- 59.Bell HC, Pellis SM, Kolb B. Juvenile peer play experience and the development of the orbitofrontal and medial prefrontal cortices. Behav Brain Res. 2010;207:7–13. doi: 10.1016/j.bbr.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 60.Muhammad A, Hossain S, Pellis S, Kolb B. Tactile stimulation during development attenuates amphetamine sensitization and structurally reorganizes prefrontal cortex and striatum in a sex-dependent manner. Behav Neurosci. 2011;125:161–174. doi: 10.1037/a0022628. [DOI] [PubMed] [Google Scholar]
- 61.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 62.Muhammad A, et al. Prenatal nicotine exposure alters neuroanatomical organization in the developing brain. Synapse. 2012 doi: 10.1002/syn21589. [DOI] [PubMed] [Google Scholar]
- 63.Frost DO, Page SC, Carroll C, Kolb B. Early exposure to haloperidol or olanzapine induces long-term alterations of dendritic form. Synapse. 2010;64:191–199. doi: 10.1002/syn.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Milstein JA, et al. Long-term neurodevelopmental sequelae of adolescent olanzapine exposure: Behavioral effects. Soc Neurosci Abst. 2010;168(36):12. [Google Scholar]
- 65.Diaz Heijtz R, Kolb B, Forssberg H. Can a therapeutic dose of amphetamine during pre-adolescence modify the pattern of synaptic organization in the brain? Eur J Neurosci. 2003;18:3394–3399. doi: 10.1046/j.0953-816x.2003.03067.x. [DOI] [PubMed] [Google Scholar]
- 66.Hamilton DA, et al. Prenatal exposure to moderate levels of ethanol alters social behavior in adult rats: Relationship to structural plasticity and immediate early gene expression in frontal cortex. Behav Brain Res. 2010;207:290–304. doi: 10.1016/j.bbr.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lawrence RC, Otero NKH, Kelly SJ. Selective effects of perinatal ethanol exposure in medial prefrontal cortex and nucleus accumbens. Neurotoxicol Teratol. 2012;34(1):128–135. doi: 10.1016/j.ntt.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mychasiuk R, et al. Is prenatal VPA exposure in rat a viable model of autism? A comprehensive behavioral and anatomical analysis. Dev Neurosci. 2012;10:268–276. [Google Scholar]
- 69.Rinaldi T, Perrodin C, Markram H. Hyper-connectivity and hyper-plasticity in the medial prefrontal cortex in the valproic Acid animal model of autism. Front Neural Circuits. 2008;2:4. doi: 10.3389/neuro.04.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology. 2008;33:901–912. doi: 10.1038/sj.npp.1301453. [DOI] [PubMed] [Google Scholar]
- 71.Ornoy A. Valproic acid in pregnancy: How much are we endangering the embryo and fetus? Reprod Toxicol. 2009;28(1):1–10. doi: 10.1016/j.reprotox.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 72.Williams G, et al. Fetal valproate syndrome and autism: Additional evidence of an association. Dev Med Child Neurol. 2001;43:202–206. [PubMed] [Google Scholar]
- 73.Abraham WC, Bear MF. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- 74.Hamilton D, Kolb B. Nicotine, experience, and brain plasticity. Behav Neurosci. 2005;119:355–365. doi: 10.1037/0735-7044.119.2.355. [DOI] [PubMed] [Google Scholar]
- 75.Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci USA. 2003;100:10523–10528. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Comeau W, Hastings E, Kolb B. Differential effect of pre and postnatal FGF-2 following medial prefrontal cortical injury. Behav Brain Res. 2007;180:18–27. doi: 10.1016/j.bbr.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 77.Thompson BL, Levitt P, Stanwood GD. Prenatal exposure to drugs: Effects on brain development and implications for policy and education. Nat Rev Neurosci. 2009;10:303–312. doi: 10.1038/nrn2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andersen SL, Navalta CP. Annual Research Review: New frontiers in developmental neuropharmacology: Can long-term therapeutic effects of drugs be optimized through carefully timed early intervention? J Child Psychol Psychiatry. 2011;52:476–503. doi: 10.1111/j.1469-7610.2011.02376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brenhouse HC, Andersen SL. Developmental trajectories during adolescence in males and females: A cross-species understanding of underlying brain changes. Neurosci Biobehav Rev. 2011;35:1687–1703. doi: 10.1016/j.neubiorev.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stewart J, Kolb B. The effects of neonatal gonadectomy and prenatal stress on cortical thickness and asymmetry in rats. Behav Neural Biol. 1988;49:344–360. doi: 10.1016/s0163-1047(88)90354-8. [DOI] [PubMed] [Google Scholar]
- 81.Kolb B, Stewart J. Sex-related differences in dendritic branching of cells in the prefrontal cortex of rats. J Neuroendocrinol. 1991;3:95–99. doi: 10.1111/j.1365-2826.1991.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 82.Juraska J. The structure of the cerebral cortex: Effects of gender and the environment. In: Kolb B, Tees R, editors. The Cerebral Cortex of the Rat. Cambridge, MA: MIT Press; 1990. pp. 483–506. [Google Scholar]
- 83.McCarthy MM, et al. The epigenetics of sex differences in the brain. J Neurosci. 2009;29:12815–12823. doi: 10.1523/JNEUROSCI.3331-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jessen HM, Auger AP. Sex differences in epigenetic mechanisms may underlie risk and resilience for mental health disorders. Epigenetics. 2011;6:857–861. doi: 10.4161/epi.6.7.16517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kolb B, Mychasiuk R, Williams P, Gibb R. Brain plasticity and recovery from early cortical injury. Dev Med Child Neurol. 2011;53(Suppl 4):4–8. doi: 10.1111/j.1469-8749.2011.04054.x. [DOI] [PubMed] [Google Scholar]
- 86.Brenhouse HC, Sonntag KC, Andersen SL. Transient D1 dopamine receptor expression on prefrontal cortex projection neurons: Relationship to enhanced motivational salience of drug cues in adolescence. J Neurosci. 2008;28:2375–2382. doi: 10.1523/JNEUROSCI.5064-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Franklin TB, Linder N, Russig H, Thöny B, Mansuy IM. Influence of early stress on social abilities and serotonergic functions across generations in mice. PLoS ONE. 2011;6:e21842. doi: 10.1371/journal.pone.0021842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kozorovitskiy Y, Hughes M, Lee K, Gould E. Fatherhood affects dendritic spines and vasopressin V1a receptors in the primate prefrontal cortex. Nat Neurosci. 2006;9:1094–1095. doi: 10.1038/nn1753. [DOI] [PubMed] [Google Scholar]
- 89.Braun K, Bock J. The experience-dependent maturation of prefronto-limbic circuits and the origin of developmental psychopathology: Implications for the pathogenesis and therapy of behavioral disorders. Dev Med Child Neurol. 2011;15(Suppl 4):14–18. doi: 10.1111/j.1469-8749.2011.04056.x. [DOI] [PubMed] [Google Scholar]