Abstract
Foot ulcers are serious complications of Diabetes Mellitus (DM) and are known to be resistant to conventional treatment. They may herald severe complications if not treated wisely. Electromagnetic radiations in the form of photons are delivered to the ulcers in laser form to stimulate healing. This study was conducted to evaluate the efficacy of Low Level Laser Therapy (LLLT) in diabetic ulcer healing dynamics. To determine mean percentage reduction of wound area in study and control groups. Settings: KLES Dr. Prabhakar Kore Hospital and Medical Research Centre, Belgaum. Study Design: Randomized-Control Study. Methods: A total of 68 patients with Type 2 DM having Meggitt-Wagner Grade I foot ulcers of atleast more than 4 weeks duration, less than 6 × 6 cm2 with negative culture were studied. Patients were randomized into two groups of 34 each. Patients in study group received LLLT with conventional therapy and those in control group were treated with conventional therapy alone. Healing or percentage reduction in ulcer area over a period of 15 days after commencement of treatment was recorded. Statistical Analysis: Unpaired Student T Test and Mann Whitney U test. Mean age of the patients was 50.94 years in control group and 54.35 years in study group (p = 0.065). There was no significant difference between control and study group with respect to mean FBS and HbA1c levels (p > 0.05), suggesting no biochemical differences between two groups. Initial ulcer area was 2608.03 mm2 in study group and 2747.17 mm2 in control group (p = 0.361). Final ulcer area was 1564.79 mm2 in study group and 2424.75 mm2 in control group (p = 0.361). Percentage ulcer area reduction was 40.24 ± 6.30 mm2 in study group and 11.87 ± 4.28 mm2 in control group (p < 0.001, Z = 7.08). Low Level Laser Therapy is beneficial as an adjunct to conventional therapy in the treatment of diabetic foot ulcers (DFU).
Keywords: Adjuvant therapies, Diabetic foot ulcers, Low Level Laser Therapy (LLLT), Wound healing
Introduction
The number of cases of diabetes mellitus (DM) worldwide is estimated to be around 150 million. This is predicted to double by 2025 with the greatest number of cases in China and India [1, 2]. Diabetic foot ulcer (DFU) is a serious complication of DM and is the single most important risk factor for lower limb amputations. More than 60% of all non-traumatic lower limb amputations are due to DFU complications [3]. Risk factors for DFUs include males, DM of more than 10 years’ duration, peripheral neuropathy, abnormal foot structure, peripheral arterial disease, smoking, previous history of ulcers or amputations, and poor glycaemic control. About 15% of patients with DM are likely to develop foot ulcers during their lifetime and about 6–40% of them may require an amputation [4].
Although the fundamental pathophysiological factors leading to DFUs remain incompletely understood, the triad of neuropathy, ischaemia, and infections is commonly considered the most important [5]. These ulcers show decrease in both angiogenic response and deficient growth factors resulting in delayed healing [6]. Non-healing DFUs are resistant to conventional treatment [7]. Several adjuvant therapies which have been tried to stimulate healing process are ultrasound, laser therapy, and other forms of photobiomodulation, electrical stimulation, hyperbaric oxygen, and vacuum-assisted closure [8, 9].
Low-level laser therapy (LLLT) also called low-intensity laser therapy (LILT) or low-energy photon therapy (LEPT) has received clearance from the United States Food and Drug Administration. The clinical efficacy of LLLT in wound healing has been reported [10]. It has been found to significantly decrease the time of wound healing [11, 12].
We conducted a study to assess the efficacy of LLLT in Indian patients with chronic DFUs [13].
Materials and Methods
This study was conducted over a period of 2 years from February 2008 to February 2010 at a tertiary level teaching hospital after obtaining ethical clearance from Institutional Ethics Committee. Type 2 DM patients with Meggitt-Wagner grade I DFUs of at least 4 weeks’ duration were included. Those with clinical signs of ischaemia and ankle brachial pressure index (ABI) less than 0.9 were excluded from the study. Sample size was 68. Patients were randomised into two groups of 34 each on the basis of computer-generated numbers. The nature of therapy to be given was topically explained to the patients and written informed consent was obtained from them before enrolment.
All patients were admitted to the surgical ward and were subjected to detailed evaluation. A complete haemogram and renal and liver function tests were carried out in all patients. Patients with fasting blood sugar (FBS) levels measured on two occasions 24 h apart between 90 and 200 mg/dL with glycosylated haemoglobin (HbA1c) levels between 6% and 9% were included. Ulcer area was calculated by obtaining the impression of ulcer floor on a sheet of cellophane paper and then transferring the imprint onto a graph paper. The ulcer size was measured on day 0 and day 15. Patients with evidence of slough were subjected to repeated surgical debridement before starting the treatment. Objective assessment of vascularity was done by careful palpation of peripheral pulses and calculation of ABI. Colour Doppler imaging of the arterial circulation of lower limbs was performed in patients with feeble or absent pulsations. Presence of osteomyelitis was determined with the help of plain radiographs and they were excluded from the study.
Systemic antibiotics were administered based on culture sensitivity reports. Insulin/oral hypoglycaemic agents (OHA) as advised by the physician/endocrinologist were used to maintain a good glycaemic control. Once adequate glycaemic and infection control had been achieved, LLLT was commenced.
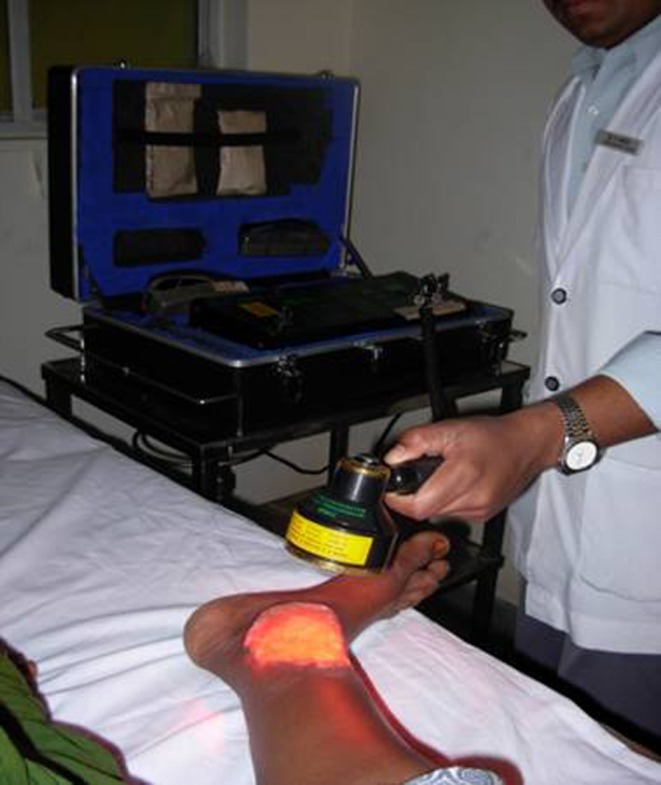
Patients in the study group received treatment with LLLT along with conventional or standard therapy, and those in control group received only conventional treatment in the form of daily wet saline or betadine dressings, antibiotic treatment, contact cast immobilization and slough excision as and when required. An LLLT device with a multidiode cluster probe (Thor International Ltd) (Fig. 1) was used in the study [8]. Figure 2 shows an LLLT cluster probe being used to treat foot ulcers. On the basis of the ulcer size, the duration of exposure was calculated to deliver 2–4 J/cm2 at 60 mW, 5 kHz, daily for 15 days. The ulcer floor and edge were irradiated. The ulcer was then covered with conventional moist dressing.
Fig. 1.
An LLLT device with a multidiode cluster probe (Thor International Ltd) used in the study
Fig. 2.

An LLLT cluster probe being used to treat foot ulcers
Pressure off-loading was carried out in patients with plantar ulcers. Healing or per cent reduction in the size of the ulcer over a period of 15 days after commencement of LLLT was recorded as the end point of the study. Simultaneously, these patients were also educated about various aspects of DM including dietary restrictions, exercise, and foot care in order to prevent recurrence. Statistical analysis of data was carried out using standard ‘t test’, and p value was calculated. Both the patients and administrators wore laser safety goggles to prevent damage to their eyes.
Results
A total of 68 patients were included in the study. Male to female ratio was 3:1. Mean age of the patients was 50.94 years in the control group and 54.35 years in the study group. No significant difference was found between two groups in the demographic characteristics and risk factors as summarized in Tables 1 and 2.
Table 1.
Demographic characteristics
| Characteristic | Control group (n = 34) | Study group (n = 34) |
|---|---|---|
| Male–female ratio | 2:1 | 2:1 |
| Mean age (years) | 50.94 ± 8.11 | 54.35 ± 6.84 |
| Occupation | ||
| Farmer | 15 (44%) | 16 (47%) |
| Housewife | 13 (38%) | 12 (35%) |
| Employed | 6 (18%) | 6 (18%) |
| Onset | ||
| Insidious/spontaneous | 11(32%) | 11 (32%) |
| Traumatic | 23 (68%) | 23 (68%) |
| Duration of ulcer at presentation | 4 weeks | 5 weeks |
Table 2.
Risk factors for diabetic foot ulcers
| Risk factors | Control group (n = 34) | Study group (n = 34) |
|---|---|---|
| Male sex | 21 (61.76%) | 22 (64.47%) |
| DM duration >10 years | 09 (26.47%) | 07 (20.58%) |
| Peripheral neuropathy | 06 (17.64%) | 09 (26.47%) |
| Smoking | 04 (11.76%) | 06 (17.64%) |
The mean FBS levels among controls were 129.80 ± 3.42 mg/dL (range 90–172 mg/dL) and 120.50 ± 4.51 (range 90–187 mg/dL) in the study group (p = 0.105). The mean HbA1c levels in the control group were 7.39 ± 0.05 (range 6.9–8%) and 7.42 ± 0.05 (range 7–8%) in the study group (p = 0.686), suggesting no biochemical differences between two groups.
Median duration of ulcer at the time of enrolment in the study was 4 weeks in control and 5 weeks in study groups. Median duration of oral hypoglycaemic agents intake was 10 years in the control group and 5 years in the study group (p = 0.51), while that of insulin intake was 6.5 months in the control group and 4 months in the study group.
Mean initial size of the ulcer was 2747.17 mm2 in the control group and 2608.03 mm2 in the study group (p = 0.361). All ulcers in both groups belonged to Meggitt-Wagner grade I and had a depth of 5 mm. There was no significant difference between the two groups (Table 3).
Table 3.
Ulcer characteristics
| Clinical features | Control group (n = 34) | Study group (n = 34) | Statistical significance |
|---|---|---|---|
| Initial ulcer area (mm2) | 2747.17 ± 603.79 | 2608.03 ± 683.14 | p = 0.361 |
| Site of the ulcer | |||
| Plantar | 20 (59%) | 18 (53%) | p = 0.625 |
| Dorsum | 14 (41%) | 16 (47%) | |
| Meggitt-Wagner grade | I | I | – |
| Depth of ulcer (mm) | 5 | 5 | – |
After completion of 15 days of therapy, the final area was 2424.75 mm2 in the control group and 1564.79 mm2 in the study group (p = 0.218). Mean reduction in ulcer area was 322.44 ± 85.84 mm2 in the control group and 1043.20 ± 266.62 mm2 in the study group, and this difference between the two groups was statistically significant (p < 0.010) (95% confidence interval = 624.91–816.73) (Table 4).
Table 4.
Outcome of the study
| Results | Control group (n = 34) | Study group (n = 34) | Statistical significance | 95% confidence interval |
|---|---|---|---|---|
| Initial ulcer area (mm2) | 2747.17 ± 603.79 | 2608.03 ± 683.14 | p = 0.361 | – |
| Final ulcer area (mm2) | 2424.75 ± 551.26 | 1564.79 ± 437.30 | p = 0.218 | – |
| Mean reduction in ulcer area (mm2) | 322.44 ± 85.84 | 1043.20 ± 266.62 | p < 0.010 | 624.91–816.73 |
Discussion
Diabetic foot ulcers pose a major healthcare problem as a significant cause of morbidity, mortality, and financial burden [14]. The healing process in DFUs is arrested in the stage of inflammation, and it is not known why it does not progress further. Decades of intense research have not unravelled the mystery of wound healing completely. However, it is evident that wound healing involves several biological processes at cellular and molecular levels. The research on wound healing targets the cellular and subcellular biomodulation [15–19]. The healing properties of LLLT are likely to be due to photobiomodulation resulting in increased granulation tissue, fibroblast proliferation, collagen synthesis, neovascularization, and early epithelialization, the important changes observed in LLLT-treated wounds. Low doses of laser are stimulative and higher doses are suppressive. Among the various non-invasive treatment modalities, LLLT is gaining increasing interest. Research findings to date based on animal, human, and in vitro studies have shown that LLLT can play a useful role in healing chronic diabetic ulcers resistant to conventional treatment [20–28].
A study by Hopkins et al. has reported results in 22 healthy subjects and shown 55% greater wound contraction in cases as compared to controls [29]. Gupta et al. have demonstrated a significantly greater reduction (p < 0.002) in the surface area of leg ulcers treated with red light and infrared light than in sham-irradiated controls. The leg ulcers were given three treatments per week for 10 weeks, by which time LLLT-treated ulcers showed an average reduction in surface area of 193.0 mm2, whereas in controls it was only 14.7 mm2 [30].
In our study, 34 ulcers treated with LLLT showed significant reduction in percentage wound area, that is, 40.24 ± 6.30 mm2 compared to 11.87 ± 4.28 mm2 in control groups (p < 0.001, Z = 7.08). These results show significant benefit following the use of LLLT.
In conclusion, the wounds in subjects treated with LLLT contracted significantly more than the wounds in the non-treated group (40.24% vs 11.87%, p < 0.001), which indicates that LLLT is an effective modality to facilitate wound contraction in patients suffering from diabetes and can be used as an adjunct to conventional mode of treatment (dressings and debridement) for healing of diabetic wounds.
References
- 1.Aboderin I, Kalache A, Ben-Shlomo Y, Lynch JW, Yajnik CS, Kuh D, et al. Life course perspectives on coronary heart disease, stroke and diabetes: key issues and implications for policy and research. Geneva: World Health Organization; 2001. [Google Scholar]
- 2.Bal A, Das AK, Pendsey S, Suresh KR, Vishwanathan V, Ambardekar P. Handbook of diabetic foot care. Bangalore: Diabetic Foot Society of India; 2005. [Google Scholar]
- 3.Brem H, Sheehan P, Rosenberg HJ, Schneider JS, Boulton AJ. Evidence-based protocol for diabetic foot ulcers. Plast Reconstr Surg. 2006;117(7):193S–209S. doi: 10.1097/01.prs.0000225459.93750.29. [DOI] [PubMed] [Google Scholar]
- 4.National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2002. Atlanta: US Dept of Health and Human Services; 2002. [Google Scholar]
- 5.Eldor R, Raz I, Ben Yehuda A, Boulton AJ. New and experimental approaches to treatment of diabetic foot ulcers: a comprehensive review of emerging treatment strategies. Diabet Med. 2004;21(11):1161–1173. doi: 10.1111/j.1464-5491.2004.01358.x. [DOI] [PubMed] [Google Scholar]
- 6.Steed DL. The role of growth factors in wound healing. Surg Clin N Am. 1997;77:575–586. doi: 10.1016/S0039-6109(05)70569-7. [DOI] [PubMed] [Google Scholar]
- 7.Millington JT, Norris TW. Effective treatment strategies for diabetic foot wounds. J Fam Pract. 2000;49(11):S40–S48. [PubMed] [Google Scholar]
- 8.Dyson M. Adjuvant therapies; ultrasound, laser therapy, electrical stimulation, hyperbaric oxygen and VAC-therapy. In: Morrison MJ, Moffatt CJ, Franks PJ, editors. Leg ulcers: a problem-based learning approach. Philadelphia: Mosby, Elsevier; 2007. pp. 429–451. [Google Scholar]
- 9.Rinaldi F, Alberetto M, Pontiroli A. The diabetic foot. General considerations and proposal of a new therapeutic and preventive approach. Diabetes Res Clin Pract. 1993;21(1):43–49. doi: 10.1016/0168-8227(93)90096-N. [DOI] [PubMed] [Google Scholar]
- 10.Baxter GD. Therapeutic lasers: theory and practice. Edinburgh: Churchill Livingstone; 1994. [Google Scholar]
- 11.Ferney R, Mauro T. Using lasers in diabetic wound healing. Diabetes Technol Ther. 1999;1(2):189–192. doi: 10.1089/152091599317404. [DOI] [PubMed] [Google Scholar]
- 12.Damme H, Limet R. The diabetic foot. Rev Med Liege. 2005;60(5–6):516–525. [PubMed] [Google Scholar]
- 13.Armstrong DG. Clinical examination of diabetic foot and identification of at risk patient. In: Morrison MJ, Moffatt CJ, Franks PJ, editors. Leg ulcers: a problem-based learning approach. Philadelphia: Mosby, Elsevier; 2002. pp. 163–176. [Google Scholar]
- 14.Shobhana R, Rama Rao P, Lavanya A, Viswanathan V, Ramachandra A. Cost burden to diabetic patients with foot complications—a study from southern India. JAPI. 2000;48:1147–1150. [PubMed] [Google Scholar]
- 15.Boulton M, Marshall J. He-Ne laser stimulation of human fibroblast proliferation and attachment in vitro. Laser Life Sci. 1986;1:125–134. [Google Scholar]
- 16.Landau Z, Schattner A. Topical hyperbaric oxygen and low energy laser therapy for chronic diabetic foot ulcers resistant to conventional treatment. Yale J Biol Med. 2001;74(2):95–100. [PMC free article] [PubMed] [Google Scholar]
- 17.Schindl A, Heinze G, Schindl M, Pernerstorfer-Schon H, Schindl L. Systemic effects of low-intensity laser irradiation on skin microcirculation in patients with diabetic microangiopathy. Microvasc Res. 2002;64(2):240–246. doi: 10.1006/mvre.2002.2429. [DOI] [PubMed] [Google Scholar]
- 18.Pereira AN, Eduardo Cde P, Matson E, Marques MM. Effect of low power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Laser Surg Med. 2002;31:263–267. doi: 10.1002/lsm.10107. [DOI] [PubMed] [Google Scholar]
- 19.Dyson M, Young S. Effect of laser therapy on wound contraction and cellularity in mice. Lasers Med Sci. 1986;1:126–130. doi: 10.1007/BF02038962. [DOI] [Google Scholar]
- 20.Brem H, Sheehan P, Boulton AJM. Protocol for treatment of diabetic foot ulcers. Am J Surg. 2004;187(5A):1S–10S. doi: 10.1016/S0002-9610(03)00299-X. [DOI] [PubMed] [Google Scholar]
- 21.Schindl M, Kerschan K, Schindl A, Schon H, Heinzl H, Schindl L. Induction of complete wound healing in recalcitrant ulcers by low-intensity laser irradiation depends on ulcer cause and size. Photodermatol Photoimmunol Photomed. 1999;15(1):18–21. doi: 10.1111/j.1600-0781.1999.tb00047.x. [DOI] [PubMed] [Google Scholar]
- 22.Maiya GA, Kumar P, Rao L. Effect of low intensity helium-neon (He-Ne) laser irradiation on diabetic wound healing dynamics. Photomed Laser Surg. 2005;23(2):187–190. doi: 10.1089/pho.2005.23.187. [DOI] [PubMed] [Google Scholar]
- 23.Basford C. Low energy laser therapy: controversies and new research findings. Lasers Surg Med. 1989;9:1–5. doi: 10.1002/lsm.1900090103. [DOI] [PubMed] [Google Scholar]
- 24.Haas AF, Isseroff RR, Wheeland RG, Rood PA, Graves PJ. Low energy helium-neon laser irradiation increases the motility of human keratinocytes. J Invest Dermatol. 1990;94:822–826. doi: 10.1111/1523-1747.ep12874679. [DOI] [PubMed] [Google Scholar]
- 25.Yu W, Naim JO, Lanzafame RJ. The effects of photo-irradiation on the secretion of TGF and PDGF from fibroblasts in vitro. Laser Surg Med Suppl. 1994;6:8. [Google Scholar]
- 26.Schindl A, Schindl M, Schon H, Knobler R, Havelec L, Schindl L. Low-intensity laser irradiation improves skin circulation in participants with diabetic microangiopathy. Diabetes Care. 1998;21(4):580–584. doi: 10.2337/diacare.21.4.580. [DOI] [PubMed] [Google Scholar]
- 27.Medrado AR, Pughese LS, Reis SR, Andrade ZA. Influence of low level laser therapy on wound healing and its biological action on myofibroblasts. Laser Surg Med. 2003;32:179–184. doi: 10.1002/lsm.10126. [DOI] [PubMed] [Google Scholar]
- 28.Koutam M, Janisch R, Veselska R. Effects of low power laser irradiation on cell proliferation. Scr Med. 2003;73(3):163–172. [Google Scholar]
- 29.Hopkins J, Todd A, Jeff G, Seegmiller G, Baxter D. Low level laser therapy facilitates superficial wound healing in humans: a triple-blind, sham-controlled Study. J Athl Train. 2004;39(3):223–229. [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta AK, Filonenko N, Salansky N, Sadder DN. The use of low energy photon therapy (LEPT) in venous leg ulcers: a double blind, placebo-controlled study. Dermatol Surg. 1998;24:1383–1386. doi: 10.1016/S1076-0512(98)00168-X. [DOI] [PubMed] [Google Scholar]


