Abstract
Iron is an essential trace element and plays a number of vital roles in biological system. It also leads the chains of pathological actions if present in excess and/or present in free form. Major portion of iron in circulation is associated with transferrin, a classical iron transporter, which prevent the existence of free iron. The fraction of iron which is free of transferrin is known as “non transferrin bound iron”. Along with the incidence in iron over loaded patient non transferrin bound iron has been indicated in patients without iron overload. It has been suggested as cause as well as consequence in a number of pathological conditions. The major organs influenced by iron toxicity are heart, pancreas, kidney, organs involved in hematopoiesis etc. The most commonly suggested way for iron mediated pathogenesis is through increased oxidative stress and their secondary effects. Generation of free oxygen radicals by iron has been well documented in Fenton chemistry and Haber–Weiss reaction. Non transferrin bound iron has obvious chance to generate the free reactive radicals as it is not been shielded by the protective carrier protein apo transferrin. The nature of non transferrin bound iron is not clear at present time but it is definitely a group of heterogenous iron forms free from transferrin and ferritin. A variety of analytical approaches like colorimetry, chromatography, fluorimetry etc. have been experimented in different research laboratories for estimation of non transferrin bound iron. However the universally accepted gold standard method which can be operated in pathological laboratories is still to be developed.
Keywords: Non transferrin bound iron, Oxidative stress, Haber–Weiss reaction, Fenton chemistry, Transferrin, Apo transferrin
Introduction
Iron is the second most abundant metal (after aluminium) and the fourth most abundant element of the earth’s crust. Biologically Iron serves both the roles; essential as well as toxic. It is essential as trace element because it is a part of the hemoproteins including hemoglobin but it is toxic if present in excess amount as it has potentiality to generate free radicals that can have deleterious effects on the biological system. The biological functions of iron are due to its ability to form diverse dexterity with organic ligands and suitable redox potential to slide between ferrous i.e. Fe(II) and ferric i.e. Fe(III) state. These essential characteristics of this element make it a dangerous entity if left unattended or unbounded. This is the reason why majority of body iron is found to be associated with proteinic and non proteinic bio molecules to shield its toxicity. In plasma, majority of iron is bound to transferrin (Tf) which is a globular protein. Non transferrin bound iron (NTBI) is the term used for the free iron present in plasma, including all the forms of iron present in serum or plasma which are not bound to Tf and the other traditional iron binding proteins like haem, apoferritin, hemosiderin etc. Unbound iron which does not have the shield in the form of protein has capacity to generate highly reactive free radical and known to cause an immense amount of damage to various organs of the body including heart, liver, pancreas, endocrine glands and erythroid cells etc., depending on final location of its deposition. Another aspect is that iron is almost a one way element and only few physiological avenues for its removal from human organism are known to exist. There is a lacunae in the literature regarding the precise way of removal and the shelf life of NTBI, though some studies state its clearance from highly vascular organs like liver and heart [1]. Even after lapse of 34 years of its first mentioning of NTBI by Hershko et al. [2] the equivocal of definition of NTBI is difficult proposition due its heterogeneous nature.
Names and Nature of NTBI
A spectrum of names is accorded to the NTBI due to its diverse biological nature. Various research workers have used different terms like “NTBI”, “free iron”, “catalytic iron pool”, “labile iron” etc. to address the same or overlapping fraction of plasma iron which is devoid of its classical carriers. According to Paffetti et al. [3] the term “free iron” was introduced to indicate low molecular weight iron free from high affinity binding plasma proteins. Breuer and Cabantchik [4] suggested that the NTBI denotes those forms of iron in serum which are bound to ligands other than Tf. The term “free iron” refers to iron loosely bound to variety of biomolecules in such a way that it retains its ability to catalyze the formation of reactive oxygen species (ROS) [5]. According to Breuer “labile serum/plasma iron” or “labile plasma iron” represent iron bound to serum albumin, citrate and other undefined negatively charged ligands [6]. The term “labile iron pool” was proposed by Greenberg and Wintrobe in 1946 and reintroduced by Jacobs in 1977 as a ‘transient iron pool’ [7]. Kruszewski [7] defined “labile iron pool” as low molecular pool of weakly chelated iron that rapidly passes through the cell. Others coined the term “catalytic iron pool” which consists of chemical forms of iron that can participate in redox cycling and are associated with oxidative stress [7–9].
Numerous researches assigned different terms for NTBI based on its chelating characteristics. For example “Bleomycin detectable iron (BDI)”, “desferrioxamine chelatable iron” (DCI) later on called as directly chelatable iron by desferrioxamine, “mobilizer dependent chelatable iron” (MDCI), “Bathophenentroline detectable iron” etc. [5, 10]. The term “BDI” have been applied to address the forms of NTBI which are detected when associated with bleomycin [10]. “Desferrioxamine-chelatable iron” (DCI) was the term used by Breuer for the forms of NTBI which are chelatable with the well known iron chelator desferrioxamine [11]. However the abbreviation “DCI” had been used by the same researcher and his colleagues later as “directly chelatable iron” to indicate the NTBI forms which are readily chelated with desferrioxamine without the assistance of mobilizer [12]. At the same time another term “MDCI” has been in use to denote the NTBI forms which can be detected only after the action of mobilizer like oxalate or nitrilo triacetic acid (NTA) [12]. The term “Bathophenenthroline detectable iron” is applicable for NTBI forms which are directly chelated by a chromogenic chelator BPS [5].
Sources and Status of NTBI in Circulation
There is 20–35 % saturation of Tf at the normal serum iron level (20 μmol/L), the only major form of non-haem iron present in plasma. In normal physiological condition the level of Tf is sufficient enough for complete scavenging of even the greater dose of free iron and ensuring its absence in internal milieu. This is the reason why levels of NTBI in normal healthy individual do not exceed 1 μmol/L, and often undetectable by most of the methods [13]. In the iron overload scenarios like thalassemia; hemochromatosis and others iron gets a chance to exist in form which is not bond to Tf [2, 14–18]. Increased levels of NTBI in the above mentioned situations is easily explainable whereas it is difficult to give a convenient explanation for the raised levels of NTBI where Tf saturation is not high [18–20]. There is ample justification that why earlier studies of NTBI were focused in the diseases where the saturation of Tf was expected to high due to iron overload. Due to high Tf saturation the surplus iron gets a chance to escape and bind to non specific ligands. It is worthy to note that presence of NTBI was also found in the certain pathological conditions wherein no iron load was found to exist. Appearance of NTBI in patients with normal Tf saturation is poses the key question that in spite of the presence of strong affinity between iron and Tf, why a fraction of iron remains to be free though there are availability of free sites in the Tf to incorporate it? Various hypotheses have been suggested in different clinical conditions, but it is simple to state the presence of free iron in the presence of low saturation Tf is due to either greater inflow or inefficient management as efficient management of iron is of high priority because it is almost one way element as mentioned earlier.
The major sources of plasma iron are dietary iron coming from mucosal absorption in gastrointestinal tract and recycling of iron from the breakdown of aged erythrocytes and macrophages. Endogenous source is more important factor as iron absorption is well regulated at the mucosal level. In hemolytic anemia, increased endogenous inflow of iron due to excess haemolysis and/or compensatory blood transfusion may cause higher saturation of Tf leading to existence of NTBI [2, 14, 15, 21]. Lee et al. [22] reported the existence of NTBI in diabetes mellitus. In diabetes mellitus glycochelates are found to play vital role in the binding of iron. Mingwei et al. suggested that glycated proteins whose concentration is high in diabetics, do not only bind to iron and copper with higher affinity but also have the tendency to extract the iron from the traditional iron carriers [23]. In case of cancer patients undergoing for chemotherapy the reasons for the existence of NTBI are due to temporary shutdown of bone marrow and associated reduced demand of iron [24–27]. The other clinical conditions in which the presence of NTBI is indicated are acute coronary syndrome, liver disease, end stage kidney disease patients who are undergoing dialysis, in chronic alcoholics, myelodysplastic syndrome etc. [20, 28–33]. In atrasferrinaemia it is pertinent that all the plasma iron molecules exist as NTBI. In the absence of Tf the NTBI level were reported to be upto 20 μmol/L, whereas in the presence of insufficient Tf concentration these levels were found to be less than 10 μmol/L [13]. The fraction of iron which is inaccessible to Tf is the key cause of the NTBI existence at low Tf saturation [20]. Oxidative stress has also been documented as a cause as well as consequence of free iron, where the stable iron compound like iron sulphur proteins, ferritin and even haem release NTBI [34–42]. As shown in Table 1 free iron has been well documented as NTBI or other relevant subfractions in various iron overload conditions like hemochromatosis and thalassemia [11, 20, 43–45].
Table 1.
Name and concentration of NTBI or its subfraction in various clinical conditions
| Clinical condition | NTBI/name of its sub-fraction | Concentration (μmol/l) |
|---|---|---|
| Genetic hemochromatosis with iron overload [43] | NTBI | 0.761 ± 0.504 |
| Genetic hemochromatosis with iron overload [43] | LPI | 0.250 ± 0.289 |
| Heredity hemochromatosis [20] | NTBI | 4.0–16.3 |
| Thalassemia major(Thailand) [11] | DCI | 1.7–8.6 |
| β Thalassemia major [44] | NTBI | 0.375 ± 0.028 |
| Diabetes mellitus [22] | NTBI | 0.62 ± 0.43 |
| Cancer patients undergoing chemotherapy [45] | NPBI | 10.6 ± 6.6 |
| End stage renal disease [20] | NTBI | 0.1–13.5 |
Bearing +3/+2 valence, iron can’t exist in absolutely free form, rather due to its cationic nature it binds with many negatively charged ligands like albumin, citrate, acetate, DNA etc. [5, 46, 47]. In the absence of precise data available presently its nature appears more heterogenic. Binding of NTBI to various molecules have been reported using different methodologies. In computer simulation studies the major form of NTBI is found to be ferric bound to citrate where as nuclear magnetic resonance analysis point out ferric bound to citrate as well as acetate in hemochromatosis patients [46, 48]. The interaction of iron is also shown with the major plasma protein albumin even when the Tf is not completely saturated [49]. Various forms of NTBI may coexist depending on degree of iron overload, cause of iron overload and its duration. Clinically it has been proven that the accessibility of different iron chelators vary in assorted clinical conditions which indirectly support the heterogeneity of NTBI. For example homochromatic sera with 80–85 % Tf saturation showed no detectable DFO-chelatable iron where as Breuer et al. [11, 46] found DCI in the similar patient group when mobilizer like oxalate was applied. In case of thalassemic sera with >90 % Tf saturation, both the forms of DCI i.e. with and without oxalate mobilization were found [20]. Pootrakul et al. [50] estimated the levels of LPI and DCI separately in iron overloaded patients and found that both are highly correlated fractions. To a small extent where they differ is 25 % lower values of LPI which may stand for minor component of DCI which is not redox active. Careful segregation of various isoforms of NTBI is very essential to establish the role of individual isoform in specific pathological condition.
Estimation of NTBI
Due to ambiguous nature of NTBI, universally accepted gold standard method is not available at present. However, various researchers apply a range of analytical approaches which target one or the other characteristics of NTBI to estimate overlapping subtractions of the large heterogeneous iron pool collectively termed as NTBI [51]. Basically there are three approaches on which methods have been developed for NTBI estimation.
Indirect estimation of NTBI with anti-tumor antibiotic bleomycin.
Chelation of NTBI with chelator followed by its separation and estimation using various analytical techniques.
Direct estimation of NTBI with iron sensitive fluorescent probe.
Indirect Estimation of NTBI with Anti-tumor Antibiotic Bleomycin
Bleomycin based method is the oldest one, which was firstly suggested by Gutteridge et al. [52]. Assay was based on ability of bleomycin to degrade DNA in the presence of ferrous iron (Fe2+), which can generate extremely reactive oxygen radicals due to its redox activity [52–54]. Iron only in ferrous form can catalyze such reaction and to facilitate the reaction all the ferric ions were converted to ferrous form by using reducing agent like ascorbate which further advances the reaction and degrades the DNA present in the reaction mixture. Such degraded DNA products were measured in the form of malonaldehyde (MDA) molecules which react with thiobarbituric acid and generate colored compounds which were read at 532 nm.
Bleomycin is the key ingredient of the assay and so the value of NTBI determined by this technique is frequently addressed as BDI [10]. The method is found to be extremely vulnerable to various factors; the important ones are the pH of the medium and the source of bleomycin [14]. To maintain specific pH usage of buffer is imperative, however much care should be taken as it can introduce artifactual iron to the reaction mixture and generate misleading results. Another important aspect is that the ascorbate which is used as reducing agent may not convert all the ferric irons to ferrous iron and can lead to false low results. The method also gets influenced by hemoglobin and applicable only when Tf saturation is >80 % [10, 55]. The procedure includes heating step for production of end point colored complex [(thiobarbituric acid) 2-malondialdehyde] which add some more negative views in the methodology [56]. Another drawback of this method is its indirect way of detection. As the degraded DNA products are measured in the form of MDA and not NTBI, any other factors which can either degrade DNA or form MDA contribute to the results. Conclusively this method is lengthy as well as tedious. Furthermore suggested chelax treatment of all the chemicals to minimize iron artifact leads to increased cost factor and procedural complexity.
Various modifications have been suggested by research workers time to time to improve this method. One of the significant alterations has been suggested by von Bonsdorff et al. [10] who made the use of the microtiter plate for sample processing which significantly decreased the demand of sample volume and made the test possible to be operated in mass analysis. Burkitt suggested using ethidium to bind or determine degraded DNA products which are later on determined with fluorescent based technique [56]. This modification is said to decrease the non specific reactions due to non specific MDA produced while heating the reaction mixture. BDI has been detected in various biological fluids like serum from patients of idiopathic hemochromatosis, thalassemia major, hematological malignancies after chemotherapy, patients with end stage renal disease, in overt diabetes and in acute coronary syndrome [10, 28, 53, 57, 58]. It is also detected in plasma of immature as well as full term infants, in synovial fluid from the rheumatoid patients and in CSF of normal subjects [52, 53, 59, 60].
Chelation of NTBI with Chelator Followed by its Separation and Estimation Using Various Analytical Techniques
This approach includes two main steps; in the first step iron is chelated with the help of chelator which is separated from the biological fluid. In the second step chelated iron is subjected to various techniques which estimate it. Different workers used various chelators like ethylenediaminetetraacetic acid (EDTA), NTA and oxalate etc. [20, 61, 62]. Hershko et al. [2] were first to utilize this approach with EDTA. Subsequently NTA found to be superior to EDTA as it exhibits minimum iron mobilization from Tf [62]. In addition to the type of chelator used, its concentration is also found to play a critical role. It has been observed that with the increase in the concentration of NTA there is also a parallel increase in the concentration of NTBI estimated [63]. In the method described by Gosriwatana et al. [19, 62, 64, 65] 80 mM NTA was suggested as compared to the other methods where lower concentrations of NTA was used. However Kolb et al. [63] suggested that 4 mM NTA is the best suitable concentration for chelation of NTBI. This chelated fraction of iron is separated from plasma protein with ultra filter of cut off of 30,000 [64]. This step is operated to minimize the donation of chelated iron to the free sites of traditional iron binding protein like apotransferrin (aTf). The separated fraction is then subjected to diverse analytical methods like high-pressure liquid chromatography (HPLC), colorimetry, atomic absorption, inductively coupled plasma (ICP) and spectroscopy [19, 26, 62, 65]. All such techniques have their own advantages and disadvantages. HPLC has many advantages especially with patients treated with desferrioxamine, however need of specialized HPLC instrument which is free of stain less steel may restrict its wide spread applicability [19, 66]. Recently Sasaki et al. suggested that an extra step to reduce iron contamination mainly in NTA and tris carbonatocobaltate may minimize the background colour and improve the sensitivity of the test when HPLC is used as detection system [67]. Paffetti et al. [3]have used the HPLC based technique for various biological fluids like plasma, amniotic fluid, bronchoalveolar lavage (BAL) and in brain tissue. Zang et al. [65] suggested the use of bathophenanthrolinedisulfonate (BPT/BPS) as a chromogen which gives coloration with iron. The similar method with some modification have been experimented and successfully compared with electro thermal atomic absorption spectroscopy [68]. Colorimetric methods appear to be attractive with minimum specialized instrumental need without compromising the reliability. However the donation of NTBI from iron-NTA complex to one of the most commonly used chromogen BPS is time consuming and increase the processing time of the assay. An interesting approach had been explored by Nilsson et al. [5] who suggested the direct application of BPS in absence of other chelators where it acts as chelator as well as chromogen. In this assay the loosely bound iron gets chelated with BPS and produce coloration. The problem of background color and fluctuation in absorbance had been diminished with filtering the reaction mixture before spectrophotometric measurement. This approach should be further studied for its elaborated application as the assay is simple, quick and requires least specialized instruments and accessories.
The major problem with the second approach is to select an appropriate type and concentration of the chelator which wipe off all the loosely and non specifically bound iron without influencing the iron bound to Tf, as a little portion of Tf bound iron acts as a big dose of NTBI and leads to its overestimation. Another drawback is due to the opposite flow of iron i.e. donation of chelated iron to free sites of Tf in place of getting entered in analytical assay which can lead to underestimation of NTBI, mainly encountered when Tf saturation is normal. Gosriwatana et al. [19] suggested the use of sodium tris carbo cobaltate to block the free iron binding sites on Tf for better reliability. This approach is extensively practiced presently but definitely confined by their labor intensive feature and higher sample volume demand.
Direct Estimation of NTBI with Iron Sensitive Fluorescent Probe
This technique determines NTBI by measuring quenching or dequenching of fluorescence. It may or may not include the mobilizer or chelator but usually does not require the separation of chelated iron fraction from the other portion of biological fluid. This approach is quite impressive in terms of least technical efforts but demand the fluorimeter as detection system. Fluorescent based technique for NTBI estimation was introduced by Breuer el al in multiwell plate format [4]. The method is one step, simple to operate, sensitive and available for 96 well plate format make it suitable for large scale analysis. The assay was based on fluorescent labeled apo transferrin (Fl-aTf) which undergoes for fluorescent quenching when it binds to iron. The assay also included 10 mM oxalate as mobilizer and 0.1 mM gallium(III) as blocking agent. After the use of Fl-aTf, the use of fluorescent labeled desferrioxamine (Fl-DFO) was successfully experimented by the same researcher to determine the “DFO chelatable iron” or DCI without the assistance of mobilizer [11]. In this method fluorescent quenching of Fl-DFO is measured after its binding with iron. The assay can significantly applicable to check the efficacy of the chelator DFO. MDCI was then analyzed with the help of mobilizer and Fl-aTf in which mobilizer like oxalate was used to wipe up all loosely bound iron, which were then analyzed by fluorescent quenching of Fl-aTF [12]. To estimate redox active fraction of NTBI, fluorogenic dihydrorhodamine (DHR) was used [12]. The targeted NTBI forms were termed as “labile plasma iron”, which generate various oxidants in the presence of a bioreductant ascorbate but the reaction gets blocked by iron chelators. The signals were determined by increase in fluorescence due to interaction of DHR with LPI. The inherited drawback of almost all fluorescent based probes is their sensitivity to environmental conditions such as serum color and turbidity, which has regularly been overcome by parallel examination of serum as control without the key ingredient. The difference in the intensity of the signal is the true indicator of the NTBI [11]. Another way is to include second fluorescent probe which is insensitive to iron. Sharma et al. [44, 69] suggested a fluorescent based method which has one more step for separation of chelated iron from the biological fluid, along with the different fluorescent probe and chelator like siderophore plus azotobactin. In the assay use of tri hydrate cobalt solution was suggested as blocking agent of aTf in place of gallium and NTA as NTBI mobilizer in place of oxalate. Quenching of azotobactins’s fluorescence is measured, which supposed to be due to NTBI. In this assay blocking agent and the mobilizer should be selected with utmost caution which should neither interfere with the attachment of Fe3+ with azotobactin nor influence on Fluorescent quenching [44]. A good number of methods experimented by various researchers, nevertheless their comparability is questioned due to lack of reliable inter laboratory analysis. One such good attempt made by Jacobs et al. [64] to measure NTBI value with different methodologies showed unacceptable difference of NTBI value in between the methods with high CV values. The high CV could be due to the measurement of overlapping sub-fractions of NTBI which are not actually identical.
Pathogenesis
Serum iron if it is not guarded by the Tf, the unbound iron (NTBI) poses a great challenge to the protective mechanism of the body. The detrimental of free iron is due to its capability to increase the load of oxidative stress. Free ferrous iron (Fe2+) may catalyses a variety of free radical oxidative reactions which in turn lead to various degenerative changes [70–72].
Fenton reaction and Haber–Weiss reactions show iron mediated generation of ROS which are as follows:
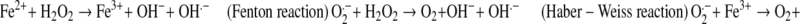 |
The oxygen metabolites including free radical species, superoxide, hydroxyl radicals and other metabolites like hydrogen peroxide and hypohalous acid are often been collectively called as reactive oxygen metabolites (ROM), or ROS or simply as oxidants. Traditional metal driven generation of oxygen derived free radicals are known to induce oxidation of proteins, lipids, lipoproteins, nucleoproteins, nucleic acids, carbohydrates and other cellular components [73]. At this point it is essential to note that all the forms of NTBI may not exhibit the aforesaid activity. So it is most important to find out the specific isoform or isoforms which do such damage.
During the oxidative stress, ROS can also release the iron from stable form like ferritin and heme which worsen the condition and continue the ongoing detrimental cascade [70, 71, 74]. NTBI is also suggested to play supporting role in growth of certain bacteria and fungi. Thus along with the increased load of reactive oxidants it makes host more susceptible against various infections [75]. Cellular uptake of NTBI is not meticulously regulated like Tf bound iron and hence could easily give rise to cellular iron overload [76].
The biological importance of formation of OH∙ through fenton chemistry has not been given much importance due to compensation of O2∙− by NO∙and formation of ONOO− instead of OH. [77]. However OH− is still commonly accepted as the main source of oxidative damage to the cell [78–80].
Role of Iron in Cardiac Disease
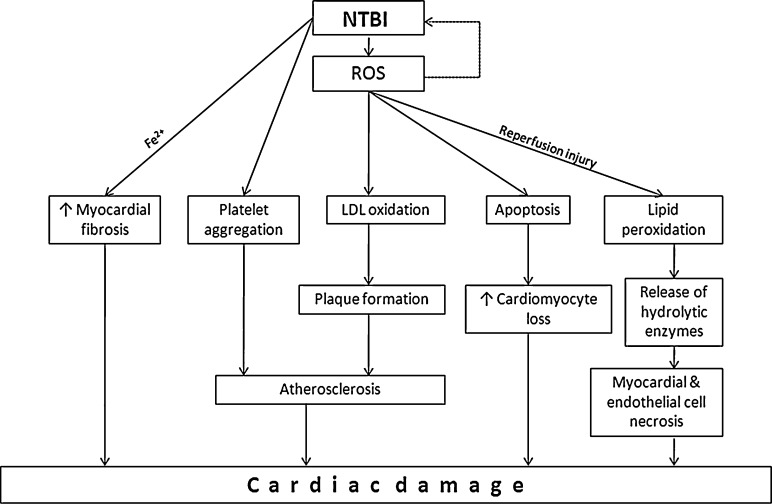
Numerous studied have reported the role of iron in cardiac diseases. The probable role of iron with cardio vascular disease (CVD) was postulated by Sullivan in 1981 which was supported by high incidences of cardiac disease in iron overload patients [81, 82]. Several studies have successfully demonstrated the correlation between the iron intake, body iron stores and cardiovascular risk in the general population [83–86]. Several pathways have been indicated to explain iron mediated heart diseases which are mainly by tissue cell loss and atherosclerosis through ROS production as shown in Fig. 1. Atherosclerosis is one of the major causes of coronary heart disease. One of the most prevalent hypotheses for its etiology is inflammation theory which describes atherosclerosis as proliferation of smooth muscle [87]. ROS known to be produced by NTBI are evident at the site of inflammation and contribute to cell damage [88, 89]. Such reactive species are identified to cause LDL oxidation which induces plaque formation [71, 90, 91]. Pratico et al. [92] demonstrated that Fe2+ could induce platelet aggregation in dose dependent manner. Iron induced oxidative damage has also been linked to increased cardiomyocyte loss due to apoptosis [93]. Altered cellular metabolism and/or iron mediated stimulation of cardiac fibroblast that may contribute to increased myocardial fibrosis. Iron is also suggested culprit for reperfusion injury through its ROS production which causes lipid peroxidation, fragility of intracellular lysozyme and release of hydrolytic enzymes and ultimately causing myocardial and endothelial cell necrosis as shown in Fig. 1 [8, 74, 94]. Experimental cell culture studies had shown that addition of NTBI to human endothelial cell culture increase the surface expression of adhesion molecules and also increase the monocyte adherence to endothelium [95, 96]. These consequences can be corrected by addition of iron chelators like desferrioxamine and dipirydyl, which decrease expression of adhesion molecules and monocyte adherence [95, 96].
Fig. 1.
NTBI induced cardiac damage through various routs
However several other studies refuse the probable correlation of iron with cardiovascular risk, which could be due to selection of improper indicators or inappropriate techniques to estimate the respective indicator [98–103].
NTBI in Diabetes Mellitus
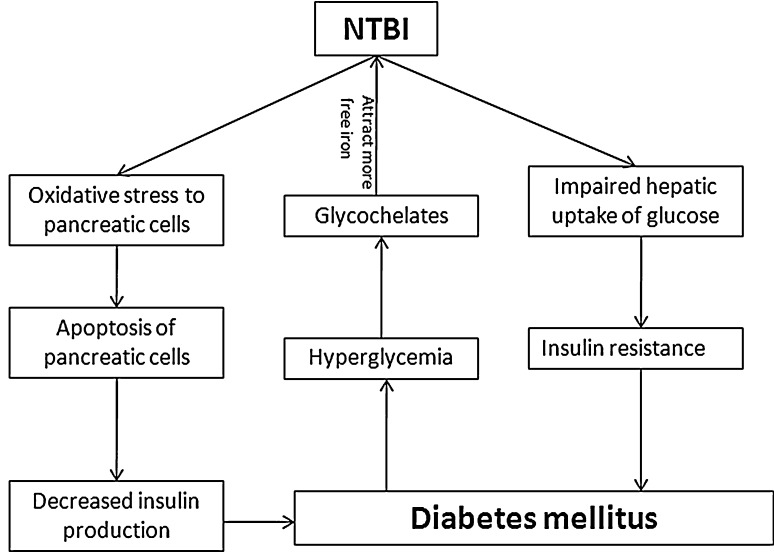
The evidence that iron overload lead to impaired glucose metabolism firstly derived from the fact that incidences of diabetes are high in hereditary hemochromatosis (HH). The exact mechanism how iron is involved in pathogenesis of diabetes mellitus is unclear but it is suggested through added oxidative stress to pancreatic cells. Higher expression of divalent metal iron transporters (DMT) additionally facilitates the entry and accumulation of iron in pancreatic cells as compared to the other cells [104]. In iron overloaded thalassemic patients insulin resistance is significantly increased which indicate the possible influence of iron on hepatic iron uptake [105–107]. In animal models it has been shown that oxidative stress causes the apoptosis of pancreatic islet cells. This obviously decreases the level of insulin production [108]. The role of iron in diabetes is depicted in Fig. 2. Along with the causes of diabetes i.e. impaired insulin production and/or its action, iron is also suggested to play vital role in development of diabetic complications [81]. This could be because of higher levels of free radicals which decrease antioxidant defense and damage the cell organelles, enzymes and increase lipid peroxidation. Iron chelator like desferrioxamine had been successfully experimented to decrease the level of A1c in non insulin dependent diabetes mellitus patients as well as in rats, which supports the link of iron in glycemic control [109]. Presence of NTBI is noticed by Lee et al. [84] in patients with type 2 diabetes mellitus. In uncontrolled diabetic patients, due to persistent high levels of reducing sugar, they get chance to react with the free amino group of proteins by non-enzymatic reactions and generate sugar bound proteins collectively known as advance glycation end products (AGE) [107]. These glycochelates are known to bind with higher amount of transitional metals like iron and copper and extract more iron from stable iron compound to increase free iron load [23]. Involvement of more iron molecules without their traditional or classical proteins may exhibit their redox activity and play vital role in development of long term complications.
Fig. 2.
Role of iron in diabetes mellitus
Iron in Renal Disease
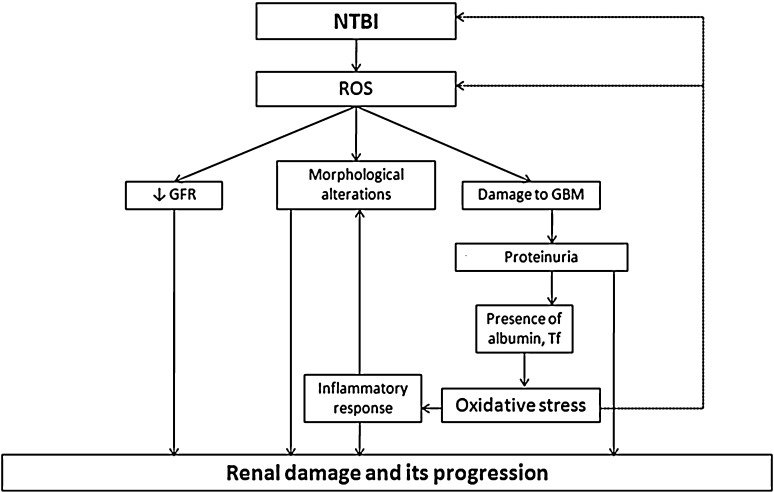
In human studies elevated iron is noted in kidney with higher urinary catalytic iron in patients with chronic kidney disease [110]. ROS produced due to NTBI are suggested as major cause of chronic kidney disease, mainly by 3 ways i.e. by damaging glomerular basement membrane (GBM), by decreasing glomerular filtration rate (GFR) and contributing in morphological changes in kidney [58]. Damage to GBM is a known cause of proteinuria, which is an early sign of renal disease. Oxidants are suggested to damage GBM by increasing the susceptibility to proteolytic damage by inactivating α1-proteinase inhibitor, which allow the activity of elastase to damage extracellular matrix. Oxidants also impair the synthesis of glomerular heparin sulfate, a proteoglycan needed to maintain the integrity of glomerular membrane. Oxidants reduce the glomerular and mesangial cell planer surface and increase myosin light chain phosphorylation. These effects could modulate the surface area of mesangial cells and modify ultrafiltration coefficient and decrease GFR. Furthermore oxidants increase the synthesis of prostaglandins and thromboxane which are suggested to cause proteinuria and/or decreased GFR. Oxidants are also suggested to participate in necrotizing crescentic glomerulonephritis which is characterized by necrosis of GBM causing marked infiltration of neutrophils and mononuclear cells [58]. In progressive kidney disease oxidants are well recognized to damage the kidney by inducing proteinuria which facilitates the protein traffic in glomerulus and kidney tubules. These proteins e.g. albumin, Tf etc. are experimentally proven to cause further oxidative damage, induce the activity of NF-κβ leading inflammatory response. On the other side protein mediated oxidative stress continues the cascade and leads to end stage renal disease [111]. The roles of NTBI and ROS have been depicted in Fig. 3. Furthermore the role of NTBI is evident by the fact that advanced oxidized protein products AOPP which are generated by the ROS due to NTBI worsen where as metal chelators improves the conditions in progressive kidney disease [112, 113].
Fig. 3.
NTBI induced renal damage and its progression
NTBI and Erythroid Cells
By causing oxidative stress NTBI are known to affect the erythroid series too. Studies suggest that the mature RBC, reticulocytes, and developing erythroid precursors take up iron through Tf independent pathway [114]. This pathway has been operated in iron overloaded patients in presence of NTBI. Such fraction instead of getting incorporated in heme synthesis induces ROS generation causing cytotoxicity in mature RBC causing intravascular hemolysis.
Increased LIP in RBC may be the result of leftover of unused iron precursors due to increased uptake from iron overloaded plasma, diminished utilization due to reduced hemoglobin production or degradation of unstable hemoglobin. In addition, RBCs may take up iron from their environment especially when the amount of iron in plasma exceeds the Tf binding capacity. Since mature RBCs are devoid of TFR this uptake is necessary of non Tf iron [115]. NTBI is also found in patients of hematologic malignancies undergoing high-dose chemotherapy, particularly those treated with myeloablative therapy and stem cell transplantation [24, 27, 55].
The existence of free iron has been traced in a number of pathological conditions by various researchers exploring different technique but sparse data are available which can correlate different NTBI isoforms their respective clinical existence and the method for its estimation. So at present time it is noteworthy to register the identity of the NTBI sub-fraction recognized by the assorted techniques, which may or may not correlate with one another. Another important aspect is to recognize the key subfraction of NTBI which has clinical diagnostic relevance. If both of these points are taken for consideration, it will help the clinician to look for the significant isoform of NTBI in specialized clinical conditions and the laboratory scientist to select a suitable method to estimate the clinically relevant sub-fraction.
References
- 1.Hider RC. Nature of nontransferrin-bound iron. Eur J Clin Invest. 2002;32:50–54. doi: 10.1046/j.1365-2362.2002.0320s1050.x. [DOI] [PubMed] [Google Scholar]
- 2.Hershko H, Graham G, Bates GW, Rachmilewitz E. Nonspecific serum iron in thalassaemia: an abnormal serum iron fraction of potential toxicity. Br J Haematol. 1978;40:255–263. doi: 10.1111/j.1365-2141.1978.tb03662.x. [DOI] [PubMed] [Google Scholar]
- 3.Paffetti P, Perrone S, Longini M, Ferrari A, Tanganelli D, Marzocchi B, et al. Non-protein-bound iron detection in small samples of biological fluids and tissues. Biol Trace Elem Res. 2006;112:221–232. doi: 10.1385/BTER:112:3:221. [DOI] [PubMed] [Google Scholar]
- 4.Breuer W, Cabantchik ZI. A fluorescence-based one-step assay for serum non-transferrin-bound iron. Anal Biochem. 2001;299:194–202. doi: 10.1006/abio.2001.5378. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson UA, Bassen M, Sävman K, Kjellmer I. A simple and rapid method for the determination of “free” iron in biological fluids. Free Radic Res. 2002;36:677–684. doi: 10.1080/10715760290029128. [DOI] [PubMed] [Google Scholar]
- 6.Breuer W, Hershko C, Cabantchik ZI. The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus Sci. 2000;23:185–192. doi: 10.1016/s0955-3886(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 7.Kruszewski M. Labile iron pool: the main determinant of cellular response to oxidative stress. Mutat Res. 2003;531:81–92. doi: 10.1016/j.mrfmmm.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 9.Kakhlon O, Cabantchik ZI. The labile iron pool: characterization, measurement, and participation in cellular processes. Free Rad Biol Med. 2002;33:1037–1046. doi: 10.1016/s0891-5849(02)01006-7. [DOI] [PubMed] [Google Scholar]
- 10.Bonsdorff L, Lindeberg E, Sahlstedt L, Lehto J, Parkkinen J. Bleomycin-detectable iron assay for non-transferrin-bound iron in hematologic malignancies. Clin Chem. 2002;48:307–314. [PubMed] [Google Scholar]
- 11.Breuer W, Ermers MJ, Pootrakul P, Abramov A, Hershko C, Cabantchik ZI. Desferrioxamine-chelatable iron, a component of serum non-transferrin-bound iron used for assessing chelation therapy. Blood. 2001;97:792–798. doi: 10.1182/blood.v97.3.792. [DOI] [PubMed] [Google Scholar]
- 12.Esposito BP, Breuer W, Sirankapracha P, Pootrakul P, Hershko C, Cabantchik ZI. Labile plasma iron in iron overload: redox activity and susceptibility to chelation. Blood. 2003;102(7):2670–2677. doi: 10.1182/blood-2003-03-0807. [DOI] [PubMed] [Google Scholar]
- 13.Anderson GJ. Non-transferrin-bound iron and cellular toxicity. J Gastroenterol Hepatol. 1999;14(2):105–108. doi: 10.1046/j.1440-1746.1999.01828.x. [DOI] [PubMed] [Google Scholar]
- 14.Graham G, Bates GW, Rachmilewitz EA, Hershko C. Non-specific serum iron in thalassaemia: quantitation and chemical reactivity. Am J Hematol. 1979;6:207–217. doi: 10.1002/ajh.2830060305. [DOI] [PubMed] [Google Scholar]
- 15.Porter JB, Abeysinghe RD, Marshall L, Hider RC, Singh S. Kinetics of removal and reappearance of nontransferrin-bound plasma iron with deferoxamine therapy. Blood. 1996;88:705–713. [PubMed] [Google Scholar]
- 16.Batey RG, Lai Chung Fong P, Shamir S, Sherlock S. A non-transferrin-bound serum iron in idiopathic hemochromatosis. Dig Dis Sci. 1980;25:340–346. doi: 10.1007/BF01308057. [DOI] [PubMed] [Google Scholar]
- 17.Aruoma OI, Bomford A, Polson RJ, Halliwell B. Nontransferrin-bound iron in plasma from hemochromatosis patients: effect of phlebotomy therapy. Blood. 1988;72:1416–1419. [PubMed] [Google Scholar]
- 18.Loreal O, Gosriwatana I, Guyader D, Porter J, Brissot P, Hider RC. Determination of non-transferrin-bound iron in genetic hemochromatosis using a new HPLC-based method. J Hepatol. 2000;32:727–733. doi: 10.1016/s0168-8278(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 19.Gosriwatana I, Loréal O, Lu S, Brissot P, Porter J, Hider RC. Quantification of non-transferrin-bound iron in the presence of unsaturated transferrin. Anal Biochem. 1999;273:212–220. doi: 10.1006/abio.1999.4216. [DOI] [PubMed] [Google Scholar]
- 20.Breuer W, Ronson A, Slotki IN, Abramov A, Hershko C, Cabantchik ZI. The assessment of serum nontransferrin-bound iron in chelation therapy and iron supplementation. Blood. 2000;95:2975–2982. [PubMed] [Google Scholar]
- 21.al-Refaie FN, Wickens DG, Wonke B, Kontoghiorghes GJ, Hoffbrand AV. Serum non-transferrin-bound iron in beta-thalassaemia major patients treated with desferrioxamine and L1. Br J Haematol. 1992;82:431–436. doi: 10.1111/j.1365-2141.1992.tb06441.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee DH, Liu DY, Jacob DR, Hai-Rim Shin JR, Song K, Lee I, et al. Common presence of non-transferrin-bound iron among patients with type 2 diabetes. Diabetes Care. 2006;29:1090–1095. doi: 10.2337/diacare.2951090. [DOI] [PubMed] [Google Scholar]
- 23.Qian M, Liu M, Eaton JW. Transition metals bind to glycated proteins forming redox active “glycochelates”: implications for the pathogenesis of certain diabetic complications. Biochem Biophys Res Commun. 1998;250:385–389. doi: 10.1006/bbrc.1998.9326. [DOI] [PubMed] [Google Scholar]
- 24.Halliwell B, Aruoma OI, Mufti G, Bomford A. Bleomycin-detectable iron in serum from leukaemic patients before and after chemtherapy. Therapeutic implications for treatment with oxidant-generating drugs. FEBS Lett. 1988;241:202–204. doi: 10.1016/0014-5793(88)81061-5. [DOI] [PubMed] [Google Scholar]
- 25.Carmine TC, Evans P, Bruchelt G, Evans R, Handretinger R, Niethammer D, et al. Presence of iron catalytic for free radical reactions in patients undergoing chemtherapy: implications for therapeutic management. Cancer Lett. 1995;94:219–226. doi: 10.1016/0304-3835(95)03852-n. [DOI] [PubMed] [Google Scholar]
- 26.Dürken M, Nielsen P, Knobel S, Finckh B, Herrnring C, Dresow B, et al. Non-transferrin-bound iron in serum of patients receiving bone marrow transplants. Free Rad Biol Med. 1997;22:1159–1163. doi: 10.1016/s0891-5849(96)00497-2. [DOI] [PubMed] [Google Scholar]
- 27.Bradley SJ, Gosriwatana I, Srichairatanakool S, Hider RC, Porter JB. Non-transferrin-bound iron induced by myeloablative chemtherapy. Br J Haematol. 1997;99:337–343. doi: 10.1046/j.1365-2141.1997.4143221.x. [DOI] [PubMed] [Google Scholar]
- 28.Lele S, Shah S, McCullough PA, Rajapurkar M. Serum catalytic iron as a novel biomarker of vascular injury in acute coronary syndromes. EuroIntervention. 2009;5:1–7. doi: 10.4244/v5i3a53. [DOI] [PubMed] [Google Scholar]
- 29.Harrison-Findik DD, Klein E, Crist C, Evans J, Timchenko N, Gollan J. Iron-mediated regulation of liver hepcidin expression in rats and mice is abolished by alcohol. Hepatology. 2007;46:1979–1985. doi: 10.1002/hep.21895. [DOI] [PubMed] [Google Scholar]
- 30.Detivaud L, Nemeth E, Boudjema K, Turlin B, Troadec MB, Leroyer P, et al. Hepcidin levels in humans are correlated with hepatic iron stores, hemoglobin levels, and hepatic function. Blood. 2005;106:746–748. doi: 10.1182/blood-2004-12-4855. [DOI] [PubMed] [Google Scholar]
- 31.Feo TM, Fargion S, Duca L, Cesana BM, Boncinelli L, Lozza P, et al. Non-transferrin-bound iron in alcohol abusers. Alcohol Clin Exp Res. 2001;25:1494–1499. doi: 10.1097/00000374-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Cortelezzi A, Cattaneo C, Cristiani S, Duca L, Sarina B, Deliliers GL, et al. Non-transferrin-bound iron in myelodysplastic syndromes: A marker of ineffective erythropoiesis? Hematol J. 2000;1:153–158. doi: 10.1038/sj.thj.6200028. [DOI] [PubMed] [Google Scholar]
- 33.Mahesh S, Ginzburg Y, Verma A. Iron overload in myelodysplastic syndromes. Leuk Lymphoma. 2008;49:427–438. doi: 10.1080/10428190701843221. [DOI] [PubMed] [Google Scholar]
- 34.Brazzolotto X, Gaillard J, Pantopoulos K, Hentze MW, Moulis JM. Human cytoplasmic aconitase (iron regulatory protein 1) is converted into its [3Fe–4S] form by hydrogen peroxide in vitro but is not activated for iron-responsive element binding. J Biol Chem. 1999;274:21625–21630. doi: 10.1074/jbc.274.31.21625. [DOI] [PubMed] [Google Scholar]
- 35.Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev. 1994;52:253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 36.Halliwell B. The role of oxygen radicals in human disease, with particular reference to the vascular system. Haemostasis. 1993;23(suppl 1):118–126. doi: 10.1159/000216921. [DOI] [PubMed] [Google Scholar]
- 37.Biemond P, Eijk HG, Swaak AJG, Koster JF. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes: possible mechanism in inflammatory diseases. J Clin Invest. 1984;73:1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abdalla DS, Campa A, Monteiro HP. Low density lipoprotein oxidation by stimulated neutrophils and ferritin. Atherosclerosis. 1992;97:149–159. doi: 10.1016/0021-9150(92)90128-4. [DOI] [PubMed] [Google Scholar]
- 39.Gutteridge JMC. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986;201:291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- 40.Puppo A, Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron: Is haemoglobin a biological Fenton catalyst? Biochem J. 1988;249:185–190. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutteridge JMC, Smith A. Antioxidant protection by haemopexin of haemstimulated lipid peroxidation. Biochem J. 1988;256:861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balla G, Jacob HS, Eaton JW, Belcher JD, Vercellotti GM. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb. 1991;11:1700–1711. doi: 10.1161/01.atv.11.6.1700. [DOI] [PubMed] [Google Scholar]
- 43.Lan C, Loréal O, Cohen T, Ropert M, Glickstein H, Lainé F, et al. Redox active plasma iron in C282Y/C282Y hemochromatosis. Blood. 2005;105:4527–4531. doi: 10.1182/blood-2004-09-3468. [DOI] [PubMed] [Google Scholar]
- 44.Sharma M, Saxena R, Gohil NK. Fluorescence assay of non-transferrin-bound iron in thalassemic sera using bacterial siderophore. Anal Biochem. 2009;394:186–191. doi: 10.1016/j.ab.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 45.Weijl NI, Elsendoorn TJ, Moison RM, Lentjes EG, Brand R, Berger HM, et al. Non-protein bound iron release during chemotherapy in cancer patients. Clin Sci (Lond) 2004;106:475–484. doi: 10.1042/CS20030271. [DOI] [PubMed] [Google Scholar]
- 46.Grootveld M, Bell JD, Halliwell B, Aruoma OI, Bomford A, Sadler PJ. Non-transferrin bound iron in plasma or serum from patients with idiopathic hemochromatosis. J Biol Chem. 1989;264:4417–4422. [PubMed] [Google Scholar]
- 47.Lovstad RA. Interaction of serum albumin with the Fe(III)-citrate complex. Int J Biochem. 1993;25:1015–1017. doi: 10.1016/0020-711x(93)90115-u. [DOI] [PubMed] [Google Scholar]
- 48.May PM, Williams DR. Computer simulation of chelation therapy. Plasma mobilizing index as a replacement for effective stability constant. FEBS Lett. 1977;78:134–138. doi: 10.1016/0014-5793(77)80290-1. [DOI] [PubMed] [Google Scholar]
- 49.Heul C, Eijk HG, Wiltink WF, Leijnse B. The binding of iron to transferrin and to other serum components at different degrees of saturation with iron. Clin Chim Acta. 1972;38:347–353. doi: 10.1016/0009-8981(72)90125-8. [DOI] [PubMed] [Google Scholar]
- 50.Pootrakul P, Sirankapracha P, Sankote J, Kachintorn U, Maungsub W, Sriphen K, et al. Clinical trial of deferiprone iron chelation therapy in beta-thalassaemia/haemoglobin E patients in Thailand. Br J Haematol. 2003;122:305–310. doi: 10.1046/j.1365-2141.2003.04412.x. [DOI] [PubMed] [Google Scholar]
- 51.Pootrakul P, Breuer W, Sametband M, Sirankapracha P, Hershko C, Cabantchik ZI. Labile plasma iron (LPI) as an indicator of chelatable plasma redox activity in iron-overloaded b-thalassemia/HbE patients treated with an oral chelator. Blood. 2004;104:1504–1510. doi: 10.1182/blood-2004-02-0630. [DOI] [PubMed] [Google Scholar]
- 52.Gutteridge JMC, Rowley DA, Halliwell B. Superoxide-dependent formation of hydroxyl radicals I in the presence of iron salts. Biochem J. 1981;199:263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gutteridge JMC, Halliwell B. Radical promoting loosely-bound iron in biological fluids and the bleomycin assay. Life Chem Rep. 1987;4:113–142. [Google Scholar]
- 54.Evans PJ, Halliwell B. Measurement of iron and copper in biological systems: bleomycin and copper-phenanthroline assays. Methods Enzymol. 1994;233:82–92. doi: 10.1016/s0076-6879(94)33010-7. [DOI] [PubMed] [Google Scholar]
- 55.Sahlstedt L, Ebeling F, Bonsdorff L, Parkkinen J, Ruutu T. Non-transferrin-bound iron during allogeneic stem cell transplantation. Br J Haematol. 2001;113:836–838. doi: 10.1046/j.1365-2141.2001.02820.x. [DOI] [PubMed] [Google Scholar]
- 56.Burkitt MJ, Milne L, Raafat A. A simple, highly sensitive and improved method for the measurement of bleomycin-detectable iron: the ‘catalytic iron index’ and its value in the assessment of iron status in haemochromatosis. Clin Sci. 2001;100:239–247. [PubMed] [Google Scholar]
- 57.Han KE, Okada S. Serum bleomycin detectable iron in patients with thalassemia major with normal range of serum iron. Acta Med Okayama. 1995;49:117–121. doi: 10.18926/AMO/30410. [DOI] [PubMed] [Google Scholar]
- 58.Shah SV. Oxidants and iron in chronic kidney disease. Kidney Int Suppl. 2004;91:50–55. doi: 10.1111/j.1523-1755.2004.09108.x. [DOI] [PubMed] [Google Scholar]
- 59.Evans PJ, Evans R, Kovar IZ, Holton AF, Halliwell B. Bleomycin-detectable iron in the plasma of premature and full-term neonates. FEBS Lett. 1992;303:210–212. doi: 10.1016/0014-5793(92)80521-h. [DOI] [PubMed] [Google Scholar]
- 60.Gutteridge JMC. Ferrous ions detected in cerebrospinal fluid by using bleomycin and DNA damage. Clin Sci. 1992;82:315–320. doi: 10.1042/cs0820315. [DOI] [PubMed] [Google Scholar]
- 61.Esposito BP, Breuer W, Slotki I, Cabantchik ZI. Labile iron in parenteral iron formulations and its potential for generating plasma nontransferrin-bound iron in dialysis patients. Eur J Clin Invest. 2002;32:42–49. doi: 10.1046/j.1365-2362.2002.0320s1042.x. [DOI] [PubMed] [Google Scholar]
- 62.Singh S, Hider RC, Porter JB. A direct method for quantification of non-transferrin-bound iron. Anal Biochem. 1990;186:320–323. doi: 10.1016/0003-2697(90)90088-q. [DOI] [PubMed] [Google Scholar]
- 63.Kolb AM, Smit NP, Lentz-Ljuboje R, Osanto S, Pelt J. Non-transferrin bound iron measurement is influenced by chelator concentration. Anal Biochem. 2009;385:13–19. doi: 10.1016/j.ab.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Jacobs EM, Hendriks JC, Tits BL, Evans PJ, Breuer W, Liu DY, et al. Results of an international round robin for the quantification of serum non-transferrin-bound iron: need for defining standardization and a clinically relevant isoform. Anal Biochem. 2005;341:241–250. doi: 10.1016/j.ab.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Zhang D, Okada S, Kawabata T, Yasuda T. An improved simple colorimetric method for quantification of non-transferrin-bound iron in serum. Biochem Mol Biol Int. 1995;35:635–641. [PubMed] [Google Scholar]
- 66.Collins KE, Collins CH, Bertran CA, Dolan J. Stainless steel surfaces in LC systems, Part 1-corrosion and erosion. LC GC Int. 2000;13:464–470. [Google Scholar]
- 67.Sasaki K, Ikuta K, Tanaka H, Ohtake T, Torimoto Y, Fujiya M, et al. Improved quantification for non-transferrin-bound iron measurement using high-performance liquid chromatography by reducing iron contamination. Mol Med Rep. 2011;4:913–918. doi: 10.3892/mmr.2011.518. [DOI] [PubMed] [Google Scholar]
- 68.Jittangprasert P, Wilairat P, Pootrakul P. Comparison of colorimetry and electrothermal atomic absorption spectroscopy for the quantification of non-transferrin bound iron in human sera. Southeast Asian J Trop Med Public Health. 2004;35:1039–1044. [PubMed] [Google Scholar]
- 69.Sharma M, Gohil NK. Interaction of azotobactin with blocking and mobilizing agents in NTBI assay. Mol BioSyst. 2010;6:1941–1946. doi: 10.1039/c004840b. [DOI] [PubMed] [Google Scholar]
- 70.Chau L. Iron and atherosclerosis. Proceedings of the national science council, Republic of China—Part B. Life Sci. 2000;24:151–5. [PubMed]
- 71.Meyers DG. The iron hypothesis: Does iron play a role in atherosclerosis? Transfusion. 2000;40:1023–1029. doi: 10.1046/j.1537-2995.2000.40081023.x. [DOI] [PubMed] [Google Scholar]
- 72.Gackowski D, Kruszewski M, Jawien A, Ciecierski M, Olinski R. Further evidence that oxidative stress may be a risk factor responsible for the development of atherosclerosis. Free Radic Biol Med. 2001;31:542–547. doi: 10.1016/s0891-5849(01)00614-1. [DOI] [PubMed] [Google Scholar]
- 73.Mohan S, Kalia K, Mannari J. Diabetic nephropathy and associated risk factors for renal deterioration. Int J diabetes Dev Ctries. 2011;32:52–59. [Google Scholar]
- 74.Horwitz L, Rosenthal E. Iron-mediated cardiovascular injury. Vasc Med. 1999;4:93–99. doi: 10.1177/1358836X9900400207. [DOI] [PubMed] [Google Scholar]
- 75.Pai B, Pai MP, Depczynski J, McQuade CR, Mercier RC. Nontransferrin-bound iron is associated with enhanced Staphylococcus aureus growth in hemodialysis patients receiving intravenous iron sucrose. Am J Nephrol. 2006;26:304–309. doi: 10.1159/000094343. [DOI] [PubMed] [Google Scholar]
- 76.Cabantchik ZI, Breuer W, Zanninelli G, Cianciulli P. LPI-labile plasma iron in iron overload. Best Pract Res Clin Haematol. 2005;18:277–287. doi: 10.1016/j.beha.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Koppenol WH. The Haber–Weiss cycle—70 years later. Redox Rep. 2001;6:229–234. doi: 10.1179/135100001101536373. [DOI] [PubMed] [Google Scholar]
- 78.Fridovich I. Oxygen toxicity: a radical explanation. J Exp Biol. 1998;201:1203–1209. doi: 10.1242/jeb.201.8.1203. [DOI] [PubMed] [Google Scholar]
- 79.Liochev SI, Fridovich I. The relative importance of HO. and ONOO− in mediating the toxicity of O∙. Free Radic Biol Med. 1999;26:777–778. doi: 10.1016/s0891-5849(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 80.Termini J, Hydroperoxide-induced DNA. Damage and mutations. Mutat Res. 2000;450:107–124. doi: 10.1016/s0027-5107(00)00019-1. [DOI] [PubMed] [Google Scholar]
- 81.Sullivan JL. Iron and the sex difference in heart disease risk. Lancet. 1981;1:1293–1294. doi: 10.1016/s0140-6736(81)92463-6. [DOI] [PubMed] [Google Scholar]
- 82.Schafer AI, Cheron RG, Dluhy R, Cooper B, Gleason RE, Soeldner JS, et al. Clinical consequences of acquired transfusional iron overload in adults. N Engl J Med. 1981;304:319–324. doi: 10.1056/NEJM198102053040603. [DOI] [PubMed] [Google Scholar]
- 83.Ascherio A, Willett WC, Rimm EB, Giovannucci EL, Stampfer MJ. Dietary iron intake and risk of coronary disease among men. Circulation. 1994;89:969–974. doi: 10.1161/01.cir.89.3.969. [DOI] [PubMed] [Google Scholar]
- 84.Lee DH, Folsom AR, Jacobs DRJ. Iron, zinc, and alcohol consumption and mortality from cardiovascular diseases: the Iowa Women’s Health Study. Am J Clin Nutr. 2005;81:787–791. doi: 10.1093/ajcn/81.4.787. [DOI] [PubMed] [Google Scholar]
- 85.van der ADL, Peeters PH, Grobbee DE, Marx JJ, van der Schouw YT. Dietary haem iron and coronary heart disease in women. Eur Heart J. 2005;26:257–62. [DOI] [PubMed]
- 86.Ramakrishnan U, Kuklina E, Stein AD. Iron stores and cardiovascular disease risk factors in women of reproductive age in the United States. Am J Clin Nutr. 2002;76:1256–1260. doi: 10.1093/ajcn/76.6.1256. [DOI] [PubMed] [Google Scholar]
- 87.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 88.Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol. 1996;303:217–220. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 89.Cuzzocrea S, Zingarelli B, Costantino G, Szabo A, Salzman AL, Caputi AP, et al. Beneficial effects of 3-aminobenzamide, an inhibitor of poly (ADP-ribose) synthetase in a rat model of splanchnic artery occlusion and reperfusion. Br J Pharmacol. 1997;121:1065–1074. doi: 10.1038/sj.bjp.0701234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leake D, Rankin S. The oxidative modi. Cation of low-density lipoproteins by macrophages. Biochem J. 1990;270:741–748. doi: 10.1042/bj2700741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knight JA. Free radicals, antioxidants, aging and disease. Washington, DC: AACC Press; 1999. [Google Scholar]
- 92.Praticó D, Pasin M, Barry OP, Ghiselli A, Sabatino G, Iuliano L, et al. Iron-dependent human platelet activation and hydroxyl radical formation: involvement of protein kinase C. Circulation. 1999;99:3118–3124. doi: 10.1161/01.cir.99.24.3118. [DOI] [PubMed] [Google Scholar]
- 93.Oudit GY, Trivieri MG, Khaper N, Husain T, Wilson GJ, Liu P, et al. Taurine supplementation reduces oxidative stress and improves cardiovascular function in an iron-overload murine model. Circulation. 2004;109:1877–1885. doi: 10.1161/01.CIR.0000124229.40424.80. [DOI] [PubMed] [Google Scholar]
- 94.Voogd A, Sluiter W, Koster JF. The increased susceptibility to hydrogen peroxide of the (post-)ischemic rat heart is associated with the magnitude of the low molecular weight iron pool. Free Radic Biol Med. 1994;16:453–458. doi: 10.1016/0891-5849(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 95.Kartikasari AE, Georgiou NA, Visseren FL, Kats-Renaud H, Asbeck BS, Marx JJ. Intracellular labile iron modulates adhesion of human monocytes to human endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:2257–2262. doi: 10.1161/01.ATV.0000147406.00871.b3. [DOI] [PubMed] [Google Scholar]
- 96.Koo SW, Casper KA, Otto KB, Gira AK, Swerlick RA. Iron chelators inhibit VCAM-1 expression in human dermal microvascular endothelial cells. J Invest Dermatol. 2003;120:871–879. doi: 10.1046/j.1523-1747.2003.12144.x. [DOI] [PubMed] [Google Scholar]
- 97.Zhang WJ, Frei B. Intracellular metal ion chelators inhibit TNF alpha-induced SP-1 activation and adhesion molecule expression in human aortic endothelial cells. Free Radic Biol Med. 2003;34:674–682. doi: 10.1016/s0891-5849(02)01375-8. [DOI] [PubMed] [Google Scholar]
- 98.Danesh J, Appleby P. Coronary heart disease and iron status: meta-analyses of prospective studies. Circulation. 1999;99:852–854. doi: 10.1161/01.cir.99.7.852. [DOI] [PubMed] [Google Scholar]
- 99.Derstine JL, Murray-Kolb LE, Yu-Poth S, Hargrove RL, Kris-Etherton PM, Beard JL. Iron status in association with cardiovascular disease risk in 3 controlled feeding studies. Am J Clin Nutr. 2003;77:56–62. doi: 10.1093/ajcn/77.1.56. [DOI] [PubMed] [Google Scholar]
- 100.Baer DM, Tekawa IS, Hurley LB. Iron stores are not associated with acute myocardial infarction. Circulation. 1994;89:2915–2918. doi: 10.1161/01.cir.89.6.2915. [DOI] [PubMed] [Google Scholar]
- 101.Corti MC, Guralnik JM, Salive ME, Ferrucci L, Pahor M, Wallace RB, et al. Serum iron level, coronary artery disease, and all-cause mortality in older men and women. Am J Cardiol. 1997;79:120–127. doi: 10.1016/s0002-9149(96)00697-2. [DOI] [PubMed] [Google Scholar]
- 102.Knuiman MW, Divitini ML, Olynyk JK, Cullen DJ, Bartholomew HC. Serum ferritin and cardiovascular disease: a 17-year follow-up study in Busselton Western Australia. Am J Epidemiol. 2003;158:144–149. doi: 10.1093/aje/kwg121. [DOI] [PubMed] [Google Scholar]
- 103.Sempos CT, Looker AC, Gillum RE, Mc-Gee DL, Vuong CV, Johnson CL. Serum ferritin and death from all causes and cardiovascular disease: the NHANES II Mortality study. Ann Epidemiol. 2000;10:441–448. doi: 10.1016/s1047-2797(00)00068-5. [DOI] [PubMed] [Google Scholar]
- 104.Andrews NC. The iron transporter DMT 1. Int J Biochem Cell Biol. 1999;31:991–994. doi: 10.1016/s1357-2725(99)00065-5. [DOI] [PubMed] [Google Scholar]
- 105.Dandona P, Hussain MA, Varghese Z, Politis D, Flynn DM, Hoffbrand AV. Insulin resistance and iron overload. Ann Clin Biochem. 1983;20:77–79. doi: 10.1177/000456328302000203. [DOI] [PubMed] [Google Scholar]
- 106.Mendler MH, Turlin B, Moirand R, Jouanolle AM, Sapey T, Guyader D, et al. Insulin resistance-associated hepatic iron overload. Gastroenterology. 1999;117:1155–1163. doi: 10.1016/s0016-5085(99)70401-4. [DOI] [PubMed] [Google Scholar]
- 107.Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 108.Jackson P, Loughrey CM, Lightbody JH, McNamee PT, Young IS. Effect of hemodialysis on total antioxidant capacity and serum antioxidants in patients with chronic renal failure. Clin Chem. 1995;41:1135–1138. [PubMed] [Google Scholar]
- 109.Redmon JB, Pyzdrowski KL, Robertson RP. No effect of deferoxamine therapy on glucose homeostasis and insulin secretion in individuals with NIDDM and elevated serum ferritin. Diabetes. 1993;42:544–549. doi: 10.2337/diab.42.4.544. [DOI] [PubMed] [Google Scholar]
- 110.Nankivell BJ, Boadle RA, Harris DCH. Iron accumulation in human chronic renal disease. Am J Kidney Dis. 1992;20:580–584. doi: 10.1016/s0272-6386(12)70222-6. [DOI] [PubMed] [Google Scholar]
- 111.Shah SV, Baliga R, Rajapurkar M, Fonseca VA. Oxidants in chronic kidney disease. J Am Soc Nephrol. 2007;18:16–28. doi: 10.1681/ASN.2006050500. [DOI] [PubMed] [Google Scholar]
- 112.Descamps-Latscha B, Witko-Sarsat V, Nguyen-Khoa T, Nguyen AT, Gausson V, Mothu V, et al. Early prediction of IgA nephropathy progression: proteinuria and AOPP are strong prognostic markers. Kidney Int. 2004;66:1606–1612. doi: 10.1111/j.1523-1755.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- 113.Lin JL, LinTan DT, Hsu KH, Yu CC. Environmental lead exposure and progression of chronic renal diseases in patients without diabetes. N Engl J Med. 2003;348:277–286. doi: 10.1056/NEJMoa021672. [DOI] [PubMed] [Google Scholar]
- 114.Prus E, Fibach E. Uptake of non-transferrin iron by erythroid cells. Anemia. 2011;2011 (Article ID 945289, 8 pages). [DOI] [PMC free article] [PubMed]
- 115.Loken MR, Shah VO, Dattilio KL, Civin CI. Flow cytometric analysis of human bone marrow II. Normal B lymphocyte development. Blood. 1987;70:1316–1324. [PubMed] [Google Scholar]