Abstract
Recent evidence suggests that sexual dimorphisms in the zebra finch song system and behavior arise due to factors intrinsic to the brain, rather than being solely organized by circulating steroid hormones. The present study examined expression of ten sex-chromosome genes in the song system of 25-day-old zebra finches in an attempt to further elucidate these factors. Increased expression in males was confirmed for nine of the genes by real-time qPCR using cDNA from individual whole telecephalons. In situ hybridization at the same age revealed specific, male-enhanced mRNA for three of the nine genes in one or more song control nuclei. These genes encode tubulin specific chaperone A, mitochondrial ribosomal protein S27, and a DNA repair protein XPACCH. Based on what is currently known about these proteins' functions and their localization to particular components of the song circuit, we hypothesize that they each may be involved in specific aspects of masculinization.
Keywords: sexual differentiation, sexual dimorphism, masculinization, songbird
INTRODUCTION
Sex differences in brain and behavior exist across a wide array of species. Those found in zebra finches are extraordinarily robust. Only adult male birds sing, learning songs from their fathers (Bottjer, 2002), and discrete brain regions that regulate aspects of this behavior are larger in males compared to females. Area X and the lateral magnocellular nucleus of the anterior nidopallium (lMAN) are components of the anterior forebrain pathway; a basal ganglia forebrain circuit largely devoted to song learning and plasticity (Bottjer et al., 1984; Scharff and Nottebohm, 1991; Williams and Mehta, 1999; Brainard and Doupe, 2000). Area X, while large in males, is not detectable in females (Nottebohm and Arnold, 1976). lMAN volume is sexually monomorphic, but nucleoli and neuron soma size are larger in males (reviewed in Nixdorf Bergweiler, 2001). HVC (proper name) and the robust nucleus of the acropallium (RA) are in the motor pathway controlling song production (Brenowitz et al., 1997). HVC and RA are about five times larger in volume in male than female zebra finches, and the projection from HVC to RA, which innervates the vocal organ (syrinx), is also substantially more robust in males (Bottjer et al., 1985; Konishi and Akutagawa, 1985; Arnold, 1997; Wade and Arnold, 2004).
The mechanisms regulating sexual differentiation of the zebra finch song circuit are unclear. While early data supported a role for estradiol in masculinizing structure and function (Arnold, 1997), new evidence implicates non-hormonal factors. Some of the most compelling information comes from a rare, naturally-occurring gynandromorphic zebra finch with lateralized male (ZZ) and female (ZW) genotype and phenotype. Data from this bird suggest that direct genetic effects mediate sexual differentiation of the neural song circuit, likely with an influence from diffusible factors such as hormones (Agate et al., 2003). Recently, microarray screens have identified several Z-chromosome genes that exhibit increased expression in particular song control nuclei in juvenile male compared to females zebra finches, including those encoding secretory carrier membrane protein 1, sorting nexin 2, and ribosomal proteins L17 and L37 (Tang and Wade, 2006; Tang et al., 2007; Tomaszycki et al., 2009; Wu et al., 2010).
The present study investigated expression of ten additional genes from our most recent screen (Tomaszycki et al., 2009) in an attempt to supplement our understanding of the genetic mechanisms underlying sexual differentiation of the song circuit. Increased whole telencephalic expression in males originally detected in this cDNA microarray study was first confirmed in a group of new 25-day-old (D25) birds by real-time qPCR. Those genes that consistently showed male-biased expression were then evaluated by in situ hybridization in males and females of the same age for localization to song control nuclei. This developmental stage is when males begin to memorize songs from their fathers, and represents a period of rapid morphological differentiation of the song circuit (Nordeen and Nordeen, 1997; Doupe et al., 2004; Wade and Arnold, 2004), allowing us to maximize our chances of discovering relevant mechanisms.
METHODS
Animals and Tissue Collection
Male and female zebra finches were reared in colony aviaries containing multiple males and females with their offspring. Animals were exposed to a 12:12 light:dark cycle, and provided ad libitum access to drinking water, seed (Kaytee Finch Feed; Chilton WI), gravel and cuttlebone. Their diets were also supplemented weekly with spinach, and egg-bread mixture, and oranges.
For both real-time qPCR and in situ hybridization, birds were rapidly decapitated at D25. For qPCR, whole telencephalons were collected and immediately frozen on dry ice. For in situ hybridization, whole brains were rapidly dissected and frozen in cold methyl-butane. In both cases, samples were stored at –80°C until processing. Gonadal sex of each animal was determined using a dissecting microscope at the time of euthanasia. All procedures were all approved by The Institutional Animal Care and Use Committee (IACUC) of Michigan State University.
Real-time qPCR
RNA from six male and six female telencephalons was extracted with Trizol (Invitrogen, Carlsbad, CA), and DNase treated on RNeasy columns (Qiagen, Valencia, CA) per manufacturer's instructions. RNA was ethanol-precipitated to increase the concentration, which was determined by spectrophotometry. RNA integrity was confirmed on 1% denaturing agarose gels. cDNA was made from the telencephalic RNA samples using the High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA) per manufacturer instructions. Primers were designed using Primer Express 2.0 (Applied Biosystems, Foster, CA; Table 1), and each pair demonstrated at least 99% efficiency in amplification using a standard curve of multiple known cDNA concentrations.
Table 1.
Primers Used for qPCR (5' to 3')
| GenBank Accession Number for cDNA | Forward | Reverse |
|---|---|---|
| CK310968* | CAAAGTTCAAACCCCAGGACAT | CCTTACCCATTAGTTCCCCTTAGC |
| CK302334* | AGGTGTGGCTGAGCTAGGGAA | TCCTAGTTCTCTCAGCTCCGCT |
| CK304617* | GCAAGTGATGGAGAATGTGGC | TTACAGATTTTGATGTCCCCTGAC |
| CK311563 | TGGACAAGTGCATCGGTTCA | TCTTTGTCGCTTTTCATCACGA |
| CK303438 | CATATCACAGCATCTCTATCAAGCAA | ATTTCAGCAACTTATTCAGACCCAT |
| CK305515 | GCAGGTGAATAGCATAAGCAAAGA | AAGCCATGTGATCATATAGCCAAA |
| CK316377 | AAAGCTGGGAAGTGGGAGGT | GTTTCTCCTTGTGCACGAACC |
| DV945585 | GAAGCCAAGCAGCCCAACT | GAGCTGCACTCTGACGCATTT |
| DV958715 | TGGATGATGCCCAGGATGTT | CGTTGGCATGCACTTCACA |
| DV957339 | TTGCCAAAAATAGCACACCCT | GCATAGTTCACACATCACTGGGAA |
| AF255390 (GAPDH) | AAACCAGCCAAGTACGATGACAT | CCATCAGCAGCAGCCTTCA |
Used for later In Situ hybridization studies
cDNA for all twelve animals, along with no-template controls, were run in triplicate with each of the ten primer sets. Each qPCR reaction used 100nM of each primer and 25ng of RNA (converted to cDNA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was run in parallel with each reaction and analyzed as a control (as in Tomaszycki et al., 2009), as its expression is not sexually dimorphic. Power SYBR green PCR Master Mix (Applied Biosystems, Foster City, CA) was used according to manufacturer's instructions. All reactions were run on an ABI Prism PE 7000 (Applied Biosystems).
The triplicate threshold cycles obtained from each animal were averaged, and sex differences were analyzed using one-tailed t-tests with Bonferroni corrections. Fold-differences between the sexes were calculated using ΔΔCT (Livak and Schmittgen, 2001), normalized using the GAPDH results.
In Situ Hybridization
Brains were coronally sectioned at 20 μm and thaw-mounted in six series onto SuperFrost Plus slides (Fisher Scientific, Hampton, NH). Tissue was stored at −80°C with dessicant until processing. Each of the ten genes of interest was initially screened on a small number of D25 males and females (n=1–3 per sex). This procedure resulted in identification of six genes with specific expression in song control nuclei such that the labeled regions stood out from the surrounding tissue (see below). Three of these genes that appeared to exhibit more labeling in males than in females as determined by visual inspection of the slides (Tubulin specific chaperone protein A [TBCA], mitochondrial ribosomal protein S27 [MRPS27], and a DNA excision repair protein [XPACCH]) were selected for more detailed investigation using larger sample sizes. For XPACCH, two adjacent sets of tissue sections (one for antisense and one for sense probes) from six individuals of each sex were used. However, due to tissue damage, one male was omitted for TBCA and one female was eliminated for MRPS27; thus each of these groups had five, rather than six, individuals. Within each gene, all of the tissue was run simultaneously.
Probes for in situ hybridization were produced from glycerol stocks (Replogle et al., 2008) of pBluescript II SK (+), and plasmid DNA was extracted using Qiagen Maxi Prep kit (Valencia, CA), and linearized with XhoI (T3) and NotI (T7). All clones were re-sequenced prior to in situ hybridization from both ends on an ABI Prism 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA). Cold transcription reactions confirmed each product's quality and correct size. T3 (anti-sense) and T7 (sense) cRNA probes were generated using the MAXIscript In Vitro Transcription Kit with T3/T7 RNA polymerases (Ambion, Austin, TX), and were labeled with 33P-UTP (Perkin Elmer, Waltham, MA).
The slides were warmed to room temperature for 15 minutes, rinsed in phosphate buffered saline (PBS), fixed in 4% paraformaldehyde for 15 minutes, and rinsed in PBS for 3 minutes. They were then incubated in 0.25% acetic anhydride in 0.1 M triethanolamine for 10 minutes, rinsed in PBS, dehydrated in a series of ethanols, and air-dried. The tissue was pre-hybridized in a solution containing 1× hybridization buffer (2.5M NaCl, 1M Tris, 0.5M EDTA, 1M DTT, 1× Denhardts, 1mg/ml yeast tRNA, and 50% dextran sulfate) and 50% formamide at 55°C for 2 hours. Slides were then hybridized overnight at 55°C with a solution containing 1× hybridization buffer, 10% dextran-sulfate, 50% formamide, and 5 × 106 cpm 33P-UTP-labeled RNA probe (sense or antisense). Hybridization buffer was removed with sequential washes in 4× SSC at room temperature, 2× SSC at 55°C for 10 minutes, and 2× SSC at room temperature for 30 minutes. Slides were incubated in 2× SSC with RNase A (20μg/ml) at 37°C for 30 minutes, then rinsed in 2× SSC at 37°C for 15 minutes and 0.1× SSC at 60°C for 10 seconds. The tissue was dehydrated in ethanol baths of increasing concentration with 0.3M ammonium acetate. Slides were air-dried and three animals per sex from sense and antisense probes were exposed to phosphor-imaging screens (Bio-Rad Kodak #170-7841, Hercules, CA) for 16 hours and scanned with a Molecular Imager FX (Bio-rad Laboratories, Hercules, CA) to confirm the expected signal in song nuclei. All slides were then dipped in NTB emulsion (Eastman Kodak, Rochester, NY) and stored in the dark at 4°C for 4 weeks. They were then developed using Kodak Professional D-19 Developer and Fixer (Eastman Kodak, Rochester, NY), and counter-stained with cresyl violet.
For all three genes, labeling appeared consistent across the two phosphor-imager screens and the emulsion-coated slides. However, for XPACCH, specific labeling was apparent in two regions on the slides that were not obvious on the imaging screens, so it was quantified there as well. As all four primary telencephalic brain regions (HVC, RA, lMAN and Area X) were analyzed for this gene, an additional region, A, was quantified as a control. As in Tang and Wade (Tang and Wade, 2006), “A” represents a portion of the arcopallium just lateral to RA that is not morphologically distinct or sexually dimorphic.
Each brain region was first located using brightfield microscopy (landmarks identified using the nissl stain), and then captured using Scion Image (NIH Image) in darkfield. The “density slice” function was used to quantify labeling on both sides of the brain in all sections containing each song nucleus of interest. The area covered by silver grains within a 264 μm × 198 μm box (placed in the center of each area) was calculated. For each anti-sense section, an adjacent section exposed to the sense probe was quantified. The sense values were then subtracted from the anti-sense values, and the resulting densities were averaged across sections within individuals.
The effect of sex on the percent area covered by silver grains within each brain region was analyzed separately for each of the genes by two-tailed t-tests. Bonferroni corrections were used to adjust α-levels accordingly. In one case in which variances were not homogenous, due to a bimodal distribution in males, a Mann-Whitney U test was also used (see below).
RESULTS
qPCR
Whole telencephalic expression was significantly increased in males compared to females for nine of the ten genes (Table 2; α =0.010). GAPDH expression did not differ between the sexes in any of the ten analyses (all t < 1.99, p > 0.075).
Table 2.
Male-Biased Gene Expression in D25 Zebra Finches
| GenBank Accession Number | Identification* | Male/Female Ratio using qPCR | qPCR t-value | qPCR p-value |
|---|---|---|---|---|
| CK310968 | Tubulin specific chaperone A | 2.16 | 8.39 | <0.001 |
| CK302334 | DNA repair protein XPACCH | 4.71 | 3.61 | <0.003 |
| CK304617 | Mitochondrial ribosomal protein S27 | 2.54 | 3.22 | <0.005 |
| CK311563 | Lsm5 protein | 2.34 | 4.52 | <0.001 |
| CK303438 | Zinc finger A20 domain | 2.24 | 6.40 | <0.001 |
| CK305515 | Autophagy protein | 2.08 | 9.37 | <0.001 |
| CK316377 | Homologue to TGF beta-inducible nuclear protein | 1.94 | 7.02 | <0.001 |
| DV945585 | Ribosomal protein S23 | 1.73 | 4.49 | <0.001 |
| DV958715 | Thioredoxin | 1.73 | 9.97 | <0.001 |
| DV957339 | Rasp21 GTPase activating protein | 1.1 | 1.01 | 0.169 |
From NCBI blast of nucleotide sequence
In Situ Hybridization
For each of the three genes of interest, labeling was generally increased in males compared to females (see phosphor images in figures), consistent with what one might expect for a Z-gene, as dosage compensation in birds is limited. However, labeling within song control nuclei was selective and specific.
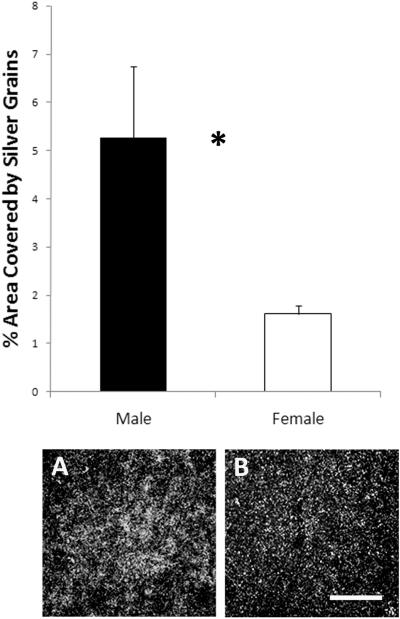
TBCA (Genbank accession number CK310968; 99% identity between our 715bp sequence and the zebra finch sequence from NCBI) expression was detected only in lMAN on the phosphor-imaging screen. Qualitative analysis of the slides for all males and females provided the same result, so only this region was quantified. The density of labeling in this area was significantly higher in males than in females (t9 = 2.71, p = 0.024; Figure 1).
Figure 1.
Labeling for the gene encoding TBCA was detected only in lMAN and was increased in males compared to females. The top panel represents means ± SEs; *p = 0.024. The photographs are darkfield images from near the center of lMAN in a (A) male and (B) female. Images are approximately twice the size of the area quantified. Scale bar for both darkfield images = 100μm.
XPACCH (CK302334; 529bp, 100% identity) was detected in Area X and RA on the phosphor-imaging screen (Figure 2). Inspection of the tissue sections revealed a similar pattern of specific labeling that defined individual regions with substantially increased expression compared to surrounding tissue in lMAN and HVC of males. Thus, these areas were also analyzed. In each of these song nuclei, labeling was greater in males compared to females (lMAN: t10 = 3.54, p = 0.005; Area X: t10 = 4.20, p = 0.002; HVC: t8 = 4.46, p = 0.002; RA: t10 = 3.80, p = 0.003; Figure 3). No effect of sex was detected in the control region, A (t10 = 2.15, p = 0.057).
Figure 2.
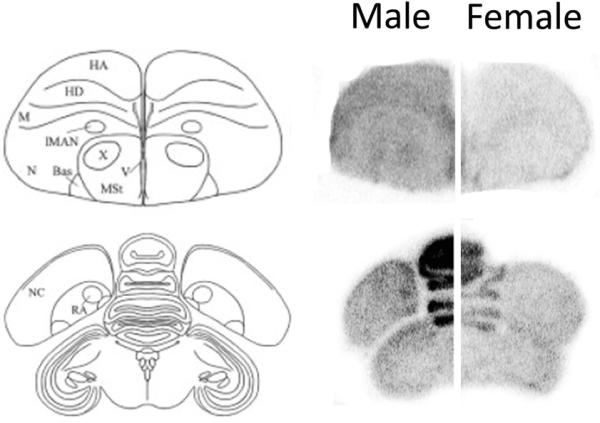
The phosphor-imaging screen detected specific, male-enhanced labeling for the gene encoding XPACCH in Area X and RA. The signal was greater overall for male compared to female brains, which can clearly be seen in the large, densely packed Purkinje cells of the cerebellum (midline layered structure in bottom images). However, only in males did it define the two song control regions.
Figure 3.
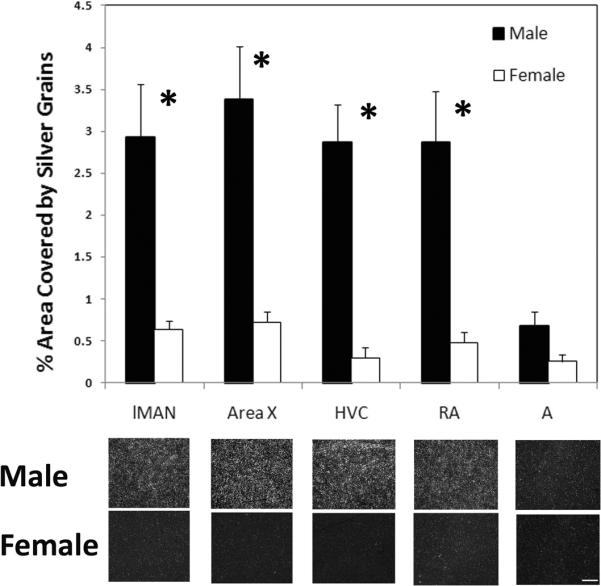
Analysis of slides indicated that the expression of the gene encoding XPACCH was specifically detected in four song control nuclei: lMAN, Area X, HVC, and RA. The last region, “A,“ represents a portion of the arcopallium just lateral to RA. A significant sex difference existed in each of the song control regions, but not in A (graph shows means ± SEs, *p≤ 0.005). Below the graphs are dark-field images depicting the center of each brain region from representative males and females. Scale bar = 100μm for all images.
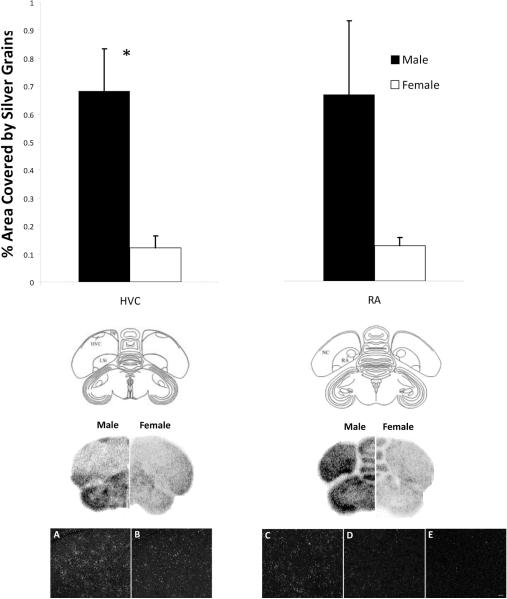
MRPS27 (CK304617; 794bp, 99% identity) was detected in HVC and RA on the phosphor-imaging screen and on the slides. Using parametric analyses, a significant effect of sex was present in HVC (t9 = 3.31, p = 0.009; Figure 4), but not RA (t9 = 1.83, p = 0.100). However, a bimodal distribution existed in the RA of males, such that two birds had silver grains covering over 1% of the analyzed area, while four birds had silver grains covering less than 0.4%. A Mann-Whitney U test detected a trend for an increase in males compared to females (U= 4.00, p= 0.045).
Figure 4.
The phosphor-imaging screen (middle panels) revealed specific, male-biased labeling of the gene encoding MRPS27 in HVC and RA. Silver grain analysis (t-tests) detected a significant effect of sex only in HVC. However, a bimodal distribution existed in the RA of males. Photographs (bottom panels) show silver grain labeling from the center of HVC (left) and RA (right). Values on the graphs (top panel) indicate means ± SEs; *p= 0.009. A and B are darkfield images depicting labeling in a male and a female, respectively. C, D and E represent the bimodal distribution of silver grains in RA of two males (C, D) and a female (E).
DISCUSSION
The present study identified selective and specific male-biased expression of three novel genes in the song control system of juvenile zebra finches: TBCA, XPACCH, and MRPS27. These genes map to the Z-chromosome, which is present in two copies in male zebra finches, but only one in females (Ellegren et al., 2007; Itoh et al., 2007). As indicated above, dosage compensation in birds is far more restricted than in mammals, so Z-chromosome genes are likely to exhibit increased expression in males compared to females. However, a variety of other direct or indirect mechanisms might cause increased expression in males compared to females. In fact, the selective nature of this pattern – the fact that sexually dimorphic TBCA, XPACCH and MRPS27 mRNAs were detected in particular song control regions and not throughout the brain – suggests that something in addition to simply their chromosomal location modulates the expression of these genes. It also supports the idea that they could contribute to particular aspects of differentiation of morphology and/or function of the song circuit.
TBCA
Enhanced expression of TBCA in D25 males was limited to lMAN. Its morphology, unlike other song nuclei, is not particularly sexually dimorphic (reviewed in Nixdorf Bergweiler, 2001). Differences do exist, however, in its projections. Both males and females undergo cell death in this area around 20 days after hatching (Bottjer and Sengelaub, 1989). Despite this loss, the number of RA projecting neurons remains intact in males while a proportion are lost in females (Nordeen et al., 1992). Retention of these projections may support cell survival in RA (Johnson and Bottjer, 1994). TBCA could facilitate maintenance of these projections in males compare to females. The gene is highly conserved among vertebrates and is essential to proper β-tubulin folding (Melki et al., 1996). Together with α-tubulin, β-tubulin forms the basic structural unit for microtubules, which are required for spindle formation during mitosis and meiosis, organelle positioning, and axonal transport (Dutcher, 2001).
Axonal transport of neurotrophins, in particular, has been implicated in the development of the songbird telencephalon (Johnson et al., 1997). Lesions of lMAN remove pre-synaptic input to RA, and cause extensive RA neuron death in juvenile male birds. Infusions of brain-derived neurotrophic factor (BDNF) directly into RA, however, suppress this process in RA. Further, lMAN neurons have the ability to anterogradely transport BDNF into RA, where TrkB (high-affinity receptor for BDNF) is expressed (Johnson et al., 1997). Thus, enhanced TBCA in the lMAN of males could contribute to this sexual differentiation by up-regulating β-tubulin and microtubule formation to increase axonal transport of neurotrophins into RA from lMAN.
This protein may also influence functional development. While females appear to learn their fathers' songs in a manner similar to males (Miller, 1979), they never produce it. Thus, increased synthesis of TBCA in lMAN might also facilitate the ability of males to learn to produce quality song. The present study documents that this specific, sexually dimorphic mRNA expression exists at D25, when birds are forming templates of their tutors' songs. It will now be important to determine both whether it extends into the later period of sensorimotor integration, as well as whether the protein is also expressed in lMAN's target, RA.
XPACCH
XPACCH was detected in Area X, lMAN, HVC, and RA, and expression was increased in males in each region. While broad, these sex differences in expression are specific, as we did not detect dimorphic labeling in a control portion of the arcopallium, A. XPACCH mediates the assembly of a pre-incision complex during DNA repair, and is the gene compromised in the recessive disorder xeroderma pigmentosum, in which the ability to repair DNA damage by ultraviolet irradiation is inhibited (Cleaver, 1969). DNA repair pathways correct mutagenic and toxic DNA damage (Nyberg et al., 2002), and accumulated DNA damage in the genome may lead to a loss in the fidelity of information transferred from DNA to protein, resulting in transcription of defective proteins that eventually cause cell death (Taddeii et al., 1997; Lu et al., 2004). While specific mechanisms associated with song system differentiation are at this point unclear, it is possible that XPACCH facilitates cell survival in males during the differentiation process. It is also possible that the increased expression in males is a result of their enhanced structure, and simply reflects an increased need for support of basic cellular processes. Further investigation of the time-course of expression in males and females will be an important step toward elucidating the function of this gene in the song circuit.
MRPS27
MRPS27 was specifically detected in two nuclei of the motor pathway. Expression was clearly enhanced in males in HVC, and a similar trend was detected in RA. It was largely driven by the high expression levels in two males. However, data from only one male was within the range of females. This result and that of the non-parametric Mann-Whitney U test, which analyzes ranks rather than absolute values, suggest that an increase in males might in fact exist. We have no obvious explanation for the variability among the males. They were raised under identical conditions, and their tissues were processed together in the in situ hybridization analysis of this gene. Because alternate sections from the brains of these birds did not exhibit a parallel pattern in analysis of TBCA or XPACCH, a biological rather than technical explanation seems likely. More work will need to be done to determine contributing factors.
MRPS27 is expressed in the mitochondrial ribosome, and thus has potential influences on the translation of proteins in cells of HVC and RA. This idea and subsequent identification of key proteins need to be evaluated. However, it is plausible as three other ribosomal proteins have been associated with song system development. RPL17and RPL37, which like MRPS27 are on the Z chromosome, as well as RPL7 (Chromosome 2), exhibit specific male-enhanced and selective expression in song nuclei (Tang and Wade, 2006; Duncan and Carruth, 2007; Tang and Wade, 2010). The challenge now is to determine the key proteins that may be regulated by these genes which can broadly influence translation.
Concluding Ideas
The present study examined sexually dimorphic expression of ten genes that were originally identified from a microarray screen. Three were selected from a preliminary in situ hybridization screen for further investigation, based on their location in one or more song nuclei and their apparent sex differences in expression. Each was localized to the song system of D25 zebra finches, and exhibited male-enhanced mRNA expression in one or more song control nuclei. Thus, their protein products may facilitate masculinization. Influences at other critical developmental stages are also possible, and work is currently underway to evaluate the time course of the expression of TBCA, XPACCH, and MRPS27.
Acknowledgements
We thank Camilla Peabody and Yu Ping Tang for technical assistance. This work was supported by the NIH, R01- MH55488.
REFERENCES
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci USA. 2003;100:4873. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. Sexual differentiation of the zebra finch song system: positive evidence, negative evidence, null hypotheses, and a paradigm shift. J Neurobiol. 1997;33:572–584. [PubMed] [Google Scholar]
- Bottjer S. Neural strategies for learning during sensitive periods of development. J Comp Phys. 2002;188:917–928. doi: 10.1007/s00359-002-0356-0. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Glaessner SL, Arnold AP. Ontogeny of brain nuclei controlling song learning and behavior in zebra finches. J Neurosci. 1985;5:1556. doi: 10.1523/JNEUROSCI.05-06-01556.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Sengelaub DR. Cell death during development of a forebrain nucleus involved with vocal learning in zebra finches. J Neurobiol. 1989;20:609–618. doi: 10.1002/neu.480200702. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal gangliañforebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Margoliash D, Nordeen KW. An introduction to birdsong and the avian song system. J Neurobiol. 1997;33:495–500. [PubMed] [Google Scholar]
- Cleaver JE. Xeroderma pigmentosum: a human disease in which an initial stage of DNA repair is defective. Proc Natl Acad Sci USA. 1969;63:428. doi: 10.1073/pnas.63.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Solis MM, Kimpo R, Boettiger CA. Cellular, circuit, and synaptic mechanisms in song learning. Ann NY Acad Sci. 2004;1016:495–523. doi: 10.1196/annals.1298.035. [DOI] [PubMed] [Google Scholar]
- Duncan KA, Carruth LL. The sexually dimorphic expression of L7/SPA, an estrogen receptor coactivator, in zebra finch telencephalon. Dev Neurobiol. 2007;67:1852–1866. doi: 10.1002/dneu.20539. [DOI] [PubMed] [Google Scholar]
- Dutcher SK. The tubulin fraternity: alpha to eta. Curr Opin Cell Biol. 2001;13:49–54. doi: 10.1016/s0955-0674(00)00173-3. [DOI] [PubMed] [Google Scholar]
- Ellegren H, Hultin-Rosenberg L, Brunstr^m B, Dencker L, Kultima K, Scholz B. Faced with inequality: chicken do not have a general dosage compensation of sex-linked genes. BMC Biol. 2007;5:40. doi: 10.1186/1741-7007-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh Y, Melamed E, Yang X, Kampf K, Wang S, Yehya N, Van Nas A, Replogle K, Band MR, Clayton DF. Dosage compensation is less effective in birds than in mammals. J Biol. 2007;6:2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson F, Bottjer SW. Afferent influences on cell death and birth during development of a cortical nucleus necessary for learned vocal behavior in zebra finches. Devel. 1994;120:13–13. doi: 10.1242/dev.120.1.13. [DOI] [PubMed] [Google Scholar]
- Johnson F, Hohmann SE, DiStefano PS, Bottjer SW. Neurotrophins suppress apoptosis induced by deafferentation of an avian motor-cortical region. J Neurosci. 1997;17:2101. doi: 10.1523/JNEUROSCI.17-06-02101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M, Akutagawa E. Neuronal growth, atrophy and death in a sexually dimorphic song nucleus in the zebra finch brain. Nature. 1985;315:145–147. doi: 10.1038/315145a0. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Melki R, Rommelaere H, Leguy R, Vandekerckhove J, Ampes C. Cofactor A Is a Molecular Chaperone Required for β-Tubulin Folding: Functional and Structural Characterization. Biochem. 1996;35:10422–10435. doi: 10.1021/bi960788r. [DOI] [PubMed] [Google Scholar]
- Miller DB. Long-term recognition of father's song by female zebra finches. Nature. 1979;280:389–391. [Google Scholar]
- Nixdorf Bergweiler BE. Lateral magnocellular nucleus of the anterior neostriatum (LMAN) in the zebra finch: neuronal connectivity and the emergence of sex differences in cell morphology. Micr Res Tech. 2001;54:335–353. doi: 10.1002/jemt.1147. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Grace A, Burek MJ, Nordeen KW. Sex-dependent loss of projection neurons involved in avian song learning. J Neurobiol. 1992;23:671–679. doi: 10.1002/neu.480230606. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Anatomical and synaptic substrates for avian song learning. J Neurobiol. 1997;33:532–548. doi: 10.1002/(sici)1097-4695(19971105)33:5<532::aid-neu4>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Nyberg KA, Michelson RJ, Putnam CW, Weinert TA. Toward maintaining the genome: DNA damage and replication checkpoints. Genetics. 2002;36 doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- Replogle K, Arnold AP, Ball GF, Band M, Bensch S, Brenowitz EA, Dong S, Drnevich J, Ferris M, George JM. The Songbird Neurogenomics(SoNG) Initiative: Community-based tools and strategies for study of brain gene function and evolution. BMC genomics. 2008;9:131. doi: 10.1186/1471-2164-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddeii F, Vulic M, Radman M, Matic I. Genetic variability and adaptation to stress. Env Stress Adapt Evol. 1997:271. doi: 10.1007/978-3-0348-8882-0_15. [DOI] [PubMed] [Google Scholar]
- Tang YP, Peabody C, Tomaszycki ML, Wade J. Sexually dimorphic SCAMP1 expression in the forebrain motor pathway for song production of juvenile zebra finches. Dev Neurobiol. 2007;67:474–482. doi: 10.1002/dneu.20354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Wade J. Sexually dimorphic expression of the genes encoding ribosomal proteins L17 and L37 in the song control nuclei of juvenile zebra finches. Brain Res. 2006;1126:102–108. doi: 10.1016/j.brainres.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YP, Wade J. Sex-and Age-Related Differences in Ribosomal Proteins L17 and L37, as well as Androgen Receptor Protein, in the Song Control System of Zebra Finches. Neurosci. 2010 doi: 10.1016/j.neuroscience.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaszycki ML, Peabody C, Replogle K, Clayton DF, Tempelman RJ, Wade J. Sexual differentiation of the zebra finch song system: potential roles for sex chromosome genes. BMC Neurosci. 2009;10:24. doi: 10.1186/1471-2202-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J, Arnold AP. Sexual Differentiation of the Zebra Finch Song System. Ann NY Acad Sci. 2004;1016:540–559. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol. 1999;39:14–28. [PubMed] [Google Scholar]
- Wu D, Tang Y, Wade J. Co-localization of Sorting Nexin 2 and androgen receptor in the song system of juvenile zebra finches. Brain Res. 2010;1343:104–111. doi: 10.1016/j.brainres.2010.04.084. [DOI] [PMC free article] [PubMed] [Google Scholar]