Abstract
Language acquisition reflects a complex interplay between biology and early experience. Psychotropic medication exposure has been shown to alter neural plasticity and shift sensitive periods in perceptual development. Notably, serotonin reuptake inhibitors (SRIs) are antidepressant agents increasingly prescribed to manage antenatal mood disorders, and depressed maternal mood per se during pregnancy impacts infant behavior, also raising concerns about long-term consequences following such developmental exposure. We studied whether infants’ language development is altered by prenatal exposure to SRIs and whether such effects differ from exposure to maternal mood disturbances. Infants from non–SRI-treated mothers with little or no depression (control), depressed but non–SRI-treated (depressed-only), and depressed and treated with an SRI (SRI-exposed) were studied at 36 wk gestation (while still in utero) on a consonant and vowel discrimination task and at 6 and 10 mo of age on a nonnative speech and visual language discrimination task. Whereas the control infants responded as expected (success at 6 mo and failure at 10 mo) the SRI-exposed infants failed to discriminate the language differences at either age and the depressed-only infants succeeded at 10 mo instead of 6 mo. Fetuses at 36 wk gestation in the control condition performed as expected, with a response on vowel but not consonant discrimination, whereas the SRI-exposed fetuses showed accelerated perceptual development by discriminating both vowels and consonants. Thus, prenatal depressed maternal mood and SRI exposure were found to shift developmental milestones bidirectionally on infant speech perception tasks.
Keywords: infancy, maternal depression, critical periods
Language, our most quintessential human characteristic, involves a complex interplay between biology and experience. At birth, infants possess an initial preparedness for language and a developmental readiness that supports learning any of the world’s languages (1–3), yet also already show privileged processing to the native language from prenatal listening experience (4–6). During the following weeks and months of life, infants become progressively attuned to the properties of their native language, including its rhythmical and segmental (e.g., consonant) information (reviewed in refs. 7 and 8). The high degree of regularity in the timing of sequential tuning to properties of the native language suggests a series of critical or sensitive periods, i.e., points in development when the system is maximally influenced by input.
The timing of onset of these sensitive periods seems to be maturationally constrained. Infants born 3 mo preterm attune to the phonetic properties (9) of their native language at the same gestational age as full-term infants, not according to the number of months of postnatal listening experience. Such maturational constraints can protect development: ensuring the optimal consecutive stabilization of the perceptual components that contribute in a step-wise fashion to language acquisition (10). However, it is known from research in other systems that highly aberrant input, and/or exposure to drugs or disease can change the timing of critical periods. For example, research with nonhuman animals shows that the timing of brain plasticity in the visual system can be shifted forward or reopened after closure by pharmacological manipulations, and that total sensory deprivation from birth (i.e., dark rearing) can delay the onset and closure of relevant critical periods (11). There are situations in which human infants are exposed to pharmacological agents and/or biological disparities that alter experience even in utero. Could such exposure shift the timing of sensitive periods in language development that typically appear to be maturationally controlled?
Prenatal exposure to maternal mood disturbances and antidepressants used to manage these disorders are exemplars of two increasingly common early life experiences that have long-term effects on behavior and development in childhood (12). During pregnancy, 15% to 20% of women experience mood disorders (e.g., depression) and between 5% and 13% of pregnant women are treated with an antidepressant drug (13, 14). Serotonin reuptake inhibitor (SRI) antidepressants are among the most commonly used drugs during pregnancy (14). Animal models have reported that perinatal antidepressant exposure impairs early 5-hydroxytryptamine (5-HT) homeostasis, influences mature cortical network function [eg, development of the auditory (15) and somatosensory cortex (16)], and affects long-term neurobehavior (17, 18). Our understanding of similar neuroanatomical and functional consequences in humans is limited. Because SRIs readily cross the placenta and the blood–brain barrier (19), concerns have been raised about the developmental consequences of altered fetal 5-HT signaling (12, 20) or other downstream consequences (21) following prenatal SRI exposure. Importantly, antenatal maternal mood disturbances also have adverse consequences on later cognitive and language development (22–24) and affect early 5-HT levels (25), leading to confusion about how to distinguish the developmental impact of SRIs from antenatal maternal mood disturbances.
Given the neurobehavioral consequences of SRI exposure and depressed maternal mood, we asked whether exposure to SRIs or to gestational maternal depression (not treated with an SRI) has an impact on the timing and precision of speech perception milestones. To provide a sensitive assay in humans, we tested infants on two perceptual tasks that have been extensively studied: (i) auditory discrimination of a nonnative consonant speech sound contrast (26) and (ii) visual discrimination of the change from one language to another while watching silent talking faces (27).
For the auditory task, we tested discrimination of the Hindi phonetic contrast between dental /da/ vs. retroflex /Da/ alveolar stops, a distinction both English- and Hindi-learning infants have been shown to discriminate as young infants. Listening experience maintains discrimination of this distinction in Hindi-learning infants, but English-learning infants who do not hear this distinction regularly show a dramatic decline in discrimination performance by 10 mo of age (26, 28). Speech perception was tested by using a modified version of the alternating/nonalternating procedure (Materials and Methods). Infants were habituated to the dental /da/ sound and then played instances of the same /da/ sound (i.e., nonalternating trial) or a new trial with alternating dental /da/ and retroflex /Da/ sounds. Discrimination in this procedure is indicated by longer looks during the alternating test trials that include the new /Da/ sound.
The visual language discrimination task tested a similar age-related decline in perceptual discrimination. Young infants visually tell apart English and French at 4 and 6 mo of age, but fail at 8 mo unless they are raised bilingual (27, 29). As in the earlier work of Weikum et al. (ref. 27 and Materials and Methods), infants were habituated to silent video clips of three bilingual speakers reciting sentences in English and then immediately shown test trials of the same three speakers silently reciting sentences in French. Discrimination in this task is indicated by a recovery in looking time during the test trials.
We tested infants at 6 mo of age, before they typically experience a decline in sensitivity to nonnative language information, and again at 10 mo, by which time typically developing infants show a decline in performance on each task. We compared three groups of infants at each age: SRI-exposed infants whose depressed mothers had been prescribed SRI medications during pregnancy, depressed-only infants whose mothers had depressive symptoms but chose not to take medication, and control infants whose mothers did not meet our criteria for depression or take psychotropic medications during pregnancy. Maternal depression at the time of testing was included as a covariate.
We predicted that infants exposed to neither depressive maternal symptoms nor SRIs would show discrimination on both tasks at 6 mo, but fail at 10 mo as their sensitive period for nonnative visual and auditory information begins to close. If SRI exposure and/or maternal mood impact the timing of sensitive period closure, the discrimination profile would be different. Acceleration would be shown by a failure to discriminate in the auditory and/or visual task already at 6 mo, whereas a delay in sensitive period closure could be shown by continued success even at 10 mo of age.
To complement our test of the timing of sensitive period closure, we also tested the participants at a time when their speech sound categories would be developing or “opening.” At 36 wk gestation, typically developing fetuses are capable of discriminating vowel sounds (e.g., /a/ and /i/) (30, 31), but no evidence exists for consonants. We tested 36 wk-gestation fetuses on discrimination of a consonant (/da/ as in “dot” vs. /ta/ as in “tot”) and a vowel (/a/ as in “ah” vs. /i/ as in “ee”) contrast. Fetal heart rate was recorded in response to stimuli presented via a speaker pointed at the mother’s abdomen. The sounds were modified and amplified to ensure transmission across the uterine wall (Materials and Methods). The previous work would predict success in the vowel but not the consonant condition. Developmental acceleration would be indicated by successful discrimination of both the vowel and consonant distinction, whereas disruption would be indicated by failure on both.
Results
Infant Age: 6 and 10 Mo.
In the first experiment comparing performance at 6 and 10 mo, 32 control infants, 21 infants of non–SRI-treated prenatally depressed mothers, and 32 infants of SRI-treated depressed mothers were analyzed. Maternal and infant characteristics are listed for all participants (Table 1). There were no significant differences across groups in the mothers’ demographics, but mothers in the SRI-exposed and depressed-only groups reported significantly higher levels of depression [i.e., Hamilton Rating Scale for depression (HAM-D) scores] prenatally and at time of testing (Table 1). There were no significant differences in the number of trials required to reach habituation criterion, number of male and female sex, or birth characteristics across the three groups of infants.
Table 1.
Participant characteristics at 6 and 10 mo
| Control |
Depressed only |
SRI-exposed |
|||||
| Characteristic | Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | P value |
| Maternal details | |||||||
| Prenatal HAM-D (56) | 3.61 ± 2.01 | 32 | 12.95 ± 4.93 | 21 | 10.67 ± 4.97 | 32 | 0.01* |
| 6 mo HAM-D (56) | 4.11 ± 3.99 | 28 | 11.42 ± 5.85 | 19 | 10.93 ± 6.58 | 28 | 0.01* |
| 10 mo HAM-D (56) | 3.57 ± 3.60 | 30 | 11.05 ± 7.58 | 19 | 10.07 ± 7.52 | 30 | 0.01* |
| Education (y) | 18.91 ± 3.65 | 32 | 17.71 ± 3.02 | 21 | 17.38 ± 3.84 | 32 | 0.21 |
| Prenatal SRI medications (median mg/d) | |||||||
| Paroxetine | — | — | — | — | 27.5 ± 9.57 | 4 | — |
| Fluoxetine | — | — | — | — | 46.67 ± 23.09 | 3 | — |
| Sertraline | — | — | — | — | 90 ± 80.23 | 5 | — |
| Citalopram | — | — | — | — | 33.33 ± 19.66 | 6 | — |
| Escitalopram | — | — | — | — | 50 ± 0 | 1 | — |
| Venlafaxine | — | — | — | — | 154.42 ± 55.39 | 13 | — |
| Alcohol (no. of drinks during pregnancy) | 4.39 ± 8.60 | 32 | 4.67 ± 6.00 | 21 | 4.69 ± 8.45 | 32 | 0.99 |
| Smoking (yes/no) | 0 ± 0 | 32 | 0.05 ± 0.22 | 21 | 0.03 ± 0.18 | 32 | 0.51 |
| Age at delivery (y) | 33.47 ± 3.88 | 32 | 36.3 ± 5.95 | 21 | 33.56 ± 5.90 | 32 | 0.11 |
| Infant details | |||||||
| Sex (M/F) | 14/18 | 32 | 12/9 | 21 | (14/18 | 32 | — |
| Birth gestational age (wk) | 39.79 ± 1.45 | 32 | 39.78 ± 1.81 | 21 | 38.96 ± 1.48 | 32 | 0.07 |
| Birth weight (g) | 3448 ± 503 | 32 | 3530 ± 441 | 21 | 3267 ± 440 | 32 | 0.11 |
| Duration of prenatal SRI exposure (d) | — | — | — | — | 255.41 ± 51.84 | 32 | — |
| Age at 6 m study (d) | 183.96 ± 5.61 | 28 | 186 ± 6.15 | 19 | 184.57 ± 5.67 | 28 | 0.45 |
| Age at 10 m study (d) | 307.03 ± 7.16 | 30 | 308.89 ± 7.47 | 19 | 306.43 ± 4.33 | 30 | 0.41 |
*Significant at P < 0.05.
Repeated-measures analyses of covariance (ANCOVAs) were used to compare trial (alternating vs. nonalternating for the auditory Hindi discrimination task, habituation vs. switch for the visual language task), and prenatal exposure group (control, depressed-only, or SRI-exposed). Concurrent postnatal maternal mood was controlled by adding the mother’s score on the HAM-D on the day of testing as a covariate. SRI exposure included all medications listed in Table 1 because our small sample sizes for each type of SRI did not permit further analysis of differential drug outcomes. Effect sizes (η2) estimating the magnitude of the difference between the variables are reported.
Hindi sound discrimination at 6 mo.
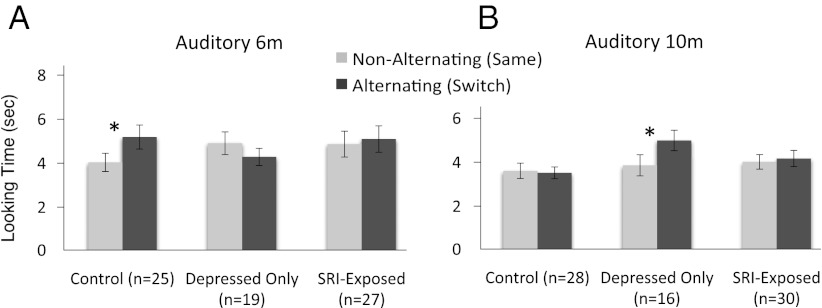
Controlling for maternal mood at 6 mo, a repeated-measures ANCOVA examining trial (alternating or nonalternating) and exposure group (control, depressed-only, SRI-exposed) revealed a significant within-subjects interaction for trial by exposure group [F(2,67) = 4.376, P = 0.016, η2 = 0.116]. When split according to exposure group at 6 mo, there was a significant main effect for trial in the control group: the infants looked significantly longer during the alternating trials [F(1,24) = 9.271, P = 0.006, η2 = 0.279]. A nonsignificant trend, but favoring the nonalternating or familiar trial, was found in the depressed-only group [F(1,18) = 2.490, P = 0.12, η2 = 0.122]. There were no significant effects in the SRI-exposed group [F(1,26) = 0.306, P = 0.585, η2 = 0.012; Fig. 1A].
Fig. 1.
Comparison of alternating (Hindi dental and retroflex) trials to nonalternating (Hindi dental) trials after being habituated to Hindi dental syllable (*P < 0.05). Error bars represent SEM. (A) Data from control (n = 25), depressed-only (n = 19), and SRI-exposed infants at 6 mo (n = 27). (B) Data from control (n = 28), depressed-only (n = 16) and SRI-exposed (n = 30) infants at 10 mo.
Hindi sound discrimination at 10 mo.
When split according to exposure group at 10 mo, there were no significant interactions or differences for infants in the control [F(1,27) = 0.058, P = 0.812, η2 = 0.002] or SRI-exposed groups [F(1,29) = 0.144, P = 0.708, η2 = 0.005], but there was a significant main effect for trial in the depressed-only group [F(1,15) = 5.886, P = 0.028, η2 = 0.282; Fig. 1B]. At this age (10 mo), infants in the depressed-only group looked longer during the alternating trials (similar to the 6-mo controls). An exploratory follow-up multivariate ANOVA of the depressed-only group at both ages revealed a significant interaction between 6 and 10 mo [F(2,33) = 8.382, P = 0.007, η2 = 0.203], confirming the change from a familiarity (i.e., nonalternating) to a novelty (i.e., alternating) preference.
Visual language discrimination at 6 mo.
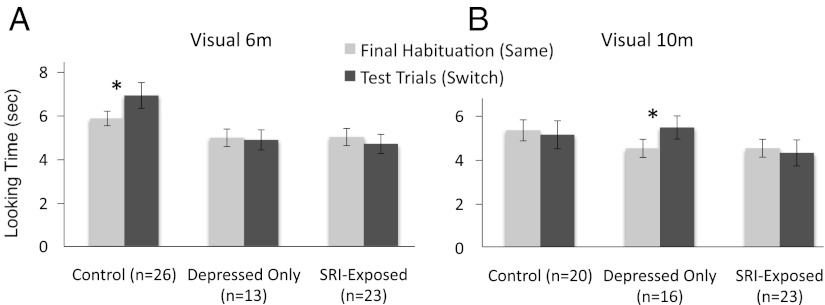
Controlling for maternal mood at 6 mo, a repeated-measures ANCOVA examining trial (final habituation vs. test), and exposure group (control, depressed-only, SRI-exposed) revealed a significant within-subjects main effect for trial [F(1,58) = 5.337, P = 0.024, η2 = 0.084] and trial-by-maternal mood interaction [F(1,58) = 5.192, P = 0.026, η2 = 0.082]. There was also a significant between-subjects main effect for exposure group [F(2,58) = 3.160, P = 0.05, η2 = 0.098]. Because there was a significant interaction for mood in the overall ANCOVA, the follow-up analyses at 6 mo split the data according to exposure group and controlled for maternal mood at 6 mo.
In the control group there was a significant difference between the final habituation and test trials [F(1,24) = 13.954, P = 0.001, η2 = 0.368] and a significant interaction between trial and maternal mood at time of study [F(1,24) = 7.434, P = 0.012, η2 = 0.237]. A follow-up linear regression analysis revealed a negative correlation between looking time to the switch and maternal depression [B(1,24) = −0.486, P = 0.012, r2 = 0.237]. No significant differences were found for the depressed-only [F(1,11) = 0.09, P = 0.926, η2 = 0.001] or SRI-exposed groups [F(1,21) = 0.613, P = 0.442, η2 = 0.028; Fig. 2A].
Fig. 2.
Comparison of final three English habituation trials to two sets of three test trials following the visual language switch to French (*P < 0.05). Error bars represent SEM. (A) Data from control (n = 26), depressed-only (n = 13), and SRI-exposed (n = 23) infants at 6 mo. (B) Data from control (n = 20), depressed-only (n = 16) and SRI-exposed (n = 23) infants at 10 mo.
Visual language discrimination at 10 mo.
When split into exposure groups and analyzed at 10 mo (controlling for maternal mood at 10 mo), there were no significant differences for the control [F(1,18) = 1.626, P = 0.218, η2 = 0.083] or SRI-exposed groups [F(1,21) = 0.031, P = 0.861, η2 = 0.001], but the depressed-only group showed a significant main effect for trial [F(1,14) = 6.023, P = 0.028, η2 = 0.301; Fig. 2B]. Like the 6-mo-old infants in the control group, the 10-mo-old infants in the depressed-only group increased their looking to the language switch.
Fetal consonant and vowel discrimination.
In the second experiment, 14 fetuses of mothers taking SRI medications during their pregnancy and 20 nonexposed fetuses were tested. With the exception of higher depressive symptoms in the SRI-treated group (based on HAM-D score), maternal demographic and health characteristics did not differ between the groups (Table 2). Independent-samples Mann–Whitney U comparisons were used to examine infant state (asleep/awake) at the time of the study and showed no significant difference between the state of the infants in the control group versus the SRI-exposed group for the consonant study (P = 0.191) or vowel study (P = 0.503).
Table 2.
Fetal participant characteristics
| Nonexposed |
SRI-exposed |
||||
| Characteristic | Mean ± SD | n | Mean ± SD | n | P value |
| Maternal details | |||||
| Education (y) | 17.4 ± 2.58 | 20 | 16.57 ± 4.31 | 14 | 0.528 |
| HAM-D score (56) at 36 wk gestation | 6.4 ± 5.11 | 20 | 10.79 ± 3.77 | 14 | 0.007* |
| Smoking (yes/no) | 0 | 20 | 0.07 ± 0.27 | 14 | 0.336 |
| Alcohol (no. of drinks for entire pregnancy) | 5.1 ± 8.34 | 20 | 4.79 ± 7.63 | 14 | 0.912 |
| Prenatal SRI medications (median mg/d) | |||||
| Paroxetine | — | — | 20 ± 0 | 2 | — |
| Fluoxetine | — | — | 20 ± 0 | 1 | — |
| Sertraline | — | — | 25 ± 0 | 1 | — |
| Citalopram | — | — | 30 ± 17.32 | 3 | — |
| Venlafaxine | — | — | 144.71 ± 69.79 | 7 | — |
| Fetal details | |||||
| Sex (M/F) | 9/11 | 20 | 5/9 | 14 | — |
| Fetal study gestational age (wk) | 36.38 ± 0.76 | 20 | 36.26 ± 0.37 | 14 | 0.521 |
| Birth gestational age (wk) | 40.28 ± 0.98 | 20 | 38.78 ± 1.64 | 14 | 0.002* |
| Duration of prenatal SRI exposure (d) | — | — | 256.07 ± 47.93 | 14 | — |
*Significant at P < 0.05.
As the sample size was not adequate for three groups, the effect of prenatal maternal mood was controlled by including it as a covariate. Heart rate deceleration during a 10-s window was used as the dependent variable following a switch in speech sound delivered from a speaker to the mother’s abdomen (Materials and Methods). It was predicted that nonexposed fetuses would discriminate the vowel contrast but not the consonant contrast.
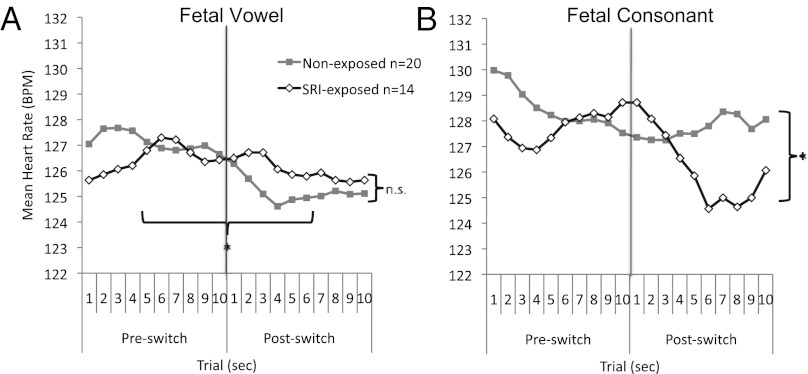
The vowel discrimination data were analyzed by using two-by-two-by-10 [group (exposed vs. nonexposed) by trial type (preswitch vs. postswitch) by test trial] repeated-measures ANCOVAs, with maternal mood score (per HAM-D) at 36 wk gestation included as a covariate. There were no significant main effects, but there was a significant interaction between trial type (preswitch vs. postswitch) and test trial [F(1,31) = 3.91, P = 0.031, η2 = 0.11] and a significant trial type-by-test trial-by-maternal mood (per HAM-D) interaction [F(1,31) = 4.18, P = 0.025, η2 = 0.12].
To probe these significant interactions, a repeated-measures ANOVA using prenatal maternal mood (per HAM-D) as a covariate was conducted across the preswitch condition test trials and postswitch condition test trials. There was no significant difference in the preswitch condition, but a change in heart rate in the postswitch condition [F(1,31) = 3.08, P = 0.05, η2 = 0.09]. Pair-wise comparisons revealed a significant decline in heart rate between trials 2 and 4 (P < 0.05), with no main effect or interaction for exposure group or maternal mood (Fig. 3A).
Fig. 3.
Mean fetal heart rate during a speech discrimination task. The y axis represents fetal heart rate in beats per minute (BPM); the x-axis represents the 10 preswitch and 10 postswitch trials. Preswitch comprises repeated presentations of the same sound, and postswitch comprises repeated presentations of the changed speech sound (*P < 0.05). (A) SRI-exposed (n = 14) and nonexposed (n = 20) fetuses at 36 wk gestation in response to a change in vowel sounds (/a/ vs. /i/). (B) SRI-exposed (n = 14) and nonexposed (n = 20) fetuses at 36 wk gestation in response to a change in consonant sounds (/da/ vs. /ta/).
The consonant data were analyzed by using the same two-by-two-by-10 [group (exposed vs. nonexposed) by trial type (preswitch vs. postswitch) by test trial] mixed ANCOVA, and again controlled for maternal mood by including the HAM-D score as a covariate. There was a significant group (exposure)-by-trial type (preswitch vs. postswitch)-by-trial interaction [F(1,31) = 3.56, P = 0.04, η2 = 0.10], with no interaction for maternal mood. Follow-up analyses controlling for maternal mood revealed no significant differences in the nonexposed group, but the exposed group showed a significant decline in heart rate between the preswitch and postswitch trials [F(1,12) = 6.66, P = 0.024, η2 = 0.36; Fig. 3B].
Discussion
This research was designed to examine whether early altered neurochemistry or maternal mental health can change the timing of critical periods in human language development. The results support an accelerated timing of perceptual attunement in SRI-exposed infants; however, postnatal maternal mood also had an impact. Whereas nonexposed infants of control mothers exhibited the typical pattern of discrimination at 6 mo and perceptual narrowing by 10 mo of age, the SRI-exposed infants already showed a more mature pattern, with failure to discriminate nonnative vowel and visual language changes at 6 mo that persisted to 10 mo. Their equivalent behavior to nonexposed fetuses on the vowel discrimination task at 36 wk in utero, coupled with their advanced perceptual capacity on the native consonant discrimination task, further supports an interpretation that failure to discriminate at 6 and 10 mo reflects a generally accelerated development of the speech perception system upon early SRI exposure.
Interestingly, maternal depression had the opposite effect. Infants in this group showed unreliable discrimination at 6 mo, with a tendency toward a familiarity rather than a novelty preference. However, at 10 mo, they reliably discriminated the nonnative speech sound difference and the change between visual English and visual French. Exposure to maternal depressed mood seems to delay stable discrimination, which ultimately extends the period of sensitivity to nonnative distinctions. Thus, exposure to maternal depression and prenatal SRI exposure appear to exhibit opposite effects on the development of infant speech perception. However, whether this reflects the impact of pre- or postnatal maternal mood remains to be determined.
A mechanistic explication of the biology by which SRIs and maternal depression disrupt critical period timing is essential. One possible explanation is that altered 5-HT levels that result from prolonged gestational antidepressant exposure could change the precision of language representation in the brain. Although direct human evidence has not been reported, exposure to SRIs early in life has been shown to alter tonotopic organization and receptive field properties in the primary auditory cortex (A1) of rats (15), suggesting that a disruption in auditory coding per se could explain a failure to discriminate speech at 6 and 10 mo by SRI-exposed infants. However, this cannot account for the finding of precocious consonant discrimination in utero by SRI-exposed fetuses. Thus, when all the findings are taken into account, the effect of early SRI exposure appears to be one of accelerated attunement to the properties of the native language.
Emerging reports from animal models suggest that early SRI exposure has a positive influence, reversing or “correcting” the adverse effects of prenatal maternal stress exposure (32–34). Although they are of interest, our results are not entirely consistent with that pattern, as, in our case, SRI exposure appears to lead to an “over-correction.” Below, we discuss how SRI exposure could influence age-related changes in speech perception.
Modulating 5-HT levels increase cortical plasticity in the adult visual system (35, 36). SRIs in particular can reopen cortical plasticity (37), but the means by which they do so has been largely undescribed. Recent insight into the cellular and molecular mechanisms underlying critical period onset and closure in the neocortex of mice now offers a more detailed mechanistic explanation. The maturation of excitatory–inhibitory local circuit balance appears pivotal (11). Any manipulation that ultimately accelerates/delays GABA circuit function will accelerate/delay critical period timing. By analogy, maturational milestones in the auditory system may also reflect a shifted excitatory–inhibitory balance. Apart from the popular action of SRIs on 5-HT transport, these drugs are notorious for their off-target effects (21). Notably, in response to submaximal GABA concentrations, fluoxetine can potentiate current flux through GABAA receptors containing the α1 subunit (38). Benzodiazepine use early in life is already known to accelerate critical period onset by this mechanism (39).
Turning to the infants of non–SRI-treated but depressed mothers, the compelling finding is their robust discrimination at 10 mo of the nonnative speech sound contrast and the visual language change. This result provides evidence for a delay of the critical period for attunement to the properties of the native language rather than a gross perceptual impairment. An organic difficulty in learning and/or performance on the habituation tasks is one explanation for unreliable discrimination performance at 6 mo. Newborns of depressed mothers show inferior performance on the Brazelton assessment (40, 41), with lower orientation scores, abnormal reflexes, inferior excitability, and withdrawal scores (42). However, the successful discrimination at 10 mo by infants born to depressed mothers not treated with an SRI may be better explained by a delayed critical period trajectory caused by early stimulus deprivation.
By analogy to dark-rearing in the visual system (11), infants of depressed mothers may not hear sufficient speech, or sufficiently engaging speech, to initiate the neurological changes that trigger critical period onset. Depressed mothers do not modify their speaking style (43, 44) to produce the exaggerated “motherese” that infants prefer (45–48), and which can highlight speech sound differences (49). Newborns of depressed mothers fail to show face/voice preference (22) whereas older infants do not learn as well from their mothers’ infant-directed speech (50). Thus, maternal depression may have resulted in subthreshold levels of appropriately engaging speech input. Further support for this possibility is provided by the significant correlation we observed between depression scores at 6 mo and performance in the visual language discrimination task even in control infants.
In summary, we have found that exposure to SRIs accelerates speech perception development, whereas exposure to maternal depression initially disrupts performance and ultimately delays perceptual narrowing by prolonging the period of sensitivity to nonnative distinctions. These findings are particularly compelling when we consider that the timing of speech perception development is typically considered to be maturationally delimited. What is unknown at this time, and of key clinical importance, is whether these small perturbations in critical period timing of core perceptual components of language acquisition have a lasting impact. To date, there are no published reports of language delay in infants or young children with SRI exposure. However, sequential timing and ordered emergence of developing systems is an essential characteristic of optimal development, as it allows more complex processes to build on first established, more foundational representations (51).
We know that, in typical language development, infants use the representations established in native speech sound discrimination to direct later word learning (10) and in rhythmical discrimination to direct later parsing of syntactic units (52). Thus, disruptions in the perceptual foundations of language could have a cascading impact on optimal language development (53). A recent population-level finding reports an intriguing association between prenatal SRI exposure and an increased risk for autism in early childhood (54), raising critical questions (55) about the long-term developmental implications of early mistiming in language development. By providing a deeper mechanistic understanding of how and when developmental trajectories are altered by early exposure to maternal mood disturbances and/or SRI exposure, more optimal outcomes can be realized for infants and their mothers.
Materials and Methods
Participants.
Informed consent was obtained from mothers recruited during their second trimester, and their infants were tested at 36 wk gestation and at 6 mo and 10 mo of age. Mothers were interviewed by trained research assistants by using the HAM-D (56) during the second and third trimesters of pregnancy and when their infants were 6 and 10 mo of age. Averages of the prenatal scores were used to classify the 6- and 10-mo-old infants into three groups: no SRIs and HAM-D scores <8 (control group), no SRIs and HAM-D scores ≥8 (depressed-only group) and SRI-exposed. The HAM-D cutoff score chosen was 8 because scores of 8 to 13 signify mild depression (57).
Auditory Task.
A total of 78 infants were tested at 6 mo, and 82 were tested at 10 mo. At 6 mo, participants were excluded for experimenter error (n = 1) and fussiness (n = 6). At 10 mo, participants were excluded for experimenter error (n = 0), fussiness (n = 4), and parental interference (n = 4).
Visual Task.
A total of 78 infants were tested at 6 mo, and 80 were tested at 10 mo. At 6 mo, participants were excluded for experimenter error (n = 0), fussiness (n = 11), parental interference (n = 2), failure to habituate (n = 1), and failure to watch the screen (n = 2). At 10 mo, participants were excluded for experimenter error (n = 1), fussiness (n = 16), parental interference (n = 1), failure to habituate (n = 2) and bilingual exposure to French (n = 1).
Fetal Task.
Fetuses of 23 mothers treated with an SRI antidepressant agent and 46 non–SRI-treated mothers were tested. Participants were excluded as a result of experimenter error (n = 1 exposed; n = 2 nonexposed), incomplete heart rate signal (n = 4 exposed; n = 7 nonexposed), and fetal movement during our window of interest (n = 4 exposed; n = 17 nonexposed). As we could not distinguish fetal heart rate responses to the speech stimuli from changes associated with fetal movement, any recorded movement that occurred (according to maternal button press) during the 10-s preswitch or 10-s postswitch period rendered the recording ineligible for inclusion in the study. There were no differences between the number of movements that occurred before or after the sound switch and no significant group differences in movements between the fetuses included for analysis and those excluded for movement or missing data.
Stimuli.
Hindi sound discrimination.
The Hindi dental /da/ and retroflex /Da/ syllables were the same as those used by Werker and Lalonde (28). They were taken from an eight-step continuum of /da/ sounds created by using the Mattingly synthesizer at Haskins Laboratories. Each syllable was 275 ms in length. As the stimuli were synthesized, the duration, pitch, and loudness were constant across all stimuli.
Visual language discrimination.
The visual speech stimuli were the same as those used by Weikum et al. (27). The faces of three bilingual (French/English) speakers were filmed while they recited sentences in both French and English. The audio was removed from the clips.
Fetal sound discrimination.
The consonant stimuli were from a database prepared by the laboratory of Colin Phillips at the University of Maryland. Alveolar /da/ and /ta/ sounds were generated by using the cascade vocal tract of the Klatt speech synthesizer for Macintosh (SenSyn; Sensimetrics). These speech sounds are identical except for the timing of voice onset between the initial consonant burst and subsequent vowel sound. Both are stop consonants produced by obstructing the airflow by placing the tongue at the alveolar ridge. However, /da/, which is “voiced,” has simultaneous onset of voicing with the release of the consonant, whereas there is a lag between the release and the onset of voicing in /ta/, making it “voiceless.” Our stimuli were 275 ms in length, the vowel followed the consonant burst immediately in the /da/ sound, and there was a 40-ms lag between the initial consonant burst and subsequent vowel in the /ta/ stimuli. Detection of the burst by the fetus is essential for the critical timing cue between voiced /da/ and voiceless /ta/ to be perceived. Because the initial burst at the release of the consonant comprises primarily high frequency information, and because high frequency information is more likely to be attenuated by the uterine wall (58), we amplified the low frequency portion of the burst (below 1,000 Hz using Adobe Audition software). The vowel stimuli /a/ and /i/ were 200 ms in length, prepared by using Mbrola software, and matched in intensity by using Adobe Audition software.
Procedure.
Hindi sound discrimination.
Infants sat on their parents’ lap facing a 32-inch flat screen television that was positioned on a table 24 inches off the floor and 36 inches from the infant. Small toys or pacifiers were permitted for infants who were fussy before the start of the study. Infants were tested with a version of the alternating/nonalternating paradigm (59). Stimuli was presented at ∼68 db using Bose Companion II speakers, and the experiment was run by using Habit X software (60) installed on a dual core Intel Xeon Mac Pro computer (Macintosh). The infants were presented with the dental /da/ sound until they habituated. Habituation was reached when their looking time to a black-and-white checkerboard on three consecutive trials decreased to 50% of their looking time during the longest habituation trial. There were two types of test trials. In “new” trials, the retroflex /Da/ sound alternated with the dental /da/ sound to which they had been habituated (i.e., /da/Da/da/Da/da/). In “old” trials, they heard only the same /da/ sound heard during habituation (i.e., /da/da/da/da/da). The interstimulus interval (ISI) was 500 ms. Each trial continued for 16 s or until the infant ended the trial by not watching the checkerboard for more than 2 s. Infants heard six test trials—three alternating and three nonalternating—and infants were randomly assigned to one of two different orders. To prevent inadvertently influencing their infant, parents wore Peltor Workstyle noise-cancelling headphones (3M) and listened to masking music played at ∼75 db.
Visual language discrimination.
Stimuli were presented by using the Habit X software and computer equipment used in the previous study. Infants were shown the faces of the three speakers silently reciting different sentences in English. The stimuli were presented seamlessly as blocks of three in which the speaker order was always the same for each infant. Infants were randomly assigned to one of four different speaker orders. When looking time in a block of three habituation trials decreased to 60% of the looking time in the longest block of three habituation trials, the program automatically started playing test trials in French. The test trials contained the same speakers in the same order so that the infants would not notice a difference as a result of speaker order and were seamlessly presented following the test trials. The test trials contained new sentences in French from each speaker. As in previous work, the test trials were repeated to ensure that the infants had adequate viewing time to detect the stimulus change. The discrimination score was average looking time during the two blocks of three trials.
Fetal sound discrimination.
A Bose Companion II speaker was positioned ∼10 cm from the mother’s abdomen on a thick pillow or large foam wedge on the mother’s lap (n = 2 exposed; n = 3 nonexposed) or, when space permitted, beside the mother (n = 12 exposed; n = 17 nonexposed). All fetuses were tested following a 50-min rest session. In an attempt to reduce experimenter bias and use a more ecologically valid method, we did not wait for each fetus to achieve a certain heart rate pattern, but instead played the stream of sounds continuously following the 50 min of rest by the mothers. The sounds were played from an iPod nano (Macintosh) at 95 ± 3 db as measured from an Extech 407735 sound meter. To provide partial masking to avoid maternal influence on fetal behavior, the mothers listened to the children’s story, “The Golden Fish” (http://storynory.com/2006/08/27/the-golden-fish/), through Peltor Workstyle noise-cancelling headphones (3M) at ∼75 db. Every fetus was exposed to 15 min of language sounds. The first 2 min contained a consonant switch and the last 2 min contained a vowel switch. Subjects were randomly assigned to one of four conditions. One consonant sound was repeated every second (ISI of 725 ms) for 1 min to familiarize the fetus to the sound and then immediately switched to repetitions of the other consonant sound for 30 s. The same protocol was followed for the vowel sounds (they were played every second; ISI of 800 ms).
Fetal heart rate was continuously monitored using a Toitu MT-325 Fetal Actocardiograph. The heart rate variability and movement patterns were used to determine the state of the fetuses at the time of testing. This would help to ensure that any effects were a result of stimuli presented and not masked by the state of the fetus. Four states were classified according to the procedure outlined by DiPietro et al. (61). Infants were classified as in quiet sleep (little or no heart rate variability and little or no movements), active sleep (moderate heart rate variability with episodic accelerations and some movements), quiet awake (a rhythmic oscillatory heart rate pattern within a wider bandwidth than quiet sleep and little or no movements), or active awake (high heart rate variability during which accelerations may be fused into tachycardia and movements). The 15 min for the language study was broken into five 3-min blocks. Each 3-min block was analyzed to determine the infant state during that portion.
The Toitu heart rate tracings were also imported into the computer program DigitizeIt (http://www.digitizeit.de/), allowing a 1-Hz recording of the fetus’s heart rate. Following the procedure of Kisilevsky et al. (62), the 10 s before (i.e., preswitch) and 10 s after the switch (i.e., postswitch) were analyzed. The data were analyzed for heart rate decelerations that occurred in response to the sound switch, indicating that the fetuses noticed the change in sound (63).
Acknowledgments
We thank the mothers and their infants who gave generously of their time; acknowledge the thoughtful work of Ursula Brain, who was central to recruitment, data collection, and management, Mary Beckingham, and Deborah Heard for data collection; and thank Ramesh Swamy for technical support and Janet DiPietro, PhD, who coded fetal state. The overall study of this cohort was funded by the Canadian Institutes of Health Research (CIHR) (T.F.O.). This study was supported by the Canadian Institutes for Advanced Research (T.K.H. and J.F.W.), the Human Frontier Science Program (RGP0018/2007 to T.K.H. and J.F.W.), and postdoctoral fellowships from CIHR (to W.M.W.) and the Michael Smith Foundation for Health Research (to W.M.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biological Embedding of Early Social Adversity: From Fruit Flies to Kindergartners,” held December 9–10, 2011, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/biological-embedding.
References
- 1.Peña M, et al. Sounds and silence: An optical topography study of language recognition at birth. Proc Natl Acad Sci USA. 2003;100:11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehler J, et al. A precursor of language acquisition in young infants. Cognition. 1988;29:143–178. doi: 10.1016/0010-0277(88)90035-2. [DOI] [PubMed] [Google Scholar]
- 3.Vouloumanos A, Werker JF. Listening to language at birth: Evidence for a bias for speech in neonates. Dev Sci. 2007;10:159–164. doi: 10.1111/j.1467-7687.2007.00549.x. [DOI] [PubMed] [Google Scholar]
- 4.Byers-Heinlein K, Burns TC, Werker JF. The roots of bilingualism in newborns. Psychol Sci. 2010;21:343–348. doi: 10.1177/0956797609360758. [DOI] [PubMed] [Google Scholar]
- 5.May L, Byers-Heinlein K, Gervain J, Werker JF. Language and the newborn brain: does prenatal language experience shape the neonate neural response to speech? Front Psychol. 2011;2:222. doi: 10.3389/fpsyg.2011.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moon C, Cooper RP, Fifer WP. Two-day-olds prefer their native language. Infant Behav Dev. 1993;16:495–500. [Google Scholar]
- 7.Kuhl PK. Early language acquisition: Cracking the speech code. Nat Rev Neurosci. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- 8.Werker JF, Gervain J. Speech perception: A foundation for language acquisition. In: Zelazo P, editor. Oxford Handbook of Developmental Psychology. New York: Oxford Univ Press; in press. [Google Scholar]
- 9.Peña M, Werker JF, Dehaene-Lambertz G. Earlier speech exposure does not accelerate speech acquisition. J Neurosci. 32:11159–11163. doi: 10.1523/JNEUROSCI.6516-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Werker JF, Yeung HH. Infant speech perception bootstraps word learning. Trends Cogn Sci. 2005;9:519–527. doi: 10.1016/j.tics.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 12.Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86:672–677. doi: 10.1038/clpt.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 14.Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. Am J Obstet Gynecol. 2007;196:544.e1–544.e5. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Simpson KL, et al. Perinatal antidepressant exposure alters cortical network function in rodents. Proc Natl Acad Sci USA. 2011;108:18465–18470. doi: 10.1073/pnas.1109353108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee LJ. Neonatal fluoxetine exposure affects the neuronal structure in the somatosensory cortex and somatosensory-related behaviors in adolescent rats. Neurotox Res. 2009;15:212–223. doi: 10.1007/s12640-009-9022-4. [DOI] [PubMed] [Google Scholar]
- 17.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 18.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 19.Kim J, et al. Stereoselective disposition of fluoxetine and norfluoxetine during pregnancy and breast-feeding. Br J Clin Pharmacol. 2006;61:155–163. doi: 10.1111/j.1365-2125.2005.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homberg JR, Schubert D, Gaspar P. New perspectives on the neurodevelopmental effects of SSRIs. Trends Pharmacol Sci. 2010;31:60–65. doi: 10.1016/j.tips.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Bianchi MT. Non-serotonin anti-depressant actions: Direct ion channel modulation by SSRIs and the concept of single agent poly-pharmacy. Med Hypotheses. 2008;70:951–956. doi: 10.1016/j.mehy.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez-Reif M, Field T, Diego M, Largie S. Depressed mothers’ newborns show longer habituation and fail to show face/voice preference. Infant Ment Health J. 2002;23:643–653. [Google Scholar]
- 23.Sohr-Preston SL, Scaramella LV. Implications of timing of maternal depressive symptoms for early cognitive and language development. Clin Child Fam Psychol Rev. 2006;9:65–83. doi: 10.1007/s10567-006-0004-2. [DOI] [PubMed] [Google Scholar]
- 24.Talge NM, Neal C, Glover V. Early Stress, Translational Research and Prevention Science Network: Fetal and Neonatal Experience on Child and Adolescent Mental Health Antenatal maternal stress and long-term effects on child neurodevelopment: How and why? J Child Psychol Psychiatry. 2007;48:245–261. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Field T, et al. Prenatal depression effects on the fetus and the newborn. Infant Behav Dev. 2004;27:216–229. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Werker JF, Tees RC. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behav Dev. 1984;7:49–63. [Google Scholar]
- 27.Weikum WM, et al. Visual language discrimination in infancy. Science. 2007;316:1159. doi: 10.1126/science.1137686. [DOI] [PubMed] [Google Scholar]
- 28.Werker JF, Lalonde CE. Cross-language speech perception: Initial capabilities and developmental change. Dev Psychol. 1988;24:672–683. [Google Scholar]
- 29.Sebastián-Gallés N, Albareda-Castellot B, Weikum WM, Werker JF. A bilingual advantage in visual language discrimination in infancy. Psychol Sci. 23:994–999. doi: 10.1177/0956797612436817. [DOI] [PubMed] [Google Scholar]
- 30.Lecanuet JP, et al. Fetal perception and discrimination of speech stimuli; demonstration by cardiac reactivity; preliminary results (Translated from French) C R Acad Sci III. 1987;305:161–164. [PubMed] [Google Scholar]
- 31.Zimmer EZ, et al. Response of the premature fetus to stimulation by speech sounds. Early Hum Dev. 1993;33:207–215. doi: 10.1016/0378-3782(93)90147-m. [DOI] [PubMed] [Google Scholar]
- 32.Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133:893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 33.Rayen I, van den Hove DL, Prickaerts J, Steinbusch HW, Pawluski JL. Fluoxetine during development reverses the effects of prenatal stress on depressive-like behavior and hippocampal neurogenesis in adolescence. PLoS ONE. 2011;6:e24003. doi: 10.1371/journal.pone.0024003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalueff AV, Olivier JD, Nonkes LJ, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci Biobehav Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Edagawa Y, Saito H, Abe K. Endogenous serotonin contributes to a developmental decrease in long-term potentiation in the rat visual cortex. J Neurosci. 2001;21:1532–1537. doi: 10.1523/JNEUROSCI.21-05-01532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kojic L, et al. Columnar distribution of serotonin-dependent plasticity within kitten striate cortex. Proc Natl Acad Sci USA. 2000;97:1841–1844. doi: 10.1073/pnas.97.4.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maya Vetencourt JF, et al. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- 38.Robinson RT, Drafts BC, Fisher JL. Fluoxetine increases GABA(A) receptor activity through a novel modulatory site. J Pharmacol Exp Ther. 2003;304:978–984. doi: 10.1124/jpet.102.044834. [DOI] [PubMed] [Google Scholar]
- 39.Fagiolini M, et al. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. doi: 10.1126/science.1091032. [DOI] [PubMed] [Google Scholar]
- 40.Abrams SM, Field T, Scafidi F, Prodromidis M. Newborns of depressed mothers. Infant Ment Health J. 1995;16:233–239. [Google Scholar]
- 41.Lundy B, Field T, Pickens J. Newborns of mothers with depressive symptoms are less expressive. Infant Behav Dev. 1996;19:419–424. [Google Scholar]
- 42.Lundy BL, et al. Prenatal depression effects on neonates. Infant Behav Dev. 1999;22:119–129. [Google Scholar]
- 43.Bettes BA. Maternal depression and motherese: Temporal and intonational features. Child Dev. 1988;59:1089–1096. doi: 10.1111/j.1467-8624.1988.tb03261.x. [DOI] [PubMed] [Google Scholar]
- 44.Kaplan PS, Bachorowski JA, Smoski MJ, Zinser M. Role of clinical diagnosis and medication use in effects of maternal depression on Infant-Directed speech. Infancy. 2001;2:537–548. doi: 10.1207/S15327078IN0204_08. [DOI] [PubMed] [Google Scholar]
- 45.Fernald A, Simon T. Expanded intonation contours in mothers’ speech to newborns. Dev Psychol. 1984;20:104. [Google Scholar]
- 46.Fernald A. Four-month-old infants prefer to listen to motherese. Infant Behav Dev. 1985;8:181–195. [Google Scholar]
- 47.Cooper RP, Aslin RN. Preference for infant-directed speech in the first month after birth. Child Dev. 1990;61:1584–1595. [PubMed] [Google Scholar]
- 48.Werker JF, McLeod PJ. Infant preference for both male and female infant-directed talk: A developmental study of attentional and affective responsiveness. Can J Psychol. 1989;43:230–246. doi: 10.1037/h0084224. [DOI] [PubMed] [Google Scholar]
- 49.Liu HM, Kuhl PK, Tsao FM. An association between mothers’ speech clarity and infants’ speech discrimination skills. Dev Sci. 2003;6:F1–F10. [Google Scholar]
- 50.Kaplan PS, Bachorowski JA, Smoski MJ, Hudenko WJ. Infants of depressed mothers, although competent learners, fail to learn in response to their own mothers’ infant-directed speech. Psychol Sci. 2002;13:268–271. doi: 10.1111/1467-9280.00449. [DOI] [PubMed] [Google Scholar]
- 51.Turkewitz G, Kenny PA. Limitations on input as a basis for neural organization and perceptual development: a preliminary theoretical statement. Dev Psychobiol. 1982;15:357–368. doi: 10.1002/dev.420150408. [DOI] [PubMed] [Google Scholar]
- 52.Gervain J, Nespor M, Mazuka R, Horie R, Mehler J. Bootstrapping word order in prelexical infants: A Japanese-Italian cross-linguistic study. Cognit Psychol. 2008;57:56–74. doi: 10.1016/j.cogpsych.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Werker JF, Tees RC. Speech perception as a window for understanding plasticity and commitment in language systems of the brain. Dev Psychobiol. 2005;46:233–251. doi: 10.1002/dev.20060. [DOI] [PubMed] [Google Scholar]
- 54.Croen LA, Grether JK, Yoshida CK, Odouli R, Hendrick V. Antidepressant use during pregnancy and childhood autism spectrum disorders. Arch Gen Psychiatry. 2011;68:1104–1112. doi: 10.1001/archgenpsychiatry.2011.73. [DOI] [PubMed] [Google Scholar]
- 55.Levitt P. Serotonin and the autisms: A red flag or a red herring? Arch Gen Psychiatry. 2011;68:1093–1094. doi: 10.1001/archgenpsychiatry.2011.98. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kearns NP, et al. A comparison of depression rating scales. Brit J Psychiatry. 1982;141:45–49. doi: 10.1192/bjp.141.1.45. [DOI] [PubMed] [Google Scholar]
- 58.Lecanuet JP, Schaal B. Sensory performances in the human foetus: A brief summary of research. Intellectica. 2002;1:29–56. [Google Scholar]
- 59.Best CT, Jones C. Stimulus-alternation preference procedure to test infant speech discrimination. Infant Behav Dev. 1998;21:295. [Google Scholar]
- 60.Cohen LB, Atkinson DJ, Chaput HH. A New Program for Obtaining and Organizing Data in Infant Perception and Cognition Studies (Version 1.0) Austin, TX: Univ Texas; 2002. [Google Scholar]
- 61.DiPietro JA, Costigan KA, Pressman EK. Fetal state concordance predicts infant state regulation. Early Hum Dev. 2002;68:1–13. doi: 10.1016/s0378-3782(02)00006-3. [DOI] [PubMed] [Google Scholar]
- 62.Kisilevsky BS, Pang LH, Hains SMJ. Maturation of human fetal responses to airborne sound in low- and high-risk fetuses. Early Hum Dev. 2000;58:179–195. doi: 10.1016/s0378-3782(00)00075-x. [DOI] [PubMed] [Google Scholar]
- 63.Richards JE. The statistical analysis of heart rate: A review emphasizing infancy data. Psychophysiology. 1980;17:153–166. doi: 10.1111/j.1469-8986.1980.tb00129.x. [DOI] [PubMed] [Google Scholar]