Abstract
Preference behaviors are often established during early life, but the underlying neural circuit mechanisms remain unknown. Adapting a unique nesting behavior assay, we confirmed a “critical period” for developing music preference in C57BL/6 mice. Early music exposure between postnatal days 15 and 24 reversed their innate bias for silent shelter, which typically could not be altered in adulthood. Instead, exposing adult mice treated acutely with valproic acid or carrying a targeted deletion of the Nogo receptor (NgR−/−) unmasked a strong plasticity of preference consistent with a reopening of the critical period as seen in other systems. Imaging of cFos expression revealed a prominent neuronal activation in response to the exposed music in the prelimbic and infralimbic medial prefrontal cortex only under conditions of open plasticity. Neither behavioral changes nor selective medial prefrontal cortex activation was observed in response to pure tone exposure, indicating a music-specific effect. Open-field center crossings were increased concomitant with shifts in music preference, suggesting a potential anxiolytic effect. Thus, music may offer both a unique window into the emotional state of mice and a potentially efficient assay for molecular “brakes” on critical period plasticity common to sensory and higher order brain areas.
Preference behaviors are shaped early in life and can last a lifetime. From classic work on chick imprinting (1) to drug vulnerability in adolescents (2, 3), neural circuits in the developing brain are especially impressionable to experience. Biases in response to acoustic signals with ethological or emotional valence over environmental noise are particularly important for social, cultural, and biological fitness. For example, infants will suckle actively to hear their mother’s voice over that of another (4) and prefer speech over nonspeech sounds (5) and native over nonnative language (6) as early as 4 d after birth. How and where such enduring preferences are instantiated in the brain remain largely unknown.
Music is a powerful tool with which to probe acoustic behavior influenced by exposure during a sensitive period. Early musical training can improve pitch processing and the remarkable ability of absolute pitch (7), verbal intelligence, and executive functioning (8), and may itself induce plasticity (9–12). Compared with infants without training, those with Kindermusik experience, which focuses on Western music with duple meter, exhibit a differential preference for this underlying hierarchical temporal structure (13) as early as 4 mo of age (14). At 9 mo of age, infants prefer human singing to instrumental music, even for familiar songs (15).
Music is an extremely complex multimodal but predominantly auditory stimulus with dynamic changes over time in a number of features, including frequency, intensity, rhythm, tempo, meter, and timbre. Although much recent work has demonstrated the presence of critical periods in the primary auditory cortex in response to relatively simple features like tones or frequency modulation (16, 17), music represents a much more complex version of the acoustic environment. Furthermore, studies in humans have revealed that musical processing involves a widespread network of brain structures in addition to auditory cortex, including the planum temporal, parietal lobe, insula, limbic circuit, nucleus accumbens, ventral tegmental area, orbitofrontal cortex, Heschl’s gyrus, premotor cortex, anterior superior-temporal gyrus, frontal lobe, and cerebellum (18).
Importantly, the choice of one type of music over another likely reflects limbic, decision-making brain regions, such as the prefrontal cortex (reviewed in 19), which is known to be engaged during judgments of kinship and close others later in life (20). Interestingly, Jouhaneau and Bagady (21) demonstrated that Swiss albino mice exposed to a certain type of music during the period from postnatal day (P) 10 to P20 later “preferred” that kind of music when given a choice during adulthood. Those exposed outside of that period were indistinguishable from controls, showing no such preference. Although no biological mechanism was offered, these results suggest that a juvenile plasticity could also explain the development of higher order features like decision-making and preference in animal models. Here, we optimized this behavioral assay to capitalize on recent advances in understanding of critical period mechanism.
Receptive field properties and tonotopic maps in primary sensory areas for vision, somatosensation, and audition exhibit precisely defined critical periods, and specific molecular players regulating their timing have now been identified (22). Critical period plasticity is initiated by a late maturation of GABAergic circuits, particularly those expressing the calcium-binding protein parvalbumin. Conversely, gradually emerging “molecular brakes” later in life actively limit plasticity in the adult brain (23). They include axonal growth-inhibiting factors, such as myelin-signaling proteins, Nogo and PirB, acting in complex with the Nogo receptor (NgR) (24), chondroitin sulfate proteoglycans (25), and epigenetic factors like histone deacetylases (26). It is therefore possible to reactivate plasticity in adult animals by removing these brakes with pharmacological or genetic manipulations (25, 26).
It remains to be determined whether these mechanisms regulating plasticity in sensory cortex can be applied to cognitive processes, such as preference for complex acoustic signals. The specific goals of this study were to (i) confirm a “critical period” for music preference in developing C57BL/6 mice by varying periods of music exposure, (ii) establish mechanisms underlying closure of this critical period by lifting of molecular brakes on plasticity in adulthood, and (iii) examine the medial prefrontal cortex (mPFC) as a region of interest for circuit changes related to modulation of preference for music.
Results
Critical Period for Acoustic Preference in Mice.
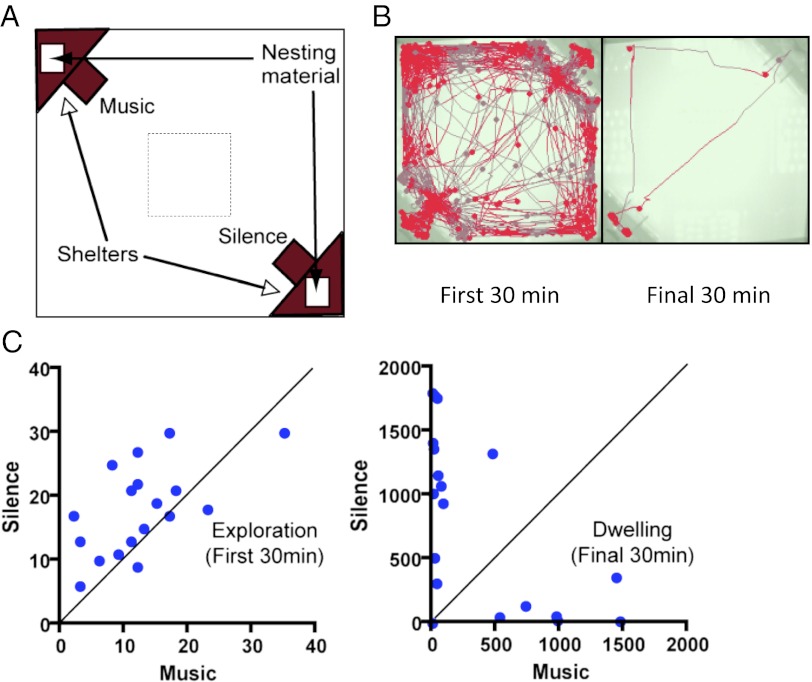
We placed WT C57BL/6J mice (WT) into an open arena with shelters in opposing corners (Fig. 1A). These shelters contained bedding material as well as acoustic stimuli (music) or remained silent. Over the course of a 3-h testing period, mice typically “explore” the arena actively (first 30 min; Fig. 1 B and C, Left) and then eventually “dwell” in a shelter of choice, where they nest (final 30 min; Fig. 1 B and C, Right). We can use the fact that mice explore the arena during the initial 30 min as an “open-field” assay. This classic measure of rodent anxiety (27) measures general anxiety levels by the number of center-square crossings (Fig. 1A, dotted) and general activity levels by measuring total distance moved during the same exploration period (initial 30 min).
Fig. 1.
Measurement of acoustic preference in mice. (A) Open 45-cm by 45-cm arena containing shelters with speakers and nesting material is monitored for 3-h trials. (B) Sample tracking of mice during the first and final 30 min of the acoustic preference tests is shown. For visual clarity, traces were plotted once every 50 samplings (out of 14 samplings per second) and superimposed on the video image of the arena setup. Dark triangular shadows in the corners of the background image depict the shelters with different acoustic stimuli. (B and C) (Left) Typically, mice will actively “explore” the arena in the first 30 min, as indicated by the number of entries into either chamber. (Right) By the final 30 min, most animals indicate their preference (in seconds) by settling into shelters (“dwell”) for extended periods of time.
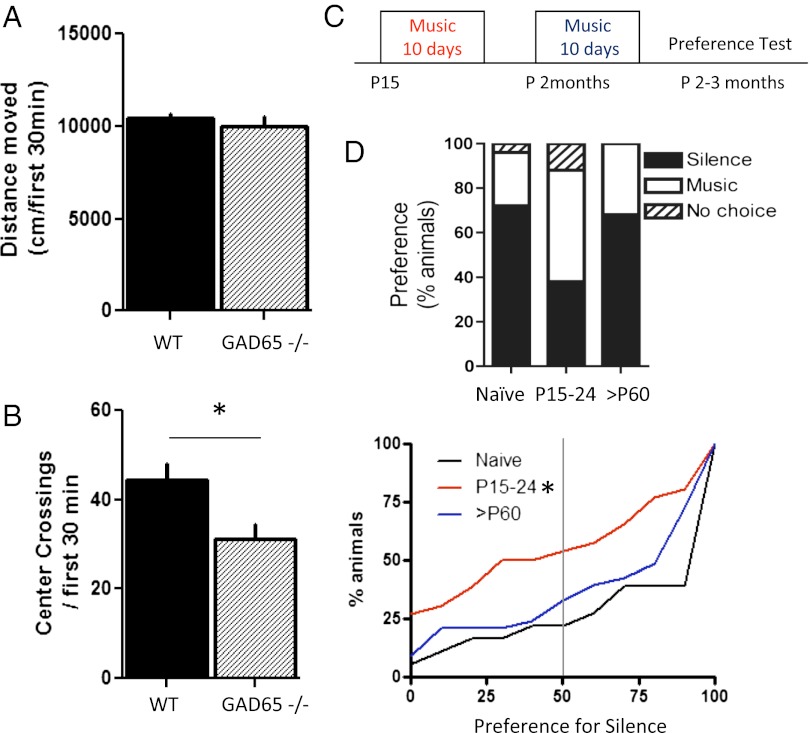
To validate our preference arena, we first tested a well-known anxiety model, in which GAD65, one of the two enzymes that synthesize GABA, is genetically deleted. In comparison to WT animals, GAD65−/− mice showed fewer center crossings (Fig. 2B), confirming their heightened anxiety as measured previously by reduced center time in standard open-field tests without shelters (28). In contrast, overall activity levels indicated by distance moved during the same period did not differ between GAD65−/− and WT mice (Fig. 2A).
Fig. 2.
Juvenile window for shaping acoustic preference. (A and B) To validate the anxiety measure in our preference test setup, a mouse model of anxiety (28), GAD65−/− mice, were tested and compared with WT (C57BL6/J) mice. GAD65−/− mice (dashed box) show a lower number of center-square crossings (B) during the first 30 min of the preference test (mean ± SEM; *Mann–Whitney U test, P < 0.05), whereas they show a similar distance moved during this time as WT mice (A). (C) Critical period for music preference in mice. (D, Upper) Percentages of naive WT mice, WT mice with exposure to music during P15–P24, or WT mice with exposure to music at >P60 that show a preference for each shelter during the final 30 min of a music vs. silence test. (D, Lower) Cumulative frequency distribution of each experimental group plotted as a function of preference for silence. The majority of naive WT mice choose the silent shelter (black curve, n = 18). This behavior can be modified by exposure to music only during a critical period in the third postnatal week (P15–24: red curve, n = 26; >P60: blue curve, n = 33) (21). The abscissa indicates a preference for the silent chamber, which was calculated by the percentage of time spent in the silent chamber over the total time spent in both chambers (final 30 min). *Kolmogorov–Smirnov test, P < 0.05.
When acoustic preference was further evaluated based on the dwelling time spent in shelters during the final 30 min, adult WT mice exhibited a characteristic “neophobic” response and settled primarily in the silent shelter (Fig. 2D, Upper, “Naive” bar and Lower, black trace). To determine whether there is a developmental plasticity in this preference, we exposed mice to music either at P15 or in adulthood (>P60) for 10 d and tested their preference between P60 and P90 (Fig. 2C).
Mice exposed to music either before P10 (21) or later as adults (Fig. 2D, >P60 and blue curve) made a similar choice for silence as in the naive group. However, animals raised in the presence of music, independent of the genre, during the third postnatal week exhibited a preference as adults for the shelter containing the previously heard music (Fig. 2D, P15–P24 and red curve; P15 vs. Naive: Kolmogorov–Smirnov test, P < 0.02; P15 vs. >P60: Kolmogorov–Smirnov test, P = 0.05). These results support the original report in albino mice that acoustic preference for music is shaped during a brief critical period (21).
Reopening Preference Plasticity in Adulthood.
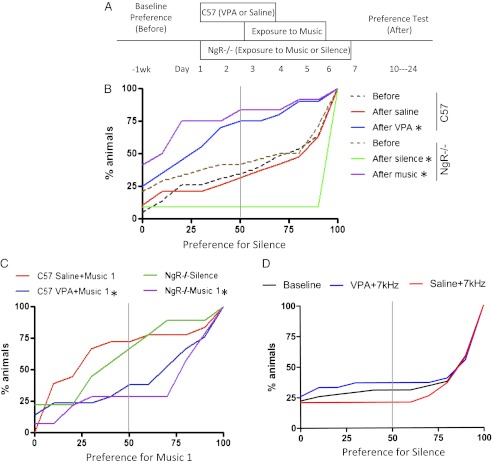
We then examined whether it is possible to reopen a window of brain plasticity in adulthood. Based on recent success in primary visual cortex (23), we focused on possible epigenetic constraints that may limit circuit rewiring. Ocular dominance plasticity in adult rats is reactivated by histone deacetylase inhibitors, such as valproic acid (VPA) or trichostatin A (26, 29). These manipulations are well-known to open chromatin structure, engaging gene expression. Removal of myelin-related signaling by gene-targeted deletion of the NgR (NgR−/−) also maintains an open-ended critical period in mice (30). Thus, we assayed both conditions paired with music or typical cage environments in adult animals (Fig. 3A).
Fig. 3.
(A) Reactivation of critical period for music preference. Adult (>P60) WT mice pretreated with VPA for 2 d or NgR−/− mice were passively exposed to music and tested for acoustic preference (as in Fig. 1) before and after exposure. (B) Note the typical preference for silence (dashed and red curves; n = 58, n = 24, and n = 19 for naive WT, NgR−/− baseline, and saline WT mice, respectively) is largely shifted in favor of exposed music in the VPA and NgR−/− groups (blue and purple curves; n = 20 and n = 12 mice, respectively). Conversely, NgR−/− mice housed in silence prefer the silent shelter even more strongly (green curve; n = 11 mice). (C) Specific preference for the previously heard music over previously unheard music in mice with reopened juvenile plasticity. The cumulative frequency distribution of each experimental group is plotted as a function of preference for previously heard music (music 1) in WT and NgR−/− groups. Adult WT mice with VPA treatment paired with music exposure (blue) show a preference for the music 1 compared with saline-treated controls (red; Kolmogorov–Smirnov test, P < 0.05). In comparison to the NgR−/− silence group (green), the NgR−/− group exposed to music 1 (purple) shows a preference for music 1 (NgR−/− music 1 vs. NgR−/− silence; Kolmogorov–Smirnov test, P < 0.03). (D) Preference shift was not observed in WT mice with VPA treatment combined with 7-kHz tone exposure following the same procedure as music exposure in A (n = 19 saline + 7-kHz vs. 27 VPA + 7-kHz tone-exposed WT mice). *P < 0.05, Kolmogorov-Smirnov test.
Strikingly, both VPA-treated and NgR−/− mice (>P60) exhibited a strong preference for the exposed music (Fig. 3B, blue curve: VPA vs. baseline Kolmogorov–Smirnov test, P < 0.01; purple curve: NgR−/− music vs. baseline Kolmogorov–Smirnov test, P < 0.004). Saline-treated controls (Fig. 3B, red curve) behaved like naive adults (Fig. 3B, dashed curves) and maintained a preference for silence. In contrast, NgR−/− mice kept in soundproof boxes shifted their preference even further toward silence (Fig. 3B, green curve: NgR−/− silence vs. baseline Kolmogorov–Smirnov test, P < 0.0001). Thus, acoustic behavior can be reshaped bidirectionally under these plastic conditions.
Specificity of Acoustic Preference.
Next, we addressed music stimulus specificity. Mice were allowed to choose between the previously heard music (music 1) and previously unheard music (music 2). Both VPA-treated and NgR−/− mice showed a preference for the music to which they had been exposed (Fig. 3C; Kolmogorov–Smirnov tests, P < 0.05 and P < 0.03 for C57 VPA + music 1 vs. saline + music 1 and NgR−/− music vs. NgR−/− silence, respectively). In a subset of NgR−/− mice, further exposure (10 d) to a second piece of music shifted preference yet again away from the initial music (Kolmogorov–Smirnov test, P < 0.04).
To determine whether preference shifts required melodic music or if just any rhythmical sound could result in preference shifts, we treated WT mice with VPA or saline following the same protocol (Fig. 2B) but exposed them instead to a repeated 5-Hz train of 7-kHz tones at 2-s intervals over a similar duration. Unlike VPA-treated, music-exposed WT mice, tone-exposed mice did not lose their innate preference for silence despite drug treatment (Fig. 3D; Kolmogorov–Smirnov test, P > 0.7). This suggests that preference shifts during reopened plasticity are specific for music.
Neural Substrate for Preference Changes.
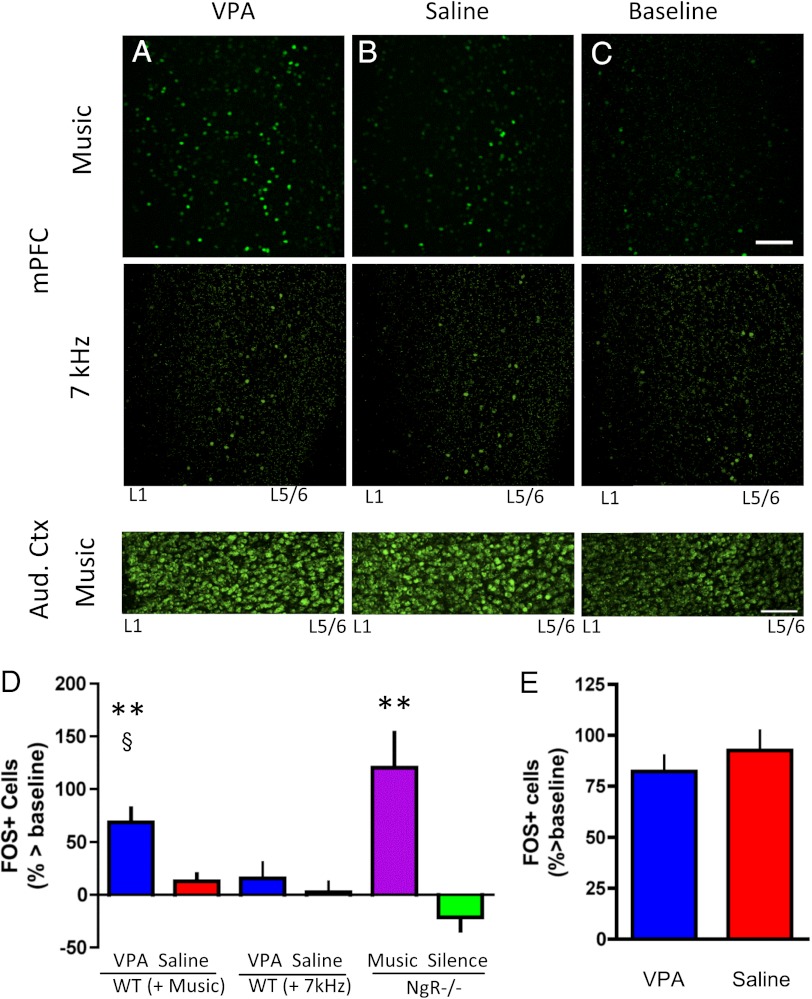
We next probed neuronal activation in response to music. We focused on the mPFC and primary auditory cortex. Immediate early gene cFOS expression in response to the previously heard music was assayed using separate sets of NgR−/− and FOS-EGFP (WT) mice. Following the same procedure as in behavioral experiments (Fig. 3A), WT mice were treated with either VPA (Fig. 4A) or saline (Fig. 4B) during continuous music or 7-kHz exposure, whereas NgR−/− mice were exposed either to music or “silence.” Mice were subsequently euthanized after a 1-h exposure to the previously heard music, 7-kHz tone, or silence (Fig. 4C) and were processed for EGFP visualization or FOS immunoreactivity.
Fig. 4.
Music engages the adult mPFC during reopened plasticity. (A–C) Representative response in FOS-EGFP mice of mPFC (Upper and Middle) and primary auditory cortex (Aud. ctx; Bottom) to brief exposure to the music (Upper), 7 kHz (Middle), or silence (C, baseline) previously heard with VPA (A) or saline (B) treatment. (Scale bar, 100 μm.) (D) Quantification of FOS+ cells in the mPFC for WT groups in A–C and NgR−/− mice previously exposed either to music or silence as in behavioral experiments. Note the increased neuronal response in the mPFC of VPA-treated, music-exposed WT, and NgR−/− music groups (n = 9 VPA + music vs. 8 saline + music: Mann–Whitney U test, **P < 0.005; VPA vs. n = 5 baseline (zero line): Mann–Whitney U test, §P < 0.03; n = 8 NgR−/− music vs. 7 NgR−/− silence: Mann–Whitney U test, **P < 0.003). In contrast, WT mice exposed to 7-kHz tones did not show an increased FOS response in the mPFC regardless of VPA (A, Middle) or saline (B, Middle) compared with silence (C, Middle; zero line) (n = 6 in each group). (E) Quantification of FOS+ cells in primary auditory cortex of WT mice in A–C (Lower). Unlike in the mPFC, VPA treatment did not yield differential FOS responses to music in comparison to saline-treated, music-exposed controls.
Both WT mice that had undergone VPA treatment paired with music (Fig. 4D, WT VPA + Music) and NgR−/− mice exposed to music (Fig. 4D, NgR−/− Music) displayed increased cFOS expression in the mPFC in comparison to saline-treated, exposed WT control (Fig. 4D, WT Saline + Music), silence WT baseline (Fig. 4D, zero line), or NgR−/− silence (Fig. 4D, green) groups (VPA + Music vs. Saline + Music: Mann–Whitney U test, P < 0.005; VPA + Music vs. WT baseline: Mann–Whitney U test, P < 0.03; NgR−/− Music vs. NgR−/− Silence: Mann–Whitney U test, P < 0.003). In contrast, 7-kHz–exposed, VPA-treated WT mice (Fig. 4D, WT VPA + 7 kHz) did not show increased FOS expression in mPFC in comparison to saline-treated counterparts or no sound baseline (Fig. 4D, zero line). Also, primary auditory cortex did not show greater FOS expression in response to the previously heard music despite VPA treatment and music exposure (Fig. 4E, blue) in comparison to their saline-treated counterparts (Fig. 4E, red).
Implications for Anxiety.
Music is reported to reduce anxiety in specific situations (“state anxiety”), such as clinical settings (31–34), and animals exposed to music exhibit anxiolytic effects (35, 36). Instead, there is limited efficacy of music for treating anxiety in adults, but it may have more potential in children and adolescents (37, 38). Therapies like music exposure may be more effective, given a certain amount of flexibility in the brain.
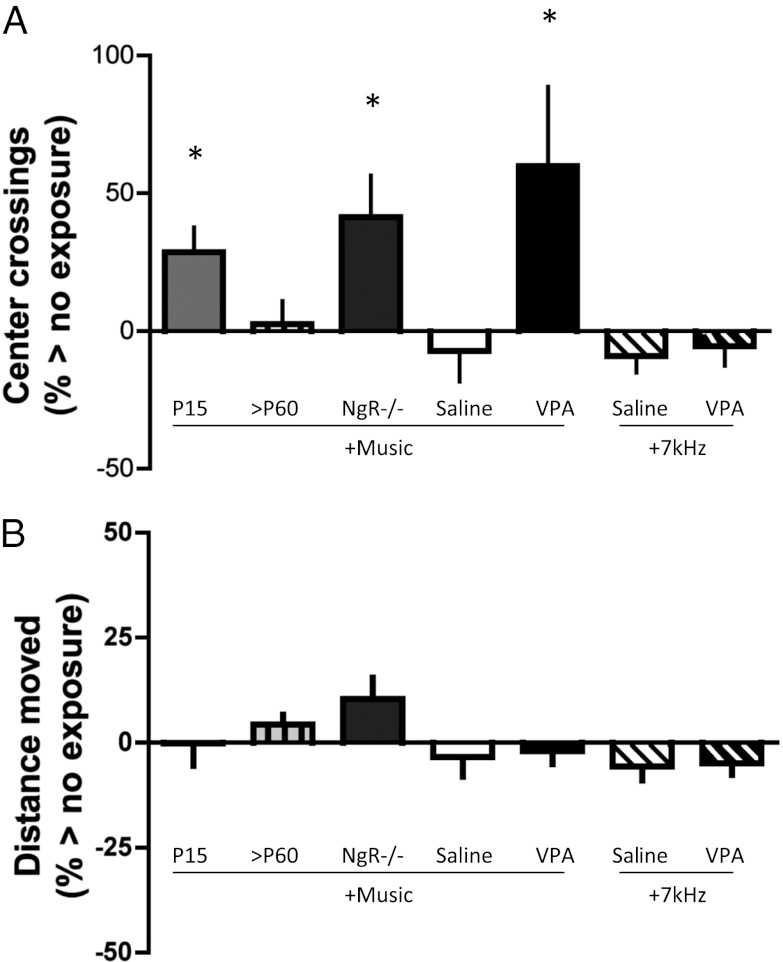
We thus examined the relationship between music combined with reopened plasticity on anxiety and overall activity level. Center-square crossings during the first 30 min were notably increased only under conditions of reopened plasticity (Fig. 5A; VPA + Music vs. Saline + Music: Mann–Whitney U test, P < 0.05; NgR−/− vs. WT >P60: Mann–Whitney U test, P < 0.03; NgR−/− vs. nonexposed NgR−/− control (zero line): Mann–Whitney U test, P < 0.02). When juvenile WT mice were exposed to music at P15 and examined for center-square crossings in adulthood, they showed similarly increased crossings in comparison to nonexposed controls (Mann–Whitney U test, P < 0.01) as well as in comparison to normal WT mice exposed as adults (>P60; Mann–Whitney U test, P < 0.05). On the other hand, WT mice with VPA treatment paired with 7-kHz exposure did not show increased center-square crossing in comparison to nonexposed conditions (zero line) or saline-treated WT mice paired with 7-kHz exposure.
Fig. 5.
Potential anxiolysis by music exposure reflects juvenile brain plasticity. (A) Increased number of center-square crossings (Fig. 1A, dashed box), indicating reduced anxiety (70), during the first 30 min measured in adult WT mice previously exposed to music at P15–P24 (light gray bar, n = 26) suggests anxiolysis in comparison to mice exposed later at >P60 (n = 18) or naive controls (zero line, n = 18). Reduced anxiety was also observed in adult NgR−/− mice (dark gray bar, n = 15) after exposure to music (in comparison to naive NgR−/− mice; zero line, n = 44) and in adult WT mice after VPA paired with music (black bar, n = 20) compared with saline-treated counterparts exposed to music (white bar, n = 19) or naive WT controls (zero line, n = 58). Note that a similar duration of tone exposure (7 kHz) in adult WT mice produced no anxiolytic effect despite VPA treatment (hatched black bar, n = 27) in comparison to saline-treated counterparts also exposed to 7 kHz (hatched white bar, n = 19). *P < 0.05 vs. non–music-exposed controls. (B) Overall distance traveled during the same first 30 min is no different across groups, regardless of exposure to music or 7-kHz tones.
To test whether increased center-square crossing in juvenile or reopened plasticity conditions was a mere reflection of increased activity, we measured overall distance traveled during the same exploration period (first 30 min) in the preference arena for each test. Unlike center-square crossings, overall distance moved was no different between plastic conditions and their corresponding controls (Fig. 5B).
Discussion
Although the hearing range of mice and humans is distinct, 1–100 kHz and 20 Hz to 20 kHz, respectively (39), we found that music created for the human ear can also lead to behavioral changes in mice. Our results demonstrated that the acoustic environment present during an open critical period can shape mPFC response. Molecular factors known to limit plasticity in sensory cortex could thus be tapped to alter music preference in adulthood. To our knowledge, this is the first report of restoring a juvenile form of brain plasticity to this circuit.
It is unknown what features of music, such as timbre, tempo, key, modulating frequency, rhythm, or some combination thereof, are integral to its efficacy in mice. Complex acoustic stimuli like music robustly engage multiple brain regions beyond primary sensory areas, including centers for arousal, emotion, and reward (40). It has been suggested that neural processing and perception of music in animals is different from that of humans in various aspects, such as processing of tonal relations (19), absolute pitch (41), and detecting mistunings within complex harmonics (41). However, certain aspects reportedly show similarity to humans, such as processing of melodic contour (42) and speaker variability in phonetic boundaries (43, 44).
Whether the development of music preference under plastic conditions would apply beyond the genres used in the present study remains to be elucidated. Some types of music influence rodent social behavior (45) and discrimination performance (46), particularly if timbre cue of music is present (47). Importantly, our results largely confirm that when pulsed tones are used with or without patterns, this effect is not observed (reviewed in 48). The specific qualities in the music stimulus that must be paired with active plasticity for achieving preference shifts can now be explored more systematically.
Our study implicates mPFC activation in preference shifts, which goes beyond basic receptive field properties traditionally shown to exhibit a critical period in the primary auditory cortex of cats (49, 50), rats (51, 52), and mice (16) or in the auditory midbrain in rats (53), chinchillas (54), and barn owls (55, 56). Our task potentially offers further insight into higher order brain processing of complex acoustic stimuli, such as music. The mPFC serves an important function in working memory (57), attention (58), and sensory gating (59). Its maturation is slow (3), which coincides with slow development in working memory (60). Conversely, impaired NgR signaling in the mPFC has been linked to psychoses (61), perhaps reflecting excessive circuit plasticity into adulthood.
Future work will investigate chemical ablation or optogenetic inactivation of the mPFC and whether overtly similar behaviors by VPA and NgR−/− could be explained by a common mechanism. Moreover, impaired synaptic rewiring during circuit development could result in long-term deficits. For example, input-specific synaptic plasticity in frontal cortex is impaired in neurodevelopmental disorders (62), and axonal organization and development in the prefrontal cortex are disrupted in autism (63). Hyperconnectivity and slow synaptic dynamics are observed in the mPFC of the Fmr1−/− mouse, a model of fragile-X syndrome, autism, and mental retardation (64). Notably, this delay in synaptic maturation arises during the second to third postnatal week of development, largely coinciding with the critical period observed here for developing acoustic preference.
Strikingly, the same time period (between P15 and P20) is also reported to be a critical period for shaping anxiety circuits (65). Knocking out 5-hydroxytryptamine (5-HT) 1A receptors increases anxious behavior, which is rescued by conditional reexpression of postsynaptic 5-HT1A receptors by P15 (but not after P21). Using fluoxetine to inhibit 5-HT transport from P4 to P21 showed supporting results (66). Music therapy is reported to produce anxiolytic effects in various clinical settings, such as in perioperative patients, patients with dementia, or mechanically ventilated patients (18, 31, 34, 67). The relationship between music, anxiety, and plasticity and a potential mediating role of the mPFC underlying this relationship warrants further investigation.
At a molecular level, WT mice exposed to music from birth (or prenatally) show increased BDNF levels, unlike those exposed only in adulthood (>P60) (35, 68). Instead, transgenic animals (BDNFMet/Met) with genetically low levels of BDNF exhibit abnormally high anxiety levels (36). Exposing them to music as adults results in an up-regulation of BDNF in the prefrontal cortex, amygdala, and hippocampus, as well as reduced anxious behavior. It remains to be seen if anxiety levels might remain low in our plastic mice without the presence of familiar music at the time of testing. It is conceivable that a “dose” of familiar music is necessary to ameliorate anxiety acutely (67). More standard anxiety tests, such as the elevated plus maze test or traditional open-field test, should be measured with or without ambient music to confirm the open-field measures.
Overall, our task reveals an innate behavioral preference that is established during a critical period shortly after hearing onset in mice (21), requiring no training and free of confounding olfactory, visual, or tactile cues. In songbirds, exposure to a tutor’s song during a sensitive developmental phase leads both sexes to prefer the songs of the tutor over other unfamiliar songs when tested in adulthood (69). Moreover, the mPFC in humans responds to close others (i.e., kinship) (20). Thus, our paradigm opens exciting possibilities for probing the brain state of mice in response to more ethologically relevant acoustic environments (e.g., ultrasonic vocalizations) experienced in their youth.
Materials and Methods
Animals.
Adult C57BL/6J mice were maintained with same-sex littermates in cages with bedding material and ad libitum access to water and food under a 12:12-h light/dark cycle. NgR−/− KO mice (back-crossed onto C57BL/6 mice for >10 generations) were provided by Z. He (Children’s Hospital Boston) from original breeders produced by Tessier-Lavigne and colleagues (70). GAD65−/− and FOS-EGFP breeders were provided by K. Obata (RIKEN Brain Science Institute, Saitama, Japan) (71) and A. Barth (Carnegie Mellon University, Pittsburgh, PA) (72), respectively, and kept under the same environmental conditions. Animal housing and experimental procedures were approved and followed the guidelines of the Harvard University Institutional Animal Care and Use Committee (AEP28-19).
Two-Choice Preference Setup.
The acoustic preference test was conducted using a Phenotyper 4500 (Noldus Information Technology), a 45-cm (width) × 45-cm (depth) × 45-cm (height) open arena with clear plastic walls viewed by means of a ceiling-mounted video camera and infrared lights and filters. Two diagonally opposing corners of the Phenotyper 4500 were chosen randomly and furnished with red, opaque plastic shelters bearing a side entrance. A small loudspeaker (1.6-cm diameter × 1-cm height) was installed on the ceiling of each shelter (6 cm high), and nesting and bedding materials were provided at the bottom (Fig. 1A). To minimize ambient noise interference, the entire test setup was placed in an anechoic sound isolation chamber [inner dimensions: 55 cm (width) × 49 cm (depth) × 66 cm (height); Industrial Acoustics Company] with an ambient light source (8 W).
Each test was initiated by placing mice in the center of the arena and was monitored for 3 h consecutively. The animals’ behavior was recorded by video camera, tracked, and analyzed using Ethovision XT software (Noldus Information Technology). Tests were conducted between 0900 and 1700 hours during the light phase to promote mouse dwelling in the shelters. All components of the test setup were wiped clean twice with Clidox solution, followed by 70% ethanol/30% purified water, and air-dried between each trial. The positions of shelters and sound playback were randomized on each trial.
Behavioral Procedure.
For the reopening of critical period experiments at P60, WT or NgR−/− mice were tested for acoustic preference to approach (first 30 min) or dwell (final 30 min) in the two-choice acoustic preference setup (Fig. 1A). One shelter played music (either first movement from Beethoven’s symphony no. 1 or no. 9, or Antonio Carlos Jobim’s “Agua de Beber”) looped continuously throughout the 3-h test duration, whereas the other shelter remained silent (music–silence test). Testing was conducted inside a sound isolation chamber, and the sound level at the entrance to the “silent” shelter was confirmed by a sound pressure level meter to be at the same level as background sound levels without music playback.
Mice were then housed with same-sex littermates in a sound isolation chamber [58.4 cm (width) × 40.6 cm (depth) × 35.6 cm (height); Industrial Acoustics Company] with ad libitum access to water and food under a 12:12-h light/dark cycle. One week after initial testing, adult C57BL/6 mice were injected (i.p.) with either VPA (200 mg⋅kg−1; Sigma–Aldrich) dissolved in saline or the same volume of saline every 12 h for 4 d. After the first 2 d of injections, they were further exposed to music (one of the three pieces above), which was played through two free-field loudspeakers [minimum sound pressure level (SPL) of 69.5 ± 2.5 dB to maximum SPL of 78 ± 1.5 dB] looped for 24 h each day over 4 d. In the case of 7-kHz control experiments, mice were exposed to looped playback of 5-Hz trains of 7-kHz tone interspersed with 2 s of silence.
In the case of adult NgR−/− mice, animals were randomly divided into two groups 1 wk after initial testing and placed in the sound isolation chamber with littermates and ad libitum access to food and water. One group was exposed to music, whereas the other was kept in silence. The music pieces and sound levels were the same as those for the WT VPA experiments above. Exposure was done between 0900 and 1700 hours each day for 7 d.
Three to four days after exposure to music or silence, both VPA and NgR−/− mice were retested in the two-choice preference setup with one shelter playing previously heard music and the other shelter silent (music–silence retest). One further week after the music–silence retest, some mice were tested a third time on a two-music preference between a shelter playing previously heard music and one with previously unheard music (Jobim or Beethoven, or vice versa). In a subset of NgR−/− mice 2 wk after the two-music test, those previously exposed to music were reexposed for 7 d to previously unheard music using the same procedure as before, after which they were tested on a two-music test between the two pieces they had heard most recently vs. previously.
To probe a developmental window (21), C57BL/6 mice were exposed at P15 with their dam or after P60 (as for adults above) to music for 10 d in the anechoic sound isolation chamber with ad libitum access to water and food. Subsequently, the P15 group was weaned and housed in a normal mouse housing facility with same-sex littermates until behavioral testing. Music–silence testing was performed between P60 and P90.
Data Analysis.
To assay preference, time spent in each shelter during the final 30 min was measured. Mice spending most of their time in open areas within the test arena but outside either shelter were labeled as having made “no choice” (Fig. 2D) and dropped from further analysis. Preference for silence or for music 1 was calculated, respectively, as 100 × (time in silent shelter/total time in both shelters) or 100 × (time in music 1 shelter/total time in both shelters). Overall activity was measured by distance (cm) moved during the first 30 min (i.e., exploration phase; Fig. 1 B and C). Anxiety was measured as an inverse of the number of crossings within the central square of the entire arena within the first 30 min of each trial. Groups of WT and NgR−/− mice were tested for anxiety with or without previous music or 7-kHz exposure combined with VPA or saline treatment (for WT) and compared within and between groups. Kolmogorov–Smirnov tests were performed for group comparisons in preference tests. Wilcoxon signed rank tests were used to compare before/after measures, and Mann–Whitney U tests (for data with nonnormal distributions) and t tests (for data with Gaussian distributions) were conducted for comparisons of two independent samples. Statistical analyses were two-tailed comparisons using SYSTAT 13 (Cranes Software International) or GraphPad Prism 4.
Visualizing FOS+ Cells.
FOS-EGFP mice were injected with either VPA or saline following the same procedure as for the behavioral experiments above for 4 d. Two days after the first injection, mice were exposed to music or 7 kHz for 4 d following the same procedure and conditions as in the behavioral experiments. NgR−/− mice were similarly exposed either to music or silence following the same procedure and conditions as in the behavioral experiments. At 3–4 d after the end of the exposure, FOS-EGFP or NgR−/− mice were perfused after 1 h of exposure to previously heard music, 7 kHz or silence (Fig. 4 A–C). Brains were removed after perfusion and then postfixed overnight at 4 °C.
FOS-EGFP brains were sectioned coronally, and the mPFC and primary auditory cortex were visualized; immunofluorescence imaging (at 488 nm) was performed on a confocal laser-scanning microscope (Olympus LX81 with Fluoview FV1000 scanner). Images were captured at a magnification of 20× using Fluoview (v1.3a). FOS+ cells were analyzed with ImageJ (National Institutes of Health) using the same threshold across sections and automatic particle count function with the same specific size and circularity settings. Brain sections of NgR−/− mice were blocked overnight at 4 °C with 0.8% Triton X-100 and 20% BSA in PBS labeled with primary antibody against FOS (molecular probes, 1:1,000) overnight at 4 °C and secondary antibody conjugated with Alexa Fluor 488 or 546 (1:1,000). NgR−/− sections were visualized, and FOS+ cells were analyzed following the same procedures as FOS-GFP sections.
Acknowledgments
We thank M. Nakamura for mouse colony maintenance, and Drs. Z. He, K. Obata, and A. Barth for NgR−/−, GAD65−/−, and FOS-EGFP breeders, respectively. This study was supported by the Human Frontiers Science Program (Grant RGP0018/2007-C), the Canadian Institutes for Advanced Research (Experience-Based Brain and Biological Development Program), and National Institute of Mental Health Grant 1P50MH094271 (to T.K.H.).
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Biological Embedding of Early Social Adversity: From Fruit Flies to Kindergartners,” held December 9–10, 2011, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/biological-embedding.
This article is a PNAS Direct Submission.
References
- 1.Bolhuis JJ. Early learning and the development of filial preferences in the chick. Behav Brain Res. 1999;98:245–252. doi: 10.1016/s0166-4328(98)00090-4. [DOI] [PubMed] [Google Scholar]
- 2.Gould TJ. Addiction and cognition. Addict Sci Clin Pract. 2010;5:4–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Kolb B, et al. Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA. 2012;109(Suppl. 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers’ voices. Science. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- 5.Colombo JA, Bundy RS. A method for the measurement of infant auditory selectivity. Infant Behav Dev. 1981;4:219–223. [Google Scholar]
- 6.Mehler J, et al. A precursor of language acquisition in young infants. Cognition. 1988;29:143–178. doi: 10.1016/0010-0277(88)90035-2. [DOI] [PubMed] [Google Scholar]
- 7.Trainor LJ. Are there critical periods for musical development? Dev Psychobiol. 2005;46:262–278. doi: 10.1002/dev.20059. [DOI] [PubMed] [Google Scholar]
- 8.Moreno S, et al. Short-term music training enhances verbal intelligence and executive function. Psychol Sci. 2011;22:1425–1433. doi: 10.1177/0956797611416999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantev C, Herholz SC. Plasticity of the human auditory cortex related to musical training. Neurosci Biobehav Rev. 2011;35:2140–2154. doi: 10.1016/j.neubiorev.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Rojo N, et al. Music-supported therapy induces plasticity in the sensorimotor cortex in chronic stroke: A single-case study using multimodal imaging (fMRI-TMS) Brain Inj. 2011;25:787–793. doi: 10.3109/02699052.2011.576305. [DOI] [PubMed] [Google Scholar]
- 11.Thaut M, McIntosh G. 2010. How music helps to heal the injured brain: Therapeutic use crescendos thanks to advances in brain science. Cerebrum. Available at http://dana.org/news/cerebrum/detail.aspx?id=26122. Accessed June 30, 2012.
- 12.Wan CY, Schlaug G. Music making as a tool for promoting brain plasticity across the life span. Neuroscientist. 2010;16:566–577. doi: 10.1177/1073858410377805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerry DW, Faux AL, Trainor LJ. Effects of Kindermusik training on infants’ rhythmic enculturation. Dev Sci. 2010;13:545–551. doi: 10.1111/j.1467-7687.2009.00912.x. [DOI] [PubMed] [Google Scholar]
- 14.Soley G, Hannon EE. Infants prefer the musical meter of their own culture: A cross-cultural comparison. Dev Psychol. 2010;46:286–292. doi: 10.1037/a0017555. [DOI] [PubMed] [Google Scholar]
- 15.Glenn SM, Cunningham CC, Joyce PF. A study of auditory preferences in nonhandicapped infants and infants with Down’s syndrome. Child Dev. 1981;52:1303–1307. [PubMed] [Google Scholar]
- 16.Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nat Neurosci. 2011;14:1189–1194. doi: 10.1038/nn.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Insanally MN, Köver H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. J Neurosci. 2009;29:5456–5462. doi: 10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin ST, et al. Mental health implications of music: Insight from neuroscientific and clinical studies. Harv Rev Psychiatry. 2011;19:34–46. doi: 10.3109/10673229.2011.549769. [DOI] [PubMed] [Google Scholar]
- 19.Levitin DJ, Tirovolas AK. Current advances in the cognitive neuroscience of music. Ann N Y Acad Sci. 2009;1156:211–231. doi: 10.1111/j.1749-6632.2009.04417.x. [DOI] [PubMed] [Google Scholar]
- 20.Krienen FM, Tu PC, Buckner RL. Clan mentality: Evidence that the medial prefrontal cortex responds to close others. J Neurosci. 2010;30:13906–13915. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jouhaneau J, Bagady A. Effect of early auditory stimulation on the choice of acoustical environment by adult Swiss albino mice (Mus musculus) J Comp Psychol. 1984;98:318–326. [PubMed] [Google Scholar]
- 22.Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- 23.Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: From molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Z, Koprivica V. The Nogo signaling pathway for regeneration block. Annu Rev Neurosci. 2004;27:341–368. doi: 10.1146/annurev.neuro.27.070203.144340. [DOI] [PubMed] [Google Scholar]
- 25.Pizzorusso T, et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 26.Silingardi D, Scali M, Belluomini G, Pizzorusso T. Epigenetic treatments of adult rats promote recovery from visual acuity deficits induced by long-term monocular deprivation. Eur J Neurosci. 2010;31:2185–2192. doi: 10.1111/j.1460-9568.2010.07261.x. [DOI] [PubMed] [Google Scholar]
- 27.Crawley JN. What’s Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. 2nd Ed. Hoboken, NJ: John Wiley & Sons; 2007. [Google Scholar]
- 28.Kash SF, Tecott LH, Hodge C, Baekkeskov S. Increased anxiety and altered responses to anxiolytics in mice deficient in the 65-kDa isoform of glutamic acid decarboxylase. Proc Natl Acad Sci USA. 1999;96:1698–1703. doi: 10.1073/pnas.96.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Putignano E, et al. Developmental downregulation of histone posttranslational modifications regulates visual cortical plasticity. Neuron. 2007;53:747–759. doi: 10.1016/j.neuron.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 30.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beccaloni AM. The medicine of music: A systematic approach for adoption into perianesthesia practice. J Perianesth Nurs. 2011;26:323–330. doi: 10.1016/j.jopan.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Han L, et al. Effects of music intervention on physiological stress response and anxiety level of mechanically ventilated patients in China: A randomised controlled trial. J Clin Nurs. 2010;19:978–987. doi: 10.1111/j.1365-2702.2009.02845.x. [DOI] [PubMed] [Google Scholar]
- 33.Kemper KJ, Danhauer SC. Music as therapy. South Med J. 2005;98:282–288. doi: 10.1097/01.SMJ.0000154773.11986.39. [DOI] [PubMed] [Google Scholar]
- 34.Nilsson U. The anxiety- and pain-reducing effects of music interventions: A systematic review. AORN J. 2008;87:780–807. doi: 10.1016/j.aorn.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Chikahisa S, et al. Exposure to music in the perinatal period enhances learning performance and alters BDNF/TrkB signaling in mice as adults. Behav Brain Res. 2006;169:312–319. doi: 10.1016/j.bbr.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Li WJ, et al. Anxiolytic effect of music exposure on BDNFMet/Met transgenic mice. Brain Res. 2010;1347:71–79. doi: 10.1016/j.brainres.2010.05.080. [DOI] [PubMed] [Google Scholar]
- 37.Jorm AF, et al. Effectiveness of complementary and self-help treatments for anxiety disorders. Med J Aust. 2004;181(7, Suppl):S29–S46. doi: 10.5694/j.1326-5377.2004.tb06352.x. [DOI] [PubMed] [Google Scholar]
- 38.Parslow R, et al. Effectiveness of complementary and self-help treatments for anxiety in children and adolescents. Med J Aust. 2008;188:355–359. doi: 10.5694/j.1326-5377.2008.tb01654.x. [DOI] [PubMed] [Google Scholar]
- 39.Heffner HE, Heffner RS. Hearing ranges of laboratory animals. J Am Assoc Lab Anim Sci. 2007;46:20–22. [PubMed] [Google Scholar]
- 40.Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klinge A, Klump GM. Frequency difference limens of pure tones and harmonics within complex stimuli in Mongolian gerbils and humans. J Acoust Soc Am. 2009;125:304–314. doi: 10.1121/1.3021315. [DOI] [PubMed] [Google Scholar]
- 42.Ruusuvirta T, Koivisto K, Wikgren J, Astikainen P. Processing of melodic contours in urethane-anaesthetized rats. Eur J Neurosci. 2007;26:701–703. doi: 10.1111/j.1460-9568.2007.05687.x. [DOI] [PubMed] [Google Scholar]
- 43.Kuhl PK, Miller JD. Speech perception by the chinchilla: Voiced-voiceless distinction in alveolar plosive consonants. Science. 1975;190:69–72. doi: 10.1126/science.1166301. [DOI] [PubMed] [Google Scholar]
- 44.Toro JM, Trobalon JB, Sebastián-Gallés N. Effects of backward speech and speaker variability in language discrimination by rats. J Exp Psychol Anim Behav Process. 2005;31:95–100. doi: 10.1037/0097-7403.31.1.95. [DOI] [PubMed] [Google Scholar]
- 45.Peretti PO, Kippschull H. Influence of five types of music on social behaviours of mice, Mus musculus. Indian J Behav. 1991;15:51–58. [Google Scholar]
- 46.Bates FC, Horvath T. Discrimination learning with rhythmic and nonrhythmic background music. Percept Mot Skills. 1971;33:1123–1126. [Google Scholar]
- 47.Poli M, Previde EP. Discrimination of musical stimuli by rats (Rattus norvegicus) Int J Comp Psychol. 1991;5:7–18. [Google Scholar]
- 48.Oswalt RM, Herrick S, Hale A. Preferences of rats given early temporal auditory patterns. Percept Mot Skills. 1973;36:907–910. doi: 10.2466/pms.1973.36.3.907. [DOI] [PubMed] [Google Scholar]
- 49.Kral A, Hartmann R, Tillein J, Heid S, Klinke R. Delayed maturation and sensitive periods in the auditory cortex. Audiol Neurootol. 2001;6:346–362. doi: 10.1159/000046845. [DOI] [PubMed] [Google Scholar]
- 50.Noreña AJ, Gourévitch B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nat Neurosci. 2006;9:932–939. doi: 10.1038/nn1720. [DOI] [PubMed] [Google Scholar]
- 51.Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nat Neurosci. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- 52.Silverman MS, Clopton BM. Plasticity of binaural interaction. I. Effect of early auditory deprivation. J Neurophysiol. 1977;40:1266–1274. doi: 10.1152/jn.1977.40.6.1266. [DOI] [PubMed] [Google Scholar]
- 53.Sanes DH, Bao S. Tuning up the developing auditory CNS. Curr Opin Neurobiol. 2009;19:188–199. doi: 10.1016/j.conb.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuhl PK, Miller JD. Speech perception by the chinchilla: Identification function for synthetic VOT stimuli. J Acoust Soc Am. 1978;63:905–917. doi: 10.1121/1.381770. [DOI] [PubMed] [Google Scholar]
- 55.Gold JI, Knudsen EI. Hearing impairment induces frequency-specific adjustments in auditory spatial tuning in the optic tectum of young owls. J Neurophysiol. 1999;82:2197–2209. doi: 10.1152/jn.1999.82.5.2197. [DOI] [PubMed] [Google Scholar]
- 56.Miller GL, Knudsen EI. Early visual experience shapes the representation of auditory space in the forebrain gaze fields of the barn owl. J Neurosci. 1999;19:2326–2336. doi: 10.1523/JNEUROSCI.19-06-02326.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goldman-Rakic PS. Architecture of the prefrontal cortex and the central executive. Ann N Y Acad Sci. 1995;769:71–83. doi: 10.1111/j.1749-6632.1995.tb38132.x. [DOI] [PubMed] [Google Scholar]
- 58.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 59.Barbas H, Zikopoulos B, Timbie C. Sensory pathways and emotional context for action in primate prefrontal cortex. Biol Psychiatry. 2011;69:1133–1139. doi: 10.1016/j.biopsych.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 60.Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport. 1999;10:2817–2821. doi: 10.1097/00001756-199909090-00022. [DOI] [PubMed] [Google Scholar]
- 61.Budel S, et al. Genetic variants of Nogo-66 receptor with possible association to schizophrenia block myelin inhibition of axon growth. J Neurosci. 2008;28:13161–13172. doi: 10.1523/JNEUROSCI.3828-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uhlhaas PJ, Singer W. The development of neural synchrony and large-scale cortical networks during adolescence: Relevance for the pathophysiology of schizophrenia and neurodevelopmental hypothesis. Schizophr Bull. 2011;37:514–523. doi: 10.1093/schbul/sbr034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zikopoulos B, Barbas H. Changes in prefrontal axons may disrupt the network in autism. J Neurosci. 2010;30:14595–14609. doi: 10.1523/JNEUROSCI.2257-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Testa-Silva G, et al. Hyperconnectivity and slow synapses during early development of medial prefrontal cortex in a mouse model for mental retardation and autism. Cereb Cortex. 2012;22:1333–1342. doi: 10.1093/cercor/bhr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leonardo ED, Hen R. Anxiety as a developmental disorder. Neuropsychopharmacology. 2008;33:134–140. doi: 10.1038/sj.npp.1301569. [DOI] [PubMed] [Google Scholar]
- 66.Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306:879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- 67.Sung HC, Chang AM, Lee WL. A preferred music listening intervention to reduce anxiety in older adults with dementia in nursing homes. J Clin Nurs. 2010;19:1056–1064. doi: 10.1111/j.1365-2702.2009.03016.x. [DOI] [PubMed] [Google Scholar]
- 68.Marzban M, et al. Effect of Mozart music on hippocampal content of BDNF in postnatal rats. Basic Clin Neurosci. 2011;2:21–26. [Google Scholar]
- 69.Riebel K, Smallegange IM, Terpstra NJ, Bolhuis JJ. Sexual equality in zebra finch song preference: Evidence for a dissociation between song recognition and production learning. Proc Biol Sci. 2002;269:729–733. doi: 10.1098/rspb.2001.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng B, et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci USA. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asada H, et al. Mice lacking the 65 kDa isoform of glutamic acid decarboxylase (GAD65) maintain normal levels of GAD67 and GABA in their brains but are susceptible to seizures. Biochem Biophys Res Commun. 1996;229:891–895. doi: 10.1006/bbrc.1996.1898. [DOI] [PubMed] [Google Scholar]
- 72.Barth AL, Gerkin RC, Dean KL. Alteration of neuronal firing properties after in vivo experience in a FosGFP transgenic mouse. J Neurosci. 2004;24:6466–6475. doi: 10.1523/JNEUROSCI.4737-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]