Abstract
This paper describes evidence that led to the concept of biological embedding and research approaches designed to elucidates its mechanisms. Biological embedding occurs when experience gets under the skin and alters human biological and developmental processes; when systematic differences in experience in different social environments in society lead to systematically different biological and developmental states; when these differences are stable and long term; and, finally, when they have the capacity to influence health, well-being, learning, or behavior over the life course. Biological embedding emerged from insights in population health on the unique characteristics of socioeconomic gradients: Ubiquity in poor and postscarcity societies alike; gradient seen regardless of whether socioeconomic status is measured by income, education, or occupation; cutting widely across health, well-being, learning, and behavior outcomes; replicating itself on new conditions entering society; and, often, showing that flatter gradients mean better overall societal outcomes. Most important, the gradient begins the life course as a gradient in developmental health, suggesting that the emergence of a multifaceted resilience/vulnerability early in life is the best place to look for evidence of biological embedding. To understand its character, the metaphor of the “archeology of biological embedding” has been used, wherein the surficial stratum of the “dig” is experience and behavior, the shallow stratum is organ system and cellular function, and the deep stratum is gene function. We are now ready to address the fundamental question of biological embedding: How do early childhood environments work together with genetic variation and epigenetic regulation to generate gradients in health and human development across the life course?
Keywords: epigenetics, social determinants of health
Population health begins with the proposition that across society, observed patterns of health are more than just the sum of individual health status and also that determinants of health in populations are not just the sum of the risk and protective factors that affect individuals (1). The sociologist Emile Durkheim (2) famously showed that the factors that drive individuals to commit suicide are not the same as those that explain why some societies have higher suicide rates than others. He called the latter “social facts” and the former “individual facts.” Similarly, the epidemiologist Geoffrey Rose (1) made a distinction between the factors that determine which individuals within a population get a disease (individual fact) and the determinants of different incidence rates of disease among different populations (social fact). For example, in every country where it has been measured, cigarette smoking is an individual risk factor for lung cancer. However, the risk appears to multiply, rather than add to, the background rate among the nonsmoking population (social fact), such that the absolute level of risk in low background rate countries (e.g., Japan) is lower than it is in high background rate countries (e.g., United Kingdom) for any given level of smoking.
Social facts are the starting point for population health inquiries. Population health identifies systematic differences in health status among well-defined population groups (e.g., by sex, ethnicity, geography, socioeconomic group, nation) and then works backward to understand the determinants of these differences. For the past 25 y, a priority in population health has been the “socioeconomic gradient.” Health, as measured by mortality or morbidity, follows a socioeconomic gradient in both developed and majority world countries, such that those populations at the top of the socioeconomic hierarchy tend to live longer, healthier lives than those in the middle, who, in turn, live longer, healthier lives than those at the bottom (3). To be sure, at the level of individuals within each socioeconomic stratum, the full range of length and healthfulness of life is found, whose explanation within the stratum will be dominated by individual facts. However, across strata, the health of individuals will be some mathematical function of the social fact (i.e., the mean health status of the individual’s socioeconomic stratum), increased or decreased by individual facts that may operate independently, synergistically, or in some contingent way with it. Although health status is ultimately a property of individuals, the population health approach described here, which led to the concept of biological embedding, started with recognizing and characterizing the social fact of the gradient and then, subsequently, working to understand how individual facts combine to explain individual level outcomes. Although biological embedding could, theoretically, have emerged from individual-based approaches, the fact is that it did not. Individual-level approaches rarely generate social facts. Similarly, the concept could, theoretically, have emerged from studying a threshold or dichotomous social fact, such as health status differences between Aboriginal and non-Aboriginal peoples. However, it did not. Dichotomies generally lead researchers to ask about the unique characteristics of the two groups rather than to think in terms of fundamental underlying causes. Historically, it was the counterintuitive shock of observing ubiquitous, persistent graded relationships between socioeconomic status (SES) and health in countries that were wealthy and had universal health care coverage that forced a fundamental rethinking of the nature of the determinants of health, out of which came the concept of biological embedding (4).
Explaining that history is one aim of this paper. First, it will define biological embedding and present an animal model that meets its conditions. Then, it will double back, tracing strands of evidence that led to the concept of biological embedding, introducing research approaches designed to elucidate its mechanisms, and providing some evidence that biological embedding is taking place in humans.
Biological embedding occurs when four conditions are met:
i) Experience, metaphorically, “gets under the skin” in ways that alter human biological and developmental (herein, biodevelopmental) processes (herein, condition BE1).
ii) Systematic differences in experience in different social environments in society (especially early in life) lead to systematically different biodevelopmental states (condition BE2).
iii) These differences are stable and long term (condition BE3).
iv) These differences have the capacity to influence health, well-being, learning, or behavior over the life course (condition BE4).
The following presents an animal model featuring each of the four conditions of biological embedding.
Animal Model of Biological Embedding
Depending on their mothers’ behavior, rat pups may receive either high or low levels of licking, grooming, and arch-backed nursing (LG-ABN). Those that receive high levels of LG-ABN during a narrow window of days in early life show differences in the function of their hypothalamic–pituitary–adrenal (HPA) axis: a low basal corticosterone level with an abrupt response to acutely stressful circumstances and an abrupt decline to baseline thereafter. Among the low–LG-ABN rats, there is a much broader range of responses, typified by higher baseline levels and a more blunted response to stressful circumstances (condition BE2). The effect occurs only by LG-ABN differences during a narrow window of days in early life, suggesting that it depends on appropriate stimulation/deprivation (condition BE1) during a highly circumscribed window of opportunity in brain development. The high–LG-ABN rat pups have reduced total lifetime secretion of corticosterone compared with the low–LG-ABN pups (condition BE3). Chronic high exposure to corticosterone, in turn, endangers selected neurons in the brain’s hippocampus (5), such that the rate of loss of hippocampal neurons is reduced in the high–LG-ABN rats over their life span. Because of cognitive functions’ sensitivity to relatively small degrees of hippocampal damage, the high–LG-ABN rats, by 24 mo of age, have been spared some of the cognitive deterioration typical of aging. Low–LG-ABN rat pups, in contrast, show a progressive deterioration in their memory, cognitive processing, and learning performance with age (condition BE4) (6). Thus, the four conditions for biological embedding are shown through systematic differences in early nurturance, as represented by the frequency of LG-ABN during a sensitive period in development, leading to differences in HPA axis function, diverging capacity for complex learning, and different rates of age-related declines in learning and memory performance (7–9).
The research gives a glimpse at a potential underlying mechanism of biological embedding. The LG-ABN paradigm operates through epigenetic mediation of gene expression, in particular, expression of the gene encoding the glucocorticoid receptor (GR) according to differences in maternal LG-ABN behavior. Although alterations in gene expression do not necessarily lead to systematic differences in metabolism and long-term outcomes, the association is compelling in this case. The epigenetic mechanism also seemed to influence higher order executive functions in the brain, such that natural low levels of maternal LG-ABN behavior were associated, among the mother’s offspring, with down-regulation of GR expression, resultant increases in HPA reactivity, and more fearful and anxious behavior. High LG-ABN, by contrast, resulted in down-regulation of HPA reactivity and bolder, less fearful behavior. These characteristics were subsequently passed on to the next generation through epigenetic or behavioral means (10, 11).
Ultimately, the validity of biological embedding depends on whether or not the four conditions fulfilled by the LG-ABN paradigm are also fulfilled in humans and if underlying mechanisms can be established. We are not yet at this point, but progress has been made. Here, we return to the fact that the concept of biological embedding was developed long before the LG-ABN paradigm was published, yet it predicted that such phenomena must occur. To understand this, it is necessary to begin where population health researchers began—with the characteristics of the socioeconomic gradient.
Socioeconomic Gradients
The socioeconomic gradient applies to a remarkably broad range of outcomes (there are exceptions: malignant melanoma has a reverse gradient, and many gynecological and breast cancers have a flat or reverse gradient) and has been replicated in wealthy and majority world societies alike. In the British Whitehall study (12), as well as in countless similar studies of health and social class, smoking, blood pressure control, and other traditional cardiovascular risk factors help to explain differences in mortality among individuals but do little to explain the stepwise rise in heart disease mortality (or a wide range of other diseases) as one goes from the highest to the lowest occupational grades of the civil service, even when health care is fully covered and living wages are paid to all.
Gradients can be contrasted with threshold effects, such as a dichotomous relationship, wherein one level of health would be associated with being “rich” and another with being “poor.” Instead, the gradient effect suggests that there are degrees of change in health associated with degrees of change in SES. The gradient cannot be explained away by reverse causation. This is demonstrated by the fact that gradients tend to be just as strong according to level of education as income. Although income can drop with ill health (i.e., reverse causation), one’s level of education cannot, such that the principal direction of association is from SES to health over the life course (13). These are shocking, counterintuitive social facts that point to the origin of conditions BE3 and BE4 for biological embedding. The following gradient characteristics strengthen its appeal as a social fact.
Replication on New Diseases.
As the major causes of death and disease have changed over the past century, the gradient has replicated itself on new diseases as they have become endemic in society, such that the overall gradient pattern has been preserved. Data from England and Wales show this during the 20th century, as major infectious causes of death were replaced by chronic diseases, such as heart disease (14). At first, heart disease displayed a different pattern, disproportionately affecting those who were privileged enough to live long enough to get it. However, by the 1950s, a socioeconomic gradient in heart disease had emerged (14). Accordingly, the gradient implies underlying mechanisms for health and disease that go beyond disease-specific pathology.
Flattening Up.
“Flatter gradients” appear to mean better average outcomes. If one plots the SES gradients in health in different societies, the lines do not fall on top of one another, suggesting that the additional gains to health from increased SES are larger in some societies than in others. At the same time, differences in health outcome at high levels of SES are far smaller than at lower levels when one compares across countries. Therefore, those societies with a “shallow” SES gradient do not get there by “pulling down” the health of the high SES groups but rather by “pulling up” the health of the lower groups, leading to higher average health status (14). (Flattening the gradient upward in steep gradient societies, such as the United States and United Kingdom could, theoretically, reduce overall mortality more than eliminating all forms of heart disease.) International comparisons have shown the flattening-up pattern for health status across the European Community (15), and also for human development outcomes (e.g., literacy and numeracy skills at 15 y) across wealthy and middle-income countries (16). The pattern of “flatter is better” is not a static difference among societies. If it were, it might be tempting to dismiss the gradient as unchangeable. Instead, when gradients have been tracked over time in different societies, the steepness of gradients is shown to change (17).
By themselves, these characteristics of the gradient are compelling but would not have led to the hypothesis of biological embedding. The principal observation that transformed interest emerged in the late 1990s, namely, that the gradient “begins the life course” as a gradient in developmental health (18).
Gradients in Developmental Health
The influence of a socially partitioned world is expressed early in the form of gradients in the key domains of child development (physical, social-emotional, and language-cognitive) before school age (condition BE2) (19). Birth cohort studies spanning decades of the life course (20) demonstrate that early experience and its attendant child developmental status influence later health, well-being, learning, and behavior outcomes (fulfilling condition BE4). This occurs through multiple life course processes:
i) There are latent effects (20, 21), whereby experiences that occur early in life influence later life course outcomes irrespective of intervening experience (condition BE4). Latent effects depend on sensitive periods in brain and biological development, which serve as windows of opportunity for early experiences to alter biological functions permanently (condition BE1). (Additional information is provided in the section of this paper describing how biological embedding works.)
ii) There are pathway effects, whereby early experiences (and the early developmental states they produce) are the first step in a chain of events that carries with it a set of “expectancies” in terms of health and development (20, 21). Social adversities often involve self-organizing feedback loops in which one traumatic event follows from others, cascading over time and giving rise to intensely negative and stressful social contexts. For example, marital conflict in the child’s family can presage divorce, which, in turn, may lead to frequent residential moves and loss of supportive social relationships for the child. By school age, the child’s social-emotional development has been adversely affected, increasing the risk for school failure and downward social mobility. Pathway effects are consistent with condition BE2 and, to the extent that path dependence emerges from early experience, with condition BE3.
iii) There are cumulative effects. Social causation often involves mundane, rather than exceptional, exposures. For example, day-to-day rearing in environments characterized by impoverished parent-child interactions, even without dramatic, catastrophic events, may be implicated in adverse outcomes over time (22, 23). Accordingly, experiences can influence outcomes in a cumulative matter; the more severe and multifaceted the experience of deprivation, the greater is the adverse effect on health and well-being over the life course (20, 21). Cumulative processes are consistent with condition BE2. They are analogous to the process of weathering in physical geography, wherein rocks, soils, and minerals break down through physical and chemical processes due to contact with the Earth’s atmosphere, biota, and waters. [Although it has not been adequately studied from a developmental standpoint, an intriguing hypothesis is that by middle age, allostatic load (24) may be a biological reflection of some combination of latent pathway and cumulative life course processes.]
By the late 1990s, the contribution of the early life course to SES gradients in a wide range of health, well-being, learning, and behavior outcomes later in life had been well established. The four conditions for biological embedding were then deduced from the characteristics of the gradient, rather than the reverse. Once defined, these conditions provided criteria for recognizing animal models of biological embedding, which is why the LG-ABN paradigm was spotted immediately after publication. Importantly, the biological plausibility of the LG-ABN paradigm only came later, with understanding of the role of epigenetics. The fact that the gradient begins the life course as a gradient in developmental health, when juxtaposed against evidence of latent pathway and cumulative effects across the life course, suggests that early life emergence of a multifaceted resilience/vulnerability is where to look for biologically plausible mechanisms in humans. However, first, consider experiences.
Experiences That Underlie Gradients in Developmental Health
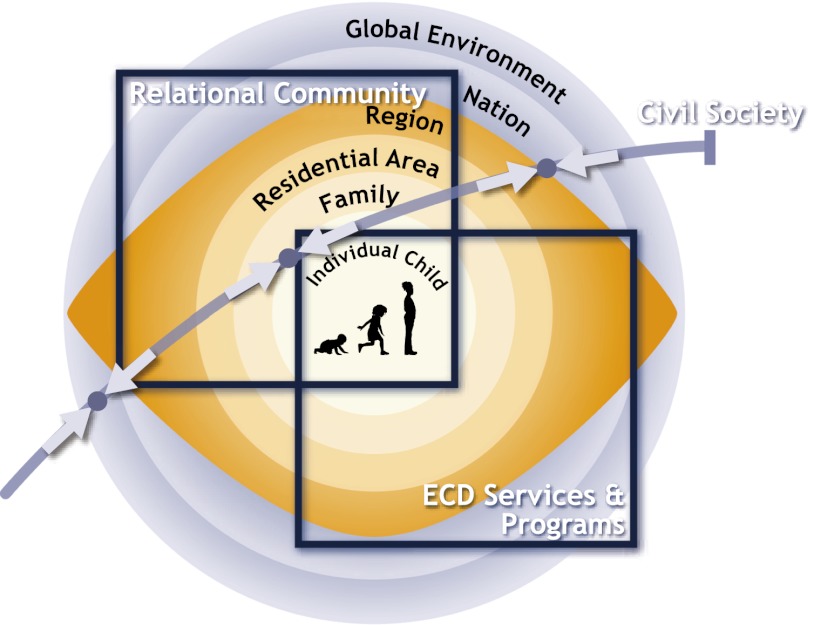
Bronfenbrenner (25) first proposed an ecological approach to the determinants of child development, spanning the intimate domain of the family to the more indirect influences from society as a whole. Ecological approaches emphasize overlapping social environments, with experiences combining in unique ways to support or undermine the developing child. The Total Environment Assessment Model of Early Child Development (Fig. 1) was developed for the World Health Organization to provide an ecological framework applicable globally (26). It features interacting and interdependent spheres of influence, each of whose effects is supported in the literature: family and dwelling; residential and relational communities; programs and services; regional, national, and global environments; and civil society. The state of early child development is conceived of as an emergent property of the interactions between the developing child and the complex of experiences that arise from these overlapping and interacting environments (27). As a social fact, the gradient in developmental health is an emergent property of systematic SES differences in the quality of experiences children derive from these environments: stimulation, support, nurturance, and opportunities for participation in daily life. The four conditions for biological embedding imply that these differences either enhance or reduce risk for a wide range of adverse outcomes across the life course by creating a generalized biodevelopmental susceptibility or resilience across multiple paths (28, 29).
Fig. 1.
Total Environment Assessment Model of Early Child Development (ECD).
How Does Biological Embedding Work?
How do early social environments and experiences “get under the skin” in a manner that meets the conditions for biological embedding? The brain should be the most critical organ for generating gradients, and brain development in the early years should affect health and well-being as an adult. Socially partitioned experiences have an opportunity to play a crucial role in the early phases of conception, prenatal, and postnatal periods of children’s development (30, 31), because sensitive periods in brain development start in the prenatal period and reach a peak in the first few years of life. Early sensory stimulation activates specific genes in different parts of the brain to differentiate neuron functions and establish sensory pathways. Sensory pathways, in turn, influence the development of neural pathways to other parts of the brain involved in coping, movement, language, and cognition, as well as those to other biological pathways, including the immune and hormone systems. Key executive functions regarding how an individual responds to social and emotional stimuli develop in the prefrontal cortex from approximately the age of 3 y to the age of 9 y, whereas neural connections to the prefrontal cortex, from centers in the midbrain that sense environmental threats, develop earlier. Thus, whereas the physiological sense of threat is developed at a very young age, the repertoire of responses to threatening circumstances may develop later (32–34). Overall early life provides a roughly ordered sequence of developmental windows of opportunity that, in turn, allow both mundane and extraordinary experiences to get under the skin at strategic time points to alter specific biological functions, which, in turn, have the capacity to alter life course trajectories.
This scenario implies that relevant activity occurs at a number of levels of biological organization. To characterize underlying mechanisms, it is necessary to move up and down these levels, from human development to neurobiology, physiological pathways, genes, and gene regulation, combining animal and human evidence to determine where there is substantial convergence, emerging suggestions of convergence, or no convergence with respect to the influences of socially partitioned environments. Here, we use the metaphor of the “archeology of biological embedding.”
Archeology of Biological Embedding
Archeology implies digging down, documenting whatever is found in each stratum, preserving findings, digging further to reveal what is below, and then integrating findings from each stratum to tell a story of a civilization. In the case of biological embedding, the surficial stratum is experience and behavior, the shallow stratum is organ system and cellular function, and the deep stratum is gene function. The current state of knowledge, however, is not akin to one dig at one site, where all layers relate to each other in a coherent way. Instead, as the following examples show, we have separate digs in different sites, with some revealing surficial, some revealing shallow, and some revealing deep strata that may or may not comprise a coherent scenario. Accordingly, the archeology of biological embedding faces the same challenges as the archeology of ancient Mesopotamia: correlating finds from different strata in different places; constructing a falsifiable model from these finds; and using the model’s predictions to choose the next, most validity-testing, dig sites.
The following gives examples of productive digs, from different sites, at the surficial, shallow, and deep levels. These models do not yet present a coherent mechanism of biological embedding, but they allow for preliminary model building.
Surficial Archeology: Population-Based Patterns of Developmental Health
Between 2000 and 2011, British Columbia completed four population-based assessments of developmental health, including more than 90% of children in school entry cohorts across the province. Developmental health was measured at the age of 5 y using the Early Development Instrument (EDI), wherein kindergarten teachers fill out a detailed checklist for each child based on five scale measures of development: physical well-being, social competence, emotional maturity, language and cognitive development, and communication and general knowledge (35). Each child is scored as “vulnerable” or “not vulnerable” on each scale, and a measure of “vulnerability on one or more scales of the EDI” is calculated as the summary measure of developmental health. When children are analyzed in groups (e.g., by neighborhood, by school), this measure is the developmental analog to child survival measures. In other words, the EDI vulnerability rate is a social fact about the state of development, by school age, among populations of children, just as the under-5-y mortality rate is a social fact about their survival. Its validity and reliability as a population-based measure have been extensively documented (36, 37).
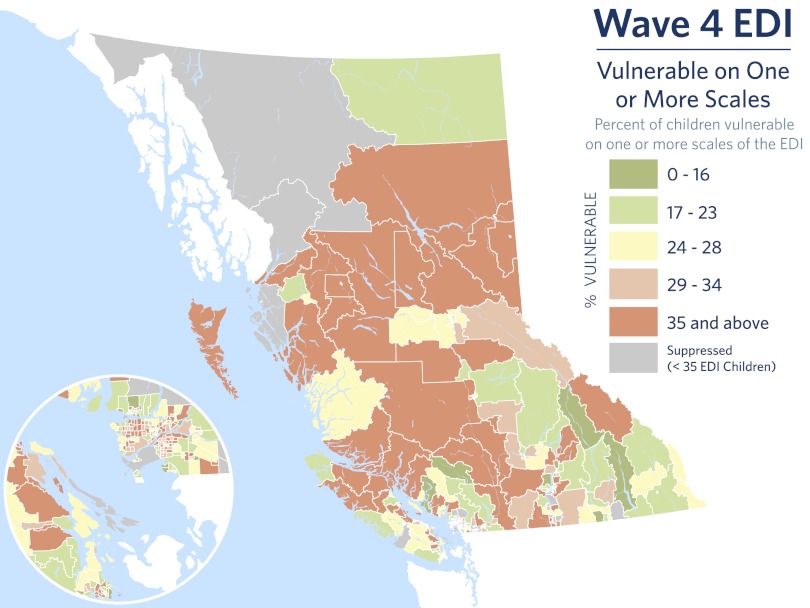
Fig. 2 maps EDI vulnerability rates for British Columbia according to the neighborhood of residence of each child (n = 460). Mapping by residential neighborhood follows logically from the ecological approach because housing markets tend to cluster families of similar SES; neighborhoods have characteristics (e.g., safety, informal social trust) that influence child development (38); are units of access (or lack thereof) to programs and services; and tend to have other stable, relevant features, such as unemployment rates and ethnocultural mix. Vulnerability ranges from low (green) to high (red): from as low as 3% to as high as 61%, a 20-fold difference. Although Fig. 2 presents only one wave of EDI, each of four waves produced a 15- to 20-fold range of vulnerability across neighborhoods, making this high level of variability a stable emergent property (http://earlylearning.ubc.ca/maps/edi/bc/). The range is much larger than predicted based on random sample surveys of child development, which rarely demonstrate socioeconomic gradients larger than threefold. Why would this be? The best answer is that ecological approaches reveal what decontextualized research conceals: Local geography defines unique combinations of factors that support or undermine child development, which are averaged out of random sample studies when children from diverse places are analyzed according to decontextualized grouping factors, such as family income. From the perspective of biological embedding, this leads to several possible interpretations. The environmental effects of specific neighborhood characteristics might be the analog of LG-ABN; the neighborhood might be a marker for characteristics of families that inhabit it, which are the proximate sources of embedding, or there might be an emergent process resulting from the mix of neighborhood and individual characteristics.
Fig. 2.
EDI vulnerability by neighborhood in British Columbia.
Across waves of EDI data collection, 45–50% of neighborhood variance in vulnerability can consistently be statistically explained by SES. To the question, “How significant is the surifical layer of biological embedding, according to gradients in developmental health,” the answer is very significant indeed. In British Columbia, one of the world’s most affluent and ethnoculturally diverse jurisdictions, the SES gradient explains approximately half of a 15- to 20-fold range in developmental vulnerability across local neighborhoods. The EDI has now been used in 15 countries of the world, including population-wide implementations in Canada and Australia and across large communities in Mexico and Chile. In each case, the patterns and strength of association described for British Columbia have been replicated (http://www.offordcentre.com/readiness/).
Shallow Archeology
With respect to shallow archeology, “candidate systems” have been identified with the capacity to transduce experience from the social environment to human biology (39). Five candidate systems meet the following criteria: It can be influenced by daily experiences (often early in life), such that differential qualities of experience can lead to a differently functioning system; it responds to such experiences throughout the life course; if dysfunctional, it has the biological capacity to influence health, well-being, learning, and/or behavior; and differential functioning of the system, to the extent that outcomes are affected, may derive from experience earlier in the life course. These systems are the HPA–cortisol axis; the autonomic nervous system; the memory, attention, and other executive functions of the prefrontal cortex; the immune system; and the social affiliation system involving the amygdala and locus coeruleus, with accompanying higher order cerebral connections, mediated by serotonin and other hormones (40). This paper cannot review all the evidence pertaining to each candidate system. Instead, separate digs into two systems are presented to exemplify emerging insights from the shallow archeology of biological embedding.
Example 1: HPA Axis
Like the rat, socially partitioned human experiences appear to be biologically embedded via HPA axis regulation. Consensus, however, has been slow to reach because of the complexities of studying human diurnal cortisol secretion, the influence of sleep/wakening, exposure to drugs and medications, and shift work. Although latent, pathway and cumulative effects of adversity on the HPA axis all seem to dysregulate the HPA axis (24, 41); dysregulation, in turn, may present as either hypo- or hypersecretion, either of which may have adverse consequences, whereas the midrange may have the most favorable outcomes (42–45). When this “U-shaped” pattern of association applies, traditional linear statistics will often provide false-negative, or inconsistent, results among studies that unwittingly sample from different parts of the “U.” Finally, methods of salivary cortisol collection and measurement have not been around long enough to allow for prospective studies from early childhood to adulthood. Notwithstanding these caveats, evidence for partial fulfillment of the four conditions of biological embedding emerges from disparate bodies of evidence.
The quality of early attachment between mother and child affects both HPA axis function and behavior, such that poorly attached toddlers have a more reactive HPA axis and less adaptive behavioral responses (33). During the late Soviet period, Romanian orphans in state-run orphanages who were neglected during the first 6 mo of life after birth tended to become high cortisol reactors and to have profound social-emotional, as well as cognitive, developmental disturbances that persisted at least into adolescence (46, 47) (condition BE1). Under much less extreme conditions in North America, social class differences in basal cortisol levels in childhood have been reported according to parental income, education, and employment status (48–50); mother’s depressive symptoms (50, 51); childhood adversity (52–54); and the stressfulness of social environments (54, 55) (condition BE2). Cortisol levels are also associated with adult SES (56–61). One study (62) found that the impact of a less advantaged socioeconomic position over a lifetime led to an approximate doubling of the proportion of extreme postwaking cortisol levels, an 8–10% increase in cumulative cortisol secretion during the early hours of the day, and a 60–91% increased risk for having dysregulated cortisol secretion patterns. Within the limitations of retrospective analysis, this study indicated that the association between cortisol secretion patterns and SES in adulthood appeared to start as an SES gradient in cortisol in childhood (addressing condition BE3).
One compelling piece of evidence for condition BE4 (i.e., embedded HPA patterns affecting long-term health outcomes) comes from comparisons between 50-y-old Swedish and Lithuanian men (58). During the final 30 y of the Soviet period, rates of heart disease between these two populations gradually diverged from parity to a fourfold higher rate in Lithuanians than Swedes. When subjected to laboratory stress tests, Swedish men’s cortisol responses were reminiscent of the high–LG-ABN rats, whereas Lithuanian men tended to have cortisol responses similar to the low–LG-ABN rats (63). The Lithuanian pattern was associated with low self-esteem, lower sense of coherence, increased reported job strain, increased “vital exhaustion,” and increased depression. Most important here, coronary heart disease risk was associated with HPA axis function but not with other heart disease risk factors (58).
Example 2: Prefrontal Cortex in Socioeconomic Context
EDI mapping in British Columbia permitted us to study prefrontal cortex development in children according to the developmental and socioeconomic context in which they spent their early years. We sampled children from two sets of neighborhoods: high socioeconomic/low vulnerability and low socioeconomic/high vulnerability; in other words, neighborhoods whose developmental state fit the SES gradient pattern (64). We investigated the relationship between SES, performance, and the neural correlates of auditory-selective attention by comparing event-related potentials (ERPs) in preadolescent children during a task in which they had to attend to two types of pure tones but ignore two other types. The hypothesis was that at comparable performance levels, higher SES children would easily ignore distracters (unattended, irrelevant tones), whereas lower SES children would attend equally to distracters and target tones. The study found that ERP waveform differences between attended and unattended tones were significant in the higher SES group but not in the lower SES group, suggesting that higher SES children had an easier time with focused attention. Despite the fact that the groups did not differ in reaction times or accuracy, electroencephalographic power analysis revealed that the higher and lower SES children recruited different neural processes to achieve the task, such that lower SES children seemed to deploy supplementary resources to attend to irrelevant information. Among lower SES children, an increase in selectivity of attention corresponded to an increase in post-ERP cortisol levels (r = 0.52), which was not seen in higher SES children (r = 0.04). These results support the notion that to process irrelevant tones fully or partially, lower SES children exerted additional executive control and monitoring on the amount of information that was let in from the “unattended channel” and, as a result, showed a positive association between selectivity of attention and cortisol levels. Lower SES children may, on average, experience more chaos, disorganization, and threat in their early environments than do higher SES children (64). If so, biological embedding of a more distractible or vigilant executive function system would be adaptive for the lower SES children in their daily lives but would likely make focused attention, a key variable for school success, more difficult for them to achieve. This example addresses conditions BE1 and BE2 but not conditions BE3 and BE4, because evidence that long-term outcomes are affected is currently lacking.
Deep Archeology
Evolved mechanisms exist that monitor early environments, including the prenatal period, to adjust set points within important brain and other biological circuits. Such adjustments produce phenotypic plasticity: the capacity of a single genome to produce a range of physically or functionally adaptive traits. The cell-level processes by which these conditional adaptations occur include changes in the expression of regulatory genes, such as developmental changes in the frequency of DNA methylation at specific CpG sites (65). These are epigenetic phenomena, that is, modification of the DNA or associated proteins, other than DNA sequence variation, that carry information during cell division (66). The same epigenetic processes, including but not limited to DNA methylation, are likely key mechanisms by which early environmental signals are transduced into conditionally adaptive changes in metabolic, endocrine, and neuroregulatory pathways. Deep archeology currently involves characterizing epigenetic modifications of the genome, studying whether or not they are responsible for developmental biases that translate into different developmental trajectories and exploring whether or not these biases and subsequent trajectories become implicated in life course outcomes (67).
First Studies of Deep Archeology of Biological Embedding in Human Populations
There is a growing body of evidence suggesting that human DNA methylation is responsive to environmental exposures during both intrauterine development and after birth in humans (68–73). Consistent with the LG-ABN paradigm, early nurturing experiences have been shown to influence epigenetic programming of the GR gene promoter in the human hippocampus (72). Several imprinted genes, including IGF2, show altered methylation levels in blood DNA of 60-y-olds who had been exposed to famine prenatally (70, 74). Early experience plausibly leaves a mark on the epigenome that, in turn, could lead to stable changes in expression of genes critical for human development and health. The challenge is to fill in the existing gaps in knowledge and logic. For example, differential gene expression, even where the function of specific genes is known, does not necessarily translate into differential health outcomes, and the function of differentially methylated genes is often unknown or partially known.
The LG-ABN paradigm revealed a gene central to a candidate system for biological embedding (i.e., the HPA axis). Such hypothesis-driven approaches have obvious value in elucidating the pathways by which experience can get under the skin; however, in humans, hypothesis-driven approaches need to be complemented by genome-wide studies of epigenetic marks. The principal reason is that much too little is known yet to make an assumption that epigenetic mechanisms of biological embedding will load on the metabolic pathways that directly govern the expression of candidate systems. It is equally plausible that epigenetic mechanisms may operate “upstream” on enabling metabolic pathways whose impact on candidate systems is indirect or preemptive. Moreover, in most cases, human brain tissue, which should be the principal site of biological embedding, will be unavailable for analysis. Instead, epigenetic epidemiology during an era of hypothesis generation will depend on such tissues as blood and buccal epithelium. Epigenetic analysis of these tissues can advance understanding of issues, such as the size, timing, and persistence of the epigenetic signature of early exposures and the consistency of profiles across populations, geographies, and time. Such first-generation insights will then frame future hypotheses. The following two recently published studies exemplify how birth cohorts, with extensive information on life course experiences over years and decades, can be exploited to create first-generation insights through genome-wide epigenetic analysis.
Deep Archeology of Biological Embedding: 1958 British Birth Cohort
This study aimed to establish whether childhood SES was associated with differential methylation of adult DNA in a birth cohort that had previously shown SES gradients in health, learning, and development across 5 decades of follow-up (75). Forty adult males from the 1958 British Birth Cohort Study were selected from SES extremes in both early childhood and middle adulthood, that is, 10 in each of four categories: low childhood (age 0–7 y)/high adulthood (age 33–45 y), low childhood/low adulthood, high childhood/high adulthood, and high childhood/low adulthood. Genome-wide methylation analysis was performed on blood DNA taken at 45 y using methylated DNA immune precipitation, and the methylation state of promoters of ∼20,000 genes and 400 micro-RNAs was mapped in triplicate. Probe methylation scores were averaged across triplicates, and differentially methylated promoter sites of selected genes were validated using pyrosequencing of bisulfite-converted DNA. Variably methylated probes (9,112 on the microarray) corresponded to 6,176 gene promoters with at least a single variable probe. Unsupervised hierarchical clustering of probes obtained from the 500 most variable promoters revealed a cluster enriched with high-SES individuals, confirming that SES differences contributed to overall epigenetic variation. Methylation levels for 1,252 gene promoters were associated with childhood SES vs. 545 promoters for adulthood SES.
Finding a methylation signature of early-life SES in adult blood DNA taken at 45 y of age is consistent with epigenetic mechanisms contributing to the association between early-life SES and adult health. Lending this claim plausibility is the fact that SES-associated methylation levels were not randomly distributed across the genome but, instead, formed clusters in large genomic regions, similar to what is seen in circumstances in which the epigenetic patterns have been associated with known pathology. Childhood SES showed the largest and most pronounced clusters, suggesting that the methylation differences between childhood SES groups were the result of systematic epigenomic organization rather than differences one might expect to see between groups of randomly selected individuals. Genes showing SES-associated promoter differential methylation were enriched for basic cell functions, including several different modes of signaling, rather than being enriched for genes operating directly at the level of the candidate systems. Importantly, hypothesis-driven studies focusing on preselected promoter regions would have missed these patterns. Instead, this study identified a set of new and different plausible routes by which early environment might become biologically embedded.
Deep Archeology of Family Stress
Do typical differences in daily experience during the early years, such as maternal and paternal experience of stress, influence DNA methylation over developmental time? Fifteen-year-old adolescents (n = 109) in a longitudinal study of child development were recruited to examine differences in DNA methylation in relation to a parent’s prospective self-reports of adversity during the adolescents’ infancy and preschool periods (76). This work produced three principal findings of relevance to biological embedding. First, maternal stress during the infancy of a child, a contributor to maladaptive developmental outcomes in children, was associated with a broad and substantial array of epigenetic marks assessed 1.5 decades later, during children’s middle adolescence. Approximately 28,000 CpG sites were interrogated in the regulatory regions of 14,000 genes. After controlling for false discovery rates related to multiple comparisons, 139 DNA methylation sites showed significantly higher levels of methylation among children whose mothers were highly stressed in the child’s first 18 mo of life, compared with those less exposed. Increased DNA methylation occurred both in genes with plausible linkages to family experiences of stress and adversity (e.g., NEUROG1) and in genes with no such linkages (e.g., PGAM2). Second, experiences of adversity among both mothers and fathers bore plausible relationships to epigenetic profiles within specific developmental time frames. Maternal stressors during infancy were most strongly predictive, but fathers’ reports of high levels of adversity during their children’s preschool years were also associated with altered methylation status in a smaller but still significant number of regulatory loci. Third, the patterning of epigenetic marks associated with early adversity varied by the sex of both parent and child, with fathers’ reported stressors being more strongly associated with girls’ epigenetic modifications but mothers’ reported stressors being related equally to both sexes. Overall, this study showed adversity-related epigenetic marks, constituting measurable epigenetic change associated with early life experience within intimate social contexts, with the potential for altering gene expression. The fact that maternal stressors in infancy were most strongly predictive of differential methylation lends plausibility to these findings.
Conclusions
This paper shows how a big social fact, the socioeconomic gradient in health, led to the hypothesis of biological embedding and its four criteria. These, in turn, led to recognizing the LG-ABN paradigm as its archetypal animal model and to considering the fundamental role of epigenetics as an underlying mechanism. In humans, elucidating underlying mechanisms is arduous, involving a process analogous to archeology: uncovering and characterizing patterns of development in the whole human, key candidate systems, and cellular/subcellular mechanisms as one digs deeper. At present, the surficial layer shows large effect sizes on early human development; however, further down, the archeology of biological embedding comprises separate digs at different depths within the human organism, with tenuous connections presently existing among them.
When it comes to the epigenetics of biological embedding in humans, the basic caveats must be acknowledged. Buccal and white blood cells are not neurons, association is not causation, DNA methylation is not gene expression, and gene expression is not the occurrence of a positive or negative health or developmental outcome. Finally, cell-level processes of biological embedding will likely be shown to include other epigenetic, as well as nonepigenetic, mechanisms. Notwithstanding these caveats, a new generation of studies is advancing the prospect that epigenetic differences may constitute a biological vestige of early exposures, potentially altering the expression of genes affecting metabolic and physiological pathways and changing trajectories of individual development.
Epigenetic modifications are a plausible basic mechanism of biological embedding, and we are now poised to ask the fundamental question that bridges from individual to social facts: How do early childhood environments work together with genetic variation and epigenetic regulation to generate gradients in health and human development across the life course? To answer this, we will need to use a broad range of data, ranging from assessments of gene polymorphisms and DNA methylation to population-level information on SES and developmental status from birth to school departure. We will need to examine the stability of epigenetic modifications from gestation to primary school entry and to generate and test hypotheses regarding differential epigenetic expression of genes for brain and biological development under different SES and nurturant conditions (77). Hopefully, such a deeper understanding of biological embedding will help to inform policies and interventions that flatten up the socioeconomic gradient in developmental health and move society in the direction of equity from the start.
Acknowledgments
I thank the Canadian Institute for Advanced Research for the opportunity to participate in this work; the Experience-Based Brain and Biological Development Program for providing an intellectual home; and, in particular, Dr. Tom Boyce and Dr. Peter Hall, whose capacity to phrase complex ideas I have liberally drawn on.
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biological Embedding of Early Social Adversity: From Fruit Flies to Kindergartners,” held December 9–10, 2011, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/biological-embedding.
This article is a PNAS Direct Submission.
References
- 1.Rose G. Strategy for Preventive Medicine. New York: Oxford Univ Press; 1992. [Google Scholar]
- 2.Durkheim E. Suicide. New York: Free Press; 1951. [Google Scholar]
- 3.Evans R, Morris B, Marmor T. Why Are Some People Healthy and Others Not?: The Determinants of Health of Populations. New York: Aldine de Gruyter; 1994. [Google Scholar]
- 4.Hertzman C. The biological embedding of early experience and its effects on health in adulthood. Ann N Y Acad Sci. 1999;896:85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky RM. Stress, the Aging Brain, and the Mechanisms of Neuron Death. Cambridge, MA: MIT Press; 1992. [Google Scholar]
- 6.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 7.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–277. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Szyf M, Weaver IC, Champagne FA, Diorio J, Meaney MJ. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrinol. 2005;26:139–162. doi: 10.1016/j.yfrne.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Szyf M. DNA Methylation Enzymology, Encyclopedia of the Human Genome. New York: Macmillan/Nature Group; 2003. [Google Scholar]
- 10.Diorio J, Meaney MJ. Maternal programming of defensive responses through sustained effects on gene expression. J Psychiatry Neurosci. 2007;32:275–284. [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver ICG, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 12.Marmot MG, et al. Health inequalities among British civil servants: The Whitehall II study. Lancet. 1991;337:1387–1393. doi: 10.1016/0140-6736(91)93068-k. [DOI] [PubMed] [Google Scholar]
- 13.Adler NE, Ostrove JM. Socioeconomic status and health: What we know and what we don’t. Ann N Y Acad Sci. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- 14.Hertzman C. Health and human society. Am Sci. 2001;89:538–545. [Google Scholar]
- 15.Wagstaff A, Paci P, van Doorslaer E. On the measurement of inequalities in health. Soc Sci Med. 1991;33:545–557. doi: 10.1016/0277-9536(91)90212-u. [DOI] [PubMed] [Google Scholar]
- 16.Siddiqi A, Kawachi I, Berkman L, Subramanian SV, Hertzman C. Variation of socioeconomic gradients in children’s development across advanced capitalist societies: Analysis of 22 OECD nations. Int J Health Serv. 2007;F37(1):63–87. doi: 10.2190/JU86-457P-7656-W4W7. [DOI] [PubMed] [Google Scholar]
- 17.Wilkins R, Berthelot J-M, Ng E. Trends in mortality by neighbourhood income in urban Canada 1971 to 1996. Health Rep. 2002;13(Suppl):1–28. [Google Scholar]
- 18.Keating D, Hertzman C, editors. Developmental Health and the Wealth of Nations. New York: Guilford; 1999. [Google Scholar]
- 19.Mustard F, Shanker S. Early Years Study 2: Putting Science into Action. Toronto: Council on Early Child Development; 2007. [Google Scholar]
- 20.Hertzman C, Power C, Matthews S, Manor O. Using an interactive framework of society and lifecourse to explain self-rated health in early adulthood. Soc Sci Med. 2001;53:1575–1585. doi: 10.1016/s0277-9536(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 21.Hertzman C, Power C. Health and human development: Understandings from life-course research. Dev Neuropsychol. 2003;24:719–744. doi: 10.1080/87565641.2003.9651917. [DOI] [PubMed] [Google Scholar]
- 22.Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. J Cogn Neurosci. 2009;21:1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- 23.Hart B, Risley TR. Meaningful Differences in the Everyday Experience of Young American Children. Baltimore: Paul H. Brookes Publishing; 1995. [Google Scholar]
- 24.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 25.Bronfenbrenner U. The Ecology of Human Development: Experiments by Nature and design. Cambridge, MA: Harvard Univ Press; 1979. [Google Scholar]
- 26.Siddiqi A, Irwin L, Hertzman C. Total Environment Assessment Model for Early Child development: Evidence Report for the World Health Organization’s Commission on the Social Determinants of Health. Vancouver: Human Early Learning Partnership; 2007. [Google Scholar]
- 27.Halfon N, Larson K, Russ S. 2010 Why social determinants? Healthcare Quarterly 14(Spec No 1):8–20. [Google Scholar]
- 28.Cassel J. The contribution of the social environment to host resistance: The Fourth Wade Hampton Frost Lecture. Am J Epidemiol. 1976;104(1):107–123. doi: 10.1093/oxfordjournals.aje.a112281. [DOI] [PubMed] [Google Scholar]
- 29.Syme SL, Berkman LF. Social class, susceptibility and sickness. Am J Epidemiol. 1976;104(1):1–8. doi: 10.1093/oxfordjournals.aje.a112268. [DOI] [PubMed] [Google Scholar]
- 30.Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: Psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talge NM, Neal C, Glover V. Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cynader MS, Frost BJ. Mechanisms of brain development: Neuronal sculpting by the physical and social environment. In: Keating D, Hertzman C, editors. Developmental Health and the Wealth of Nations. New York: Guilford; 1999. pp. 153–184. [Google Scholar]
- 33.Gunnar MR, Nelson CA. Event-related potentials in year-old infants: Relations with emotionality and cortisol. Child Dev. 1994;65:80–94. [PubMed] [Google Scholar]
- 34.Knudsen EI, Heckman JJ, Cameron JL, Shonkoff JP. Economic, neurobiological, and behavioral perspectives on building America’s future workforce. Proc Natl Acad Sci USA. 2006;103:10155–10162. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janus M, Offord DR. Reporting on readiness to learn in Canada. ISUMA: Can J Policy Res. 2000;1:71–75. [Google Scholar]
- 36.Guhn M, Janus M, Hertzman C, editors. Special Issue: The Early Development Instrument. Early Education and Development. Vol 18. Philadelphia: Lawrence Erlbaum Associates; 2007. [Google Scholar]
- 37.Guhn M, Zumbo BD, Janus M, Hertzman C. Validation theory and research for a population-level measure of children’s development, wellbeing, and school readiness. Soc Indic Res. 2011;103:179–191. [Google Scholar]
- 38.Kohen DE, Brooks-Gunn J, Leventhal T, Hertzman C. Neighborhood income and physical and social disorder in Canada: Associations with young children’s competencies. Child Dev. 2002;73:1844–1860. doi: 10.1111/1467-8624.t01-1-00510. [DOI] [PubMed] [Google Scholar]
- 39.Keating DP. Social interactions in human development: Pathways to health and capabilities. In: Hall P, Lamont M, editors. Successful Societies: Institutions, Cultural Repertoires and Population Health. New York: Cambridge Univ Press; 2009. pp. 53–81. [Google Scholar]
- 40.Keating DP. Society and early child development. In: Keating DP, editor. Nature and Nurture in Early Child Development. New York: Cambridge Univ Press; 2011. pp. 245–292. [Google Scholar]
- 41.McEwen BS. Effects of adverse experiences for brain structure and function. Biol Psychiatry. 2000;48:721–731. doi: 10.1016/s0006-3223(00)00964-1. [DOI] [PubMed] [Google Scholar]
- 42.Belanoff JK, Gross K, Yager A, Schatzberg AF. Corticosteroids and cognition. J Psychiatr Res. 2001;35:127–145. doi: 10.1016/s0022-3956(01)00018-8. [DOI] [PubMed] [Google Scholar]
- 43.Davis EP, Bruce J, Gunnar MR. The anterior attention network: Associations with temperament and neuroendocrine activity in 6-year-old children. Dev Psychobiol. 2002;40(1):43–56. doi: 10.1002/dev.10012. [DOI] [PubMed] [Google Scholar]
- 44.Haley DW, Weinberg J, Grunau RE. Cortisol, contingency learning, and memory in preterm and full-term infants. Psychoneuroendocrinology. 2006;31:108–117. doi: 10.1016/j.psyneuen.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herbert J, et al. Do corticosteroids damage the brain? J Neuroendocrinol. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- 46.Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from romanian orphanages. Dev Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- 47.Kreppner JM, et al. Normality and impairment following profound early institutional deprivation: A longitudinal follow-up into early adolescence. Dev Psychol. 2007;43:931–946. doi: 10.1037/0012-1649.43.4.93. [DOI] [PubMed] [Google Scholar]
- 48.Lupien SJ, et al. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- 50.Lupie SJ, King S, Meaney MJ, McEwen BS. Can poverty get under your skin? basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- 51.Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biol Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- 52.Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann N Y Acad Sci. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- 53.Evans GW, Kim P. Childhood poverty and health: Cumulative risk exposure and stress dysregulation. Psychol Sci. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 54.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 55.Flinn MV, England BG. Social economics of childhood glucocorticoid stress response and health. Am J Phys Anthropol. 1997;102(1):33–53. doi: 10.1002/(SICI)1096-8644(199701)102:1<33::AID-AJPA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 56.Brandtstädter J, Baltes-Götz B, Kirschbaum C, Hellhammer D. Developmental and personality correlates of adrenocortical activity as indexed by salivary cortisol: Observations in the age range of 35 to 65 years. J Psychosom Res. 1991;35:173–185. doi: 10.1016/0022-3999(91)90072-v. [DOI] [PubMed] [Google Scholar]
- 57.Steptoe A, et al. Socioeconomic status and stress-related biological responses over the working day. Psychosom Med. 2003;65:461–470. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- 58.Kristenson M, Eriksen HR, Sluiter JK, Starke D, Ursin H. Psychobiological mechanisms of socioeconomic differences in health. Soc Sci Med. 2004;58:1511–1522. doi: 10.1016/S0277-9536(03)00353-8. [DOI] [PubMed] [Google Scholar]
- 59.Kunz-Ebrecht SR, Kirschbaum C, Steptoe A. Work stress, socioeconomic status and neuroendocrine activation over the working day. Soc Sci Med. 2004;58:1523–1530. doi: 10.1016/S0277-9536(03)00347-2. [DOI] [PubMed] [Google Scholar]
- 60.Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom Med. 2006;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- 61.Cohen S, et al. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- 62.Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Life-time socio-economic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32:824–833. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 63.Sapolsky RM. Social subordinance as a marker of hypercortisolism. Some unexpected subtleties. Ann N Y Acad Sci. 1995;771:626–639. doi: 10.1111/j.1749-6632.1995.tb44715.x. [DOI] [PubMed] [Google Scholar]
- 64.D’Angiulli A, Herdman A, Stapells D, Hertzman C. Children’s event-related potentials of auditory selective attention vary with their socioeconomic status. Neuropsychology. 2008;22:293–300. doi: 10.1037/0894-4105.22.3.293. [DOI] [PubMed] [Google Scholar]
- 65.Russo VEA, Cove DJ, Edgar LG, Jaenisch R, Salamini F, editors. Development: Genetics, Epigenetics and Environmental Regulation. Berlin: Springer; 1999. [Google Scholar]
- 66.Feinberg AP. Epigenetics at the epicenter of modern medicine. JAMA. 2008;299:1345–1350. doi: 10.1001/jama.299.11.1345. [DOI] [PubMed] [Google Scholar]
- 67.de Kloet ER, Fitzsimons CP, Datson NA, Meijer OC, Vreugdenhil E. Glucocorticoid signaling and stress-related limbic susceptibility pathway: About receptors, transcription machinery and microRNA. Brain Res. 2009;1293:129–141. doi: 10.1016/j.brainres.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 68.Terry MB, et al. Genomic DNA methylation among women in a multiethnic New York City birth cohort. Cancer Epidemiol Biomarkers Prev. 2008;17:2306–2310. doi: 10.1158/1055-9965.EPI-08-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Borghol N, Lornage J, Blachère T, Sophie Garret A, Lefèvre A. Epigenetic status of the H19 locus in human oocytes following in vitro maturation. Genomics. 2006;87:417–426. doi: 10.1016/j.ygeno.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Heijmans BT, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Oberlander TF, et al. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3(1):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 72.McGowan PO, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Waterland RA, et al. Season of conception in rural gambia affects DNA methylation at putative human metastable epialleles. PLoS Genet. 2010;6:e1001252. doi: 10.1371/journal.pgen.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tobi EW, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borghol N, et al. Associations with early-life socio-economic position in adult DNA methylation. Int J Epidemiol. 2012;41:62–74. doi: 10.1093/ije/dyr147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Essex MJ, et al. 2011 Epigenetic Vestiges of Early Developmental Adversity: Childhood Stress exposure and DNA Methylation in Adolescence. Child Dev, 10.1111/j.1467-8624.2011.01641.x. [Google Scholar]
- 77.Hertzman C, Boyce T. How experience gets under the skin to create gradients in developmental health. Annu Rev Public Health. 2010;31:329–347, 3p following 347. doi: 10.1146/annurev.publhealth.012809.103538. [DOI] [PubMed] [Google Scholar]