Abstract
Our study aims to review the literature on the management of pyogenic liver abscess, focusing on the choice of drainage. A case series of our experience with clinicopathological correlation is presented to highlight the indication and outcome of each modality of drainage. Intravenous antibiotic is the first line, and mainstay, of treatment. Drainage is necessary for large abscesses, equal to or larger than 5 cm in size, to facilitate resolution. While percutaneous drainage is appropriate as first-line surgical treatment in most cases, open surgical drainage is prudent in cases of rupture, multiloculation, associated biliary, or intra-abdominal pathology. Percutaneous drainage may help to optimize clinical condition prior to surgery. Nevertheless, in current good clinical practices, the choice of therapy needs to be individualized according to patient’s clinical status and abscess factors. They are complementary in the management of liver abscesses.
Keywords: Pyogenic liver abscess, Ultrasound-guided percutaneous drainage
Introduction
Pyogenic liver abscess is a burning problem of tropical countries and remains a formidable diagnostic and therapeutic problem. If left untreated, the disease invariably runs a lethal course. The management of this disease varies considerably from surgeon to surgeon. Pyogenic liver abscess is a condition with significant mortality. The most common presenting clinical symptoms are upper abdominal pain, high-grade fever, nausea, and vomiting. Loss of appetite, jaundice, and respiratory symptoms are less frequent clinical features. These clinical features are variable depending on the size of the abscess, general health of the patient, associated diseases, and complications. The most common sign is right hypochondrial tenderness frequently with guarding and hepatomegaly. Some patients may present with jaundice, ascites, or pleural effusion. In majority of the cases, the underlying cause could not be identified. Biliary tract disease is reported to be the most frequent cause followed by portal circulation, arterial circulation, cryptogenic, and trauma [1]. It may be due to bacterial or parasitic invasion of liver [2]. Majority of abscesses are multiple which are due to biliary system and arterial circulation and subdiaphragmatic and are noted in the right lobe of liver. Solitary or single abscess is due to portal circulation, cryptogenic, and trauma. Early studies by Oschner et al. recommended open surgical drainage as the treatment of choice [3]. For the past two decades, advances in the imaging field coupled with ultrasound-guided percutaneous needle aspiration and drainage brought dramatic changes in the pattern of treatment for pyogenic liver abscess. The aim of our study was to determine etiopathology, clinical, radiological, and bacteriological characteristics of the condition and to review its management strategies.
Material and Methods
A total of 400 patients with pyogenic liver abscess were managed in the Department of Surgery, Surat Municipal Institute of Medical Education and Research Hospital, Surat, from July 2007 to December 2010. Medical records of all the patients were maintained. The cases were collected from the emergency unit and surgical outdoor department of the hospital. All the patients were sent to the radiology department for confirmation of diagnosis on ultrasound. The chest X-ray was also performed. Ultrasound-guided percutaneous needle aspiration and drainage was performed in the radiology department. Other investigations included complete blood picture, liver function tests, and hemagglutination tests. Abscesses smaller than 5 cm sizes were managed by parenteral antibiotic therapy, while those above 5 cm size were planned to be managed by ultrasound-guided percutaneous aspiration/drainage. In general, broad-spectrum antibiotics were given intravenously after initial work-up. In cases of pyogenic liver abscess, fluoroquinolones or a second-generation cephalosporin was given intravenously along with metronidazole. Percutaneous catheter drainage with an 8 F pigtail catheter inserted under ultrasonography (USG) guidance was performed in patients with liver abscesses more than 10 cm in size, without significant septations and liquefaction or those patients who did not respond to two sessions of percutaneous aspiration and again presented with liver abscess. The catheter was removed 24 h after the outcome became nil, and patients were discharged with oral antibiotics. Surgical drainage was reserved for the patients who presented with burst liver abscess or those who had concurrent intra-abdominal pathology requiring open surgical management. The patient’s clinical condition was closely observed and follow-up imaging with USG was performed every 15–20 days till the abscess resolved or became less than 2 cm in size.
Result
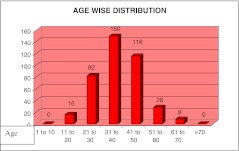
In our study, out of 400, 320 patients (80%) were males and 80 patients (20%) were females; male to female ratio was 4:1. The patients age was ranging from 10 to 70 years, and mean age was 35 years (Fig. 1).
Fig. 1.
Age-wise distribution [COMP: Delete the text “AGE WISE DISTRIBUTION” from the artwork]
Majority of patients 390 (97%) with pyogenic liver abscess presented with upper abdominal pain. High-grade fever was noted in 296 (74%) patients, vomiting and nausea in 200 (50%) patients, while loss of appetite in 198 (49.5%) patients (Fig. 2).
Fig. 2.
Symptomatology [COMP: Delete “SYMPTOMATOLOGY” from the artwork]
On examination, tenderness in right hypochondrium (RHC) was the predominant sign (95%), followed by localized guarding (47%), whereas 26% patients were presented with hepatomegaly. About 4.5% patients presented with signs of toxemia (Fig. 3).
Fig. 3.
Signs on examination
Majority of liver abscesses (83%) were found in the right lobe of liver. About 4.5% of liver abscesses were confined to the left lobe only. Both lobes were involved in 12.5% of patients. In present series, more than 50% of abscesses were solitary at the time of presentation. Multiple abscesses were found in 21% of patients. Majority of patients (68.5%) presented with partially liquefied abscess. About 23% patients presented with liquefied liver abscess. In present series, 41.5% patients had hemoglobin less than 10 g%, 80.5% patients had abnormal total leucocytic count, and 12.5% patients had hyperbilirubinemia. We had performed pus culture study in 264 cases of which only 44 reports came with positive microbial reports. Here, 45% cases were positive for Klebseilla and 32% shown Escherichia coli. Other organisms were Staphylococcus (9%), Bacteroides (9%), and Citrobacter (5%) (Table 1)
Table 1.
Main laboratory investigations
| Investigations | Number of cases | Percentage (%) |
|---|---|---|
| Hemoglobin (<11 g%) | 166 | 41.5 |
| Total leucocytic count (<4,000/mm3) | 10 | 2.5 |
| Total leucocytic count (>11,000/mm3) | 312 | 78 |
| Total bilirubin (>2 g%) | 50 | 12.5 |
| Prothrombin time (>16) | 138 | 34.5 |
Of all the pyogenic liver abscesses, 56% were cryptogenic in origin. The rest 44% were biliary tract (15.5%), portal pyemia (15%), or hematogenous (13.5%) in origin (Fig. 4).
Fig. 4.
Etiology
Intravenous antibiotic therapy (cephalosporin or fluoroquinolones combination with metronidazole and aminoglycoside) started to all patients. A total of 130 patients (32%) were improved completely by this regime. Remaining 270 patients required intervention in different forms as shown in Table 2.
Table 2.
Surgical management
| Number of cases (n = 270) | Percentage (%) | |
|---|---|---|
| Percutaneous aspiration | 212 | 78.52 |
| Percutaneous catheter | 24 | 8.88 |
| Aspiration + intercostal drainage (ICD) | 14 | 5.18 |
| Pleural tapping + laparotomy | 6 | 2.22 |
| Laparotomy | 14 | 5.18 |
| Total | 270 |
Of the patients who were aspirated, 59% required single aspiration and 41% patients required more than one aspiration. One patient was aspirated five times before complete resolution of his liver abscess (Table 3).
Table 3.
Aspiration versus percutaneous catheter insertion in relation to abscess size
| Aspiration group (n = 226) | Catheter group (n = 24) | |
|---|---|---|
| Mean size (cm) | 6.87 | 11.5 |
| Number of patients with abscess no. 1:2:3 | 67:29:17 | 11:01:00 |
Mean abscess size in the aspiration group was 6.87 cm and that in the catheter group was 11.5 cm. Among the aspiration group of patients, 59.3% had solitary abscess, while 25.66 and 15.04% had two and multiple liver abscesses.
Among the catheter group, 91.66% of patients had solitary large abscess. About 91% of liver abscesses were confined to liver at the time of presentation. Others were ruptured in the pleural (4%), peritoneal (4%), or both the cavities (1%) (Table 4).
Table 4.
Complications
| Number of cases | Percentage (%) | |
|---|---|---|
| Ruptured liver abscess | 20 | 5 |
| Pyothorax | 20 | 5 |
| Death | 6 | 1.5 |
Rupture of abscess into pleural or peritoneal cavity was the major complication (5%), and 1.5% of patients expired during or after the treatment.
Discussion
In this study, the most significant clinical features of pyogenic liver abscess were upper abdominal pain with high-grade fever and hepatomegaly as reported by other authors [4–7]. Oschner et al. [2] and Norman et al. [6] showed that clinical features of pyogenic liver abscess were non-specific and thus a high index of suspicion was required for the early diagnosis of the disease.
The majority of liver abscesses have an underlying source that must be controlled before successful treatment of the abscess is possible. The present series showed maximum incidence of the disease in middle-aged patients (31–50 years). About 56% of the total abscesses were cryptogenic in origin, while 15.5, 15, and 13.5% were of biliary, portal, and hematogenous in origin, respectively. Historically, liver abscess developed in young, otherwise healthy patients with an intro-abdominal infection. Oschner reported a peak incidence in the fourth decade [2], which corresponds to this study, but there is a gradual trend of increase in the age of patients with liver abscess, probably due to aggressive treatment of acute appendicitis and other intra-abdominal infections of the young which prevents the abscess from occurring. This has resulted in an older age group and a greater proportion of poor risk patients with liver abscess and a change of etiology from portal phlebitis toward biliary tract diseases. With a large number of cryptogenic abscesses in this (56%) and other reports [8, 9], a better understanding of the underlying pathogenesis is needed. The incidence has been reported from 4% [10] to nearly 60% [2, 11].
The most common laboratory abnormality was altered total leucocytic count, either more than 11,000 (78%) or less than 4,000 (2.5%). The total leucocytic count of less than 4,000 was found in patients with burst liver abscess and signs of toxemia at the time of admission. Hemoglobin less than 11 g% was found in 41.5% of patients. Hyperbilirubinemia was present in 12.5% cases.
Qualified and well-experienced sonographers play an important role for quick diagnosis; as a result the mortality rate of pyogenic liver abscess has been significantly reduced from 40 to 10–25% in past two decades [12–14]. The improvements in results are due to improved imaging qualities and effective antimicrobial agents such as third-generation cephalosporin. These improvements apparently decline the role of open surgery, which was considered as primary treatment of the condition. More sophisticated modalities such as MRI T1 and T2 weighted can differentiate the abscess from other hepatic lesions [15].
The X-ray chest showed elevated dome of right hemidiaphragm. It was always abnormal and may point to the right upper quadrant as a source of abnormality [16]. About 6% patients presented with blunting of costophrenic angles, either unilateral (5.5%) or bilateral (0.5%). About 5% patients presented with features of pulmonary consolidation. Four patients had chest X-ray findings suggestive of pulmonary Koch’s. All these pathologies in the lung field can lead to liver abscess via hematogenous route.
The characteristics of liver abscess found at radiologic imaging were noted. The mean size of liver abscess was 6.87 cm (range 2–15 cm). The majority of patients had solitary liver abscesses (56%). Most of the abscesses occurred in the right lobe (84%). Nine patients (4.5%) had liver abscess in the left hepatic lobe, and 25 patients (12.5%) had bilobar disease. In our study, there is a high percentage of liver abscesses via hematogenous and portal routes (28.5%). There is a preferential blood supply to the right hepatic lobe through the large right branch of portal vein. This explains the high incidence of the right lobe liver abscess in our study.
Intravenous antibiotics were given to all the patients, and this was the only treatment for 65 patients (32.5%). The most commonly used antibiotics were fluoroquinolones, the third-generation cephalosporins with metronidazole. If appropriate antibiotics are given for 4–6 weeks, according to the antimicrobial sensitivity of the cultured microorganism, it can be curative for abscesses measuring less than 5 cm in diameter [17].
Percutaneous needle aspiration in combination with systemic antibiotics is safe and effective treatment, and it should be considered as first-line treatment [18]. This mode of management is highly appreciable in our study. About 56% of diagnosed patients (113 out of 200) were treated with this mode of regime, and the result was satisfactory. These patients had abscess larger than 5 cm, and the lesion was noted in the locations suitable for aspiration. From remaining, 12 cases with very large liver abscesses (10 cm or more) underwent percutaneous drainage therapy. The ultrasound-guided drainage remains a preferred radiological management of percutaneous needle aspiration to date.
Percutaneous needle aspiration was successful in 111 (55.5%) of 200 patients after one (n = 66), two (n = 41), three (n = 3) aspirations (i.e., 59.46, 36.93, and 2.7%, respectively). Only one patient required aspiration for five times. This particular patient had liquefied liver abscess whose abscess cavity regressed completely after every session of percutaneous aspiration followed by symptomatic relief. Enver and Amir also reported similar success rates after one, two, and three aspirations (i.e., 60, 35, and 5%, respectively) [19].
Percutaneous needle aspiration with systemic antibiotics as a modality of treatment was compared with continuous catheter drainage, and it was found that mean abscess size in the aspiration group was 6.87 cm and that in the catheter group was 11.5 cm. Majority of abscesses treated by aspiration were solitary (59.3%), but two (25.66%) or more abscesses (17%) were also found in contrast to the catheter group where about 92% of patients had solitary abscesses. The mean hospital stay in the aspiration group (4.63 days) was also less than that of the catheter group (7 days). In two early randomized studies [20, 21], in which use of continuous catheter drainage was compared with repeated needle aspiration in the management of liver abscess, recommendations for the first-line percutaneous treatment differed. Razak et al. showed a significantly higher success rate in the catheter drainage group. However, only 11 of their 50 patients had a confirmed diagnosis of pyogenic liver abscess. In addition, they had limited their aspiration attempts to two times, which was likely to reduce the overall success rate of needle aspiration (60%) compared with catheter drainage (100%). Yu and colleagues [21] performed a randomized trial involving 64 patients of pyogenic liver abscess. Percutaneous needle aspiration was repeated if there was either lack of clinical improvement or lack of size reduction of the abscess cavity. Those investigators concluded that percutaneous needle aspiration was probably as effective as continuous percutaneous catheter drainage.
Thus, both these management modalities of percutaneous aspiration and catheter drainage gave satisfactory results, but advantage of one over the other modality could not be decided. However, it is noted that needle aspiration is cheaper, less invasive, and needs less postprocedure care, and it has the advantage to drain multiple liver abscesses in one session [21]. Diagnostic aspiration should be performed as soon as the diagnosis is made. It can be performed under ultrasonographic [22] (if small or superficial) or CT guidance and is usually followed by placement of a drainage catheter. Multiple abscesses necessitate CT-guided aspiration [22]. Recently, it has been shown that using multidetector CT to analyze abscesses could identify factors predictive of percutaneous drainage failure [23]. In addition to abscess size, other criteria for percutaneous drainage include continued pyrexia after 24–48 h of adequate medical treatment, and clinical or ultrasonographic features suggestive of impending perforation [24].
Surgical exploration and open drainage of liver abscess was performed in cases presenting with intraperitoneal rupture of liver abscess. This was done in 10 patients (7.4%). Mohammad Aslam et al. [5] did surgery in 7.14% of patients, which corresponds to the rate of surgical intervention in our study.
The most common causative organism isolated in abscess culture were Klebseilla species (7.57%), E. coli (5.3%), and anaerobes (1.51%), in accordance with the previous studies [9, 17, 25]. A recent nationwide report from South Korea also showed that the proportion of K. pneumoniae infection increased dramatically in that country over time from approximately 0% in 1955–1969 to 78.2% during 2004–2005 [26]. However, majority of samples for abscess culture were sterile (83.33%). This could be due to the widespread use of early empirical antibiotics in the present study.
The low mortality rate (1.5%) in our study can be explained by the early detection and prompt management by the USG-guided percutaneous techniques. The mortality rate in developed countries ranges from 2 to 12%. Independent risk factors for mortality include need for open surgical drainage, the presence of malignancy, and the presence of anaerobic infection [27, 28].
Conclusion
In conclusion, our experience with patients of percutaneous needle aspiration under USG guidance is helpful, effective, and cost-effective for multiple or solitary abscesses of less than 10 cm. Percutaneous needle aspiration under USG guidance is combined with administration of systemic antibiotics and metronidazole. Patients treated by this technique recover faster and duration of hospital stay is less. Less medical or nursing care is required by aspiration. Success rate is more than 90%. It is minimally invasive and readily acceptable to most of the patients and easy to perform and without any complications. Patients treated with catheter drainage have a longer duration of hospital stay. The technique requires expertise and is costlier than percutaneous aspiration, but is useful in selected cases.
References
- 1.Lee KT, Wong SR, Sheen PC. Pyogenic liver abscess. Dig Surg. 2001;18:459–465. doi: 10.1159/000050194. [DOI] [PubMed] [Google Scholar]
- 2.Oschner A, DeBaker M, Murray S. Pyogenic abscess of liver. II. An analysis of forty seven cases with review of literature. Am J Surg. 1938;40:292–319. doi: 10.1016/S0002-9610(38)90618-X. [DOI] [Google Scholar]
- 3.Mehnaz A, Mohsin S (1991) Liver abscess in children not an uncommon problem. JPMA 273–275 [PubMed]
- 4.Miedema BW, Dineen P. The diagnosis and treatment of pyogenic liver abscess. Ann Surg. 1984;200(3):328–334. doi: 10.1097/00000658-198409000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugti QA, Baloch MA, Wadood AU, Mulghani AH, Azeem B, Ahmed J. Pyogenic liver abscess: demographic, clinical, radiological and bacteriological characteristics and management strategies. GJMS. 2005;3:1. [Google Scholar]
- 6.Norman DC, Yoshikawa TT. Intra-abdominal infection: diagnosis and treatment in the elderly patient. Gerontology. 1984;30:327–328. doi: 10.1159/000212652. [DOI] [PubMed] [Google Scholar]
- 7.McFadzean AJS, Chang KPS, Wong CC. Solitary pyogenic abscess of the liver treated by closed aspiration and antibiotics: a report of 14 consecutive cases with recovery. Br J Surg. 1953;41:141–152. doi: 10.1002/bjs.18004116606. [DOI] [PubMed] [Google Scholar]
- 8.Stain SC, Yellin AK, Donovan AJ, et al. Pyogenic liver abscess: modern treatment. Arch Surg. 1991;126:991–996. doi: 10.1001/archsurg.1991.01410320077010. [DOI] [PubMed] [Google Scholar]
- 9.Lee KT, Sheen PC, Chen JS, et al. Pyogenic liver abscess—multivariate analysis of risk factors. World J Surg. 1991;15:372–377. doi: 10.1007/BF01658732. [DOI] [PubMed] [Google Scholar]
- 10.Rubin RH, Swartz MN, Malt R. Hepatic abscess: changes in clinical, bacteriological and therapeutic aspects. Am J Med. 1974;57:601–610. doi: 10.1016/0002-9343(74)90012-6. [DOI] [PubMed] [Google Scholar]
- 11.Bourne WA. The diagnosis of pyogenic liver abscess. Lancet. 1954;2:1093–1094. doi: 10.1016/S0140-6736(54)90651-0. [DOI] [PubMed] [Google Scholar]
- 12.Bergamini TM, Larson GM, Malangoni MA, et al. Liver abscess: review of a 12-year experience. Am Surg. 1987;53:596–599. [PubMed] [Google Scholar]
- 13.Chiu CT, Lin DY, Wu CS, et al. A clinical study of pyogenic liver abscess. J Formos Med Assoc. 1990;86:571–576. [Google Scholar]
- 14.Yang CC, Chen CY, Lin XZ, et al. Pyogenic liver abscess in Tiwan: emphasis on gas-forming liver abscess in diabetics. Am J Gastroenterol. 1993;88:1911–1915. [PubMed] [Google Scholar]
- 15.Balci NC, Semelka RC, Noone TC, et al. Pyogenic hepatic abscesses MRI findings on T-1 and T2 weighted and serial gadolinium-enhanced gradient echo images. J MRI. 1999;9:285–290. doi: 10.1002/(sici)1522-2586(199902)9:2<285::aid-jmri20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 16.Seeto RK, Rockey DC. Pyogenic liver abscess, changing, etiology, management and outcome. Medicine (Baltimore) 1996;75:99–113. doi: 10.1097/00005792-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Lok KH, Li KF, Li KK, Szeto ML. Pyogenic liver abscess: clinical profile, microbiological characteristics, and management in a Hong Kong hospital. J Microbial Immunol Inf. 2008;41:483–490. [PubMed] [Google Scholar]
- 18.Ch Yu S, Hg Lo R, Kan PS, Metreweli C. Pyogenic liver abscess treatment with needle aspiration. Clinical Radiol. 1997;52:912–916. doi: 10.1016/S0009-9260(97)80223-1. [DOI] [PubMed] [Google Scholar]
- 19.Zerem E, Hadzic A. Sonographically guided percutaneous catheter drainage versus needle aspiration in the management of pyogenic liver abscess. AJR. 2007;189:W138–W142. doi: 10.2214/AJR.07.2173. [DOI] [PubMed] [Google Scholar]
- 20.Razak CL, Gupta S, Jain S, et al. Percutaneous treatment of liver abscesses: needle aspiration versus catheter drainage. AJR. 1998;170:1035–1039. doi: 10.2214/ajr.170.4.9530055. [DOI] [PubMed] [Google Scholar]
- 21.Yu SC, Ho SS, Lau WY, et al. Treatment of pyogenic liver abscess: prospective randomized comparison of catheter drainage and needle aspiration. Hepatology. 2004;39:932–938. doi: 10.1002/hep.20133. [DOI] [PubMed] [Google Scholar]
- 22.Giorgio A, Stefano G, Sarno A, et al. Percutaneous needle aspiration of multiple pyogenic abscesses of the liver: 13-year single-center experience. AJR Am J Roentgenol. 2006;187(6):1585–1590. doi: 10.2214/AJR.05.1104. [DOI] [PubMed] [Google Scholar]
- 23.Liao WI, Tsai SH, Yu CY, Huang GS, Lin YY, Hsu CW, Hsu HH, Chang WC. Pyogenic liver abscess treated by percutaneous catheter drainage: MDCT measurement for treatment outcome. Eur J Radio. 2011; [Epub ahead of print]. [DOI] [PubMed]
- 24.Malik AA, Bari SU, Rouf KA, Wani KA. Pyogenic liver abscess: changing patterns in approach. World J Gastrointest Surg. 2010;2(12):395–401. doi: 10.4240/wjgs.v2.i12.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zibari GB. Pyogenic liver abscess. Surg Infect. 2000;1:15–21. doi: 10.1089/109629600321254. [DOI] [PubMed] [Google Scholar]
- 26.Chung DR, Lee SS, Lee HR, Kim HB, Choi HJ, Eom JS, et al. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect. 2007;54:578–583. doi: 10.1016/j.jinf.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Chen SC, Huang CC, Tsai SJ, et al. Severity of disease as main predictor for mortality in patients with pyogenic liver abscess. Am J Surg. 2009;198:164. doi: 10.1016/j.amjsurg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Lok KH, Li KF, Li KK, Szeto ML. Pyogenic liver abscess: clinical profile, microbiological characteristics, and management in a Hong Kong hospital. J Microbiol Immunol Infect. 2008;41:483. [PubMed] [Google Scholar]